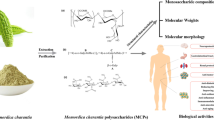
Abstract
The root of Angelica sinensis (Oliv.) Diels, a well-known Chinese herbal medicine, has been used historically as hematopoietic and anti-inflammatory agents for thousands of years. Recent phytochemistry and pharmacological studies have proved that polysaccharides are one of the major active ingredients in A. sinensis. It has been demonstrated that ASPs (A. sinensis polysaccharides) had various important biological activities, such as hematopoietic, hepatoprotective, hypoglycemic, anti-inflammatory, antitumor, and antioxidant activities. The purpose of this present review is to appraise previous and current literatures on the extraction, purification, structural characterization and biological activities of ASPs. In addition, the structure–activity relationship will be further explored and discussed. We believe that this review will provide a useful bibliography for the investigation, production, and application of ASPs in functional foods and therapeutic agents. Moreover, this review also highlights the challenges of investigation and future considerations for holistic utilization.

Similar content being viewed by others
Introduction
The root of Angelica sinensis (Oliv.) Diels (A. sinensis), belonging to Umbelliferae family, is identified as a multi-species of about 100 and A. sinensis is largely cultivated in the temperate regions, particularly in Africa, Asia, parts of South America [1, 2]. The A. sinensis has been cultivated for 2000 years in China, mainly distributed in the northwest region (Shaanxi Province, Gansu Province) and southwest region (Yunnan Province, Sichuan Province) [3, 4]. It is also known as “Dang Gui” in China and was first described in the oldest medical material book “Shennong's Classic of Material Medica (300 BCE-200 CE)” [5]. The root was also identified as an excellent herbal medicine in “Compendium of Materia Medica (1552–1578)”, and the taste and nature are slightly sweet, neutral, and warm [3, 6]. It has been used as a tonic and hematopoietic agent for the treatment of gynecological diseases, including menstrual disorders, amenorrhea, dysmenorrheal and relaxing bowel [7, 8]. Modern pharmacological studies have shown that A. sinensis has hematopoietic, immunomodulatory, antitumor, antioxidant and hepatoprotective activities, and gastrointestinal protective and anti-inflammatory effects [2, 9]. These beneficial effects have been partly attributed to the different and complicated bioactive components of the root of A. sinensis which contains over 70 chemical compounds, including carbohydrates, organic acids, essential oils, phenolic compounds, vitamins, protein, amino acids, and other constituents [10, 11].
A. sinensis contains a high amount of carbohydrates, which are a group of reducing sugars and polysaccharides. As we know, plant polysaccharides have become a new research hotpot in recent years for their high bioactivity and low toxicity [12], such as Ziziphus jujuba Mill. polysaccharides [13], Dioscorea spp. polysaccharides [14], Lycium barbarum polysaccharides [15], and Panax ginseng C. A. Meyer polysaccharides [16], as well as Angelica sinensis polysaccharides (ASPs) [4]. Recent phytochemical and pharmacological studies have demonstrated that the diverse activities mentioned above, including hematopoiesis [17, 18], immunomodulation [19, 20], antitumor [21, 22], antioxidant [23, 24], and radioprotection [3, 25], are mainly attributed to the polysaccharides extracted from the root of A. sinensis.
To our knowledge, no review concerning ASPs is available. Therefore, we systematically summarized the related research findings of the past two decades and provided comprehensive insights into the physicochemical properties, structural characteristics, pharmacological effects and application prospects of A. sinensis polysaccharides, providing detailed reference for the study about their structure–bioactivity relationships, the exploration of commercial applications and potential uses in functional foods as well.
Procedures of extraction and purification
Among the functional components of A. sinensis, polysaccharides are the most important active constituents as they exert different biological effects and potential health benefits [4, 26]. Adequate procedures have been designed to obtain pure A. sinensis polysaccharides which could be chemically characterized and accurately tested in vitro and in vivo assays [27, 28].
In general, different procedures were usually used in ASPs extraction (Fig. 1). First, the dried roots were ground to a fine powder and defatted by refluxing with a chloroform: methanol (2:1) mixture. Oligosaccharides and small coloring molecules were further removed by refluxing with 80% ethanol at room temperature. The filtered and dried residues were then used to extract the water-soluble polysaccharide mixture using different methods [29]. Basically, moderate extraction conditions were adopted to separate the outer layer of the cell wall from the inner layer, without altering polysaccharide materials [30]. Traditional hot or boiling water extraction is the classical and probably the most convenient method in the laboratory and industrial extraction of plant polysaccharides. It is widely used to extract ASPs. Hot water (80–100 °C for 120–180 min, with a solvent-to-material ratio of 5–10, over two or five extraction rounds) was proved to be the most proper extraction solvent to prepare ASPs. Ai et al. analyzed the influence of different parameters, including extraction time, extraction temperatures, solid/liquid ratio and number of extraction cycles on ASPs yields using an orthogonal array experimental design (OAD) model and found that the maximum polysaccharide yields were obtained in 180 min, 4 cycles with a 1:6 solid/liquid ratio mix in boiling water [31]. According to a recent study, the acidic extraction for ASPs after water extraction was also used to obtain different polysaccharide fractions. For instance, Sun et al. (2016) extracted polysaccharides from the A. sinensis root using pH 4.50 solutions at 85 °C, allowing the structures of these fractions to be analyzed by methylation, GC–MS and NMR [3].
The A. sinensis has rigid cell walls and some polysaccharides are structural components of the cell walls or exist in the cell. Therefore, breaking up cell wall of A. sinensis could release polysaccharides easily and improve the extraction yield. Some different technologies could improve the extraction efficiencies, including microwave-assisted treatments and ultrasonic processing [32]. Zhao et al. identified the optimum operational conditions for maximizing polysaccharides yield through response surface methodology as ultrasound power 180 W, ultrasound time 45 min using a water: material ratio (w:w) of 7:1 at 90 °C. Under these conditions, 6.96% of ASPs were achieved [33]. Meanwhile, Tian et al. reported that up to 21.89 ± 0.21% of ASPs were obtained under the optimum extraction conditions as water/raw material ratio 43.31 mL/g, ultrasonic time 28.06 min, power 396.83 W, proving the contribution of ultrasound in polysaccharide extraction [25]. Another extraction method, microwave-assisted extraction, was also frequently used. Li et al. extracted ASPs using distilled water under optimal extraction conditions, including a microwave power of 500 W, microwave time of 20 min, water: material ratio (w:w) of 15:1 at room temperature [34]. In summary, different assisted extraction methods or combined process have been developed to improve the extraction efficiency of ASPs, shorten the processing time and reduce solvent consumption and energy requirements.
