Abstract
Herein, we report the construction of a colorimetric probe used to detecting Cr3+ ions in aqueous solution based on functionalized gold nanoparticles. We investigated 4-mercaptobenzoic acid, 4-nitrobenzenethiol, and a mixture of 4-mercaptobenzoic acid and 4-nitrobenzenethiol as ligands for Cr3+ ions to functionalize the gold nanoparticles, respectively. The results showed that the three probes were all aggregated in the presence of Cr3+ ions, which induces a color change from ruby to violet. Moreover, gold nanoparticles modified with 4-mercaptobenzoic acid exhibit a higher response toward Cr3+ than the two other probes, which can be detected by the naked eye and UV-vis absorption spectroscopy. The detection time was rapid (within 25 min). A linear relationship was obtained from 20 to 25 μM between the ratio of the absorbance observed at 635 nm and 520 nm (A635 nm/A520 nm) with the limit of detection was 5 × 10−6 M. This method exhibited excellent selectivity for Cr3+ ions over other tested heavy metal ions, anions, and organic molecules in the absence of another shielding reagent of metal ion. The system was successfully utilized to detect Cr3+ ions in simulated samples.
Similar content being viewed by others
Introduction
Chromium is extensively applied in various fields, including steel works (Gomez et al. 2006; Parlayici, et al. 2019), electroplating, tanning industry, and chemical industries. This extensive use has resulted in environmental pollution (Shuang et al. 2017; Wei, et al. 2014). Cr3+ is an essential trace nutrient that is regulated at normal levels for human’s health (Gómez et al. 2006). An abnormal level of Cr3+ affects DNA’s function for protein synthesis and damages biomacromolecules (Glinsman et al. 1966; Wei et al. 2014); it can also induce diabetes and cardiovascular disease (Anderson 1986; Chen et al. 2011). Therefore, the detection of Cr3+ ions in drinking water is of great significance to ensure human health. Traditional analytical technologies mainly utilize atomic absorption spectrometry (Mashhadizadeh et al. 2013), chemiluminescence (Yang et al. 2003), high-performance liquid chromatography (Cathum et al. 2002), fluorescence spectroscopy (Chen et al. 2014), and electrochemical methods (Wei et al. 2007). These are expensive, time-consuming, and require complicated instrumentation. Therefore, the development of a simply and selective methods for the detection Cr3+ ions is urgently required. Metal nanoparticles have been widely utilized for biosensing due to their excellent optical properties (Guo et al. 2011; Du, et al. 2013). Gold nanoparticles (AuNPs) functionalized with organic molecules exhibit a colorimetric change according to their aggregation and dispersion (Guo et al. 2014; Upadhyay, et al. 2018). Herein, we have investigated the single/collaborative behavior of 4-mercaptobenzoic acid (4-MBA) and 4-nitrobenzenethiol (4-NPT) for functionalizing AuNPs in Cr3+ ions detection. 4-MBA and 4-NBT contained a -SH group, which interacts with AuNPs via a covalent bond. Additionally, the -NO2 group in 4-NBT and -COOH group in 4-MBA are both modified on the surface of the AuNPs together. These groups have a strong affinity to metal ions (Hemmateenejad et al. 2015; Lin, et al. 2002). Functionalized AuNPs are aggregated in the presence of Cr3+ ion via the ion-templated chelation, resulting in an appreciable color changes that can be detected by the color by the naked eyes and UV-vis spectroscopy.
Experimental
Materials
All chemicals used were of analytical grade. Hydrogen tetrachloroaurate hydrate (HAuCl4•4H2O) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai China). 4-Mercaptobenzoic acid was purchased from Tokyo Chemical Industry Co., Ltd (Japan). 4-Nitrobenzenethiol and melamine were obtained from Sigma-Aldrich (USA). Cr(NO3)3•9H2O and other metal ions were purchased from Beijing Chemical Company (Beijing, China). All the solutions were prepared using purified water with a resistance of 18 MΩ•cm. UV–Vis absorption spectroscopy was recorded on a UV-2550 spectrophotometer (Shimadzu, Japan), using a 1-cm pathlength quartz cuvettes for measurements. Transmission electron microscopy (TEM) was performed on an H-7500 instrument (Hitachi, Japan) operated at 80 kV.
