Abstract
Background
Plants-microbial technology has been regarded as a popular and applied new technique for the remediation of cadmium (Cd) polluted soils.
Methods
Therefore, a pot experiment was conducted to explore the effect of Serendipita indica (S. indica) on soybean growth and the soil enzyme activities, pH and Cd chemical fractions in the soil in 0, 3, 6, and 9 mg·kg−1 Cd concentrations.
Results
Results reflected that compared to uninoculated treatment, S. indica inoculation can still enhance the dry weight (66.57%) and shoot length (90.35%) and promote the net photosynthesis rate (72.18%), transpiration ratio (80.73%), and stomatal conductance (119.05%) of soybean under 9 mg·kg−1 Cd stress. Furthermore, the soil pH, phosphatase (116.39%), and catalase (4.17%) activities in the S. indica treatments were increased under 3 mg·kg−1 Cd concentration. Meanwhile, S. indica could shift Cd from exchangeable fraction to other stable fractions, primarily decreased Cd contamination degree/risk in 6 mg·kg−1 Cd concentration.
Conclusions
This work suggested that S. indica may be a potential biotechnology for low Cd-contaminated farmland, S. indica can not only alleviate Cd phytotoxicity and promote plant growth but also improve soil quality in Cd-contaminated soils.
Graphical Abstract

Similar content being viewed by others
Background
Since the twentieth century, science and technology have developed rapidly, promoted economic development, and improved people’s living standards; at the same time, mankind has also paid a heavy dear [1]. Due to the discharge of industrial waste, the improper use of fertilizers, and the development of mining, the soil is seriously polluted by heavy metals, such as Cd [2]. Heavy metal Cd cannot be biodegraded and will flow with the food chain, threatening the life safety of plants, animals, and even humans [3, 4]. Cd contamination degree/risk depends on its concentration and fraction. Exchangeable Cd fraction is more toxic than other Cd fractions [5]; the content of Cd exchangeable fraction increases with the decrease of soil pH [6]. Moreover, Cd seriously affects the growth of soybean and potentially leads to severe impacts on public health [7, 8].
Soybean (Glycine max (Linn.) Merr.), as the main crop in Asian countries, is one of the best sources of plant protein. And it can fix nitrogen by endosymbiotic nodule bacteria and improve soil fertility [9]. Furthermore, oxalate accumulation induced by Cd contributes to the inhibition of root growth in soybean. Overexpression of wild soybean oxalyl-CoA synthetase can reduce oxalate accumulation and increase Cd tolerance of hairy soybean roots [10]. Therefore, soybean has application prospects in the safe production of Cd soil, while microbial-plant combined technology has the effect of expanding the Cd tolerance of soybean.
The root endophytic fungus, Serendipita indica (formerly known as Piriformospora indica), can colonize the roots of plenty of plant species and promote the intake of nutrients. Besides, the antioxidant defense system of the plant can be enhanced by the fungus, which has a vital role in resistance against biotic and abiotic stresses [11]. S. indica can increase plant photosynthesis, such as leaf transpiration rate and net photosynthetic rate, and improve antioxidant defense capabilities, such as proline and glutathione content to promote plant growth to resist Cd stress [12, 13]. S. indica can not only increase soil urease and phosphatase activities to improve plant utilization efficiency of soil nutrients but also increase soil catalase to relieve the toxic effects of hydrogen peroxide [14]. Moreover, S. indica can accumulate As in the plant roots and restrict their movement to aerial parts [15]. Therefore, we hope that with the help of S. indica, Cd can be fixed in the soil and not easily absorbed by plants; even if plants absorb Cd, it can be accumulated in the non-edible parts, so that the edible parts of plants have no or low Cd concentrations.
The symbiotic relationship between fungi and host plants can produce a synergistic effect on Cd phytoremediation. Therefore, in this research, we hypothesized that S. indica could promote soybean growth and transform Cd into a fraction, which is not easily utilized by plants under high Cd stress, thus reducing the risk of Cd flowing into the food chain. We conducted pot experiments to determine the effects of Cd stress and S. indica on soybean growth, soybean physiological characteristics, the enzyme activities, and the accumulation of Cd in the soil. We also analyzed the ecological risk of soybean soil to reveal the application prospect of S. indica in the Cd-contaminated farmland.
Materials and methods
Experimental set-up
The pot experiment was carried out in the greenhouse on the Hulan campus of Heilongjiang University and conducted in a 2 × 4 factorial design with a completely randomized factorial design: Fungal factor: uninoculated (control) treatments (− S) and inoculated treatments (+ S); Cd factor: 0 mg·kg−1 (NH), 3 mg·kg−1 (LH), 6 mg·kg−1 (MH), and 9 mg·kg−1 (HH). Each treatment was repeated 10 times for a total of 80 pots.