The A. sinensis polysaccharides are heteropolysaccharides and contain impurities and different fractions as reported [35, 36]. After extraction, purification is essential for the research of chemical structure of plant polysaccharides [37]. As widely used purification techniques, ion-exchange chromatography (DEAE-Sephadex A-25, DEAE-Spharose Fast Flow, and DEAE-Sepharose CL-6B) is used to separates neutral and acidic polysaccharides by gradient salt elution, while gel filtration column chromatography (Superdex 200, Sephacryl S series, and Sepharose CL-6B) is often used to separate ASPs of different molecular weights [27, 38]. For example, Sun et al. (2005) fractionated ASPs through ion-exchange chromatography on a column of DEAE-Sepharose CL-6B (D 2.6 cm × 30 cm) eluted with NaCl gradient (0–1 M). And it was found that neutral ASPs were rich in glucose, galactose, arabinose, galacturonic acid in different ratio, while the acidic polysaccharide fractions (ASP1, ASP2, ASP3) eluted with NaCl solution ranging from 0.2 to 0.6 M mainly consisted of galacturonic acid along with rhamnose, arabinose and galactose, indicating the pectic polysaccharide [39]. Good results and higher yield should be based on proper extraction and purification methods [40].
Structure elucidation
The structural features of ASPs, such as molecular weights, monosaccharide compositions, types of glycosyl linkage, and conformational features, have been investigated by chromatography technology, spectrum analysis and other chemical analysis. Some studies on the structures as well as bioactivities of ASPs were summarized and illustrated in Table 1 and Fig. 2.
Schematic structures of Angelica sinensis polysaccharides. a A. sinensis polysaccharides (ASP1), b A. sinensis polysaccharides (ASP2), c A. sinensis polysaccharides (ASP2) [46]. d A new water-soluble polysaccharide (APS-1II) [27]. e An acidic polysaccharide (WASP3) [3]. f The predicted chemical structure of ASP [28]. g A soluble polysaccharide (JASP-1A) [47]. h The chemical structure of ASP [56]. i The predicted structure of APS-1d [38]. j The structure of ASP [57]
Molecular weights
HPLC and high-performance gel permeation chromatography (HPGPC) were widely applied to determine the average molecular weights of ASPs [41, 42]. Molecular weights ranging within 104–105 Da were reported in ASPs extracted from A. sinensis roots under different experimental conditions. In addition, different fractions from the same source could be also different. For instance, Zhao et al. demonstrated that the molecular weights of two polysaccharides from the root of A. sinensis were 4.90 × 104 (APS-1a) and 6.54 × 104 (APS-3a) Da, respectively [43].
Monosaccharide compositions
Monosaccharide compositions analyses are usually performed with complete acid hydrolysis to cleave glycosidic linkages for derivatization, detection, and quantification with HPLC and GC [44, 45]. Due to the differences in sources, different monosaccharide compositions of ASPs were reported, and the majority of these polysaccharides were composed of mannose, rhamnose, arabinose, glucose, galactose and galacturonic acid with various molar ratios. For example, in a study, Xing et al. (2013) obtained three polysaccharides (ASP1, ASP2 and ASP3) with Sephadex G-50 and analyzed them by GC. Data indicated that the three fractions showed quite notable difference in monosaccharide compositions (Table 1) [46]. Meanwhile, Zhou et al. [47] studied the monosaccharide compositions of crude and neutral polysaccharides (JASP and JASP1A) isolated from A. sinensis collected in Gansu Province and found that JASP consisted of glucose, glucuronic acid, galactose and arabinose in a molar ratio of 1.7:1.0:5.02:1.85, while JASP1A only contained rhamnose, galactose and arabinose [47].
Glycosidic linkage types and positions
To analyze the detailed structure of plant polysaccharide, it is necessary to clarify not only the monosaccharide composition/molecular weights, but also the manner of attachment of various monosaccharides as well as branches [14]. The combination of instrumental techniques, including GC–MS and nuclear magnetic resonance (NMR), with chemical analysis such as periodate oxidation, Smith degradation, partial acid hydrolysis and enzyme digestion was applied to investigate the structures of ASPs [48, 49]. In a previous study, the preliminary structure of APS-1II was successfully identified. According to GC–MS, 1D and 2D NMR results (1H NMR, 13C NMR, TOCSY, NOESY, HMBC), Liu et al. (2019) concluded that it had a glucoarabin backbone, mainly composed of 1,3-Araf and 1,6-Glcp with an Ara: Glc ratio of ~ 5:1, and branching occurred from the C-5 of 1,3-Araf residues [27]. In another study, Wang et al. (2007) conducted a preliminary separation of A. sinensis polysaccharide (ASDII-3–3), and according to the results of infrared spectroscopy, GC–MS and 13C NMR, ASDII-3–3 was proved to have a backbone composed of (1 → 2)-linked-Rhaf and (1 → 4)-linked Galp with branches composed of (1 → 5)-linked-Araf terminated with Ara residues and (1 → 4)-linked-Xylp terminated with Manp residues [50].
An early publication in 1999 of Zhang and Huang first reported that A. sinensis polysaccharides As-IIIa was a linear α-glucan composed of only α-(1 → 6)-glucan, and As-IIIb had a repeating unit consisting of (1 → 4) and (1 → 6) glycosidic bonds [51]. In another study mentioned above, the structural features of a water-soluble polysaccharide (ASD-PII) which was purified by DEAE-Sephadex A-25 column were investigated by methylation analysis and GC–MS analysis, and deduced to be a backbone as → 1)Fruf (2 → and → 6)Glcp(1 → with highly branched structure [55].
