Abstract
Background
Invasive mechanical ventilation is lifesaving in the setting of severe acute respiratory failure but can cause ventilation-induced lung injury. Advances in extracorporeal CO2 removal (ECCO2R) technologies may facilitate more protective lung ventilation in acute respiratory distress syndrome, and enable earlier weaning and/or avoid invasive mechanical ventilation entirely in chronic obstructive pulmonary disease exacerbations. We evaluated the in vitro CO2 removal capacity of the novel PrismaLung+ ECCO2R device compared with two existing gas exchangers.
Methods
The in vitro CO2 removal capacity of the PrismaLung+ (surface area 0.8 m2, Baxter) was compared with the PrismaLung (surface area 0.35 m2, Baxter) and A.L.ONE (surface area 1.35 m2, Eurosets) devices, using a closed-loop bovine blood–perfused extracorporeal circuit. The efficacy of each device was measured at varying pCO2 inlet (pinCO2) levels (45, 60, and 80 mmHg) and blood flow rates (QB) of 200–450 mL/min; the PrismaLung+ and A.L.ONE devices were also tested at a QB of 600 mL/min. The amount of CO2 removed by each device was assessed by measurement of the CO2 infused to maintain circuit equilibrium (CO2 infusion method) and compared with measured CO2 concentrations in the inlet and outlet of the CO2 removal device (blood gas analysis method).
Results
The PrismaLung+ device performed similarly to the A.L.ONE device, with both devices demonstrating CO2 removal rates ~ 50% greater than the PrismaLung device. CO2 removal rates were 73 ± 4.0, 44 ± 2.5, and 72 ± 1.9 mL/min, for PrismaLung+, PrismaLung, and A.L.ONE, respectively, at QB 300 mL/min and pinCO2 45 mmHg. A Bland–Altman plot demonstrated that the CO2 infusion method was comparable to the blood gas analysis method for calculating CO2 removal. The resistance to blood flow across the test device, as measured by pressure drop, varied as a function of blood flow rate, and was greatest for PrismaLung and lowest for the A.L.ONE device.
Conclusions
The newly developed PrismaLung+ performed more effectively than PrismaLung, with performance of CO2 removal comparable to A.L.ONE at the flow rates tested, despite the smaller membrane surface area of PrismaLung+ versus A.L.ONE. Clinical testing of PrismaLung+ is warranted to further characterize its performance.
Similar content being viewed by others
Background
Patients with severe acute hypoxemic and/or hypercapnic respiratory failure require invasive mechanical ventilation (IMV) to facilitate gas exchange and to support breathing. While IMV may be lifesaving in this setting, it is associated with significant short- and long-term side effects. Consequently, there is considerable interest in developing strategies such as extracorporeal CO2 removal (ECCO2R), which can facilitate CO2 removal [1], or extracorporeal membrane oxygenation (ECMO), which, in addition, provides oxygenation in instances of severe hypoxemic respiratory failure [2]. These approaches may enable reductions in the intensity and/or the duration of IMV in these patients.
In patients with severe hypoxemia, particularly those with acute respiratory distress syndrome (ARDS), the loss of alveolar ventilation capacity due to alveolar consolidation, edema and/or collapse contributes to the need for ventilatory support [3]. The discovery that high tidal and minute ventilation strategies can cause harm—termed “ventilator-induced lung injury” (VILI )[4,5,6]—has led to the use of lung “protective” ventilation (LPV) strategies, where low tidal volumes (4–8 mL/kg of per body weight [PBW ][7] versus 10–15 mL/kg of PBW in conventional mechanical ventilation [MV ][6]) decrease lung stretch, reduce VILI [8], and can potentially improve survival and reduce mortality in patients with acute lung injury and ARDS [6, 9]. Amato et al. showed that lower driving pressure was the physical variable that best correlated with survival in patients with ARDS [10]; higher positive end-expiratory pressure (PEEP), lower peak and plateau pressures, and lower respiratory rate, may also be associated with improved survival [11, 12].
The use of lower tidal and minute volumes with LPV strategies is limited by the resultant respiratory acidosis [13,14,15]. The rationale to integrate ECCO2R into the management of severe ARDS is to allow more protective ventilation, i.e., providing very low tidal volumes (VT) (less than 6 mL/kg PBW) with conventional MV, while avoiding extreme levels of respiratory acidosis. Arterial CO2 tensions are generally maintained in the range 45–60 mmHg rather than targeting normocapnia with this approach [16]. The potential for use of ECCO2R in patients with ARDS has been evidenced in a number of clinical studies [17,18,19], indicating it may be an effective strategy in ARDS management and a viable option to further reduce tidal and minute volumes in these patients [15, 16].
In patients with acute exacerbations of chronic obstructive pulmonary disease (aeCOPD), where hypercapnia is predominant, non-invasive positive pressure ventilation (NIV) is used as a first-line strategy in order to avoid MV [20]. Use of NIV has been reported to reduce mortality by approximately 70% [21]; however, in some patients, additional assistance is required to prevent the need for intubation [22]. NIV fails in almost 40% of cases, and patients must undergo endotracheal intubation and IMV to restore adequate gas exchange [22,23,24,25]. There is increasing clinical evidence supporting the use of low-flow, partial ECCO2R for patients experiencing aeCOPD who are failing support with NIV [22], avoiding the need for IMV and/or decreasing the length of time on the ventilator [26].