Glycine max (Linn.) Merr. (Soybean) was selected from Heinong 48 strain, and the seeds were purchased from seed station, Harbin, China. Plump and consistent seeds were picked for surface sterilization by soaking in 70% ethanol for 2 min and 4% sodium hypochlorite for 10 min, then washed 3 times with double distilled water.
Serendipita indica (S. indica) strain was donated by Prof. Wu Chu of Changjiang University, and was preserved and propagated in the laboratory, and was cultured in Petri dishes on a Hill & Käfer medium at 30 ± 1 °C in the dark for 14 days. The plugs of S. indica (10 mm) were taken from the edge of the fungus culture plates (≈ 7.3 × 104 spore/plug). The fungal plugs of − S treatments were autoclave sterilized at 100 °C for 1 h.
The soil of pot experiment was collected from Nangang District of Harbin City, Heilongjiang Province, PR China. It is a typical black soil (physicochemical properties: soil pH 7.8, organic matter content 24.3 g kg−1, total nitrogen 1.8 g·kg−1, available nitrogen 50.3 mg·kg−1, available potassium 213.1 mg·kg−1, available phosphorus 9.2 mg·kg−1, total nitrogen 740.18 mg·kg−1, total phosphorus 906.52 mg·kg−1, total potassium 824.07 mg·kg−1) [16].
Air-dried soil was sieved with a 2 mm sieve and autoclave sterilized 3 times at 100 °C for 1 h to eliminate native arbuscular mycorrhizal fungal propagules and other microorganisms. After that, Cd (0, 3, 6, and 9 mg·kg−1) factors were added in the form of CdCl2·5H2O aqueous solution. Then the soil samples were incubated at 20 °C for 30 days to make the Cd evenly distributed and stabilized. The final measured Cd contents were 0.97, 3.56, 5.88, and 8.65 mg·kg−1.
Each experimental plastic pot (30 × 15 × 15 cm3) was filled with 5 kg soil, five soybean seeds. Underneath each seed was a (un)sterilized fungal plug. Soybean was watered according to soil moisture content and growth period, and plant samples were harvested after 120 days of planting (June 4–October 4, 2018).
The plant samples were rinsed with distilled water and wiped with filter paper. Then stem length and photosynthetic physiological parameters were determined and finally dried at 75 °C for 48 h for the determination of dry weight and Cd content. The soil samples were sieved with a 0.15 mm sieve and stored. Then they were air-dried for the determination of soil pH, Cd content, and Cd fractions, and samples for the soil enzyme activity were stored at 4 °C.
Infection rate
After 60 days of planting, 3 soybean root samples were selected from each treatment to determine the infection rate, stained with 0.05% trypan blue using the method of Phillip [17], and observed and photographed under a light microscope. The grid-line intersect method was used to evaluate the infection rate [18].
Photosynthetic physiology
Using an ultra-light portable photosynthetic system (CI-340, CID Inc., USA) to determine the net photosynthetic rate (Pn), stomatal conductance (Tr), intercellular CO2 concentration (Ci), and transpiration rate (Gs) of soybean leaves in the third round of blooming stage from 9:00 am to 11:00 am on July 20, 2018. Three plants with the same size were measured in each treatment, and each plant was repeated three times.
Soil pH and enzyme activity
Potentiometric method (water:soil = 1:1) was used to measure soil pH [19]. The urease activity was assayed by phenol sodium colorimetric method, and the results are expressed as the number of milligrams of NH3-N released in 1 g of soil after incubation at 37 ℃ for 24 h (mg·g−1) [20]. The sucrase activity was measured by 3, 5-dinitrosalicylic acid colorimetry (DNS method), and the results are expressed as the number of milligrams of glucose hydrolyzed in 1 g of soil after incubation at 37 °C for 24 h (mg·g−1) [21]. The phosphatase activity was assayed by sodium diphenyl phosphate colorimetry method, and the results are expressed as the number of milligrams of phenol released in 1 g of soil after incubation at 37 °C for 24 h (mg·g−1) [22]. The catalase activity was determined by UV spectrophotometry (240 nm), and the results are expressed as the number of milligrams of hydrogen peroxide consumed in 1 g of soil after incubation at 20 °C for 0.5 h (mg·g−1) [23].
Cd content and Cd chemical fractions content
Wet digestion method was used for plant samples, and electrothermal method for soil samples [24]. And then iCAP Q ICP-MS (Thermo Fisher Scientific, Waltham, MA, USA) was used [25]. According to Tessier method [26], all the soil samples were extracted in sequence to determine the soil Cd fractions. Cd fractions were named exchangeable fraction (MgCl2, pH = 7); carbonate fraction (NaOAc, pH = 5); reducible iron and manganese fraction (NH4OH + HCl); organic matter bound fraction (HNO3 + H2O2, H2O2, NH4OAc); and residual fraction (HF + HNO3 + H2O2), respectively. The supernatant liquid was separated from the solid phase by centrifugation at 1917 g for 20 min after each extraction step to determine the concentrations of Cd (mg kg−1).