Also, it should be noted that the molecular structures of A. sinensis polysaccharides are closely related to their inherent local biodiversity (genomic variety, geographic origin, and environmental conditions), which was reflected in the previous reports.
Conformational features
Chain conformation is also an important parameter of plant polysaccharides because of its close relationship with bioactivity [44], so efforts have been made to illuminate this parameter of ASPs. For example, a study by Wang et al. [23] described the conformation of ASP-24 by SEM 1000-fold amplification as a compact surface structure [23]. In addition, it is reported that the ASP might have a higher value of apparent viscosity due to the intermolecular association of hydrophobic segments in the different size molecules [58].
If there is more detailed information available, it is more well founded for us to build the structure–activity relationship of ASPs. However, unfortunately, reports about the spatial structures of these polysaccharides are quite inadequate and need more extensive investigation by advanced technologies, such as NMR spectroscopy, static and dynamic light scattering, fluorescence spectroscopy, etc.
Biological activities
With the exploitation and application of traditional Chinese medicine (TCM) in recent years, a considerable attention has been paid to ASPs owing to their diverse and potent bioactivities such as hematopoietic, hepatoprotective, hypoglycemic, anti-inflammatory, antitumor, antioxidant and other activities according to the in vitro and in vivo experiments. The multiple bioactivities of ASPs and underlying mechanisms are listed in Table 1.
Hematopoietic activity
ASPs are important bioactive component, and have been used for treating anemia and gynecological disorders. It has been reported that ASP could stimulate hematopoietic cells and muscle tissues and enhance hematopoietic growth factors [59].
Wang et al. [60] found that ASPs could enhance hypoxic induction of erythropoietin (EPO) in Hep3B cell, with a mechanism that involved the stabilization of HIF-2α protein. ASPs rescued the inhibition of EPO induced by TNF-α through blocking GATA binding protein 2 (GATA2) and NF-κB activation. The restoration of EPO production and EPOR mRNA expression with ASPs treatment activated EPOR downstream JAK2/STAT5 and PI3K/Akt signaling, induced their target genes, such as B-cell lymphoma-extralarge (Bcl-xL), Fam132b and TFRc, and increased Bcl-2/Bax ratio in bone marrow-derived mononuclear cells of CKD rats. Meanwhile, it was found that in a rat model of adenine-induced anemia, oral administration of ASPs could correct anemia and alleviate renal damage and inflammation [60] (Fig. 3).
In addition, it is reported that ASPs could inhibit inflammatory hepcidin in both HepG2 cell and ACD (Anemia of chronic disease) rats by blocking the IL-6/STAT3 and BMP/SMAD pathways. Besides, ASP inhibited NF-κB p65 activation via the IκB kinases (IKKs-) IκBα pathway, thereby reducing the secretion of interleukin-6 (IL-6) and TNF-α in ACD rats (Fig. 4a) [61]. Zhang et al. showed that crude polysaccharides extracted from the root of Angelica sinensis (Oliv.) were mainly composed of arabinose, glucose, and galactose in a molar ratio of 1:2.5:7.5, with an average molecular weight of 72,900 Da. Studies indicated that ASPs could considerably decrease hepcidin expression via down-regulating the expression of JAK1/2, phospho-JAK1/2, phospho-SMAD1/5/8, phospho-ERK1/2 while promoting the expression of SMAD7 in the liver, thus benefiting the treatments of diseases caused by hepcidin over-expression (Fig. 5) [18]. All these findings mentioned above confirmed the hematopoietic activity of ASPs. So as to improve the hematopoietic activity, polysaccharides through intestinal microbiota fermentation might be a promising research direction in the future.
Hepatoprotective activity
Many investigations have confirmed the direct hepatoprotective effect of ASPs. For example, Wang et al. (2016) explored the mechanisms of ASPs-mediated hepatoprotective effects in type 2 diabetic BALB/c mice and found that ASPs could stimulate insulin secretion, promote hepatic glycogen synthesis, regulate adipokine release, reduce liver fat accumulation, and attenuate liver injury [62]. Moreover, ASPs upregulated the expression of PPARγ and liver insulin signaling proteins, including IRS-2, PI3K, Akt, p-Akt and GLUT2, increased anti-apoptotic protein Bcl-2, decreased pro-apoptotic protein Bax expressions, and protected the mice against hepatic damage (Fig. 4b) [62].
In addition, in a previous study, it was shown that 125 μg/mL ASPs pretreatment noticeably decreased pro-inflammatory cytokines (TNF-α, IFN-γ, IL-2 and IL-6), reduced MDA and ROS levels, enhanced SOD activity, and attenuated Caspase-3-dependent apoptosis by Caspase-8 and JNK-mediated pathway, inhibited the activation of IL-6/STAT3 and NF-κB signaling pathways in ConA-induced liver damage in mice (Fig. 4c) [63].
Meanwhile, a novel approach using biochemical parameters coupled with metabolomics based on GC–MS was designed to explain the hepatoprotective effect mechanism of ASP. In a study, pathological change, SOD activity, MDA content, ALT, AST, and γ-GT all had certain degrees of recovery after 120 mg/kg/day ASP intervention. In addition, the regulative effect on nine potential biomarkers in the liver homogenate (malic acid, fumaric acid, glycine, succinic acid, hexadecenoic acid, octadecanoic acid, valine, linoleic acid, arachidonic acid) and ten potential biomarkers in the plasma (fumaric acid, alanine, malic acid, glycine, citric acid, arachidonic acid, octadecanoic acid, hexadecenoic acid, linoleic acid, valine) were found to explain the hepatoprotective mechanism of ASPs [64]. The pharmacokinetics of ASPs including the digestion, absorption, and utilization after oral administration, still needs investigation in the future.