Preparation of AuNPs functionalized with 4-MBA and 4-NPT
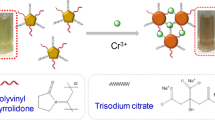
We utilized the method of the chemical reduction of HAuCl4 to prepare citrate-capped AuNPs (Ji, et al. 2007). Briefly, 15 mL of 38.8 × 10−3 mol L−1 solution of trisodium citrate was rapidly added into 150 mL 1.0 × 10−3 mol L−1 solution of HAuCl4 heated at reflux under vigorously magnetic stirring. The mixed solution was heated under reflux with stirring for another 30 min to produce a ruby red colored solution. Then, we used 4-MBA, 4-NTP, and a 4-MBA/4-NTP mixture were used as capping agents to functionalize the AuNPs. Typically, stock solutions containing 1 × 10−4 M 4-MBA, 4-NTP, and 4-MBA/4-NTP were prepared using purified water. 1.0 mL of 10−4 M the above-mentioned solutions was added into 100 mL of the AuNPs solution, and the resulting mixture was equilibrated at the room temperature for 30 min to ensure the self-assembly of 4-MBA and 4-NBT on the surface of the AuNPs.
Detection of Cr3+ ions
The colorimetric detection of Cr3+ was carried out using functionalized AuNPs. Typically, 300 μL of an aqueous solution of Cr3+ prepared at different concentrations was mixed with 2.7 mL of the AuNPs solution. Subsequently, the mixture was incubated for 10 min at the room temperature, and the absorbance and absorbance ratio (A635 nm/A520 nm) were recorded. Meanwhile, the effect of pH value on detection of Cr3+ was investigated. We employed 0.1-M sodium hydroxide (NaOH) or 0.1-M hydrochloric acid (HCl) to adjust pH value of the solution of 4-MBA modified AuNPs from 6 to 10. Then, 300 μL of aqueous solutions of Cr3+ ions were added into 2.7 mL of the 4-MBA-AuNPs solution with the various pH value, respectively. Finally, the process of the mixture was the same as detection of Cr3+ ions. The final concentration of Cr3+ ions were all 20 μM.
Selectivity Cr3+ ions
The selectivity of detection for Cr3+ was investigated. 150 μL of an aqueous solution of Cr3+ and 150 μL of an aqueous solution of interferents were mixed with 2.7 mL of the 4-MBA-AuNPs solution. The final concentration of Cr3+ and interferents were all 1 × 10−4 M. Then, the process of the mixture was the same as detection of Cr3+ ions.
Result and discussion
Sensing mechanism
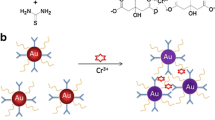
Our detecting system contained three key parts: The target ion (Cr3+), probe (functionalized Au NPs), and signal readout (UV-vis absorption and colorimetric reaction). The probe is aggregated in the presence of Cr3+ ions, which is accompanied by a color change. We have proposed a mechanism for this phenomenon in Fig. 1. The surface of the AuNPs linked with 4-MBA and 4-NPT, containing the carboxyl group and nitro group. The two groups have a strong affinity to Cr3+ ions (Hemmateenejad et al. 2015; Lin et al. 2002), which induced the neighboring AuNPs to become closer and finally aggregated with one another. This results in an appreciable change in their color and absorption properties.