Transport factor (TF) and bioconcentration factor (BCF) of Cd
The larger the TF or BCF of Cd, the stronger the Cd enrichment or transfer ability of the plant [27]:
where Ctissue is the Cd content of each part of the plant and Csoil is the soil Cd content,
where Cshoot is the Cd content in the aerial parts of plants and Croot is the Cd content of plant root.
Assessment of soil pollution degree
Single factor pollution index method [28]:
where Pi is the single factor pollution index of Cd, Ci is the measured value of Cd, and Si is the evaluation standard value of Cd. Pi < 1 means no pollution; Pi > 1 means pollution, and pollution level increases with increasing Pi value.
Farmland soil pollution index:
where \(S\genfrac{}{}{0pt}{}{i}{1}\) is the Cd content absorbed by crops in theory, \(S\genfrac{}{}{0pt}{}{i}{o}\) is the limit value of Cd content in the national vegetable standard (0.2 mg·kg−1), and \(S\genfrac{}{}{0pt}{}{i}{bio}\) is the bioavailable content of Cd in soil.
Concentration risk of farmland soil pollution:
where \(S\genfrac{}{}{0pt}{}{i}{2}\) is the risk value of Cd content that may be absorbed by plants, \(S\genfrac{}{}{0pt}{}{i}{T}\) is total Cd content in farmland soil, and \(S\genfrac{}{}{0pt}{}{i}{R}\) is the residual Cd content in farmland soil.
Data analysis
The experimental data were statistically analyzed by Statistical Product and Service Solutions 22.0 (SPSS Inc., Chicago, IL, USA). Two-way analysis of variance (ANOVA) was used to statistically analyze the significance of each treatment. The primary factors (S. indica and Cd levels) and their interactions were evaluated. Duncan's Multiple Range Test was used to compare the mean values of 0.05 and 0.01 probability levels; alternatively, Dunnett's T3 test was used. Data are expressed as the means of replicates ± standard error (SE) (n ≥ 3). Redundancy analysis (RDA) in CANOCO for Windows (version 5.0, Microcomputer power, Ithaca, NY, US; ter Braak et Šmilauer, 2019) was used to draw the relationships between variables. Graphs were drawn by Origin 8.0 (OriginLab, Northampton, MA, USA). Spearman correlations were used to examine the relationships between the parameters. Structural equation modeling (SEM) was applied to investigate the effect of soil characteristics on plant growth. The SEM analysis was performed via the robust maximum likelihood evaluation method using the software AMOS 22.0 (AMOS IBM, USA).
Results
Establishment of the symbiotic relationship
Serendipita indica could infect soybean roots, especially establish symbiotic relationships with mature soybean root (Fig. 1). The spores and hyphae of S. indica can be observed under a light microscope. The hyphae were white and nearly transparent, and the spores were pear shape as shown in Fig. 1. The results showed that with the NH, LH, MH, and HH treatments, the colonization rate of + S treatments was 83.81%, 67.46%, 47.62%, and 34.92% (Table 1). This indicated that the root infection rate decreased with the increase of Cd content in the soil and reached the lowest level in the HH treatment.
Soybean growth
Cd treatment and fungal treatment had significant main effects and interaction on soybean growth parameters (P < 0.01). The limitation of Cd on plant growth, stem length, and dry weight decreased with increasing Cd concentration, while S. indica could alleviate the survival pressure of soybean (Table 1). Compared with -S treatment, NH, LH, MH, and HH treatments with S. indica increased soybean plant height by 11.00%, 13.52%, 31.52%, and 90.35%, while dry weight increased by 137.35%, 116.60%, 84.07%, and 66.57%, respectively. The stem length (63.92 cm) and dry weight (43.91 g) of soybean reached the highest value under NH with S. indica. This indicated that S. indica could promote soybean growth under different levels of Cd stress and the promoting effect on plant height increased and biomass decreased with the increase of Cd concentration.
Soybean photosynthesis
Cd treatment and fungal treatment had significant main effects and interaction on photosynthetic physiological parameters of soybean (P < 0.01). Data from Table 2 revealed that with the increase of soil Cd content, the Pn, Tr, Ci, and Gs of soybean were reduced, and Pn (6.65 umol/m2/s), Tr (1.68 mmol/m2/s), and Gs (257.80 mmol/m2/s) reached the lowest value in the HH without S. indica treatment. S. indica significantly increased Pn, Tr, and Gs, compared with the control treatment, whereas it decreased Ci (P < 0.05). S. indica led to a maximum increase of Pn (72.18%), Tr (80.73%), and Gs (119.05%) in the HH treatment, and maximum reduction of Ci (12.93%) in the NH treatment, compared with the -S treatment. This indicated that S. indica could enhance the photosynthesis of soybean to promote growth and resist Cd resistance.
