Abstract
Background
Over the past decade, the great impact of agricultural crop diseases has generated considerable economic losses and has compromised the production of edible crops at a time when the world population is only expected to rise, leading to the search for new pest management strategies. Besides that, the environmental impact resulting from the continued use of chemical pesticides has led to the search for natural and sustainable alternatives. One of the existing solutions that currently stands out for its effectiveness is the use of bioactive plant extracts. This study aims to evaluate the antimicrobial activity of propyl propane thiosulfinate (PTS) and propyl propane thiosulfonate (PTSO), two organosulfur compounds (OSCs) derived from Allium cepa, against a wide range of target bacteria and fungi. To this end, various in vitro procedures were conducted as well as soil sanitization tests using sterile substrate inoculated with soil-borne pathogens. In addition, this study also evaluates the pesticidal activity of both compounds through in vitro mortality and repellence tests.
Results
PTS and PTSO revealed inhibition activity on all the pathogens tested, belonging to different taxonomic groups. Moreover, both significatively reduced the population of bacteria and fungi in soil. The quantification of active substances in soil carried out in parallel to the microbial quantification showed that their use reduces the risk of residue accumulation since they break down quickly when applied. The set of antimicrobial tests performed demonstrated that the antifungal effect of both compounds is higher than the bactericidal effect. Lastly, PTS and PTSO showed a concentration-dependent significant biocidal and repellent effect against aphids.
Conclusions
The results presented in this work demonstrate that both PTS and PTSO have a significant antimicrobial and pesticidal activity against the great majority of phytopathogens tested, being a promising tool to improve pest management in crops.
Graphical Abstract

Similar content being viewed by others
Background
The global human population is predicted to number between 9.4 and 10.1 billion in 2050, an increase by about 1.5 billion compared to 2020 [80]. Around 80% of agricultural production is dedicated to human nutrition [24]. This includes not only the direct use of agricultural products as food, but also the use of crops and other vegetal matter to feed animals, which are in turn intended for human consumption. Keeping in mind the estimated population growth, the production of edible crops might need to increase by up to 119% [20]. This imposes a serious challenge, which involves adopting changes to ensure the transformation of agricultural and food systems toward greater sustainability, and to reduce waste and spoilage [54].
Plant diseases caused by biotic factors are the main responsible for the decrease in crop productivity; in fact, pests and pathogens bring about 40 billion dollars losses a year worldwide [77], which means reductions between 21 and 30% globally in major crops [68]. Plant pathogen control will be even more challenging as climate change conditions progress [11]. Since the environment has a great impact on plant pathogenesis [70], global warming is directly related to disease incidence and severity [81]. Higher temperatures are correlated with soil degradation and less water availability, and foster the emergence of new pathogens and a changeable geographic distribution [67].
Over the last several decades, synthetic agrochemicals have contributed to increase food production worldwide through controlling crop diseases, but with a severe environmental impact [65]. Their application has not only gradually disrupted biological control by natural enemies, but also caused disease outbreaks and the development of resistance [57]. Moreover, synthetic pesticides severely damage non-target organisms, such as pollinators, and human health [73].
In this regard, plant-derived secondary metabolites are receiving increasing attention and gradually replacing synthetic biocides and soil disinfectants from disease management protocols [85]. Many products based on antimicrobial phytochemicals isolated from plants have been developed over the past few years as novel eco-friendly non-synthetic plant protection measures [55]. The extraction of phytochemicals from medicinal and fragrant plants is quite common [35, 58]; however, the extraction of bioactive compounds from by-products from the food industry or second-class plant material, such as grape cane waste [63] and pepper leaves [56], through clean extraction methodologies has become an innovative strategy that contributes to the revaluation of agricultural waste and support circular economy [71].
In recent years, the functional properties of organosulfur compounds (OSCs) obtained from onion (Allium cepa) and garlic (Allium sativum), such as antioxidative, immunomodulatory and antimicrobial activity, have been deeply studied [61]. OSCs are secondary metabolites that are biosynthesized by the plant as a defence mechanism against biotic and abiotic stressors [61]. Garlic bulbs are rich in alliin (S-allyl cysteine sulfoxide) and in a lower degree methiin (S-methyl-l-cysteine sulfoxide), while onion bulbs contain methiin but also isoalliin (S-propenyl-l-cysteine sulfoxide) and propiin (S-propyl-l-cysteine sulfoxide) [61]. Cysteine sulfoxides are natural constituents of fresh bulb tissue, non-volatile and odourless [10, 66]. The disruption of the bulb tissue triggers an enzymatic reaction carried out by alliinase, that catalyses the conversion of these precursors to thiosulfinates [62], volatile compounds to which the antimicrobial activity of Allium genus plants are mainly attributed and bear the primary responsibility for their organoleptic properties [41, 60].
According to the existing literature, the antimicrobial effect of thiosulfinates is primarily due to their ability to inhibit thiol-containing enzymes by oxidizing protein cysteine or glutathione residues [8]. Enzymes containing thiol include the main enzymes of microbial metabolism as well as bacterial enzymes of the acetyl-CoA-forming system, and RNA polymerase [8]. In onion, propiin turns into propyl-propane thiosulfinate (PTS), a labile compound that changes into dipropyl disulphide and propyl-propane thiosulfonate (PTSO) through dismutation or disproportionation reactions [27].
Whereas bioactive properties of Allicin—that represent about 75% of thiosulfinates in garlic—has been thoroughly investigated in several fields of study, from antimicrobial therapy for human infections to integrated pest management [15, 19], information regarding the antimicrobial activity of PTS and PTSO from onion is limited and focused on the potential of thiosulfinates against human and animal infections. Recent studies have shown broad-spectrum antibacterial activity of PTS and PTSO and its gaseous form against clinical isolates of bacteria and Candida species that are resistant to at least one group of antibiotics [74, 75]. In a previous study we demonstrated the in vitro and in planta antifungal activity of volatile organosulfur compounds PTS and PTSO from onion against Verticillium dahliae [23], the most devastating soil-borne pathogenic fungi affecting olive trees [51]. Moreover, the same study showed the potential of both compounds as soil sanitizers, as they reduced V. dahliae population in an artificially infested substrate. As previously mentioned, PTS and PTSO are volatile compounds [42]. Owing to their low molecular weight (< 300 g/mol), they can diffuse through plant cell membranes and soil, playing a key role in the functioning of the whole ecosystem [34]. The study of the active properties of their gaseous phase is thus of interest to back up their use against phytopathogens and in pest management systems, especially in the present context in which the search for alternatives to conventional pesticides has become one of the main focuses of modern agriculture research [33].
Within this context, the aim of the present study was to evaluate the bactericidal, fungicidal, pesticidal and repellent activity of PTS and PTSO obtained from low-quality onions not suitable for human consumption, through in vitro methodologies and performing soil sanitization trials.
Materials and methods
Compounds and reagents
Standardized fractions of PTS and PTSO at 20% were supplied by DOMCA SA (Granada, Spain). Both compounds were obtained from onions that had been discarded as they were not suitable for human consumption, following the methodology described by Hu et al. [30] to obtain the allyl derivatives form garlic. Summarizing, onions were chopped and immersed in a solution of Ethanol (70%) in a percentage equivalent to four times their weight. The extraction was carried out for 2 weeks at room temperature, then the mixture was filtered, and the solution was concentrated and extracted with Ethyl acetate (EtOAc). The EtOAc extract was concentrated and fractionated by 2 sequential column chromatography’s, taking the trichloromethane (CHCl3) fraction from the first column, and then using EtOAc/hexane as mobile phase in the second column to obtain purified PTS and PTSO. All reagents were purchased from Sigma-Aldrich Química S.L. (Spain), unless otherwise stated.