Optimal ligand for the functionalized AuNPs
According to the previous studies (Zhang et al. 2015), 4-MBA and 4-NTP have higher coordination properties with Cr3+ ions. To choose the optimal ligand in our study, we also utilized 4-MBA and 4-NTP to functionalize the AuNPs for the sensitively detecting of Cr3+ ions. The Fig. 2 shows that the 4-MBA-AuNPs exhibit a higher response than 4-NTP-AuNPs and 4-MBA/4-NTP-AuNPs. The 4-NTP and the mixture of 4-MBA and 4-NTP modified AuNPs have lower sensitivity than that of 4-MBA-AuNPs. This result indicates that 4-NTP decreases the sensitivity of the probe toward the detection of Cr3+ ions. Therefore, we chose 4-MBA as the ligand to functionalize the AuNPs for the detection of Cr3+. Meanwhile, the FT-IR spectra recorded for pure 4-MBA and the 4-MBA modified AuNPs (4-MBA-AuNPs) are shown in Fig. 3a. When comparing these FT-IR spectra, the characteristic absorption peak of –SH at 2524 cm−1 in pure 4-MBA had disappears in the FT-IR spectrum of 4-MBA-AuNPs. This indicates that 4-MBA had been successfully modified onto the surface of the AuNPs via the –SH group in 4-MBA, which is similar to that previously report in the literature (Zhou et al. 2011). Besides, the 4-MBA-AuNPs has the stable optical properties (Fig. 3b), which is conducive to analysis for Cr3+.
The 4-MBA modified AuNPs are stable and well-dispersed (Fig. 4a). The mean size of 4-MBA-AuNPs was estimated to be 20 nm from their size distribution using TEM image (Fig. 4). Direct evidence for the Cr3+-induced aggregation of the 4-MBA-AuNPs could be further supported by TEM measurements. Figure 3 shows the TEM images of the 4-MBA-AuNPs in the absence and presence of a 4 × 10−6 M aqueous solution of Cr3+ ions. In the absence of Cr3+, the 4-MBA-AuNPs were well-dispersed in the aqueous solution. On the other hand, 4-MBA-AuNPs were aggregated when added to an aqueous solution of Cr3+ ions (Fig. 4b). These results clearly indicate that the addition of trace amounts Cr3+ ions can readily lead to the aggregation of the modified AuNPs.
Effect of pH
The pH condition for colorimetric detection of Cr3+ was optimized over the range from 6.0 to 10.0. As shown in Fig. 5, when the concentration of Cr3+ was 20 μM, the modified AuNPs show obvious UV–vis spectroscopy absorption changes at pH 6. However, at the higher pH, the absorption showed a little change. Therefore, pH 6 was selected for further experiments considering the preferable sensitivity.
Limit of detection
The color change of the 4-MBA modified AuNPs induced by Cr3+ can be detected using UV-vis absorption spectroscopy. Upon the addition of Cr3+, the absorbance of the 4-MBA-AuNPs observed at 520 nm decreased, and a new absorption band appears at 635 nm appeared. Increasing the concentrations of Cr3+ led to the absorbance at 635 nm increasing and a concomitant decrease in the SPR peak observed at 520 nm (Fig. 6). Accordingly, the color of the AuNPs progressively changes from ruby red to purple and finally to blue. The ration of the absorbance observed at A635 and A520 nm was used for the quantitative analysis of Cr3+. A linear correlation was observed between the absorption ratio (A635 nm/A520 nm), and the Cr3+ concentration within the range of 20–25 μM. The limit of detection was 5 × 10−6 M (S/N).
To further find the performance of the proposed method, a comparison with other methods is shown in Table 1. Table 1 indicated that the proposed method exhibited lower detection limit.
The optimal of reaction time
The detection time is a key factor that needs to be determined. We studied the kinetics of the detection step (Fig. 7). From 0 to 10 min, the A635nm /A520nm value increased with a steep slope; after 10 min, the value reached a plateau. Thus, our detection can be completed in less than 30 min, indicating it is a rapid probe.
Selective detection of Cr3+ using 4-MBA modified AuNPs and simulated samples detection
In comparison with the laboratory-made samples, real aqueous solution contains more and real interferents, which affect the detection results. Therefore, the selectivity of the sensor is important towards the detection Cr3+. We studied the selectivity of the sensor against several interferents in an aqueous solution, including Al3+, Ca2+, Fe2+, K+, Mg2+, Mn2+, Na+, Zn2+, NO3−, SO42−, carbamide, and glucose (Fig. 8). Upon the addition of the interferents, the absorbance at 520 nm showed a slight change; however, a new absorption band did not appear. Therefore, no obvious effect on our sensor system was observed. Clearly, 4-MBA-AuNPs showed high selectivity for Cr3+ over the interferents studied. The spectral and color changes observed for 4-MBA-AuNPs upon the addition of Cr3+ can be explained well by the aggregation of the AuNPs via the coordination interaction between Cr3+ and 4-MBA. The 4-MBA-AuNPs were stabilized in the solution because the 4-MBA ligands on the surface of the AuNPs protected them from aggregation. 4-MBA has –COOH groups, which can be used to bind with the metal ions.