Soil enzyme activities and pH
The activities of soil urease, sucrase, phosphatase, and catalase decreased with the increase of Cd stress (Fig. 2). S. indica could significantly increase the soil enzyme activities, especially the activities of soil urease (53.48%) and sucrase (100.43%) increased most in the NH treatment (P < 0.05), and the activities of phosphatase (116.39%) and catalase (4.17%) increased most in the LH treatment (P < 0.05). In addition, the increase of Cd content decreased the soil pH, and the soil pH of S. indica inoculated treatments were significantly higher than the − S treatments (P < 0.05). The soil pH in the + S treatments ranged from 7.68 to 7.96 and 7.60 to 7.85 in the − S treatments. This indicated that S. indica could increase the soil enzyme activities and pH value under different Cd stress levels.
Transport factor (TF) and bioconcentration factor (BCF)
Soybean could absorb Cd in the soil, and the stronger the Cd stress, the more the Cd content flowing into soybean plants. S. indica could reduce the Cd content in the aboveground part (ABG-Cd), but the underground part (UND-Cd) enriched more Cd content than the uninoculated treatments (Table 3). Under Cd stress, S. indica could reduce ABG-Cd by up to 16.70% in the HH treatment, while UND-Cd accumulated 7.56% more than that of MH without S. indica treatment. In addition, the bioconcentration factor (BCF) increased with increasing Cd stress. S. indica could also reduce the BCF of the aboveground part of soybean under Cd stress and increase the BCF of the underground part. Under Cd stress and with the help of S. indica, BCF (ABG) reached the lowest value of 0.51 in the HH, while BCF (UND) reached the highest value of 1.35 in the LH treatment. Moreover, transport factor (TF) increased with the increase of Cd stress in the LH, MH, and HH treatments. S. indica could reduce TF, and even the TF in HH with S. indica treatment (0.52) was the same as the lowest value of -S treatment (in the LH treatment). This indicated that S. indica was able to reduce the flow of Cd to the aboveground part and confine Cd to the root of soybean.
Cd fraction in soil
Unlike the residual Cd content in plants, S. indica was able to reduce Cd content in soil (Fig. 3B). The effect of S. indica on residual Cd in soil showed a trend of increasing first and then decreasing, and the highest effect was achieved in the MH treatment, with a significant decrease of 21.64%. Also, the effect of S. indica was achieved by reducing the exchangeable fraction, and it was most obvious under moderate Cd stress, with a decrease of 41.76%. At the same time, Fig. 3B also shows that the presence of S. indica increased the content of carbonate fraction(CAR-Cd) and reducible iron and manganese fraction (RIM-Cd). These are shown more clearly in Fig. 3A. The proportions of CAR-Cd, RIM-Cd, ORG-Cd, and RES-Cd were generally increased. This suggested that S. indica can reduce the soil Cd content and convert soil Cd from a fraction that was easily available to plants to more stable chemical fractions that was fixed in the soil.
Effects of Serendipita indica and Cd stress on the percent (A) and content (B) of soil Cd fractions. Bars topped by the same letter do not differ significantly at P < 0.05 according to Duncan’s Multiple Range Test (n ≥ 3). The abbreviations in the legend are the initial letter combinations of Cd fractions
Assessment of Cd pollution
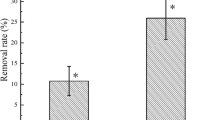
The single factor pollution index and the farmland soil pollution index can reflect the degree of soil pollution. Their changing trends are the same as the residual soil Cd content. Both of them showed a positive correlation with the level of Cd stress. Table 4 shows that the single factor pollution index and farmland soil pollution index of the S. indica inoculation treatments were significantly reduced, and the effect of the MH treatment was the most obvious, which decreased by 21.64% and 23.82%, respectively. In addition, the trend of concentration risk of farmland soil pollution is the same as the pollution index, and S. indica can minimize soil pollution risk (11.62%) in MH treatment. This showed that S. indica can reduce the pollution degree and pollution risk of soil, and the effect is obvious under the pollution degree of 6 mg kg−1 Cd.
Correlation analysis of soybean growth index and environmental factors
According to Fig. 4A, principal component analysis (PCA) was used to analyze the indicators affecting plant growth. Statistical axis 1, 2, 3 eigenvalues were 0.9117, 0.0706, and 0.0103. The first principal component that seriously affects plant growth was CAT, pH, exchangeable fraction, etc., the second principal component was Ci, and the third principal component was photosynthetic physiological indicators. Figure 4B shows that S. indica can improve growth physiology and photosynthetic physiology and soil enzyme activities, while reducing Cd in soil. Figure 4A, C shows that there was a positive correlation between growth physiological indicators, photosynthetic physiological indicators, and soil enzyme activities, and there was a positive correlation between plant Cd content, soil Cd content, and fractions. There was a negative correlation between the indicators of two groups, which were positively correlated. Structural equation model (SEM) results showed that soil pH had a significant effect on Cd content, which had a significant effect on soil enzyme activity, and plant growth was significantly affected by photosynthesis, soil enzyme activity, and Cd content (Fig. 4D).