Phytopathogens strains and growth media used
Bacteria and fungi used in this study were obtained from the Spanish Collection of Type Cultures (CECT), the German Collection of Microorganisms and Cell Cultures (DSMZ), the plant pathogen collection of DMC Research and the culture collection of the Department of Crop Protection, Institute for Sustainable Agriculture, Spanish National Research Council (Córdoba, Spain), which are listed in Table 1. Each phytopathogen grew on a specific culture medium and time, indicated by the corresponding culture collection. For the antimicrobial activity tests against pathogenic bacteria, Mueller–Hinton Agar and Mueller–Hinton broth supplied by Scharlau (Barcelona, Spain) were used as culture media [18]; for the in vitro antimycotic test, Rose-Bengal agar supplied by Scharlau and RPMI-1640 medium with l-glutamine [17] supplied by Labclinics (Barcelona, Spain) were used.
Insects
Adult individuals of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae) were supplied by TECNOVA Technological Center (Almería, Spain). The individuals were reared on courgette leaves at the TECNOVA Experimental Center greenhouse for future experiments.
Soil
The soil used in this study was superficially collected from an olive grove in Linares, Jaen (30U 444908.38 4209274.77 UTM WGS84), owned by the cooperative DCOOP, the world's largest producer of olive oil. This soil was chosen because no biocide product had been applied on the farm in the last two years, as it was the control farm of an experimental field trial.
The soil was dried in an oven at 50 °C and passed through a 2 mm pore sieve to remove plant material, soil macrofauna and stones [9]. Then, it was stored in polyethylene bags for future analysis and characterized according to the procedures previously described. Soil pH, which was determined in a 1:1 water suspension according to the international standard (International Society of Soil Science, ISSS), was 8.71 ± 0.01, that is to say, moderately basic according to the criteria established by the United States Department of Agriculture [48]. Moreover, the soil, classified as sandy loam, contained 6.5 ± 0.22% fine silt, 4.9 ± 0.85% coarse silt, 21.5 ± 0.07% clay, 67.1 ± 0.72% sand (determined through Robinson pipette method [64]), and 1.13% organic matter (soil organic matter fractionation was measured according to [79]). Lastly, this soil presented a maximum Water Holding Capacity (mWHC) of 0.414 g H2O per g soil dry matter (determined by the Keen—box method [37]). Based on this parameter, it was determined that the soil had 95.46% dry matter of field-moist soil and a water content of natural moist soil of 0.04 g water/g dry matter.
In vitro antimicrobial activity against pathogenic bacteria
The antibacterial activity of organosulfur compounds PTS and PTSO was evaluated by performing different testing procedures. The disk diffusion method proposed by Bauer et al. [1] and modified by Calvo and Asensio [14] was used to evaluate the antibacterial activity. Agar plates were inoculated using bacterial suspension adjusted to 106 CFU/ml, so that the growth after incubation was confluent. Sterile 6 mm cellulose disks (Whatman® antibiotic test discs, Buckinghamshire, UK) impregnated with 20 µl of PTS or PTSO at 5, 10 and 25 µg/µl were placed in the centre of inoculated agar plates. The inhibition zone of bacterial growth was measured after 48 h incubation.
Determination of the minimum bactericidal concentration (MBC) was performed by the broth microdilution method, following the guidelines of the Clinical and Laboratory Standards Institute (CLSI) collected in the standard Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically [18], to establish the lowest concentration of each antimicrobial agent that reduces the viability of the initial inoculum by 99.9%. 1:2 decreasing dilution were prepared from an initial solution of each compound at 10,000 µg/ml so that the following concentrations were obtained: 5000; 2500; 1250; 625; 312.5; 156.25; 78.125; 39.06; 19.53; and 9.76 µg/ml. Each dilution was inoculated with bacterial suspension so that the final concentration in each well was 105 CFU/ml, and incubated overnight at room temperature. As positive control, a mix of ampicillin and streptomycin (100,000 and 25,000 µg/ml, resp.) was used. As negative control, liquid media without antimicrobial agent was inoculated with bacteria. Bacterial growth was tested by culturing in agar plates, and the lowest concentration of PTS/PTSO in which no growth was observed was established as the MBC.
The antibacterial activity of the gaseous phase of PTS and PTSO was assessed through a previously described procedure [23]. Bacterial suspensions adjusted to 106 CFU/ml were spread on agar plates. Sterile 6 mm cellulose disks were placed, not in the centre of the plate, but in the centre of the lid of the petri dish, and they were impregnated with 20 µl of PTS or PTSO solutions. The same PTS and PTSO concentrations as in the disk diffusion assay were used, i.e., 5, 10 and 25 µg/µl. Plates were incubated for 48 h and subsequently growth inhibition zones were measured. All in vitro assays were performed in duplicate.
In vitro antimicrobial activity against pathogenic fungi
The antifungal activity of PTS and PTSO was evaluated following the same methodology described for bacteria, using the appropriate liquid and solid media indicated in section “Phytopathogens strains and growth media used”. The disk diffusion method and the gas phase activity test were carried out with no modifications, with the exception of the incubation time, which was 5 days. Regarding the determination of the Minimum Fungicidal Concentration (MFC), the broth microdilution method was also carried out according to the standard reference method for broth dilution antifungal susceptibility testing of filamentous fungi of the CLSI, which does not differ from that described for the determination of MBC [17]. For the positive control, natamycin (50,000 µg/ml) was used instead of ampicillin and streptomycin.
Moreover, in a fourth trial, the influence of both organosulfur compounds on mycelial growth was determined. Different volumes of PTS and PTSO at 20% were added to Rose-Bengal medium to obtain supplemented agar plates at 25, 50, 100, 250 and 500 µg/ml. Each fungal strain was grown on Rose-Bengal agar for 3 days. From these cultures, agar plugs of 5 mm diameter were obtained, which were distributed among the supplemented agar plates [47]. In addition, non-supplemented plates with 5 mm agar plugs from each strain tested were incubated as control of fungal growth. For 17 days of incubation the diameter of the mycelium over time was measured, compared to the mycelial growth of each fungus when growing on non-supplemented Rose Bengal agar plates. Each experiment was repeated twice.
In vitro activity against aphids
In this study, the contact toxicity and repellent activity were evaluated for a liquid blend of PTS and PTSO in proportion 1:1 (w/w) at different concentrations (5000; 2500; 1000; and 500 µg/ml). Since the treatments were prepared in water, blank control included only water.
The contact toxicity of PTS and PTSO was assessed by the leaf immersion method, as previously reported [69]. Circular cuttings of courgette leaves of 55 mm diameter were immersed in the treatment and control solutions for 5 s, air-dried and placed on 60 mm diameter petri dishes. Twenty-four adults were transferred to each treated leave cutting in petri dish using a brush. Decis® Protech, a deltamethrin-based pyrethroid insecticide purchased from Bayer CropScience S.L. (Barcelona, Spain) (Ref 84942464) was used as positive control at the dose indicated on the label. The plates were wrapped with Parafilm® purchased from amcor (Valencia, Spain) to prevent the aphids to scape, and maintained in a climate chamber at 25 ± 1 °C, 75 ± 5 relative humidity and Light:Dark photoperiod of 14:10 [89]. Mortality was recorded after 24 h. An aphid was considered dead if it did not move its legs when touching its abdomen with a brush and if the body turned black [86, 87].
The repellent activity was assessed by a choice assay in 90 mm diameter petri dishes [72]. N, N-diethyl-meta-toluamide (DEET), an active ingredient used in many repellent products, was purchased from Sigma–Aldrich at 97% (Ref. D100951) and diluted to 2000 µg/ml as positive control [32]. Circular cuttings of courgette leaves of 25 mm diameter were immersed in the treatment and control solutions for 5 s and dried at room temperature, as in the previous trial. A treated leaf and a negative control leaf were placed in each petri dish on a moist filter paper disk to maintain humidity [88]. Then, 24 adults were introduced into each petri dish using a brush. The parafilm-sealed petri dishes were maintained in the conditions previously indicated. The repellent effect was observed after 24 h and expressed as percentage of repellence according to the following formula [59]:
where C is the number of aphids on the control leaf, and T is the number of aphids on the treated leaf. Each experiment was performed in triplicate.