Advances in extracorporeal device technologies have made selective ECCO2R devices a less invasive and more feasible option than ECMO, with several devices clinically available that utilize blood flow rates between 180 mL/min and 1700 mL/min [27]. However, these devices were historically designed for use as oxygenators for ECMO treatment in the neonatal or pediatric setting, rather than being optimized for CO2 removal [28]. Here, we describe a newly developed ECCO2R device, the PrismaLung+ (Additional file 1: Figure S1), created specifically for CO2 removal. We compared the in vitro CO2 removal rates during low blood flow (QB 200–450 mL/min) of three devices: PrismaLung+ (Baxter), PrismaLung (Baxter), and Eurosets A.L.ONE (Eurosets), and during a QB of 600 mL/min for the PrismaLung+ and A.L.ONE devices [29,30,31]. We hypothesized that PrismaLung+ with a membrane surface area of 0.8 m2 provides significantly higher CO2 removal rates than PrismaLung (surface area 0.35 m2), whereas we expected similar performance for PrismaLung+ and the A.L.ONE device (surface area 1.35 m2), since with increasing membrane surface area, the low blood flow rates limit CO2 removal.
Methods
Experimental set-up
In vitro experimentation to determine CO2 removal rates was performed using three different ECCO2R devices: PrismaLung+ (Baxter), PrismaLung (Baxter), and Eurosets A.L.ONE (Eurosets) (Table 1). The devices were selected as they had the same membrane composition, i.e., polymethylpentene hollow-fiber mats, in order to remove this potential source of variability from the experiments. Five test devices of each type were investigated. The total surface areas of the gas exchange membranes are PrismaLung+0.8 m2, PrismaLung, 0.35m2, and A.L.ONE, 1.35 m2.
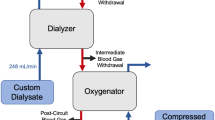
Test media bovine blood parameters were adjusted as listed in Table 2. NaCl and NaHCO3 solutions were used to adjust the required ranges of blood parameters. The experimental setup was a closed-loop circuit in which a continuous CO2 infusion balanced the CO2 removal from the test gas exchanger to establish a steady-state condition and allowing the CO2 removal rate to be determined (Fig. 1). The total amount of blood used in the circuit was approximately 600–700 mL. The test setup comprised the following: blood reservoir, 250 ml Duran glass bottle (Schott AG, Germany) with a temperature sensor Pt100 (Technetics, Germany); tubing (Promedt, Germany) with inserted septum as sample port and valves; 2 × peristaltic blood pumps (made in-house, Baxter, Germany); datalogger for sensor read-out mikromec® logger (Technetics, Germany); control loop: gas exchanger for CO2 input, PrismaLung (Baxter, Germany) (closed at gas outlet with plugs), a Thermax blood warmer bag (Baxter, France) inside an in-house made holder, and water bath with thermostat EH (Julabo, Germany); CO2 gas bottle ≥ 99.5% purity (Linde, Germany) including pressure regulator and gas tubing; CO2 mass flow regulator GSC-A9TA-BB22 (Vögtlin, Switzerland); 2 × pressure sensor, PE2 bar (Technetics, Germany); Test loop: sweep gas mass flow regulator GSC-C9TA-BB12 (Vögtlin, Switzerland); 3 × pressure sensor, PE1 bar (Technetics, Germany); syringe pump 540270 (TSE Systems, Germany); compressed air as sweep gas (in-house) including pressure regulator and tubing. Blood samples were analyzed with an ABL 90 blood gas analyzer (Radiometer, Germany).
Before use, an integrity test of the CO2 infusion circuit was performed, after which the whole test setup, including test devices, was primed with saline or dialysate solution (e.g., Prismasol 2, Baxter) to remove all air. The setup was then filled with bovine blood and all saline or dialysate solution was replaced. The test circuit comprised a central reservoir filled with 200–300 mL of blood as well as two loops. The control loop had a gas exchanger connected to a CO2 supply, which was used to achieve the targeted pinCO2 levels. In addition, a blood warming system, where the blood warmer bag was submerged in a water bath, was used to maintain blood pool temperature at 37 ± 1 °C. The control loop was fed through the central reservoir then connected to the test loop in which the test device was attached, and CO2 removal was determined. Loss of water due to evaporation through the membrane and into the sweep gas was balanced by infusion of reverse osmosis water. The sodium concentration was kept constant throughout experimentation, as analyzed by the blood gas analyzer, to maintain a constant water flow.
Measurements/data collection
When the test was initiated, the blood flow of the control loop was set to 500 mL/min and the sweep gas flow was set at the targeted rate. The CO2 inlet flow was adjusted in a stepwise fashion to maintain pinCO2 at the targeted value and to reach steady-state conditions (constant values for pinCO2, CO2 inflow rate, CO2 removal rate). The pCO2 value was measured by blood gas analysis, after samples were taken at the blood inlet of the test device. Following an equilibration time of at least 13 minutes, during which CO2 removal from the test circuit was demonstrated to be balanced by CO2 addition to the control loop, CO2 removal rate was determined based on the CO2 inflow rate. If blood samples were taken, a syringe with a volume of 0.5–1 mL was used. On average, no more than 2–3 blood sample measurements were necessary to confirm a steady state, which is below 0.5% of the total circuit blood volume.