Hypoglycemic activity
Non-starch polysaccharides could decrease levels of blood sugar through different mechanisms of action. In a previous study [65], fasting blood glucose (FBG) levels were reduced after a 4-week oral administration of ASPs in streptozotocin (STZ)-induced diabetic BALB/c mice. The hepatic glycogen (HG) and muscle glycogen (MG) concentrations increased, while insulin resistance (IR)-related inflammatory factors IL-6 and TNF-α in serum reduced in STZ-induced diabetic mice. Meanwhile, researchers found that ASPs could improve the dyslipidemia conditions, and reduce elevated serum total cholesterol (TC), triglyceride (TG) concentrations.
Zhang et al. [66] previously reported the protective effects of ASPs on pancreatic islets of T2DM mice. And they demonstrated that the hypoglycemic effect of ASPs might be closely attached to the promotion of insulin secretion and the inhibition of apoptosis in pancreatic β-cells via blocking both extrinsic and intrinsic pathways (Fig. 4d).
In another study, Chen [67] reported that oral administration of ASPs (20 and 100 mg/kg body weight) for 21 days resulted in a significant reduction in blood glucose levels coupled with the improvement of plasma insulin level in alloxan-induced diabetic rats, which might be associated with the repair and regeneration of the damaged islet B cell [67]. These studies implied that ASPs had hypoglycemic activity therapeutic potential.
Anti-inflammatory activity
Inflammation is subsequent response of the body's immune system to injury, infection and stress. Polysaccharides from plant have attracted increasing attentions of researchers around the world as for their safety and anti-inflammatory activity [68]. For example, Li et al. [69] reported that 4-week oral administration of ASP exhibited significant effect in decreasing pro-inflammatory cytokines (IL-6 and TNF-α) in complete Freund’s adjuvant (CFA)-induced arthritic rats besides its activity in improving anemia [69].
Meanwhile, the protective effects of ASP on dextran sulfate sodium-induced ulcerative colitis were investigated by Cheng et al. [70]. It was found that ASP could considerably ameliorate the symptoms of weight loss, disease activity index score, and colon shortening caused by dextran sulfate sodium, and markedly suppress the myeloperoxidase activity in colon tissues. ASP inhibited inflammatory cytokine expressions, decreased apoptosis of intestinal epithelial cells and promoted tight junction protein expression in enteritis mice. Meanwhile, they could protect the intestinal barrier function and accelerate the healing of chronic ulcers [70]. The protective effects of ASP were highly associated with the attenuation of oxidative stress and apoptosis (two important pathogenic factors in the induction of ulcerative colitis) in colonic cells [11]. Although ASPs from various species showed potent anti-inflammatory activity, the effective factors and mechanisms deserves further investigation, especially in intestinal microbiota degradation.
Antitumor activity
The plant polysaccharides could exert antitumor activity through different mechanisms, which included preventing oncogenesis, improving immune response, inducing tumor cells apoptosis, and inhibiting tumor cells proliferation [71]. Fu et al. [72] previous reported that APS could induce apoptosis via regulation of the Janus kinase (JAK)/signal transducers and activators (STAT) of transcription pathway in breast cancer cell [72].
In addition, Cao et al. [73] reported that the total polysaccharide (APS-1d) isolated from A. sinensis could decrease HeLa cell proliferation while induce apoptosis in a concentration- and time-dependent manner in vitro, and inhibit tumor growth significantly in athymic nude mice, which primarily involved the activation of the intrinsic mitochondrial pathway [73].
Besides, Ren et al. found that ASPs could inhibit tumor growth in mice xenografted with mouse breast cancer cell line and mouse hepatocellular carcinoma cell line. In vivo experiments showed that ASPs could potently regulate hepcidin expression in liver and serum and decrease iron burden in liver, spleen and grafted tumors in mouse model, and markedly suppress the expression of interleukin-6 (IL-6), JAK2, p-STAT3, and p-SMAD1/5/8 in liver, which provided evidence for the potential use of ASP in cancer treatment in patients with iron overload [74]. ASPs have a good antitumor activity and little toxic side effect, and whether it can directly induce tumor cells is yet to be further verified.
Antioxidant activity
The antioxidant activity of ASPs is frequently carried out through various assay methods in vivo and in vitro. In a study mentioned above, researchers investigated the antioxidant activity of ASPs in vitro and demonstrated that ASPs had certain antioxidant activities in suppressing superoxide anion radicals, hydroxyl radicals, and 2,2-diphenyl-1-picryhydrazyl (DPPH) radicals in a dose-dependent manner [75].
In another study, oral ASPs administration at a dosage levels of 150 mg/kg 3 times a day before meals for 3 months in middle-aged women subjects indicated that the superoxide dismutase, catalase, glutathione peroxidase, reduced glutathione, and lipid peroxidation levels were significantly enhanced while serum vascular cell adhesion molecule-1, interleukin-1 beta, interleukin-6, and tumor necrosis factor levels were considerably reduced compared to the control group, which was comparable to effects of Tai Chi exercise [76].
In addition, Yang et al. [77] found that APF3 (A. sinensis polysaccharide fraction) could effectively inhibit H2O2-caused decrease of cell viability, lactate dehydrogenase leakage and malondialdehyde formation, and reduce H2O2-caused decline of superoxide dismutase activity and glutathione depletion. Studies revealed that 100 μg/mL APF3 could effectively protect macrophages by inhibiting the release of excess NO and reactive oxygen species induced by high concentrations of H2O2 [77].
Other bioactivities
In addition to the above-mentioned ones, other biological activities of ASPs, including anti-aging, immunomodulation, anti-radiation, were also explored and reported in recent years.
For instance, Qian et al. previous reported that ASP treatment could suppress pulmonary fibrosis in rats and fibrogenesis in alveolar type II epithelial (RLE-6TN) cell via a DANCR/AUF1/ FOXO3 regulatory axis, thus benefiting the treatment of idiopathic pulmonary fibrosis (IPF) [78].
Besides, ASPs showed anti-aging activity by attenuating excessive activation of Wnt/β-catenin/TCF-4 signaling in the D-gal-induced senescence of Sca-1+HSC/HPCs significantly and inhibiting the phosphorylation of GSK-3β which inactivated β-catenin degradation. The antiaging mechanism could be involved in GSK3β interacted with Notch signaling and PI3K/Akt signaling in stem cell aging [79].