Soil sanitization
The study of the persistence of phytopathogenic microorganisms in soil was carried out by microcosm systems [22]. The ability of a powder blend of PTS and PTSO in proportion 1:1 (w/w) to reduce the population of a pathogenic microorganism artificially inoculated in soil was determined against the bacterium A. tumefaciens and the fungus F. oxysporum, both pathogens that inhabit the soil, where they can survive for long periods [49, 90]. Two different concentrations of active substances, 100 µg/g (50 µg/g of each one) and 500 µg/g (250 µg/g of each one), were tested; and the efficacy of a treatment based on a single application was compared with the efficacy of a treatment consisting of 3 applications of the same dose separated in time. In addition, non-inoculated soil was used as sterility control, while untreated inoculated soil was used to follow up microbial growth. Finally, as positive control, the assay included a study group of inoculated soil that was treated with soil fumigant Metam sodium (C2H4NNaS2) (EPA Reg. No. 45728-16) [76]. Metam sodium 42.1% aqueous solution was purchased from Eastman Chemical Company (Madrid, Spain), diluted in sterile water and set to 60 µg/g, in accordance with the recommended application rates [44]. Each study group consisted of four replicates. Table 2 shows the experimental design of the assay and details of the different groups of study.
The experimental microcosm unit consisted of a polypropylene box with a drainage system, of 28 cm length × 5 cm width × 17 cm height and 1.75 l capacity. The soil was autoclaved through 4 cycles of 20 min at 121 °C in a steam sterilizer (Raypa, Terrasa, Spain) for 4 successive days [38], interspersed with incubations at 4 °C, to eliminate vegetative forms by heat shock [45]. After drying in an oven at 50 °C, 700 g of sterile soil were introduced into each microcosm unit on a bed of sterile gravel to facilitate drainage and prevent soil compaction. Subsequently, soil was inoculated with 140 ml of microbial suspension previously adjusted to 109 CFU/ml, so that the soil moisture was adjusted to 60% of the water holding capacity (WHC) [82]. The negative control group was inoculated with 140 ml of distilled water. Microcosms were then placed in a room at 25 °C (optimal growth temperature of the two phytopathogens used), where they were kept until the end of the trial. Four days after inoculation, every study group was sampled to establish the starting microbial population. Next, the treatments were applied to the corresponding group at the appropriate dose. Microbial population was quantified 1, 2, 4, 7, 11, 15, 31 and 45 days after treatment. The second and third application of the PTS/PTSO powder treatment was added to the corresponding microcosm units 10 and 30 days after the first application. At each enumerated date, 25 g of soil were diluted in 225 ml of buffered peptone water (Scharlau). A lab paddle blender (MASTICATOR, UIL, Barcelona, Spain) was used to homogenize the samples. Serial dilutions were prepared from the supernatant, cultured in the appropriate solid medium, and incubated at 25 °C for 3 days [21]. In the cases in which no microbial growth was observed on the plate, to confirm the absence of microorganisms a pre-enrichment step was carried out in a non-selective nutrient medium. Microorganism population was expressed as Log 10 CFU/g soil.
PTS and PTSO concentrations achieved by each application protocol were assessed by High-performance liquid chromatography using a UV detector (HPLC–UV). Fifty grams of soil were mixed with 100 ml of acetone, homogenized with vortex for 1 min and extracted in a sonication bath for 10 min. Supernatant was separated from the soil by filtration and the process of extraction was repeated adding 20 millilitres of acetone to the solid residue. Then, the supernatant from both extractions was evaporated until dryness in a vacuum rotator and reconstituted with 10 ml of methanol (MeOH) vortexing for 30 s. Finally, the extract was filtered through a nylon filter of 0.2 µm (Sigma–Aldrich, Darmstadt, Germany) and injected into the HPLC system. For the PTS and PTSO determination, an Agilent 1260 Infinity LC (Agilent Technologies, Santa Clara, CA, USA) system was used. The separation of the compounds was accomplished using a Zorbax Eclipse Plus RRHD (50 × 2.1 mm, 1.8 mm) column at 25 °C, and the gradient and mobile phases described by Sorlozano-Puerto et al. [75]. Wavelength of detection was set at 200 nm. A calibration curve using PTS and PTSO standards was made for the quantification.
Statistical treatment
GraphPad prism 8.0 software (GraphPad Software Inc., San Diego, California) was used for statistical analysis. The data obtained in the in vitro antimicrobial activity assays were analyzed using descriptive statistics. Shapiro–Wilk normality tests were used to determine normal distribution of all data subjected to ANOVA. A one-way ANOVA test supplemented with Tukey’s post hoc test was used to compare every treatment and control of the in vitro assays against aphids with each other. Repeated measures two-way ANOVA test supplemented with Dunnett’s post-hoc test was used for evaluation of statistically significant inhibition of mycelial growth and to establish significant differences between microorganism survival in treated soil and the positive control, considering different treatments and time points. Differences were considered statistically significant when p < 0.05.
Results
In vitro assessment of antibacterial activity
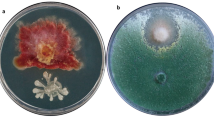
The antibacterial activity of PTS and PTSO was tested against different bacteria involved in infectious processes of agricultural crops. As shown in Table 3, both compounds displayed antimicrobial activity against all the bacterial strains included in the study in various degrees. Moreover, in most cases the bacteriostatic effect rises as the concentration of the product increases, being considerably more modest in the case of P. syringae. Regarding the volatility-linked activity assay, PTS and PTSO inhibited growth of all bacteria tested without coming into direct contact with either the medium or the microorganism, but rather by diffusion of their gas phase, as shown in Fig. 1. Xanthomonas campestris, C. m. michiganensis and A. tumefaciens were the most sensitive, showing growth inhibition zones of at least 40 mm in all cases (Tables 3 and 4). Erwinia persicina and specially P. syringae were, on the other hand, the most resistant strains to PTS and PTSO in both tests, with the smallest inhibition zones among the strains studied. Furthermore, in both agar tests PTSO displayed a greater capacity to inhibit the growth of P. savastanoi, P. syringae, C. m. michiganensis and A. tumefaciens than PTS. Whereas the results of the diffusion and volatility tests in agar appear to suggest that PTSO may have higher antibacterial capacity, the results obtained in the MBC test indicated that it was significantly more active than PTS. As shown in Table 5, the lower MBC of PTS is 156.25 µg/ml (Me = 312.5 µg/ml), while MBC data for PTSO ranges from 156.25 to 19.53 µg/ml (Me = 78.125 µg/ml).
In vitro antimycotic activity
As for antifungal activity, all phytopathogenic fungi used in this study were sensitive to both organosulfur compounds in a dose dependent manner according to the results of the disk-diffusion method, presented in Table 6. Table 7 details the results obtained in the MFC determination. In all cases, the MFC of PTSO for each strain was at least one dilution lower than the corresponding concentration of PTS. The highest values obtained, which ranges from 625 to 156.25 µg/ml, correspond to the two Penicillium species used. On the other hand, A. alternata, F. oxysporum and F. graminearum were the most sensitive, displaying PTSO MFC values of 39.06, 19.53 and 9.76 µg/ml, respectively. The fungicidal activity of the gas phase of the compounds was also demonstrated, since the volatility test generated growth inhibition halos whose diameters were similar to the halos obtained by the agar diffusion test (Table 8 and Fig. 2).