For each test device, at all requested test parameters (9 settings of varying QB and pinCO2, Table 3 in the Appendix), measurements were taken at the inlet and outlet, with samples taken in triplicate. PO2 inlet values (160–183 mmHg) indicated that the blood used in this study was oxygen saturated. All devices were tested at QB 200, 300, and 450 mL/min, with additional testing of the PrismaLung+ and A.L.ONE devices at QB 600 mL/min and pinCO2 45 mmHg. Test conditions are outlined in Table 2.
The primary method utilized for measuring CO2 removal was the infusion method, which was validated using the blood gas analysis method. In the infusion method, normalized CO2 removal rate (JCO2(inf)) was determined based on the CO2 input flow rate at equilibrium, controlled by sample taking and analysis at the inlet. The blood gas analysis method utilized the same setup as the infusion method, but blood samples were taken additionally after the test device, at the outlet. Only samples, where measured pCO2 and/or pH were inside the reportable range (i.e., pCO2 > 12 mmHg, pH < 7.85) were used to validate the data from the infusion method.
Prior to the series of experiments, the suitability of the analyzer, with respect to its intended use, was verified through the measurement principles of the blood gas analyzer [32, 33], to ensure that the device was able to measure bovine parameters. Furthermore, the same device was used for all replicates. Measurements taken included the analysis of ctHb (g/dL), pH, pCO2 (mmHg), pO2 (mmHg), and FMetHb (%) (Table 3 in the Appendix). Following experimentation, an integrity test of the circuit was again performed to confirm its CO2 gas integrity.
Data analysis
Mean values were determined from triplicate measurements.
In the infusion method, JCO2(inf) was determined based on CO2 input flow rate, using the following equation:
where QCO2 is the CO2 input flow within the control loop, pinCO2(ref) is the target inlet pCO2 of 45, 60, or 80 mmHg, and pinCO2(inlet) is the actual partial pressure of CO2 in the blood reservoir or gas exchanger inlet. pinCO2 was normalized to reduce variability in measurements resulting from small deviations from target pinCO2 values (± 10%).
QCO2 values are referred to in mL/min under normal conditions (0 °C, 1013 mbar) and are re-calculated where appropriate, applying the ideal gas equation:
where Q (T) is the volumetric flow at a defined temperature (T, in °C). The temperatures used for calculation ranged from 0 to 37 °C. Note: the atmospheric pressure is assumed to be constant at 1013 mbar.
In addition, using the more commonly utilized blood gas analysis method, the normalized CO2 removal rate (JCO2(BGA)) was determined according to the following equation:
where Vm is the temperature-dependent molar volume; QB is the blood flow within the test loop; ctCO2 is the total blood concentration of CO2 (given by the blood gas analyzer, derived from pH, pCO2, saturation of oxygen sO2, and hemoglobin concentration); pinCO2(ref) is the target inlet pCO2 of 45, 60, or 80 mmHg; and pinCO2(inlet) is the partial pressure of CO2 in blood.
CO2 removal rates were additionally calculated in units of mmol/min to remove any dependency of reported values upon pressure and reference temperature.
Statistical analysis
A total of 5 test runs were performed for each device and parameter settings. Data are expressed as mean ± SD and the normal distribution of the data sets was assessed using the Kolmogrov–Smirnov Test (α = 0.05). CO2 removal performance results were compared using an ANOVA test with p values of < 0.05 considered as indicating a significant difference. Bland–Altman analysis was used to compare the two different performance test methods, generated using Sigmaplot software [34, 35]. A linear regression comparing both methods was additionally used. A paired t test was used to compare data obtained via the infusion and blood gas analysis methods.
Results
CO2 removal rates
Performance across the different ECCO2R devices
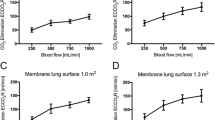
The CO2 removal rates of the different devices were analyzed using the infusion method at pinCO2 levels (45, 60, and 80 mmHg) and QB (200, 300, and 450 mL/min), at 37 °C (Fig. 2 a–c, Table 4 in the Appendix). The A.L.ONE and PrismaLung+ devices provided comparable CO2 removal rates across the range of different test conditions (p > 0.05, not significant). For both devices, removal rates were significantly higher than those observed with the PrismaLung device (p < 0.05).
CO2 removal performance as a function of pinCO2 and QB. Assessed using the infusion method for the three devices at 37 °C, across the blood flow rates (QB) 200, 300, and 450 mL/min at pinCO2 levels of a 45 mmHg, b 60 mmHg, and c 80 mmHg. pinCO2, partial pressure of carbon dioxide at the inlet; QB, blood flow rate. Data are plotted as mean values ± SD. *p > 0.05 PrismaLung+ vs. A.L.ONE; †p < 0.05 PrismaLung+ vs. PrismaLung
CO2 removal rates at an increased blood flow rate of 600 mL/min were additionally evaluated for the PrismaLung+ and A.L.ONE devices only and were comparable for both devices (p > 0.05) (Additional file 2: Figure S2). At a pinCO2 of 45 mmHg, at 37 °C, the mean CO2 removal rates at a blood flow rate of 600 mL/min were 106 ± 3.8 mL/min and 106 ± 5.8 mL/min for the PrismaLung+ and A.L.ONE devices, respectively.