Correlation analysis between the indicators. A Redundancy analysis of soybean growth index and environmental factors; B S. indica contribution rate (data were represented by degree of increase/decrease compared to uninoculated S. indica); C Correlation matrix; D Structural Equation Modeling (SEM). EXC exchangeable fraction; CAR carbonate fraction; RIM reducible iron and manganese fraction; ORG organic matter bound fraction; RES-Cd residual fraction; S-Cd: residual content in soil; DW dry weight; SH shoot height; URE urease; PHO phosphatase; SUC sucrase; CAT catalase; Tr transpiration ratio; Gs stomatal conductance; Pn net photosynthesis rate; Ci: intercellular CO2 concentration
Discussion
S. indica can improve soybean growth under Cd stress
S. indica can be successfully colonized in soybean roots, and the increase of Cd concentration in soil led to the decrease of soybean root colonization rate [12], which can be attributed to the toxicity of Cd to soybean improves the immunity of soybean under stress.
Excessive Cd can limit the nutrient absorption, photosynthesis, growth, and development of plants [29]. In this research, the addition of exogenous Cd decreased the dry weight and plant height of soybean (Table 1). However, S. indica increased the dry weight and plant height to promote soybean growth (Table 1, 2), which are consistent with the findings of Wu [30].
On one hand, S. indica might improve the activities of soil enzymes via a dilution effect and makes the nutrients (N/ P/ K) in the soil easy to be absorbed by soybeans to promote growth (Fig. 2) [31]. On the other hand, S. indica can effectively improve the stability of Cd, reduce the absorption of Cd by plants, and limit the flow of Cd from the ground to the ground, thereby slowing the damage received by soybean physiology (Table 3; Fig. 3).
This protection is also reflected in photosynthetic physiology. Our results expounded that Cd could decrease the stomatal conductance, prevent CO2 from entering soybean leaves, and lead to a decrease in Pn (Table 2), which was the consistent with the conclusion of Liu et al. [32]. S. indica can promote the utilization of light and CO2 in soybean by promoting stomatal conductance (Table 2, Fig. 4B), thereby increasing the net photosynthetic rate and increasing the accumulation of biomass to compensate for the energy consumed against Cd.
S. indica can promote soil enzyme activities and increase pH under Cd stress
Among the different soil enzymes, soil urease, sucrase, and phosphatase are often used to evaluate the nutrient absorption of plants and organic matter transformation, and catalase was often used to evaluate the detoxification ability of soil ecosystem [33]. In this study, we observed that Cd inhibited the activity of soil enzymes and this inhibition was enhanced with increasing Cd content in soil (Fig. 2). However, S. indica can increase the activity of soil enzymes, which was similar to the conclusion of Xiao et al. [34]. And our results showed that S. indica inoculation significantly increased the soil phosphatase and catalase activities in the moderate Cd stress (Fig. 4B).
On one hand, the reason for S. indica to play a role might be that S. indica promoted the growth of soybean (Table 2), stimulated the secretion of plant root metabolites, and directly enhanced soil enzyme activity. On the other hand, S. indica stimulated soil microorganisms, which increased the biomass and activity of microorganisms and indirectly increased the activity of soil enzymes [35].
In addition, there was a positive correlation between soil enzyme activity and soil pH (Fig. 4). That is, the decrease of soil pH could represent the adverse effect of Cd on soil enzyme activity [36]. The increase of Cd content led to more organic acids (the citric and malic acids) secreted by plant roots, decreased soil pH, and increased metal availability, making plants absorb more heavy metals (Fig. 4) [37, 38]. Our study found that the soil pH decreased under the influence of different Cd concentrations, while S. indica increased the soil pH (Fig. 2E). This may be related to the fact that S. indica reduces the content of soil organic acids and effectively inhibits the process of soil acidification. Soil enzyme activity and soil pH were closely related to heavy metal ecotoxicity [39].
S. indica can reduce soil pollution degree and risk
The Cd fractions in soil and the proportion of various fractions are the key factors to determine its impact on the environment and the surrounding ecosystem [40]. Generally, soil pH, organic matter, and redox conditions all affected the fractions of heavy metals in soil, but pH is the most important factor. It was found that the decrease of pH value by only 0.2 unit will lead to the increase of exchangeable Cd by 3 ~ 5 times [41, 42]. Our results showed that the application of S. indica to soils could contribute to higher pH, especially under the treatment of high Cd, the soil pH recovered from 7.60 to 7.68 (Fig. 2E), which lead the decrease of exchangeable fraction content.