To complete the in vitro assessment of antimycotic activity, the influence of both organosulfur compounds on the mycelial growth was studied. The results of the mycelial growth inhibition test, represented in Figs. 3 and 4, also indicates that most fungal species were sensitive to at least the two highest concentrations evaluated (250 and 500 µg/ml), showing significant inhibition (p < 0.05). In the case of F. graminearum, which, as in the MFC test, turned out to be the most sensitive, all concentrations of both products completely inhibited the mycelial growth (p < 0.0001), with the exception of PTSO at 25 µg/ml. Moreover, the treatments of 50 and 100 µg/ml of PTS and PTSO successfully controlled the development of P. cinnamomi, Phyllosticta spp and F. graminearum in agar plates. On the other hand, only the exposure to PTS and PTSO at 500 µg/ml achieved a significant reduction of P. digitatum and G. candidum growth (p < 0.05). Penicillium expansum was the most resistant fungal strain, since none of the evaluated concentrations of PTS and PTSO significantly inhibited mycelial development. Although in the case of the other fungal strains no differences were observed between compounds, the effectiveness of PTSO was lower against Phyllosticta spp, F. oxysporum and F. graminearum.
Mycelial growth of fungi on agar plates supplemented with PTS (left column) and PTSO (right column) at 25, 50, 100, 250 and 500 µg/ml over time compared to control, using the Dunnett test at a 95% confidence level. Values are means with SD in bars. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 respect to control. 1 PTS 2 PTSO a G. candidum b A. alternata c Phyllosticta spp. d P. cinnamomi
Mycelial growth of on agar plates supplemented with PTS (left column) and PTSO (right column) at 25, 50, 100, 250 and 500 µg/ml over time compared to control, using the Dunnett test at a 95% confidence level. Values are means with SD in bars. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 respect to control. 1 PTS 2 PTSO e F. oxysporum f F. graminearum g P. expansum. h P. digitatum
In vitro activity against aphids
Insecticidal and repellent capacity of PTS and PTSO were assessed against adult aphids and presented in Fig. 5. In the contact toxicity assay, the mortality rate of the aphid population after 24 h of exposure to impregnated leaves was investigated. We found that the exposure of A. gossypii to both compounds significantly reduced the population at all concentrations evaluated with respect to the negative control (p < 0.05). The average mortality of aphids treated with 500 and 1000 µg/ml of PTS and 500 µg/ml of PTSO were 17, 19 and 19% respectively, which is lower than the mortality rate of the population treated with the insecticide used as positive control (28%). On the other hand, the death of individuals treated with PTS ≥ 2500 µg/ml and PTSO ≥ 1000 µg/ml equalled or exceeded the mortality rate of the positive control. However, none of the treatments showed significant differences compared to the positive control, except for the group of aphids treated with PTSO at 5000 µg/ml which registered a significant increase of mortality, reaching 42% after 24 h of exposure (p < 0.05).
In vitro activity of different concentrations of PTS and PTSO against A. gossypii: a Mortality rate due to contact toxicity expressed as percentage, b Repellent activity expressed as percentage. Each panel includes a group in which the leaves cuttings were immersed in water (C −), in a commercial biocide/pesticide (C +) and in PTS or PTSO at 500; 1000; 2500 and 5000 µg/ml. For both panels, bars with different letters indicate significant differences according to Tukey test (p < 0.05)
Moreover, whereas the insecticidal capacity of PTSO was found to be higher than that of PTS, the behaviour of both compounds was quite homogeneous in terms of repellent activity. At concentrations of 500 and 1000 µg/ml, PTS and PTSO demonstrated a repellent action of 53–56% and 39–47%, respectively. Even though these treatments showed no significant differences, neither between them nor with respect to the positive control (39% repellence), the responses were stronger with higher concentrations, displaying a significant increase of the repellent activity with respect to the control (p < 0.05).
Antimicrobial effect and PTS/PTSO quantification in soil
When non-treated, A. tumefaciens and F. oxysporum generated growth curves according to what was expected, reaching the concentration of 108 CFU/g of soil at the end of the study. Although Metam sodium and the two concentrations of PTS and PTSO tested significantly reduced the population of A. tumefaciens and F. oxysporum when compared to the untreated soil (p < 0.05), the behaviours of the bacterium and the fungus in the presence of the antimicrobials were very unlike. As shown in Fig. 6, within 24 h of exposure A. tumefaciens density were narrowed 3 logarithmic units (from 107 to 104 CFU/g) and, surprisingly, thereupon the population remained steady till the end of the assay. In contrast to the bacterial response, F. oxysporum was drastically affected by all the treatments during the first 11 days of sampling. As illustrated in Fig. 7, from day 15, the progressive recovery of the fungus was observed in soil treated with Metam sodium and a single application of PTS and PTSO at 100 and 500 µg/g, being less pronounced with 500 µg/g. During the 45 days of the assay, F. oxysporum could not be isolated from the soil treated with 3 applications of PTS and PTSO at both 100 and 500 µg/g, which suggests a greater efficacy of the treatments based on repeated lower doses compared to those including a single initial dose at a higher concentration.
Density of A. tumefaciens in soil expressed as Log10 CFU/g of soil over time. Significant reductions in the population of each treated microcosm in comparison with the non-treated group (control) were established according to the Dunnett test at a 95% confidence level. Values are means with SD in bars. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 respect to control
Density of F. oxysporum in soil expressed as Log10 CFU/g of soil over time. Significant reductions in the population of each treated microcosm in comparison with the non-treated group (control) were established according to the Dunnett test at a 95% confidence level. Values are means with SD in bars. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 respect to control
Figure 8 shows the concentrations of PTS and PTSO detected in soil by HPLC–UV. In each of the treatments, both compounds evolved in the same way over time. Due to their volatility, neither PTS nor PTSO remained at the established concentration in soil 1 h after being applied, time at which the first determination was made ([PTS] = 16.04 ± 0.36 and 112.20 ± 0.1168 µg/g instead of 50 and 250 µg/g) ([PTSO] = 37.02 ± 0.54 and 159.20 ± 2.11 µg/g instead of 50 and 250 µg/g). The steady reduction in PTS and PTSO concentration explains why the treatment based on 3 applications of 100 µg/g of active compounds obtained better results than those based on 1 single dose of 500 µg/g for both, A. tumefaciens and F. oxysporum.
Additionally, although the doses administered of PTS and PTSO for each application were equal (50 µg/g or 250 µg/g of each, depending on the total concentration of active substance), PTSO was shown to be somewhat more stable since its concentration decreases less drastically.
Discussion
In this work, PTS and PTSO have demonstrated broad antimicrobial activity against phytopathogens that represent a significant threat to many economically important crops, including 6 bacterial and 8 fungal strains.
Organosulfur compounds PTS and PTSO antimicrobial activity had been previously studied against a wide range of human and animal pathogens, including those affecting the poultry industry and aquaculture [2, 12, 74]. Despite of its instability, allicin significant microbicidal effect has been demonstrated on soil-borne plant pathogenic fungi [4] and, to a lesser extent, on phytopathogenic bacteria [84]. However, there is a very little information available that refers to the bioactive properties of PTS and PTSO against phytopathogens and plant pests, and that supports their application within the framework of plant health. Hayat et al. [28] found that potent broad-spectrum biofungicidal effect of Alliaceae extracts might not only be attributed to allicin but also to other organosulfur compounds, stressing the importance of identify such compounds and their bioactivity. Previous reports have suggested that onion essential oils, and particularly PTS and PTSO, have potential applications in post-harvest preservation [7, 53]. The present investigation is so far the first study to characterize the activity of these compounds in vitro against a wide range of target organisms implicated in plant disease breakouts.
Our results indicate that PTSO is more active than PTS against bacteria, what was also reported by Sorlozano-Puerto et al. [74]. Similarly, the higher fungicidal activity of PTSO was also observed in comparison to PTS but, interestingly, no differences were appreciated between both compounds with regard to the fungistatic effect, which was assessed through the mycelial growth inhibition method. This has already been described by our group in previous research on the antifungal activity of PTS and PTSO against the soil-borne pathogenic fungus V. dahliae [23]. Additionally, according to the results presented in this study, both compounds preserve the antimicrobial activity in their gas phase. However, when compared the MFC and MBC values of PTS and PTSO, it was found that their antifungal effect was higher than their antibacterial effect. These results are consistent with the data obtained in previous in vitro investigations in which the antimicrobial capacity of PTS and PTSO was examined against yeasts and bacteria for their potential use in human therapy [75].