As the volume flow of gases, i.e., the CO2 removal rate, is temperature- and pressure-dependent, data were calculated at standard reference conditions, 0 °C and 25 °C (STP as defined by IUPAC), in addition to the physiological conditions, 37 °C, for the PrismaLung+ device at a pinCO2 of 45 mmHg and QB range of 200–450 mL/min (Fig. 3a). Results illustrate the dependence of CO2 removal on the chosen reference temperature. By definition, calculation of CO2 removal rates in mmol/min across QB 200–450 mL/min, at a pinCO2 of 45 mmHg, is independent from any reference temperature (Fig. 3b).
CO2 removal performance as a function of temperature. Assessed using the infusion method for a PrismaLung+ at a pinCO2 of 45 mmHg and QB 200–450 mL/min, referenced to 0, 25, or 37 °C, and b for all tested gas exchangers at 37 °C, at a pinCO2 of 45 mmHg, across QB 200–450 mL/min, in units of mmol/min. pinCO2, partial pressure of carbon dioxide at the inlet; QB, blood flow rate. Data are plotted as mean values ± SD
Pressure drop levels
To examine the blood flow resistance for each device, pressure drop was analyzed at the blood side, for all pinCO2 levels (45, 60, and 80 mmHg). Pressure drop was observed to be largest for the PrismaLung, and lowest for the A.L.ONE device, being 17 (±3), 24 (±4), and 38 (±8) mmHg (±SD) for PrismaLung; 11 (±6), 17 (±7), and 25 (±7) mmHg (±SD) for PrismaLung+; and 7 (±3), 13 (±4), and 19 (±4) mmHg (±SD) for the A.L.ONE device, at blood flow rates of 200, 300, and 450 mL/min, respectively (Fig. 4). All comparisons for the PrismaLung+ versus PrismaLung, and PrismaLung+ versus A.L.ONE devices, were significantly different (p < 0.05), except for the PrismaLung+ versus A.L.ONE devices at a QB of 300 mL/min (p > 0.05). The differences seen across the devices are likely driven by the variances in the surface areas, with PrismaLung having the smallest surface area (0.35 m2), followed by PrismaLung+ (0.8 m2) and then A.L.ONE (1.35 m2).
Analysis using the infusion and blood gas methods
The CO2 removal performances were analyzed using the infusion and blood gas analysis methods. However, at the lower blood flow rates, many of the outlet samples were below the measuring range of pCO2, among others, using the blood gas analyzer method. A comparison of the two methods using a Bland–Altman analysis (Fig. 5a) and linear regression analysis (Fig. 5b) across the different pinCO2 levels (45, 60, and 80 mmHg) and blood flow rates (200, 300, and 450 mL/min), using valid data within the reportable range of the blood gas analyzer used, indicated a linear relationship between the data obtained by the two methods, suggesting comparability. Statistical analysis revealed that CO2 removal performance values obtained with the infusion method were, on average, 4.2 mL greater than the values obtained with blood gas analysis (p < 0.05). In addition, the difference was shown to be independent of the test conditions and a constant offset between the two methods. The infusion method was used for the analysis of the full data set as the two methods are similar in terms of validity.
Comparison of CO2 removal performance using the infusion and blood gas analysis methods. Assessed across all tested devices and parameter settings at 37 °C. a Bland–Altman analysis to demonstrate the relationship between the BGA method and the CO2 input method. Mean = 4.2 mL/min, SD = 4.4 mL/min, Limits of Agreement (95%) = − 4.4 mL/min, 12.9 mL/min. b Linear regression analysis comparing the two test methods: BGA method and CO2 input method. Slope not significantly different from 1.0. BGA, blood gas analysis; SD, standard deviation
Discussion
In this in vitro study, the CO2 removal performance of the new PrismaLung+ device was comparable to the A.L.ONE device, with both devices demonstrating CO2 removal rates ~ 50% greater than the PrismaLung device. The performance of the three devices was consistent over a range of blood pinCO2 levels and at flow rates from 200 to 450 mL/min, with both the PrismaLung+ and A.L.ONE devices also performing comparably at the higher flow rate of 600 mL/min. CO2 removal data obtained with the CO2 infusion method were comparable to those obtained with the blood gas analysis method. The resistance to blood flow across the test device, as measured by pressure drop, varied as a function of blood flow rate, being greatest for the PrismaLung, intermediate for the PrismaLung+, and lowest for the A.L.ONE device, most likely driven by the differences in the surface areas. Taken together with prior clinical studies of ECCO2R devices [17,18,19, 22], these findings suggest that the PrismaLung+ may be an effective device and further testing in the clinical setting is warranted.
Rationale for CO2 removal
ECCO2R technologies may have important roles in the management of patients with ARDS and patients with aeCOPD. ECCO2R can help facilitate lung-protective strategies by enabling very low VT (< 6 mL/kg PBW) ventilation [36, 37]. The safety and feasibility of ECCO2R has been demonstrated in multiple studies [16, 18, 19] of patients with ARDS, with reduced lung injury and benefits in terms of pulmonary inflammation with low VT ventilation [17]. Several studies also support the use of ECCO2R in patients with aeCOPD requiring ventilatory support [38,39,40,41,42].