To assess the applicability of this colorimetric sensor for the analysis of real samples, an aqueous solution of Cr3+ was added into the interferents solution to prepare simulated samples that were then detected utilizing our Cr3+-induced colorimetric method. The results were shown that the simulated samples induced the colorimetric reaction that can be quantified by using UV-vis absorption spectroscopy (Fig. 9). This indicates that the colorimetric detection of Cr3+ is a practical tool for the determination of Cr3+ ions in real samples.
Conclusions
In conclusions, we studied 4-MBA, 4-NTP, and a mixture of 4-MBA and 4-NTP as ligand to modify the AuNPs for detection of Cr3+ions. The results showed that 4-MBA modified AuNPs exhibit a higher response and selectivity toward Cr3+ via the carboxyl group in 4-MBA. The detection can be observed by the naked eye or the UV–Vis absorption spectroscopy. The detection can be completed within 25 min over a linear range from 20 to 25 μM with the detection of limit of 5 μM. Therefore, our probe achieved rapid and sensitivity detection of Cr3+.
Availability of data and materials
Research data have been provided in the manuscript.
Abbreviations
- 4-MBA:
-
4-Mercaptobenzoic acid
- 4-NTP:
-
4-Nirothiophenol
- AuNPs:
-
Gold nanoparticles
References
Anderson RA. Chromium metabolism and its role in disease processes in man. Clin Physiol Biochem. 1986;4:31–41.
Bothra S, Kumar R, Sahoo SK. Pyridoxal conjugated gold nanoparticles for distinct colorimetric detection of chromium(III) and iodide ions in biological and environmental fluids. New J Chem. 2017;41:7339–46.
Cathum S, Brown C, Wong W. Determination of Cr3+, CrO
42–, and Cr2O72– in environmental matrixes by high-performance liquid chromatography with diode-array detection (HPLC–DAD). Anal Bioanal Chem. 2002;373:103–10.Chen H, Chen J, Wang L, Zhou C, Ling B, Fu J. A sensitive method for determination of trace amounts of chromate (III) with terbium (III) sodium hexametaphosphate chelate as fluorescent probe. Luminescence. 2011;26:434–8.
Chen M, Cai HH, Yang F, Lin D, Yang PH, Cai J. Highly sensitive detection of chromium (III) ions by resonance Rayleigh scattering enhanced by gold nanoparticles. Spectrochim Acta A. 2014;118:776–81.
Chwastowska J, Skwara W, Sterlinska E, Pszonicki L. Speciation of chromium in mineral waters and salinas by solid-phase extraction and graphite furnace atomic absorption spectrometry. Talanta. 2005;66:1345–9.
Gómez V, Callao MP. Chromium determination and speciation since 2000. TrAC, Trends Anal Chem. 2006;25:1006–15.
Glinsman Wh, Mertz W. Effect of trivalent chromium on glucose tolerance. Metabolism. 1966;15:510.
Gomez V, Callao MP. Chromium determination and speciation since 2000. TrAC, Trends Anal Chem. 2006;25:1006–15.
Guo Y, Wang Z, Qu W, Shao H, Jiang X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron. 2011;26:4064–9.
Guo Y, Zhang Y, Shao H, Wang Z, Wang X, Jiang X. Label-free colorimetric detection of cadmium ionsin rice samples using gold nanoparticles. Anal Chem. 2014;86:8530–4.
Hemmateenejad B, Safavi A, Honarasa F. Determination of nanoparticles concentration by multivariate curve resolution. Chemometrics Intellig Lab Syst. 2015;141:88–93.