On the basis of our findings, the persistence of a soil-borne pathogenic bacterium and fungus in soil when treated with PTS and PTSO was assessed. As acknowledged by Arnault et al. [4], the potential of thiosulfinates contained in Allium species to be employed in pest management and, in particular, to serve as biofumigants, has yet to be investigated. From the results obtained in this study it can be concluded that PTS and PTSO have a significant effect on soilborne pathogens. Furthermore, we have established a correlation between the antimicrobial effect of both compounds and their persistence in soil. PTS and PTSO quantification in soil by HPLC–UV demonstrated that both rapidly volatilize in varying degrees, being PTSO more stable than PTS. Due to their high volatility, the concentration of PTS and PTSO in soil decreases immediately after the application, which allows the microorganism to recover. It can therefore be concluded that their antimicrobial activity in soil is not only linked to the concentration applied initially but, more importantly, to their persistence in soil, which highlights the importance of establishing an optimal application protocol based on repeated doses over time. What is more, despite not being the objective of this study, the evaluation of the antifungal activity of PTS and PTSO in olive plantlets carried out in a previous work of our group evidenced the absence of phytotoxicity, not observing any effect on the appearance or development of the plants [23]. The absence of phytotoxicity and the non-permanence of the compounds in soil supports the safety profile of PTS and PTSO as well as their use as a sustainable alternative for pre- and post-plant soil fumigation. However, further studies on how these compounds influence plant physiology and stress response mechanisms are needed.
Additionally, the results obtained in the soil sanitization assay indicate that PTS and PTSO antifungal activity is higher than their antibacterial activity, which had previously been suggested according to the in vitro antimicrobial activity tests performed in this investigation. While the high permeability of volatile organosulfur compounds through the phospholipid membranes and their ability to interact with thiols containing compounds may explain their antimicrobial activity [28, 50], the higher permeability of the fungal chitin cell wall compared to the peptidoglycan cell wall of bacteria support our findings that PTS and PTSO influence fungal growth to a greater extent [39, 43]. Another explanation might be the interaction of PTS and PTSO with small secreted cysteine-rich proteins (SSCPs), specific of the secretomes of filamentous and dimorphic fungi [25]. These molecules present a broad functional versatility and, although most of them remain unclassified, it has been demonstrated that SSCPs have an essential role in fungal reproduction and dispersal [13, 86, 87]. They are also involved in environmental interactions, fungi-plant interactions and substrate colonization [26, 83]. Lu and Edwards [46] functionally characterized SSCPs of F. oxysporum by their detection in the secretome by nano LC–MS/MS and the subsequent identification in infected wheat heads through gene expression profiling, thus demonstrating the role of SSCPs in the pathogenesis of the disease caused by F. oxysporum in wheat.
According to several studies, antimicrobial plant derived compounds might also possess insecticidal and repellent activity within their bioactive properties [72]. Jiang et al. [32] found that linalool is responsible for the insecticidal and repellent activities of Cinnamomum camphora essential oil against cotton aphids. Similarly, other authors showed that glucosinolates extracted from Tropaeolum tuberosum and capsaicinoids extracted from Capsicum Chinese can successfully control Aphis cytisorum [16]. Insecticidal and repellent activity of volatile compounds of onion and garlic had already been established against aphid Myzus persicae by contact toxicity test and choice test using commercial essential oils [29]. In addition, a non-standardized blend of aqueous extracts of onion and garlic was effectively used as an alternative pesticide that was able to significantly reduce aphids’ population of different species infecting date palm trees, as reported by Ali Al-Shuraym [3]. In this study we have also demonstrated the effect of PTS and PTSO against the aphid species A. gossypii. Our in vitro results showed that the mortality rates achieved by both compounds equals that of commercial chemical insecticide from a concentration of 0.25% PTS and 0.1% PTSO. What is more, the repellent capacity exhibited by 0.1% PTS and PTSO significantly exceed the effect of DEET. While PTSO shows greater biocidal activity against A. gossypii, the greater volatility of PTS compared to PTSO, as demonstrated by HPLC–UV determination, explains why PTS has a greater repellent capacity.
Bioactive compounds from plant material with proven antibacterial, antifungal and insecticidal activity are an important natural source for the development of new environmentally safe plant protection products [5, 31]. Onion is one of the most cultivated and consumed vegetables worldwide [78]. Taking into account those that do not reach the consumer due to their low quality and the inedible parts, onion processing creates massive wastes that are a rich source of bioactive compounds [6, 52]. Therefore, to orientate the valorisation of onion solid wastes towards the formulation of novel and sustainable products suitable for agricultural production could not only reduce the environmental impact but also cover important needs for the agrifood sector [36, 40].
Even thought our results suggest that PTS and PTSO from A. cepa display strong activity against pathogenic microorganisms and aphids, and provide useful information that support their use for crop disease control, the present work has not evaluated how these organosulfur compounds influence soil microbial populations, natural enemies or pollinators. Therefore, further studies should focus on the analysis of the effect of PTS and PTSO on soil microbiome and non-target species.
Conclusions
PTS, and specially PTSO, showed antimicrobial effect against a wide range of bacteria and fungi infecting plants. This study revealed that the degree of efficacy of PTS and PTSO depend on the target species, being more effective against the fungal strains evaluated. Despite their rapid volatilization from soil, the combination of these compounds successfully controlled soilborne microorganism population. Both of them had similar, if not more, biocidal and repellent effect than commercial fumigants. Although PTS and PTSO will be further evaluated in field experiments for potential control of pathogen populations in crops, these results encourage their use for the development of sustainable biopesticides that contribute to environmental health. Moreover, both metabolites are found to be promising candidates for Integrated Pest Management, whose bases include sustainable pest control, reduction of pesticide residues and the use of natural resources.
Availability of data and materials
All data generated during this study are included in this manuscript.
Abbreviations
- PTS:
-
Propyl-propane thiosulfinate
- PTSO:
-
Propyl-propane thiosulfonate
- OSCs:
-
Organosulfur compounds
- EtOAc:
-
Ethyl acetate
- CECT:
-
Spanish collection of type cultures
- DSMZ:
-
German collection of microorganisms and cell cultures
- WHC:
-
Water holding capacity
- MBC:
-
Minimum bactericidal concentration
- CLSI:
-
Clinical and Laboratory Standards Institute
- MFC:
-
Minimum fungicidal concentration
- DEET:
-
N, N-diethyl-meta-toluamide
- HPLC–UV:
-
High-performance liquid chromatography UV detector
- MeOH:
-
Methanol
- SSCPs:
-
Small secreted cysteine-rich proteins
References
Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6.
Aguinaga-Casañas MA, Mut-salud N, Falcon-Piñeiro A, Alcaraz-Martinez Á, Guillamón E, Baños A. In vitro antiparasitic activity of propyl-propane-thiosulfinate (PTS) and propyl-propane-thiosulfonate (PTSO) from Allium cepa against Eimeria acervulina sporozoites. Microorganisms. 2022. https://doi.org/10.3390/microorganisms10102040.
Ali Al-Shuraym L. The impact of the onion-garlic extracts to control date palm aphids in Saudi Arabia. J Saudi Soc Agric Sci. 2022;21(8):546–51. https://doi.org/10.1016/j.jssas.2022.03.004.
Arnault I, Fleurance C, Vey F, Fretay GDu, Auger J. Use of Alliaceae residues to control soil-borne pathogens. Ind Crops Prod. 2013;49:265–72. https://doi.org/10.1016/j.indcrop.2013.05.007.
Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46(2):446–75. https://doi.org/10.1016/j.fct.2007.09.106.
Benítez V, Mollá E, Martín-Cabrejas MA, Aguilera Y, López-Andréu FJ, Cools K, Terry LA, Esteban RM. Characterization of industrial onion wastes (Allium cepa L.): dietary fibre and bioactive compounds. Plant Foods Hum Nutr. 2011;66(1):48–57. https://doi.org/10.1007/s11130-011-0212-x.
Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). Lwt. 2004;37(2):263–8. https://doi.org/10.1016/j.lwt.2003.09.001.
Beshbishy A, Wasef L, Elewa Y, Al-Sagan A, Abd El-Hack M, Taha A, Abd-Elhakim Y. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 2020;12(3):872.
Bhavya VP, Anil Kumar S, Alur A, Shivanna M, Shivakumar KM. Changes in soil physical properties as a result of different land use systems with depth. Int J Curr Microbiol Appl Sci. 2018;7(1):323–7. https://doi.org/10.20546/ijcmas.2018.701.035.
Breu W. Allium cepa L. (Onion) Part 1: Chemistry and analysis. Phytomedicine. 1996;3(3):293–306. https://doi.org/10.1016/S0944-7113(96)80069-9.
Burdon JJ, Zhan J. Climate change and disease in plant communities. PLoS Biol. 2020;18(11):1–7. https://doi.org/10.1371/journal.pbio.3000949.
Cabello-Gómez JF, Aguinaga-Casañas MA, Falcón-Piñeiro A, González-Gragera E, Márquez-Martín R, del Agraso M, Bermúdez L, Baños A, Martínez-Bueno M. Antibacterial and antiparasitic activity of propyl-propane-thiosulfinate (PTS) and propyl-propane-thiosulfonate (PTSO) from Allium cepa against gilthead sea bream pathogens in in vitro and in vivo studies. Molecules. 2022. https://doi.org/10.3390/molecules27206900.
Cai F, Gao R, Zhao Z, Ding M, Jiang S, Yagtu C, Zhu H, Zhang J, Ebner T, Mayrhofer-Reinhartshuber M, Kainz P, Chenthamara K, Akcapinar GB, Shen Q, Druzhinina IS. Evolutionary compromises in fungal fitness: hydrophobins can hinder the adverse dispersal of conidiospores and challenge their survival. ISME J. 2020;14(10):2610–24. https://doi.org/10.1038/s41396-020-0709-0.
Calvo MA, Asensio JJ. Evaluación de la eficacia de productos antimicrobianos en la alimentación animal. Anaporc. 1999;192:142–6.
Choo S, Chin VK, Wong EH, Madhavan P, Tay ST, Yong PVC, Chong PP. Review: antimicrobial properties of allicin used alone or in combination with other medications. Folia Microbiol. 2020;65(3):451–65. https://doi.org/10.1007/s12223-020-00786-5.
Claros Cuadrado JL, Pinillos EO, Tito R, Mirones CS, Gamarra Mendoza NN. Insecticidal properties of capsaicinoids and glucosinolates extracted from capsicum chinense and tropaeolum tuberosum. Insects. 2019. https://doi.org/10.3390/insects10050132.
CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (3rd ed.). 2017.
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically (11th ed.). 2018. www.clsi.org.
Curtis H, Noll U, Störmann J, Slusarenko AJ. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol Mol Plant Pathol. 2004;65(2):79–89. https://doi.org/10.1016/j.pmpp.2004.11.006.
Davies CR, Wohlgemuth F, Young T, Violet J, Dickinson M, Sanders JW, Vallieres C, Avery SV. Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biol Rev. 2021;36:15–26. https://doi.org/10.1016/j.fbr.2021.01.003.
Deberdt P, Perrin B, Beaudu RC, Hortsys UPR, Agro PDR. Pdis-07-11-0601. May, 687–692. 2012.
Del Papa MF, Pistorio M, Balagué LJ, Draghi WO, Wegener C, Perticari A, Niehaus K, Lagares A. A microcosm study on the influence of pH and the host-plant on the soil persistence of two alfalfa-nodulating rhizobia with different saprophytic and symbiotic characteristics. Biol Fertil Soils. 2003;39(2):112–6. https://doi.org/10.1007/s00374-003-0690-6.
Falcón-Piñeiro A, Remesal E, Noguera M, Ariza JJ, Guillamón E, Baños A, Navas-Cortes JA. Antifungal activity of propyl-propane-thiosulfinate (PTS) and propyl-propane-thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: in vitro and in planta assays. J Fungi. 2021. https://doi.org/10.3390/jof7090736.
FAO. The future of food and agriculture—Alternative pathways to 2050. 2018. http://www.fao.org/3/I8429EN/i8429en.pdf.
Feldman D, Yarden O, Hadar Y. Seeking the roles for fungal small-secreted proteins in affecting saprophytic lifestyles. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.00455.
Frías M, González C, Brito N. BcSpl1, a cerato-platanin family protein, contributes to Botrytis cinerea virulence and elicits the hypersensitive response in the host. New Phytol. 2011;192(2):483–95. https://doi.org/10.1111/j.1469-8137.2011.03802.x.
Guillamón E, Andreo-Martínez P, Mut-Salud N, Fonollá J, Baños A. Beneficial effects of organosulfur compounds from Allium cepa on gut health: a systematic review. 2021. Foods. https://doi.org/10.3390/foods10081680.
Hayat S, Cheng Z, Ahmad H, Ali M, Chen X, Wang M. Garlic, from remedy to stimulant: Evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars; allicin containing aqueous garlic extracts trigger antioxidants in Cucumber. Front Plant Sci. 2016;7:1–15. https://doi.org/10.3389/fpls.2016.01235.
Hori M. Settling inhibition and insecticidal activity of garlic and onion oils against Myzus persicae (SULZER) (Homoptera: Aphididae). Appl Entomol Zool. 1996;31(4):605–12. https://doi.org/10.1303/aez.31.605.
Hu Q, Yang Q, Yamato O, Yamasaki M, Maede Y, Yoshihara T. Isolation and identification of organosulfur compounds oxidizing canine erythrocytes from garlic (Allium sativum). J Agric Food Chem. 2002;50(5):1059–62. https://doi.org/10.1021/jf011182z.
Isman MB. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem Rev. 2020;19(2):235–41. https://doi.org/10.1007/s11101-019-09653-9.
Jiang H, Wang J, Song L, Cao X, Yao X, Tang F, Yue Y. Gc×Gc-tofms analysis of essential oils composition from leaves, twigs and seeds of cinnamomum camphora l. presl and their insecticidal and repellent activities. Molecules. 2016. https://doi.org/10.3390/molecules21040423.
Jiao X, Takishita Y, Zhou G, Smith DL. Plant associated rhizobacteria for biocontrol and plant growth enhancement. Front Plant Sci. 2021. https://doi.org/10.3389/fpls.2021.634796.
Kaddes A, Fauconnier ML, Sassi K, Nasraoui B, Jijakli MH. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules. 2019;24(6):1–16. https://doi.org/10.3390/molecules24061065.
Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10(10):813–29. https://doi.org/10.2174/0929867033457719.
Katsampa P, Valsamedou E, Grigorakis S, Makris DP. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box-Behnken experimental design and kinetics. Ind Crops Prod. 2015;77:535–43. https://doi.org/10.1016/j.indcrop.2015.09.039.
Keen BA, Raczkowski H. The relation between the clay content and certain physical properties of a soil. J Agric Sci. 1921;11(4):441–9. https://doi.org/10.1017/S0021859600004469.
Kelsey JW, Slizovskiy IB, Peters RD, Melnick AM. Sterilization affects soil organic matter chemistry and bioaccumulation of spiked p, p′-DDE and anthracene by earthworms. Environ Pollut. 2010;158(6):2251–7. https://doi.org/10.1016/j.envpol.2010.02.011.