In addition, Yang et al. [80] investigated the immunomodulatory activities of an A. sinensis polysaccharide (AP) in vitro. It was found that AP promoted the proliferation of total spleen cell, macrophages and T cell. Besides, AP upregulated IL-2 and IFN-γ, while decreased IL-4 on both protein and gene levels. The treatment of AP also remarkably increased the percentage of CD4+ T cell in total spleen cell, while decreased that of CD8+ T cell slightly. Studies indicated that AP had immunomodulatory activity by regulating the expression of Th1- and Th2-related cytokines. Furthermore, the time–effect relation of cytokines response suggested that cells involved in nonspecific immunity, such as macrophages and natural killer cells, were primary activated and helper T cells were secondarily affected after AP treatment [80].
Anti-radiation is another activity attracts extensive attention about ASPs. It is reported that under gamma radiation treatment, compared with the irradiated controls, 7 days pre-treatment of pectic polysaccharide, namely ASP3, exhibited effective protective effect on leucocytes and lymphocytes of mice against radiation-induced damage at a dosage of 200 mg/kg d body weight when mice were exposed to a 60Co source with a dose of 3.0 Gy at a uniform dose rate of 80.0 cGy/min [39]. In another study, researchers found that two homogeneous polysaccharides, APS-1a and APS-3a, could improve the thymus and spleen index, increase the number of red blood cell (RBC), and white blood cell (WBC) in peripheral blood as well as the cellularity of bone marrow cell numbers in irradiated mice, protect mice against radiation-induced micronucleus formation in bone marrow, demonstrating their radio-resistance activity [43]. Because of the complex structure of ASPs, the mechanism of their pharmacological effects and the structure–activity relationship are still unknown.
Structure–activity relationship
The bioactivities of polysaccharides have close relationship with their structures, including their monosaccharide compositions, glycosidic linkages types and positions, molecular weights, chain conformations, etc. ASPs are no exceptions, however, few reports about their structure–activity relationship are available, which needs further investigations. But still some indications can be inferred as follows.
ASPs with radio-protective activity tend to be richer in galacturonic acid, galactose and arabinose in their molecular composition, which could be inferred from those three mentioned above, among which APS-1a was composed of galactose, arabinose and glucose in a relatively molar ratio of 57.34, 27.67 and 14.98%, and APS-3a consisted of galactose, arabinose and glucose in a relatively molar ratio of 84.54, 6.50, and 8.96%, while ASP3 mainly consisted of galacturonic acid (58.27%), galactose (24.93%), arabinose (10.5%) and rhamnose (1.87%) [39, 43].
ASPs with high β-galactoside content seems to possess high antitumor activity as the latter could specifically bind to Galectins (Gal) which play important roles in anti-apoptosis in various cancer cells. For example, Liu et al. [81] reported that a heterogeneous polysaccharide (APS-2I) and its galactosidase digested fraction named G-4 showed higher affinity to Gal-3 with dissociation constant (Kd) of 9.35 ± 0.3 μM and 1.97 ± 0.7 μM, respectively, and exhibited growing apoptosis inducing effect on leukemia cells. The mechanism involved inhibiting the anti-apoptotic effect of Gal-3 and activating the caspase-9 and caspase-3 in leukemia cells by APS-2I and G-4. Structure characterization and comparison indicated that the contents of Gal, GalA and Man as well were important for the anti-leukemia bioactivity of APS-2I, but a high proportion of Araf was not essential. In addition, glycosidic linkages, including T-Galp and 1, 4-Galp could be of great importance in forming active conformation in APS-2I [81].
The influence of molecular weight on the functions of ASPs appears to be controversial. It was reported that high molecular weight might play a crucial role in the formation and maintenance of active spatial conformation of ASPs [4]. Nevertheless, other studies demonstrated that the bioactivity of ASPs have no direct connection with their molecular weights, and sometimes the relationship between them could be quite complex, being related with the object of action and the solubility of ASPs [46, 81].
It is definite that chain conformation means a lot in determining the bioactivities of ASPs, but precise demonstration about this is absent and massive work should be done to uncover the mysterious veil, which will exactly benefit our understanding of the mode of action of ASPs.
Conclusions
Most polysaccharides derived from plants are nontoxic and do not cause any significant side effects. A. sinensis polysaccharides could be effectively isolated and purified with various extraction methods. The ASPs have a variety of biological activities including hematopoietic, antitumor, antioxidant, hepatoprotective, hypoglycemic, and anti-inflammatory activities, indicating their potential broad contribution in disease treatment in the future. As for their chemical structure, efforts have been paid to illuminate their monosaccharide composition, molecular weight, primary structure and high order structure. Facing the complexity of ASPs structure, we could use a variety of methods, including computer-aided liquid nuclear magnetic resonance analysis technology, computer molecular docking and molecular dynamics simulation technology, and 3D emerging technologies, to improve the accuracy of polysaccharide structure characterization in the future. However, the relationships of ASPs between their structure and functions have not been well established yet. Further research on the exact structure–bioactivity relationships is required, which would provide guidance for the structural modifications of ASPs to achieve their maximum effects. Meanwhile, to better determine the effects of ASP metabolites on human health, in vivo studies in animals and clinic should be conducted.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lu CC, Liu M, Shang WR, Yuan Y, Li MX, Deng XX, Li HJ, Yang KH. Knowledge mapping of Angelica sinensis (Oliv.) Diels (Danggui) research: a scientometric study. Front Pharmacol. 2020;11:294.
Wei WL, Zeng R, Gu CM, Qu Y, Huang F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J Ethnopharmacol. 2016;190:116–41.
Sun YL. Preparation, structural features and radioprotective effect of polysaccharides from Angelica sinensis (Oliv.) Diels. Jiangnan University, Wuxi, China. 2006.
Jin ML, Zhao K, Huang QS, Xu CL, Shang P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: a review. Carbohyd Polym. 2013;89(3):713–22.
Wang QK. Effect of injected Angelica polysaccharide on non-specific immunity in grouper, Epinephelus Malabaricus. Qingdao: Ocean University of China; 2012.