The exchangeable fraction of Cd is highly mobile and highly toxic, while carbonate fraction and reducible iron–manganese fraction are relatively stable components [43]. Our results showed that S. indica reduced the content of exchangeable fraction, caused Cd was difficult to enter into plants (Table 1, 2; Fig. 3, 4), which may be the reason for S. indica weakened the toxicity of Cd to soybean physiology and relieved the growth stress of soybean [44]. In addition, this reduction in the exchangeable fraction content caused by S. indica addition also reduced the degree and risk of soil contamination (Table 3), and the effect was most pronounced in the Cd 6 mg·kg−1 stress [45].
Conclusions
Collectively, our findings suggested that soil Cd pollution leads to plant physiological dysfunction and soil quality deterioration, and this negative effect increased with the enhancement of Cd stress. S. indica inoculation not only enhanced plant growth and photosynthetic physiology to mitigate the negative effects of Cd, especially in Cd 9 mg·kg−1 stress, but also promoted soil enzyme activity and increase soil pH. In addition, S. indica inoculation can convert the Cd exchangeable fraction, which is readily available to plants, into a more stable fraction immobilized in the soil solid phase, thereby reducing the negative impact of Cd on plant physiology and soil contamination degree/risk. These effects of S. indica were most obvious under the Cd stress of 6 mg·kg−1. Therefore, we proposed that S. indica can be used as a microbial fertilizer in Cd-contaminated farmland soil.
Availability of data and materials
All datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Cd:
-
Cadmium
- S. indica :
-
Serendipita indica
- SEM:
-
Structural equation modeling
- TF:
-
Transport factor
- BCF:
-
Bioconcentration factor
- DW:
-
Dry weight
- SH:
-
Shoot length
- URE:
-
Urease
- PHO:
-
Phosphatase
- SUC:
-
Sucrase
- CAT:
-
Catalase
- Tr:
-
Transpiration ratio
- Gs:
-
Stomatal conductance
- Pn:
-
Net photosynthesis rate
- Ci:
-
Intercellular CO2 concentration
- EXC:
-
Exchangeable fraction
- CAR:
-
Carbonate fraction
- RIM:
-
Reducible iron and manganese fraction
- ORG:
-
Organic matter bound fraction
- RES-Cd:
-
Residual fraction
- S-Cd:
-
Residual content in soil
References
Da-Le N, Hoang TTH, Phung VP, Nguyen TL, Rochelle-Newall E, Duong TT, Pham TMH, Phung TXB, Nguyen TD, Le PT, Pham L, Nguyen TAH, Le TPQ. Evaluation of heavy metal contamination in the coastal aquaculture zone of the red river delta (Vietnam). Chemosphere. 2022;303: 134952. https://doi.org/10.1016/j.chemosphere.2022.134952.
Luo XH, Wu C, Lin YC, Li WC, Deng M, Tan JQ, Xue SG. Soil heavy metal pollution from Pb/Zn smelting regions in China and the remediation potential of biomineralization. J Environ Sci. 2023;125:662–77. https://doi.org/10.1016/j.jes.2022.01.0291001-0742.
Mombo S, Laplanche C, Besson P, Sammartino S, Schreck E, Dumat C, Capowiez Y. Metal soil pollution differentially affects both the behaviour and exposure of A-caliginosa and L-terrestris: a mesocosm study. Biol Fertil Soils. 2018;54:319–28. https://doi.org/10.1007/s00374-017-1261-6.
Zhou QX, Liu YX, Li T, Zhao HZ, Alessi DS, Liu WT, Konhauser KO. Cadmium adsorption to clay-microbe aggregates: implications for marine heavy metals cycling. Geochim Cosmochim Acta. 2020;290:124–36. https://doi.org/10.1016/j.gca.2020.09.002.
Kang XR, Geng N, Li X, Yu JP, Wang H, Pan H, Yang QG, Zhuge YP, Lou YH. Biochar alleviates phytotoxicity by minimizing bioavailability and oxidative stress in foxtail millet (Setaria italica L,) cultivated in Cd- and Zn-contaminated soil. Frontiers in Plant Science. 2022;13:782963. https://doi.org/10.3389/fpls.2022.782963.
Ma P, Tian T, Dai ZY, Shao TY, Zhang W, Liu MD. Assessment of Cd bioavailability using chemical extraction methods, DGT, and biological indicators in soils with different aging times. Chemosphere. 2022;296:133931. https://doi.org/10.1016/j.chemosphere.2022.133931.
Zhi Y, Sun T, Zhou Q, Leng X. Screening of safe soybean cultivars for cadmium contaminated fields. Sci Rep. 2020;10(1):12965. https://doi.org/10.1038/s41598-020-69803-4.
Rossi L, Zhang WL, Schwab AP, Ma XM. Uptake, accumulation, and in planta distribution of coexisting cerium oxide nanoparticles and cadmium in Glycine max (L.) Merr. Environ Sci Technol. 2017;51:12815–24. https://doi.org/10.1021/acs.est.7b03363.