Khan MA, Zhihui C. Influence of garlic root exudates on cyto-morphological alteration of the hyphae of Phytophthora capsici, the cause of Phytophthora blight in pepper. Pak J Bot. 2010;42(6):4353–61.
Kumar M, Barbhai MD, Hasan M, Punia S, Dhumal S, Radha A, Rais N, Chandran D, Pandiselvam R, Kothakota A, Tomar M, Satankar V, Senapathy M, Anitha T, Dey A, Sayed AAS, Gadallah FM, Amarowicz R, Mekhemar M. Onion (Allium cepa L.) peels: a review on bioactive compounds and biomedical activities. Biomed Pharmacother. 2022;146:112498. https://doi.org/10.1016/j.biopha.2021.112498.
Kyung KH. Antimicrobial properties of allium species. Curr Opin Biotechnol. 2012;23(2):142–7. https://doi.org/10.1016/j.copbio.2011.08.004.
Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112(1–2):3–22. https://doi.org/10.1016/j.chroma.2005.12.016.
Lemar KM, Turner MP, Lloyd D. Garlic (Allium sativum) as an anti-Candida agent: a comparison of the efficacy of fresh garlic and freeze-dried extracts. J Appl Microbiol. 2002;93(3):398–405. https://doi.org/10.1046/j.1365-2672.2002.01707.x.
Li J, Huang B, Wang Q, Li Y, Fang W, Han D, Yan D, Guo M, Cao A. Effects of fumigation with metam-sodium on soil microbial biomass, respiration, nitrogen transformation, bacterial community diversity and genes encoding key enzymes involved in nitrogen cycling. Sci Total Environ. 2017;598:1027–36. https://doi.org/10.1016/j.scitotenv.2017.02.058.
Louie A, VanScoy BD, Brown DL, Kulawy RW, Heine HS, Drusano GL. Impact of spores on the comparative efficacies of five antibiotics for treatment of Bacillus anthracis in an in vitro hollow fiber pharmacodynamic model. Antimicrob Agents Chemother. 2012;56(3):1229–39. https://doi.org/10.1128/AAC.01109-10.
Lu S, Edwards MC. Genome-wide analysis of small secreted cysteine-rich proteins identifies candidate effector proteins potentially involved in fusarium graminearum-wheat interactions. Phytopathology. 2016;106(2):166–76. https://doi.org/10.1094/PHYTO-09-15-0215-R.
Ma D, Cui X, Zhang Z, Li B, Xu Y, Tian S, Chen T. Honokiol suppresses mycelial growth and reduces virulence of Botrytis cinerea by inducing autophagic activities and apoptosis. Food Microbiol. 2020;88:103411. https://doi.org/10.1016/j.fm.2019.103411.
Marañes Corbacho A, Sánchez Garrido JA, de Haro Loza S, Sánchez Gómez S, Lozano Cantero FJ. Análisis de Suelos; metodología e interpretación (J. A. Sánchez Garrido (Ed.); 1° Edition). 1994.
Michielse CB, Van Wijk R, Reijnen L, Manders EMM, Boas S, Olivain C, Alabouvette C, Rep M. The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 2009. https://doi.org/10.1371/journal.ppat.1000637.
Miron T, Rabinkov A, Mirelman D, Wilchek M, Weiner L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim Biophys Acta. 2000;1463(1):20–30. https://doi.org/10.1016/S0005-2736(99)00174-1.
Montes-Osuna N, Mercado-Blanco J. Verticillium wilt of olive and its control: what did we learn during the last decade? Plants. 2020;9(6):1–31. https://doi.org/10.3390/plants9060735.
Mourtzinos I, Prodromidis P, Grigorakis S, Makris DP, Biliaderis CG, Moschakis T. Natural food colorants derived from onion wastes: application in a yoghurt product. Electrophoresis. 2018;39(15):1975–83. https://doi.org/10.1002/elps.201800073.
Mylona K, Garcia-Cela E, Sulyok M, Medina A, Magan N. Influence of two garlic-derived compounds, propyl propane thiosulfonate (Pts) and propyl propane thiosulfinate (ptso), on growth and mycotoxin production by fusarium species in vitro and in stored cereals. Toxins. 2019;11(9):1–16. https://doi.org/10.3390/toxins11090495.
Nelson R. International plant pathology: past and future contributions to global food security. Phytopathology. 2020;110(2):245–53. https://doi.org/10.1094/PHYTO-08-19-0300-IA.
Pane C, Caputo M, Francese G, Manganiello G, Scalzo RL, Mennella G, Zaccardelli M. Managing rhizoctonia damping-off of rocket (Eruca sativa) seedlings by drench application of bioactive potato leaf phytochemical extracts. Biology. 2020;9(9):1–18. https://doi.org/10.3390/biology9090270.
Pane C, Fratianni F, Parisi M, Nazzaro F, Zaccardelli M. Control of Alternaria post-harvest infections on cherry tomato fruits by wild pepper phenolic-rich extracts. Crop Prot. 2016;84:81–7. https://doi.org/10.1016/j.cropro.2016.02.015.
Park IK, Kim J, Lee YS, Shin SC. In vivo fungicidal activity of medicinal plant extracts against six phytopathogenic fungi. Int J Pest Manag. 2008;54(1):63–8. https://doi.org/10.1080/09670870701549665.
Pavela R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—a review. Plant Protect Sci. 2016;52(4):229–41. https://doi.org/10.17221/31/2016-PPS.
Pavela R, Vrchotová N, Šerá B. Repellency and toxicity of thr ee impatiens species (Balsaminaceae) extracts on Myzus persicae Sulzer (Homoptera: Aphididae). J Biopest. 2009;2(1):48–51.
Poojary MM, Putnik P, Bursać Kovačević D, Barba FJ, Lorenzo JM, Dias DA, Shpigelman A. Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. J Food Compos Anal. 2017;61:28–39. https://doi.org/10.1016/j.jfca.2017.04.007.
Putnik P, Gabrić D, Roohinejad S, Barba FJ, Granato D, Mallikarjunan K, Lorenzo JM, Bursać Kovačević D. An overview of organosulfur compounds from Allium spp.: from processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019;276:680–91. https://doi.org/10.1016/j.foodchem.2018.10.068.
Ramirez DA, Locatelli DA, Torres-Palazzolo CA, Altamirano JC, Camargo AB. Development of garlic bioactive compounds analytical methodology based on liquid phase microextraction using response surface design. Implications for dual analysis: cooked and biological fluids samples. Food Chem. 2017;215:493–500. https://doi.org/10.1016/j.foodchem.2016.07.170.
Rayne S, Karacabey E, Mazza G. Grape cane waste as a source of trans-resveratrol and trans-viniferin: high-value phytochemicals with medicinal and anti-phytopathogenic applications. Ind Crops Prod. 2008;27(3):335–40. https://doi.org/10.1016/j.indcrop.2007.11.009.
Robinson GW. A new method for mechanical analysis of soil and other dispersion. J Agric Sci. 1992;12:306–21.
Rongai D, Milano F, Sciò E. Inhibitory effect of plant extracts on conidial germination of the phytopathogenic fungus Fusarium oxysporum. Am J Plant Sci. 2012;03(12):1693–8. https://doi.org/10.4236/ajps.2012.312207.
Rose P, Whiteman M, Moore K, Zhun Y. Allium: the chemistry of potential therapeutic agents. Nat Prod Rep. 2005;22:351–68.
Rossati A. Global warming and its health impact. Int J Occup Environ Med. 2017;8(1):7–20. https://doi.org/10.15171/ijoem.2017.963.
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3(3):430–9. https://doi.org/10.1038/s41559-018-0793-y.