Lower flow ECCO2R devices
Devices with reduced blood flow requirements will, by design, be less efficient at removing CO2 than higher flow devices, but they do have several advantages. The PrismaLung+ device in this study has a design tailored to specifically remove CO2. The lack of a need for a heat exchanger inside the device allows for reduced size and weight, given that a heat exchanger is available for use next to the machine during treatment. The new PrismaLung+ device has the lowest ratio of blood volume to membrane surface of the tested devices reducing the risks associated with large extracorporeal blood volumes. The removal of the heater also allows for a streamlined design, which should reduce the potential for pooling and low flows of blood within the device. Further aspects of the device design, including the fluid path and dimensional parameters, have been developed to enable an intended operating blood flow of 200 to 450 mL/min. Namely blood flow velocity distribution was calculated to avoid stagnant areas or areas with very low blood flow velocity and to ensure that channeling of the blood did not occur. The residual volume space is smaller than other devices, minimizing the space for blood to clot. The ratio of CO2 removal rate to blood volume of the PrismaLung+ device allows for optimized performance at these flow rates. It has been shown that an extracorporeal CO2 removal rate of 51 ± 26 mL/min was associated with an increase in PaCO2 from 43 ± 8 to 53 ± 9 mmHg when applying low tidal volume ventilation (VT = 4 mL/kg) in patients with mild-to-moderate ARDS [43]. Whereas a mean CO2 removal of 81 ± 9 mL/min enabled a reduction in VT to 4.29 ± 9 mL/min without an increase in PaCO2 of more than 10% [19]. Therefore, larger CO2 removal rates are desirable to allow ultraprotective ventilation in ARDS patients without a significant increase in PaCO2. Furthermore, it is assumed that a reduced interaction between blood and foreign material, i.e., a preferably small device, may potentially support biocompatibility [44]. The streamlined design of Prismalung+ might require less anticoagulation, which entails a lower risk of bleeding complications in patients, as there is less potential for pooling and low flows of blood within the device. This hypothesis needs to be investigated in future studies.
An advantage of lower flow ECCO2R devices is that smaller bore catheters can be used. A second advantage is that they may be integrated with other organ support strategies familiar to critical care physicians and nurses, such as continuous renal replacement therapy (CRRT), making these approaches much more feasible in the busy critical care environment. The potential to integrate ECCO2R into continuous renal replacement circuits may improve the risk/benefit ratio for hypercapnic patients with acute kidney injury (AKI) [45]. If effective, such devices could also be used in patients that do not have AKI, given the familiarity of the critical care team with this equipment. A feasibility study demonstrated that the use of a low-flow ECCO2R device managed with an RRT platform easily and safely enabled very-low-tidal-volume ventilation with moderate increase in PaCO2 in patients with mild-to-moderate ARDS [43].
Comparison of CO2 removal by different devices
In this study, the new PrismaLung+ device performed similarly to the A.L.ONE device, with both devices demonstrating CO2 removal rates ~ 50% greater than the PrismaLung device. While the increase in CO2 removal observed with PrismaLung+ compared with PrismaLung can, at least in part, be explained by an increase in membrane surface area from 0.35 m2 to 0.8 m2, the similarities observed for PrismaLung+ and A.L.ONE occurred despite an increase in surface area, suggesting a more complex explanation. Recent data from Karagiannidis and colleagues suggest that the capability of different ECCO2R devices to eliminate CO2 is dependent upon a dynamic interplay within the device between the surface area available for gas exchange and the blood flow rate [44]. Devices with gas exchange membrane surface areas ranging from 0.35 m2 (e.g., PrismaLung) up to 1.3 m2 (e.g., A.L.ONE) are currently used in clinical practice [19, 22, 26, 46,47,48]. Furthermore, recent in vitro and in silico studies suggest that CO2 removal rate can increase with increasing blood flow rate [49, 50], in line with the observations we report here. Our study also confirms the findings from Karagiannidis and colleagues [44], as both surface area and blood flow rates govern the rate of CO2 removal; however, an increase in the surface area above a certain threshold has limited impact on CO2 removal when low blood flows are applied, as is the case with the A.L.ONE device. Larger membrane surface areas are thought to result in greater levels of CO2 removal at higher blood flow rates, with a smaller pressure drop across the gas exchanger [44]. In our in vitro study, pressure drop values across the three devices were relatively low, with levels of up to 25 mmHg with PrismaLung+ (surface area 0.8 m2) at a blood flow rate of 450 mL/min. Some challenges do exist for devices with larger membrane surface areas and those that require larger priming volumes, as they may have increased thrombotic potential due to increased interaction with an artificial surface [44]. A lower blood flow rate combined with a larger surface area may lead to more clotting events due to the increased time blood spends passing through the membrane [43, 51]. Furthermore, in the clinic, larger priming volumes can negatively affect exposure time and the hemolysis index [51], potentially resulting in increased blood loss due to clotting events and the device having to be replaced. We did not observe clotting events; however, this was not a focus of the study and would require investigation in the clinic.
These findings highlight the potential for lower flow devices with higher surface areas to remove CO2 from blood. The data also demonstrate the restrictions of conventional diffusive CO2 removal determined by blood flow rates. To further enhance CO2 removal at low blood flow rates novel systems, such as approaches involving acidification of bloo d[52] or bicarbonate dialysi s[53], need to be investigated.