Gu PF, Xu SW, Zhou SZ, Liu ZG, Sun YQ, Ou N, Hu YL, Liu JG, Wu Y, Wang XW, Wang DY. Optimization of angelica sinensis polysaccharide-loaded poly (lactic-co-glycolicacid) nanoparticles by RSM and its immunological activity in vitro. Int J Biol Macromol. 2018;107:222–9.
Lv JL, Chen LH, Duan JA, Liu JW. Research progress of structures and pharmacological activities of phthalides from Angelica sinensis. China J Chin Materia Medica. 2016;41(2):167–76.
Chao WW, Lin BF. Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chin Med. 2011;6:29.
Yang TH. Purification and immunomodulating activities of polysaccharide from Angelica sinensis. The Fourth Military Medical University, Xi`an, China. 2003.
Hook ILI. Danggui to Angelica sinensis root: are potential benefits to European women lost in translation? A review. J Ethnopharmacol. 2014;152(1):1–13.
Wong VK, Yu L, Cho CH. Protective effect of polysaccharides from Angelica sinensis on ulcerative colitis in rats. Inflammopharmacology. 2008;16:162–7.
Yang Y, Wang HX, He JR. Research progresses of pharmacokinetics of polysaccharides. Acta Pharm Sin. 2014;49(4):443.
Ji XL, Peng Q, Yuan YP, Shen J, Xie XY, Wang M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill): a review. Food Chem. 2017;227:349–57.
Huang R, Xie JH, Yu Y, Shen MY. Recent progress in the research of yam mucilage polysaccharides: isolation, structure and bioactivities. Int J Biol Macromol. 2020;155:1262–9.
Masci A, Carradori S, Casadei MA, Paolicelli P, Petralito S, Ragno R, Cesa S. Lycium barbarum polysaccharides: extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018;254:377–89.
Ji XL, Hou CY, Shi MM, Yan YZ, Liu YQ. An insight into the research concerning Panax ginseng C. A. Meyer polysaccharides: a review. Food Rev Int. 2020. https://doi.org/10.1080/87559129.2020.177136.
Younas F, Aslam B, Muhammad F, Mohsin M, Raza A, Faisal MN, Shamshud-Ul-Hassan, Majeed W. Haematopoietic effects of Angelica sinensis root cap polysaccharides against lisinopril-induced anaemia in albino rats. Pharm Biol. 2017; 55(1):108–113.
Zhang Y, Cheng Y, Wang N, Zhang QL, Wang KP. The action of JAK, SMAD and ERK signal pathways on hepcidin suppression by polysaccharides from Angelica sinensis in rats with iron deficiency anemia. Food Funct. 2014;5(7):1381–8.
Pan SK, Jiang LF, Wu SJ. Stimulating effects of polysaccharide from Angelica sinensis on the nonspecific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immun. 2018;74:170–4.
Yang XB, Zhao Y, Li GL, Wang ZZ, Lv Y. Chemical composition and immuno-stimulating properties of polysaccharide biological response modifier isolated from Radix Angelica sinensis. Food Chem. 2008;106(1):269–76.
Yang J, Shao XJ, Jiang J, Sun Y, Wang LZ, Sun LR. Angelica sinensis polysaccharide inhibits proliferation, migration, and invasion by downregulating microRNA-675 in human neuroblastoma cell line SH-SY5Y. Cell Biol Int. 2018;42(7):867–76.
Cheng Y, Zhou JL, Li Q, Liu Y, Wang KP, Zhang Y. The effects of polysaccharides from the root of Angelica sinensis on tumor growth and iron metabolism in H22-bearing mice. Food Funct. 2016;7(2):1033–9.
Wang Y, Li X, Chen XT, Zhao P, Qu Z, Ma D, Zhao CC, Gao WY. Effect of stir-frying time during Angelica Sinensis Radix processing with wine on physicochemical, structure properties and bioactivities of polysaccharides. Process Biochem. 2019;81:188–96.
Zhang S, He B, Ge JB, Li HB, Luo XY, Zhang H, Li YH, Zhai CL, Liu PA, Liu X, Fei XT. Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int J Biol Macromol. 2010;47(4):546–50.
Sun YL, Ma GG, Tang J. Studies on radioprotection effect of Angelica sinensis polysaccharides on subchronic radiation injured mice. J Chin I Food Sci Technol. 2009;9(4):33–7.
Wen Y, Fu ZY, Lai Y, Meng DS. Research progress of pharmacological action of angelica polysaccharides. Chin Med Her. 2012;9(30):27–30.
Liu WJ, Li WY, Sui Y, Li XQ, Liu CQ, Jing H, Zhang HM, Cao W. Structure characterization and anti-leukemia activity of a novel polysaccharide from Angelica sinensis (Oliv.) Diels. Int J Biol Macromol. 2019;121:161–72.
Cao P, Sun JL, Sullivan MA, Huang X, Wang HX, Zhang Y, Wang N, Wang KP. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int J Biol Macromol. 2018;111:1133–9.
Ji XL, Hou CY, Guo XD. Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): a review. Int J Biol Macromol. 2019;135:637–46.
Kakar MU, Naveed M, Saeed M, Zhao SC, Rasheed M, Firdoos S, Manzoor R, Deng YL, Dai RJ. A review on structure, extraction, and biological activities of polysaccharides isolated from Cyclocarya paliurus (Batalin) Iljinskaja. Int J Biol Macromol. 2020;156:420–9.
Ai ST, Fan XD, Fan LF, Sun Q, Liu Y, Tao XF, Dai KR. Extraction, and chemical characterization of Angelica sinensis polysaccharides and its antioxidant activity. Carbohyd Polym. 2013;94(2):731–6.
Miao JN, Regenstein JM, Qiu JQ, Zhang JQ, Zhang XP, Li HX, Zhang H, Wang ZY. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-a review. Int J Biol Macromol. 2010;150:102–13.
Zhao Y, Shi YY, Yang HX, Mao LJ. Extraction of Angelica sinensis polysaccharides using ultrasound-assisted way and its bioactivity. Int J Biol Macromol. 2016;88:44–50.