Sayed S, Soliman MM, Al-Otaibi S, Hassan MM, Elarrnaouty SA, Abozeid SM, El-Shehawi AM. Toxicity, deterrent and repellent activities of four essential oils on Aphis punicae (Hemiptera: Aphididae). Plants. 2022;11(3):1–13. https://doi.org/10.3390/plants11030463.
Scholthof KBG. The disease triangle: pathogens, the environment and society. Nat Rev Microbiol. 2007;5(2):152–6. https://doi.org/10.1038/NRMICRO1596.
Sciubba F, Chronopoulou L, Pizzichini D, Lionetti V, Fontana C, Aromolo R, Socciarelli S, Gambelli L, Bartolacci B, Finotti E, Benedetti A, Miccheli A, Neri U, Palocci C, Bellincampi D. Olive mill wastes: a source of bioactive molecules for plant growth and protection against pathogens. Biology. 2020;9(12):1–20. https://doi.org/10.3390/biology9120450.
Semerdjieva I, Zheljazkov VD, Radoukova T, Dincheva I, Piperkova N, Maneva V, Astatkie T, Kačániová M. Biological activity of essential oils of four juniper species and their potential as biopesticides. Molecules. 2021;26(21):1–17. https://doi.org/10.3390/molecules26216358.
Soriano G, Petrillo C, Masi M, Bouafiane M, Khelil A, Tuzi A, Isticato R, Fernández-Aparicio M, Cimmino A. Specialized metabolites from the allelopathic plant retama raetam as potential biopesticides. Toxins. 2022;14(5):1–12. https://doi.org/10.3390/toxins14050311.
Sorlozano-Puerto A, Albertuz-Crespo M, Lopez-Machado I, Ariza-Romero JJ, Baños-Arjona A, Exposito-Ruiz M, Gutierrez-Fernandez J. In vitro antibacterial activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate derived from allium spp. Against gram-negative and gram-positive multidrug-resistant bacteria isolated from human samples. BioMed Res Int. 2018. https://doi.org/10.1155/2018/7861207.
Sorlozano-Puerto A, Albertuz-Crespo M, Lopez-Machado I, Gil-Martinez L, Ariza-Romero JJ, Maroto-Tello A, Baños-Arjona A, Gutierrez-Fernandez J. Antibacterial and antifungal activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate, two organosulfur compounds from Allium cepa: in vitro antimicrobial effect via the gas phase. Pharmaceuticals. 2021;14(1):1–17. https://doi.org/10.3390/ph14010021.
Pruett SB, Peyton Myers L. Toxicology of metam sodium. J Toxicol Environ Health Part B. 2001;4(2):207–22. https://doi.org/10.1080/10937400117071.
Syed Ab Rahman SF, Singh E, Pieterse CMJ, Schenk PM. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018;267:102–11. https://doi.org/10.1016/j.plantsci.2017.11.012.
Top Onion Producing Countries. Atlas Big. 2022. https://www.atlasbig.com/en-in/countries-by-onion-production.
Tyurin IV. Analitical procedure for a comparative study of soil humus. Trudy Pochr Inst Dokuchaev. 1951;33:5–21.
United Nations. World Population Prospects 2019: Data Booklet [PDF]. 2019. Date of access: 12 December 2019, retrieved from: https://population.un.org/wpp/Publications/Files/WPP2019_DataBooklet.pdf. Department of Economic and Social Affairs Population Division, 1–25. https://population.un.org/wpp/Publications/Files/WPP2019_DataBooklet.pdf.
Velásquez AC, Castroverde CDM, He SY. Plant-pathogen warfare under changing climate conditions. Curr Biol. 2018;28(10):R619–34. https://doi.org/10.1016/j.cub.2018.03.054.
Vischetti C, Casucci C, Perucci P. Relationship between changes of soil microbial biomass content and imazamox and benfluralin degradation. Biol Fertil Soils. 2002;35(1):13–7. https://doi.org/10.1007/s00374-001-0433-5.
Viterbo A, Chet I. TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol Plant Pathol. 2006;7(4):249–58. https://doi.org/10.1111/j.1364-3703.2006.00335.x.
Wallock-Richards D, Doherty CJ, Doherty L, Clarke DJ, Place M, Govan JRW, Campopiano DJ. Garlic revisited: antimicrobial activity of allicin-containing garlic extracts against Burkholderia cepacia complex. PLoS ONE. 2014;9(12):1–13. https://doi.org/10.1371/journal.pone.0112726.
Zefzoufi M, Smaili A, Fdil R, Rifai LA, Faize L, Koussa T, Makroum K, Ben Ali A, Tabyaoui M, Mouzdahir A, Sraidi K, Faize M. Composition of essential oil of Moroccan Dysphania ambrosioides and its antimicrobial activity against bacterial and fungal phytopathogens. J Plant Pathol. 2020;102(1):47–58. https://doi.org/10.1007/s42161-019-00371-x.
Zhao C, Ma C, Luo J, Niu L, Hua H, Zhang S, Cui J. Potential of cucurbitacin b and epigallocatechin gallate as biopesticides against Aphis gossypii. Insects. 2021;12(1):1–16. https://doi.org/10.3390/insects12010032.
Zhao Z, Cai F, Gao R, Ding M, Jiang S, Chen P, Pang G, Chenthamara K, Shen Q, Bayram Akcapinar G, Druzhinina IS. At least three families of hyphosphere small secreted cysteine-rich proteins can optimize surface properties to a moderately hydrophilic state suitable for fungal attachment. Environ Microbiol. 2021;23(10):5750–68. https://doi.org/10.1111/1462-2920.15413.
Zheljazkov VD, Cantrell CL, Semerdjieva I, Radoukova T, Stoyanova A, Maneva V, Kačániová M, Astatkie T, Borisova D, Dincheva I, Salamon I. Essential oil composition and bioactivity of two juniper species from Bulgaria and Slovakia. Molecules. 2021;26(12):1–25. https://doi.org/10.3390/molecules26123659.
Zhou BG, Wang S, Dou TT, Liu S, Li MY, Hua RM, Li SG, Lin HF. Aphicidal activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione s-transferases activities in Myzus persicae (Hemiptera: Aphididae). J Insect Sci. 2016. https://doi.org/10.1093/jisesa/iev163.
Zupan J, Muth TR, Draper O, Zambryski P. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 2000;23(1):11–28. https://doi.org/10.1046/j.1365-313X.2000.00808.x.
Acknowledgements
We would like to acknowledge the work of Jose Manuel García-Madero in editing the text and TECNOVA Technological Center (Almería, Spain) for providing the aphids.
Funding
This research has been carried out within the project GRUPO OPERATIVO SALUD- OLIVAR from the Spanish Rural Development Program (2014–2020) funded by the Spanish Ministry of Agriculture, Fisheries and Food and co-financed by 80% by the European Agricultural Fund for Rural Development (FEADER) (Total investment 384.830,96 EUR). It has also received funding from European Union’s Horizon 2020 research and innovation program under Grant agreement no. 887281 (BIOVEXO). This study is part of the Industrial Doctorate of the doctoral student Ana Falcón Piñeiro, granted by the State Research Agency of the Spanish Government, with the following reference: DIN2019-010792.
Author information
Authors and Affiliations
Contributions
AB and DG designed the study. AF-P, AB and AKM conducted in vitro analyses. AF-P and DG-L conducted experiments with aphids. AF-P and MDC-Y performed soil sanitization assays. LG-M and JMdlT conducted HPLC–UV determination. BB-D and SL-P provided soil samples and data. AB supervised the experiments. AF-P analyzed data and wrote the manuscript. AB, DG and EG reviewed the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Falcón-Piñeiro, A., García-López, D., Gil-Martínez, L. et al. PTS and PTSO, two organosulfur compounds from onion by-products as a novel solution for plant disease and pest management. Chem. Biol. Technol. Agric. 10, 76 (2023). https://doi.org/10.1186/s40538-023-00452-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00452-1