Study limitations and considerations
We used the infusion analysis rather than the more commonly accepted blood gas analysis method to determine the CO2 removal rates. This was done because of the limitations of the blood gas analysis method, because test conditions utilizing lower blood flow produced CO2 results below the reportable range of the analyzer. The blood gas analysis method was used to determine CO2 removal rates when test results where within the reportable range of the blood gas analyzer. The results obtained with the blood gas analysis method demonstrated the validity of the infusion method. The comparison of the valid data obtained by blood gas analysis versus the infusion method by slope analysis indicated that these two data sets are comparable (Fig. 5). It is important to note that the comparative data indicated that a small amount of gas loss from the test setup was likely, as for example, if the reservoir and/or tubing are not fully gas-tight. This is demonstrated by the data being slightly off the line of identity, the offset of the mean, as shown in the Bland–Altman diagram (4.2 mL/min) and the paired t test.
The general suitability of the blood gas analyzer, with respect to its intended use, was verified through the measurement principles of the analyzer [32, 33], to ensure that the ABL90 device used here could measure bovine blood parameters. In addition, the ABL devices from Radiometer have been routinely used to perform experiments on blood from different species [54,55,56,57].
We used a flow rate that would be achievable by a monitor that the PrismaLung+ device is intended to be used on in the clinic, namely 200 to 450 mL/min, allowing us to characterize the device in conditions comparable to mild-to-moderate hypercapnia where the device would be used. The A.L.ONE device is designed to run at higher blood flow rates than the flow rates used in this study; therefore, conditions perhaps did not favor the CO2 removal rate of the device, despite the high surface area in comparison with PrismaLung+ [31].
Further methodological limitations to consider include the use of bovine blood for experimentation, as it is easier to obtain than human blood and well-accepted for use in in vitro studies. It should be noted that as the blood was obtained from healthy animals, levels of blood components will be different from those for ICU patients; however, levels were consistent across experiments. Furthermore, a high dose (5 U/mL) of heparin was used that is higher than that routinely used in the clinic; this dose was selected to ensure no clotting occurred during transportation from the slaughterhouse and during the in vitro experiment and is not expected to impact CO2 removal. Given the in vitro nature of the data, caution should be exercised when translating these data to the clinical setting, and further studies are needed to explore coagulation in the clinic.
Here, and in similar studies, the CO2 removal rates are stated in units of mL/min, with dependency upon pressure and chosen reference temperature. To remove the dependency upon these parameters, units of mmol/min would be more appropriate and comparable when reporting CO2 removal rates. Despite this, the units of mmol/min are not standard and are not used in the clinical setting.
ECCO2R uses similar gas-exchange principles as ECMO, but the main goal is to remove CO2 in those with sufficient oxygenation and at lower blood flow rates than ECMO [58]. Oxygenation and O2 transfer rates were not a focus in this study. In the clinical setting, venous sO2 levels are expected to be around 70% and therefore the testing of fully oxygen-saturated blood in this setting may slightly underestimate CO2 removal performance. This will require further experimental confirmation.
Conclusions
In summary, at the flow rates tested, PrismaLung+ performed more effectively than PrismaLung for CO2 removal, with comparable performance to A.L.ONE, despite the smaller surface area. The PrismaLung+ may be an effective device for reducing CO2 levels, and further testing in the clinical setting is warranted.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
acute kidney injury
- ARDS:
-
acute respiratory distress syndrome
- BGA:
-
blood gas analysis
- COPD:
-
chronic obstructive pulmonary disease
- ECCO2R:
-
extracorporeal CO2 removal
- ECMO:
-
extracorporeal membrane oxygenation
- IMV:
-
invasive mechanical ventilation
- IUPAC:
-
International Union of Pure and Applied Chemistry
- LPV:
-
lung-protective ventilation
- MV:
-
mechanical ventilation
- NIV:
-
non-invasive positive pressure ventilation
- PBW:
-
per body weight
- pCO2 :
-
partial pressure of carbon dioxide (CO2)
- pinCO2 :
-
partial pressure of carbon dioxide (CO2) at the inlet
- PEEP:
-
positive end-expiratory pressure
- Q B :
-
blood flow rate
- RRT:
-
renal replacement therapy
- SD:
-
standard deviation
- STP:
-
standard temperature and pressure
- VILI:
-
ventilator-induced lung injury
- V T :
-
tidal volume
References
Baker A, Richardson D, Craig G (2012) Extracorporeal carbon dioxide removal (ECCO2R) in respiratory failure: an overview, and where next? JICS 13:232–237
Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Brechot N, Merceron S, Luyt CE, Trouillet JL, Chastre J, Leprince P, Combes A (2013) Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 39:838–846
Moran I, Zavala E, Fernandez R, Blanch L, Mancebo J (2003) Recruitment manoeuvres in acute lung injury/acute respiratory distress syndrome. Eur Respir J 22:37s–42s