Li YP, Wu WA, Yang JX, Cheng K, Smith P, Sun JF, Xu XR, Yue Q, Pan GX. Exploring the environmental impact of crop production in China using a comprehensive footprint approach. Sci Total Environ. 2022;824:153898. https://doi.org/10.1016/j.scitotenv.2022.153898.
Xian PQ, Cai ZD, Cheng YB, Lin RB, Lian TX, Ma QB, NianH. Wild soybean Oxalyl-CoA synthetase degrades oxalate and affects the tolerance to cadmium and aluminum stresses. Int J Mol Sci. 2020;21(22):8869. https://doi.org/10.3390/ijms21228869.
Chen CY, Huang PH, Yeh KW, Wang SJ. Colonization of Piriformospora indica enhances insect herbivore resistance of rice plants through jasmonic acid- and antioxidant-mediated defense mechanisms. J Plant Interactions. 2022;17(1):9–18. https://doi.org/10.1080/17429145.2021.2008031.
Sun RT, Zhang ZZ, Feng XC, Zhou N, Feng HD, Liu YM, Harsonowati W, Hashem A, Abd-Allah EF, Wu QS. Endophytic fungi accelerate leaf physiological activity and resveratrol accumulation in Polygonum cuspidatum by up-regulating expression of associated genes. Agronomy-Basel. 2022;12(5):1220. https://doi.org/10.3390/agronomy12051220.
Saeed-ur-Rahman Khalid M, Kayani SI, Tang KX. The ameliorative effects of exogenous inoculation of Piriformospora indica on molecular, biochemical and physiological parameters of Artemisia annua L. under arsenic stress condition. Ecotoxicol Environ safety. 2020;206:111202. https://doi.org/10.1016/j.ecoenv.2020.111202.
Yang L, Zou YN, Tian ZH, Wu QS, Kuca K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci Hortic. 2020;277:109815. https://doi.org/10.1016/j.scienta.2020.109815.
Shukla J, Mohd S, Kushwaha AS, Narayan S, Saxena PN, Bahadur L, Mishra A, Shirke PA, Kumar M. Endophytic fungus Serendipita indica reduces arsenic mobilization from root to fruit in colonized tomato plant. Environ Pollut. 2022;298: 118830. https://doi.org/10.1016/j.envpol.2022.118830.
Phillips JM. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. 1970. https://doi.org/10.1016/S0007-1536(70)80110-3.
Institute of Soil Science, Chinese Academy of Sciences. Soil physicochemical analysis. Shanghai Scientific and Technical Publishers, 1978
Giovannetti M, Mosse B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980;84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x.
Carrigan RA. Methods of determination of soil pH. Proceedings soil science society of Florida.1940; 25–39.
Zantua MI, Bremner JM. Comparison of methods of assaying urease activity in soils. Soil Biol Biochem. 1975;7:291–5. https://doi.org/10.1016/0038-0717(75)90069-3.
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;3:14–26.
Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem. 1969;1(4):301–7. https://doi.org/10.1016/0038-0717(69)90012-1.
Trasar-Cepeda C, Camiñaa F, Leirós MC, Gil-Sotres F. An improved method to measure catalase activity in soils. Soil Biol Biochem. 1999. https://doi.org/10.1016/S0038-0.
Tokalioglu S. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012;134(4):2504–8. https://doi.org/10.1016/j.foodchem.2012.04.093.
Grunke K, Stark HJ, Wennrich R, Franck U. Determination of traces of heavy metals (Mn, Cu, Zn, Cd and Pb) in microsamples of teeth material by ETV-ICP-MS. Fresenius J Anal Chem. 1996;354:5–6. https://doi.org/10.1007/s0021663540633.
Tessier A, Campbell P, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem. 1979;51:844–51.
Mackay D. Correlation of bioconcentration factors. Environ Sci Technol. 1982;16(5):274–8. https://doi.org/10.1021/es00099a008.
Li WX, Zhang XX, Wu B, Sun SL, Chen YS, Pan WY, Zhao DY, Cheng SP. A comparative analysis of environmental quality assessment methods for heavy metal-contaminated soils. Environ Sci Technol. 2008;18(3):344–52. https://doi.org/10.1016/S1002-0160(08)60024-7.
Cornu JY, Bussiere S, Coriou C, Robert T, Nguyen C. Changes in plant growth, Cd partitioning and xylem sap composition in two sunflower cultivars exposed to low Cd concentrations in hydroponics. Ecotoxicol Environ Saf. 2020;205:111145. https://doi.org/10.1016/j.ecoenv.2020.111145.
Wu MY, Wei Q, Xu L, Li HZ, Oelmüller R, Zhang WY. Piriformospora indica enhances phosphorus absorption by stimulating acid phosphatase activities and organic acid accumulation in Brassica napus. Plant Soil. 2018;432:333–44. https://doi.org/10.1007/s11104-018-3795-2.