Liu JL, Liu X, Liu S. Study on microwave-assisted extraction of polysaccharide from Angelica sinensis. J Anhui Agri Sci. 2012;40(34):16573–5.
Zhang Y, Zhou T, Wang HJ, Cui Z, Cheng F, Wang KP. Structural characterization, and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohyd Polym. 2016;147:401–8.
Sun YL, Cui SW, Tang J, Gu XH. Structural features of pectic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohyd Polym. 2010;80:544–50.
Yu P, Sun HS. Purification of a fucoidan from kelp polysaccharide and its inhibitory kinetics for tyrosinase. Carbohyd Polym. 2014;99:278–83.
Cao W, Li XQ, Liu L, Yang TH, Li C, Fan TH, Jia M, Lv ZG, Mei QB. Structure of an anti-tumor polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohyd Polym. 2006;66(2):149–59.
Sun YL, Tang J, Gu XH, Li DY. Water-soluble polysaccharides from Angelica sinensis (Oliv. Diels: preparation, characterization and bioactivity. Int J Biol Macromol. 2005;36(5):283–9.
Shi L. Bioactivities, isolation and purification methods of polysaccharides from natural products: a review. Int J Biol Macromol. 2016;92:37–48.
Ma P, Sun CY, Li WJ, Deng WW, Adu-Frimpong M, Yu JN, Xu XM. Extraction and structural analysis of Angelica sinensis polysaccharide with low molecular weight and its lipid-lowering effect on nonalcoholic fatty liver disease. Food Sci Nutr. 2020. https://doi.org/10.1002/fsn3.1581.
Li WY, Li P, Yan HW, Sun L, Cao W. Research progress in the structure of polysaccharide derived from Angelica sinensis (Oliv.) Diels. Northwest Pharm J. 2015;30(3):321–5.
Zhao L, Wang Y, Shen HL, Shen XD, Nie Y, Wang Y, Han T, Yin M, Zhang QY. Structural characterization and radioprotection of bone marrow hematopoiesis of two novel polysaccharides from the root of Angelica sinensis (Oliv.) Diels. Fitoterapia. 2012;83(8):1712–20.
Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA. Structure-function relationships of immunostimulatory polysaccharides: a review. Carbohydr Polym. 2015;132:378–96.
Yan JK, Wang WQ, Wu JY. Recent advances in Cordyceps sinensis polysaccharides: mycelial fermentation, isolation, structure, and bioactivities: a review. J Funct Foods. 2014;6:33–47.
Xing LP. Study on structure identification and anti-tumor activity in vitro of three uniform molecular weight-Angelica polysaccharides. Wuhan: Huazhong University of Science and Technology; 2013.
Zhou T. Isolation, structural identification and biological acitivity of polysaccharides from Angelica sinensis. Wuhan: Huazhong University of Science and Technology; 2018.
Abdel-Akher M, Hamilton JK, Montgomery R, Smith F. A new procedure for the determination of the fine structure of polysaccharides. J Am Chem Soc. 1952;74(19):4970–1.
Zhang R, Zhang XX, Tang YX, Mao JL. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohyd Polym. 2020;228:115381.
Wang SC, Shi SS, Cui J, Li L, Wang ZT, Hu ZB. Isolation and structure analysis of a hetero polysaccharide from Angelica sinensis (Oliv) Diels. Chin Pharm J. 2007;42(16):1255–6.
Zhang LW, Huang RD. Purification, characterization and structure analysis polysaccharide As-IIIa and As-IIIb from Angelica sinensis. Acta Laser Biol Sin. 1999;8(2):123–6.
Chen RX, Xu GY, Wang HY, Xu HZ, Liu YM. Isolation and structure analysis of polysaccharides XC-1 from Angelica sinensis (Oliv.). Chem. 2010;64:372–4.
Cao W, Li XQ, Liu L, Wang M, Fan HT, Li C. Structural analysis of water-soluble glucans from the root of Angelica sinensis (Oliv.) Diels. Carbohyd Res. 2006;341:1870–7.
Cao W, Li XQ, Liu L, Yang TH, Li C, Fan HT. Structure of an anti-tumor polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohyd Polym. 2006;66(2):149–59.
Xu GY, Chen Y, Chen RX. Selective acid hydrolysis condition for the composition and linkage with a fructofuranosyl backbone of a polysaccharide from Angelica sinensis (Oliv) Diels. Chinese Chem Lett. 2006;7(10):1369–72.
Zhuang C, Wang YJ, Zhang YK, Xu NW. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int J Biol Macromol. 2018;115:281–6.
Wang KP, Chen ZX, Zhang Y, Wang PP, Wang JH, Dai LQ. Molecular weight and proposed structure of the Angelica sinensis polysaccharide-iron complex. Chin J Chem. 2008;26:1068–74.
Wang Y, Li X, Zhao P, Qu Z, Bai DT, Gao XX, Zhao CC, Chen J, Gao WY. Physicochemical characterizations of polysaccharides from Angelica Sinensis Radix under different drying methods for various applications. Int J Biol Macromol. 2019;121:381–9.
Sarver SD, Nahar L. Natural medicine: the genus Angelica. Curr Med Chem. 2004;11:1479–500.
Wang KP, Wu J, Xu JY, Gu SS, Li Q, Cao P, Li MM, Zhang Y, Zeng F. Correction of anemia in chronic kidney disease with Angelica sinensis polysaccharide via restoring EPO production and improving iron availability. Front Pharmacol. 2018;9:803.
Wang KP, Wu J, Cheng F, Huang X, Zeng F, Zhang Y. Acidic polysaccharide from Angelica sinensis reverses anemia of chronic disease involving the suppression of inflammatory hepcidin and NF-κB activation. Oxid Med Cell Longev. 2017;2017:7601592.
Wang KP, Tang ZH, Zheng ZM, Cao P, Shui WZ, Li Q, Zhang Y. Protective effects of Angelica sinensis polysaccharide against hyperglycemia and liver injury in multiple low-dose streptozotocin- induced type 2 diabetic BALB/c mice. Food Funct. 2016;7(12):4889–97.