Pinhu L, Whitehead T, Evans T, Griffiths M (2003) Ventilator-associated lung injury. Lancet 361:332–340
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ (2017) An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 195:1253–1263
Schilling T, Kozian A, Huth C, Buhling F, Kretzschmar M, Welte T, Hachenberg T (2005) The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 101:957–965
Needham DM, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Sevransky JE, Dennison Himmelfarb CR, Desai SV, Shanholtz C, Brower RG, Pronovost PJ (2012) Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ 344:e2124
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755
Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, Brochard L, Clarkson K, Esteban A, Gattinoni L, van Haren F, Heunks LM, Kurahashi K, Laake JH, Larsson A, McAuley DF, McNamee L, Nin N, Qiu H, Ranieri M, Rubenfeld GD, Thompson BT, Wrigge H, Slutsky AS, Pesenti A (2016) Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 42:1865–1876
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A (2016) Epidemiology, Patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800
Hickling KG, Henderson SJ, Jackson R (1990) Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 16:372–377
Hickling KG, Walsh J, Henderson S, Jackson R (1994) Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 22:1568–1578
Fanelli V, Costamagna A, Ranieri VM (2014) Extracorporeal support for severe acute respiratory failure. Semin Respir Crit Care Med 35:519–527
Combes A, Fanelli V, Pham T, Ranieri VM (2019) Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med 45:592–600
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM (2009) Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 111:826–835
Bein T, Weber-Carstens S, Goldmann A, Muller T, Staudinger T, Brederlau J, Muellenbach R, Dembinski R, Graf BM, Wewalka M, Philipp A, Wernecke KD, Lubnow M, Slutsky AS (2013) Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ʻconventionalʼ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 39:847–856
Fanelli V, Ranieri MV, Mancebo J, Moerer O, Quintel M, Morley S, Moran I, Parrilla F, Costamagna A, Gaudiosi M, Combes A (2016) Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care 20:36
Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA, Mannino D, Sciurba FC, Holguin F (2012) Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med 185:152–159
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333:817–822
Del Sorbo L, Pisani L, Filippini C, Fanelli V, Fasano L, Terragni P, Dell'Amore A, Urbino R, Mascia L, Evangelista A, Antro C, D’Amato R, Sucre MJ, Simonetti U, Persico P, Nava S, Ranieri VM (2015) Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 43:120–127
Stefan MS, Nathanson BH, Higgins TL, Steingrub JS, Lagu T, Rothberg MB, Lindenauer PK (2015) Comparative effectiveness of noninvasive and invasive ventilation in critically ill patients with acute exacerbation of chronic obstructive pulmonary disease. Crit Care Med 43:1386–1394
Conti G, Antonelli M, Navalesi P, Rocco M, Bufi M, Spadetta G, Meduri GU (2002) Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 28:1701–1707
Confalonieri M, Garuti G, Cattaruzza MS, Osborn JF, Antonelli M, Conti G, Kodric M, Resta O, Marchese S, Gregoretti C, Rossi A (2005) A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 25:348–355
Braune S, Sieweke A, Brettner F, Staudinger T, Joannidis M, Verbrugge S, Frings D, Nierhaus A, Wegscheider K, Kluge S (2016) The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med 42:1437–1444
Abrams D, Roncon-Albuquerque R Jr, Brodie D (2015) Whatʼs new in extracorporeal carbon dioxide removal for COPD? Intensive Care Med 41:906–908
Van Ommen CH, Neunert CE, Chitlur MB (2018) Neonatal ECMO. Front Med (Lausanne) 5:289
Godet T, Combes A, Zogheib E, Jabaudon M, Futier E, Slutsky AS, Constantin JM (2015) Novel CO2 removal device driven by a renal-replacement system without hemofilter. A first step experimental validation. Anaesth Crit Care Pain Med 34:135–140
Winiszewski H, Aptel F, Belon F, Belin N, Chaignat C, Patry C, Clermont C, David E, Navellou JC, Labro G, Piton G, Capellier G (2018) Daily use of extracorporeal CO2 removal in a critical care unit: indications and results. J Intensive Care 6:36
Eurosets (2018) Adult oxygenators catalogue
Radiometer (2018) User Manual blood gas analyzer ABL90 FLEX
Zijlstra WG, Buursma A (1997) Spectrophotometry of Hemoglobin: Absorption Spectra of Bovine Oxyhemoglobin, Deoxyhemoglobin, Carboxyhemoglobin, and Methemoglobin. Comp Biochem Physiol B: Biochem Mol Biol 118:743–749
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Giavarina D (2015) Understanding Bland Altman analysis. Biochem Med (Zagreb) 25:141–151
Morelli A, Del Sorbo L, Pesenti A, Ranieri VM, Fan E (2017) Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med 43:519–530
Trahanas JM, Lynch WR, Bartlett RH (2017) Extracorporeal support for chronic obstructive pulmonary disease: a bright future. J Intensive Care Med 32:411–420
Burki NK, Mani RK, Herth FJF, Schmidt W, Teschler H, Bonin F, Becker H, Randerath WJ, Stieglitz S, Hagmeyer L, Priegnitz C, Pfeifer M, Blaas SH, Putensen C, Theuerkauf N, Quintel M, Moerer O (2013) A novel extracorporeal CO(2) removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 143:678–686
Mani RK, Schmidt W, Lund LW, Herth FJ (2013) Respiratory dialysis for avoidance of intubation in acute exacerbation of COPD. ASAIO J 59:675–678