Ji XH, Song XN, Li J, Bai YB, Yang WS, Peng XG. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J Am Chem Soc. 2007;129:13939–48.
Du JJ, Yin SY, Jiang L, Ma B, Chen XD. A colorimetric logic gate based on free gold nanoparticles and the coordination strategy between melamine and mercury ions. Chem Commun. 2013;49:4196–8.
Lin W, Zhou YS, Zhao Y, Zhu QS, Wu C. Cr3+/COO- complexation induced aggregation of gelatin in dilute solution. Macromolecules. 2002;35:7407–13.
Manjubaashini N, Thangadurai DT, Bharathi G, Nataraj D. Rhodamine capped gold nanoparticles for the detection of Cr3+ ion in living cells and water samples. J Lumin. 2018;202:282–8.
Mashhadizadeh MH, Amoli-Diva M. Atomic absorption spectrometric determination of Al3+ and Cr3+ after preconcentration and separation on 3-mercaptopropionic acid modified silica coated-Fe3O4 nanoparticles. J Anal At Spectrom. 2013;28:251–8.
Panda S, Pati PB, Zade SS. Twisting (conformational changes)-based selective 2D chalcogeno podand fluorescent probes for Cr(III) and Fe(II). Chem Commun. 2011;47:4174–6.
Parlayici S, Avci A, Pehlivan E. Electrospinning of polymeric nanofiber (nylon 6,6/graphene oxide) for removal of Cr (VI): synthesis and adsorption studies. J Anal Sci Technol. 2019;10:13.
Shuang L, Ren G, Fang C, Wu H, Qu F, Shuang L, Ren G, Fang C. Gold nanoparticles based colorimetric probe for Cr(III) and Cr(VI) detection. Colloids Surf Physicochem Eng Aspects. 2017;535:215–24.
Upadhyay Y, Bothra S, Kumar R, Sahoo SK. Smartphone-assisted colorimetric detection of Cr3+ using vitamin B6 cofactor functionalized gold nanoparticles and its applications in real sample analyses. 2018;3:6892–6.
Wei H, Yu J, Wang R, Chen J, Shi YP. “Green” colorimetric assay for the selective detection of trivalent chromium based on Xanthoceras sorbifolia tannin attached to gold nanoparticles. Anal Methods-UK. 2014;6:5720.
Wei ZW, Nian Bing L, Qun LH. Simultaneous determination of chromium(III) and cadmium(II) by differential pulse anodic stripping voltammetry on a stannum film electrode. Talanta. 2007;72:1733–7.
Yang WP, Zhang ZJ, Wei D. Simultaneous, sensitive and selective on-line chemiluminescence determination of Cr(III) and Cr(VI) by capillary electrophoresis. Anal Chim Acta. 2003;485:169–77.
Zhang ZK, Zhou Y, Yang JK, Wang PL, Su XO, Zhao H, He YJ, Cao ZQ, Luo MQ. Colorimetric detection of Cr3+ in aqueous solution based on cofunctionalized silver nanoparticles modified with 4-nitrobenzenethiol and 4-mercaptobenzoic acid. Nano. 2015;10:1550095.
Zhou Y, Zhao H, He YJ, Ding N, Cao Q. Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles. Colloid Surf. A-Physicochem. Eng. Aspect. 2011;391:179–83.
Acknowledgements
Not applicable.
Funding
The project is funded by the Youth Fund of Education Department of Hebei Province (No. QN2019230) and Research Fund for Doctoral Programs of Hebei University of Science and Technology (No. 1181267).
Author information
Authors and Affiliations
Contributions
ZKZ, YML, and RJL designed the experiment. ZKZ and YXJ carried out the experimental studies and collection, analysis, and interpretation of data. ZKZ wrote the manuscript. QQL helped to draft and revise the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Ye, X., Liu, Q. et al. Colorimetric detection of Cr3+ based on gold nanoparticles functionalized with 4-mercaptobenzoic acid. J Anal Sci Technol 11, 10 (2020). https://doi.org/10.1186/s40543-020-00209-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-020-00209-7