Wang KP, Song ZZ, Wang HJ, Li Q, Cui Z, Zhang Y. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. Int Immunopharmacol. 2016;31:140–8.
Ji P, Wei YM, Sun HG, Xue WX, Hua YL, Li PL, Zhang WQ, Zhang L, Zhao HF, Li JX. Metabolomics research on the hepatoprotective effect of Angelica sinensis polysaccharides through gas chromatography-mass spectrometry. J Chromatogr B. 2014;973:45–54.
Wang KP, Cao P, Shui WZ, Yang QX, Tang ZH, Zhang Y. Angelica sinensis polysaccharide regulates glucose and lipid metabolism disorder in prediabetic and streptozotocin-induced diabetic mice through the elevation of glycogen levels and reduction of inflammatory factors. Food Funct. 2015;6(3):902–9.
Zhang Y, He ZH, Liu XC, Chen ZH, Sun JL, Wu ZJ, Yang XW, Chen XT, Tang ZH, Wang KP. Oral administration of Angelica sinensis polysaccharide protects against pancreatic islets failure in type 2 diabetic mice: pancreatic β-cell apoptosis inhibition. Food Funct. 2019;54:361–70.
Chen J. On effects and mechanism of Angelica sinensis polysaccharides on glucose metabolism in experimental diabetic rats. J Wuhan I Technol. 2010;9:93–5.
Hou CY, Chen LL, Yang LZ, Ji XL. An insight into anti-inflammatory effects of natural polysaccharides. Int J Biol Macromol. 2020;153:248–55.
Li MM, Zhang Y, Wu J, Wang KP. Polysaccharide from Angelica Sinensis suppresses inflammation and reverses anemia in complete freund’s adjuvant-induced rats. Curr Med Sci. 2020;40(2):265–74.
Cheng F, Zhang Y, Li Q, Zeng F, Wang KP. Inhibition of dextran sodium sulfate-induced experimental colitis in mice by Angelica Sinensis polysaccharide. J Med Food. 2020;23(6):584–92.
Wu L, Sun J, Su X, Yu Q, Yu Q, Zhang P. A Review about the development of fucoidan in antitumor activity: progress and challenges. Carbohydr Polym. 2016;154:96–111.
Fu ZY, Li YD, Yang SS, Ma CX, Zhao RR, Guo H, Wei HP. Angelica sinensis polysaccharide promotes apoptosis by inhibiting JAK/STAT pathway in breast cancer cells. Trop J Pharm Res. 2019;18(11):2247–53.
Cao W, Li XQ, Wang X, Fan HT, Zhang XN, Hou Y, Liu SB, Mei QB. A novel polysaccharide, isolated from Angelica sinensis (Oliv.) Diels induces the apoptosis of cervical cancer HeLa cells through an intrinsic apoptotic pathway. Phytomedicine. 2010;17:598–605.
Ren F, Li J, Wang YL, Wang YX, Feng SJ, Yuan ZQ, Qian XL. The effects of Angelica Sinensis polysaccharide on tumor growth and iron metabolism by regulating hepcidin in tumor-bearing mice. Cell Physiol Biochem. 2018;47(3):1084–94.
Tian SY, Hao CC, Xu GK, Yang JJ, Sun RG. Optimization conditions for extracting polysaccharide from Angelica sinensis and its antioxidant activities. J Food Drug Anal. 2017;25(4):766–75.
Jiang J, Guo YJ, Niu AJ. Extraction, characterization of Angelica sinensis polysaccharides and modulatory effect of the polysaccharides and Tai Chi exercise on oxidative injury in middle-aged women subjects. Carbohyd Polym. 2019;77(2):384–8.
Yang XB, Zhao Y, Zhou YJ, Lv Y, Mao JL, Zhao P. Component and antioxidant properties of polysaccharide fractions isolated from Angelica sinensis (Oliv.) Diels. Biol Pharm Bull. 2007;30(10):1884–90.
Qian WB, Cai XR, Qian QH, Wang DL, Zhang L. Angelica Sinensis polysaccharide suppresses epithelial-mesenchymal transition and pulmonary fibrosis via a DANCR/AUF-1/FOXO3 regulatory axis. Aging Dis. 2020;11(1):17–30.
Mu XY, Zhang YY, Li J, Xia JY, Chen XB, Jing PW, Song XY, Wang L, Wang YP. Angelica Sinensis polysaccharide prevents hematopoietic stem cells senescence in D-galactose-induced aging mouse model. Stem Cells Int. 2017;2017:3508907.
Yang TH, Jia M, Meng J, Wu H, Mei QB. Immunomodulatory activity of polysaccharide isolated from Angelica. Int J Biol Macromol. 2006;39:179–84.
Liu WJ, Xiao KM, Ren L, Sui Y, Chen J, Zhang T, Li XQ, Cao W. Leukemia cells apoptosis by a newly discovered heterogeneous polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohyd Polym. 2020;241:116279.
Acknowledgements
We are grateful to the platform of College of Food and Bioengineering, Zhengzhou University of Light Industry.
Funding
This research was financially supported by the Natural Science Foundation of Henan Province (212300410297), Basic Research Plan of Higher Education School Key Scientific Research Project of Henan Province (21A550014), Doctoral Research Foundation of Zhengzhou University of Light Industry (2020BSJJ015), National Key R&D Program of China (2018YFD0901003), High Level Innovation Teams of Guangxi Colleges & Universities/Outstanding Scholars Program (Guijiaoren [2018] 35) and Guangxi Key Laboratory of Polysaccharide Materials and Modification, Guangxi University for Nationalities (Nanning 530008, P. R. China).
Author information
Authors and Affiliations
Contributions
Chunyan Hou, Writing-review & editing. Xiaolong Ji: Supervision, Writing-review & editing. Minsong Yin, Ping Lan: Writing-review & editing. Huiru Wang: Writing-review & editing, Visualization. Hui Nie: Project administration, Funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hou, C., Yin, M., Lan, P. et al. Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agric. 8, 13 (2021). https://doi.org/10.1186/s40538-021-00214-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-021-00214-x