Bonin F, Sommerwerck U, Lund LW, Teschler H (2013) Avoidance of intubation during acute exacerbation of chronic obstructive pulmonary disease for a lung transplant candidate using extracorporeal carbon dioxide removal with the Hemolung. J Thorac Cardiovasc Surg 145:e43–e44
Kluge S, Braune SA, Engel M, Nierhaus A, Frings D, Ebelt H, Uhrig A, Metschke M, Wegscheider K, Suttorp N, Rousseau S (2012) Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 38:1632–1639
Abrams DC, Brenner K, Burkart KM, Agerstrand CL, Thomashow BM, Bacchetta M, Brodie D (2013) Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thoracc Soc 10:307–314
Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A (2018) Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care 22:122
Karagiannidis C, Strassmann S, Brodie D, Ritter P, Larsson A, Borchardt R, Windisch W (2017) Impact of membrane lung surface area and blood flow on extracorporeal CO2 removal during severe respiratory acidosis. Intensive Care Med Exp 5:34
Fanelli V, Cantaluppi V, Alessandri F, Costamagna A, Cappello P, Brazzi L, Pugliese F, Biancone L, Terragni P, Ranieri VM (2018) Extracorporeal CO2 removal may improve renal function of patients with acute respiratory distress syndrome and acute kidney injury: an open-label, interventional clinical trial. Am J Respir Crit Care Med 198:687–690
Forster C, Schriewer J, John S, Eckardt KU, Willam C (2013) Low-flow CO2 removal integrated into a renal-replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit Care 17:R154
Allardet-Servent J, Castanier M, Signouret T, Soundaravelou R, Lepidi A, Seghboyan JM (2015) Safety and efficacy of combined extracorporeal CO2 removal and renal replacement therapy in patients with acute respiratory distress syndrome and acute kidney injury: the pulmonary and renal support in acute respiratory distress syndrome study. Crit Care Med 43:2570–2581
Hermann A, Riss K, Schellongowski P, Bojic A, Wohlfarth P, Robak O, Sperr WR, Staudinger T (2015) A novel pump-driven veno-venous gas exchange system during extracorporeal CO2-removal. Intensive Care Med 41:1773–1780
Svitek RG, Federspiel WJ (2008) A mathematical model to predict CO2 removal in hollow fiber membrane oxygenators. Ann Biomed Eng 36:992–1003
May AG, Jeffries RG, Frankowski BJ, Burgreen GW, Federspiel WJ (2018) Bench Validation of a Compact Low-Flow CO2 Removal Device. Intensive Care Med Exp 6:34
Karagiannidis C, Hesselmann F, Fan E (2019) Physiological and Technical Considerations of Extracorporeal CO2 Removal. Crit Care 23:75
Scaravilli V, Kreyer S, Belenkiy S, Linden K, Zanella A, Li Y, Dubick MA, Cancio LC, Pesenti A, Batchinsky AI (2016) Extracorporeal Carbon Dioxide Removal Enhanced by Lactic Acid Infusion in Spontaneously Breathing Conscious Sheep. Anesthesiology 124:674–682
Cove ME, Vu LH, Ring T, May AG, Federspiel WJ, Kellum JA (2019) A Proof of concept study, demonstrating extracorporeal carbon dioxide removal using hemodialysis with a low bicarbonate dialysate. ASAIO J 65:605–613
Yilmaz O, Karapinar T (2019) Evaluation of the i-STAT analyzer for determination of ionized calcium concentrations in bovine blood. Vet Clin Pathol 48:31–35
Neves RC, Stokol T, Bach KD, McArt JAA (2018) Method comparison and validation of a prototype device for measurement of ionized calcium concentrations cow-side against a point-of-care instrument and a benchtop blood-gas analyzer reference method. J Dairy Sci 101:1334–1343
Morel H, Jaffrin MY, Paullier P, Legallais C (2009) In vitro tests and modelization of bicarbonate kinetics and mass transfers during online hemodiafiltration. Int J Artif Organs 32:482–491
Knobloch H, Becher G, Decker M, Reinhold P (2008) Evaluation of H2O2 and pH in exhaled breath condensate samples: methodical and physiological aspects. Biomarkers 13:319–341
Cove ME, MacLaren G, Federspiel WJ, Kellum JA (2012) Bench to bedside review: Extracorporeal carbon dioxide removal, past present and future. Crit Care 16:232
Acknowledgements
Editorial support for the development of this manuscript was provided by Daniel Johnson PhD, Ailsa Bennett PhD, Ruth Brown PhD, and Siobhán Ahern PhD, SciMentum (Nucleus Global), with funding provided by Baxter.
Funding
This study was sponsored by Baxter.
Author information
Authors and Affiliations
Contributions
MR and SV performed the in vitro experiments. All other authors conceived and designed the experimental layout, evaluated and analyzed the data, supported the writing of the manuscript, and studied and discussed the literature and experimental results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. IH, JG, MS, KH, DP, MR, SV are full-time employees of Baxter International. J.L. has received consulting fees from Baxter International.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Cross section of the PrismaLung+ device
Additional file 2: Figure S2.
CO2 removal rates for the PrismaLung+ and A.L.ONE devices. Assessed at 37 °C, QB 600 mL, and a pinCO2 of 45 mmHg. pinCO2, partial pressure of carbon dioxide at the inlet; QB, blood flow rate. Data are plotted as mean values ± SD. p > 0.05 PrismaLung+ vs. A.L.ONE, not significantly different. Results are the mean of 4 tested devices
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hospach, I., Goldstein, J., Harenski, K. et al. In vitro characterization of PrismaLung+: a novel ECCO2R device. ICMx 8, 14 (2020). https://doi.org/10.1186/s40635-020-00301-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-020-00301-7