Yun P, Xu L, Wang SS, Lana S, Sergey S, Zhang WY. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoot. Plant Growth Regul. 2018;86:323–31. https://doi.org/10.1007/s10725-018-0431-3.
Liu H, Yang L, Li N, Zhou C, Han X. Cadmium toxicity reduction in rice (Oryza sativa L.) through iron addition during primary reaction of photosynthesis. Ecotoxicol Environ Safety. 2020;200:110746. https://doi.org/10.1016/j.ecoenv.2020.110746.
Wang L, Zou R, Li YC, Tong ZH, You M, Huo WM, Chi KY, Fan HL. Effect of wheat—Solanum nigrum L. intercropping on Cd accumulation by plants and soil bacterial community under cd contaminated soil. Ecotoxicol Environ Safety. 2020. https://doi.org/10.1016/j.ecoenv.2020.111383.
Xiao Y, Zhao Z, Chen L, Li Y. Arbuscular mycorrhizal fungi mitigate the negative effects of straw incorporation on trifolium repens in highly Cd-polluted soils. Appl Soil Ecol. 2021;157: 103736. https://doi.org/10.1016/j.apsoil.2020.103736.
Mosaddeghi MR, Hosseini F, Hajabbasi MA, Sabzalian MR, Sepehri M. Chapter two—Epichloë spp. and Serendipita indica endophytic fungi: functions in plant-soil relations. Adv Agronomy. 2022. https://doi.org/10.1016/bs.agron.2020.09.001.
Haddad SA, Tabatabai MA, Loynachan TE. Effects of liming and selected heavy metals on ammonium release in waterlogged agricultural soils. Biol Fertil Soils. 2017;53:153–8. https://doi.org/10.1007/s00374-016-1163-z.
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut. 2011;159:84–91. https://doi.org/10.1016/j.envpol.2010.09.019.
Yang X, Qin J, Li J, Lai Z, Li H. Upland rice intercropping with solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J Hazard Mater. 2021;406: 124325. https://doi.org/10.1016/j.jhazmat.2020.124325.
Heidari E, Mohammadi K, Pasari B, Rokhzadi A, Sohrabi Y. Combining the phosphate solubilizing microorganisms with biochar types in order to improve safflower yield and soil enzyme activity. Soil Sci Plant Nutr. 2020;66:255–67. https://doi.org/10.1080/00380768.2019.1704180.
Pan Y, Fu YQ, Liu SJ, Ma TF, Tao XQ, Ma Y, Fan S, Dang Z, Lu GN. Spatial and temporal variations of metal fractions in paddy soil flooding with acid mine drainage. Environ Res. 2022. https://doi.org/10.1016/j.envres.2022.113241.
Zhu H, Chen C, Xu C, Zhu Q, Huang D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical china. Environ Pollut. 2016;219:99–106. https://doi.org/10.1016/j.envpol.2016.10.043.
Meng J, Cui Z, Zhang H, Zhang J, Shan S. Combined effects of arbuscular mycorrhizae fungus and composted pig manure on the growth of ryegrass and uptake of Cd and Zn in the soil from an e-waste recycling site. Environ Sci Pollut Res. 2021;28(10):12677–85. https://doi.org/10.1007/s11356-020-11215-y.
Zhang J, Hua P, Krebs P. Influences of land use and antecedent dry-weather period on pollution level and ecological risk of heavy metals in road-deposited sediment. Environ Pollut. 2017;228:158–68. https://doi.org/10.1016/j.envpol.2017.05.029.
Wei T, Li X, Li H, Gao H, Guo JK, Li YT, Ren XH, Hua L, Jia HL. The potential effectiveness of mixed bacteria-loaded biochar/activated carbon to remediate Cd, Pb co-contaminated soil and improve the performance of pakchoi plants. J Hazard Mater. 2022;435:129006. https://doi.org/10.1016/j.jhazmat.2022.129006.
Wang XH, Fan XX, Wang WD, Song FQ. Combined effects of inoculating Serendipita indica on soybean growth and soil health under Cd stress. Res Square Priprint. 2021;10:11.
Acknowledgements
The authors thank Professor Wu Chu for the strain of Serendipita indica.
Funding
The authors are grateful for the financial support of the Natural Science Foundation of Heilongjiang Province (TD2019C002), Heilongjiang Provincial Key Research and Development Plan Guidance Project (GZ20210009) and National Natural Science Foundation of China (31971527).
Author information
Authors and Affiliations
Contributions
XW and FS designed experiments; XW carried out experiments; XW, XF, and WW analyzed experimental results; XW and FS wrote and edited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflict of interest regarding the publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Fan, X., Wang, W. et al. Use of Serendipita indica to improve soybean growth, physiological properties, and soil enzymatic activities under different Cd concentrations. Chem. Biol. Technol. Agric. 9, 66 (2022). https://doi.org/10.1186/s40538-022-00331-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-022-00331-1