Abstract
Background
Identifying the prevalence of adenoid hypertrophy (AH) and craniofacial factors associated with this condition requires studies with random sampling from the general population, and multiple criteria can be used for assessing AH on lateral cephalometric radiograph (LCR). The present analysis represents the first report performed according to these requirements in a large cross-sectional sample of children.
Methods
LCRs of 517 12-year-old children (286 males, 231 females) randomly selected from the general population were retrospectively retrieved. AH was defined using three criteria (At/Nd, Ad-Ba/PNS-Ba, 1-Npaa/Npa), and twelve craniofacial variables were measured (SNA, SNB, ANB, Wits, Cd-Gn, MnP^SN, MxP^MnP, TPFH/TAFH, OPT^SN, C2ps-C4pi^SN, H-CV, H-FH). Skeletal characteristics were compared between children with and without AH using Mann–Whitney U test. Binary logistic regression (adjusted for sex and skeletal growth) was used to independently quantify the association between craniofacial factors and AH.
Results
The prevalence of children with AH was 17.6% (according to At/Nd), 19.0% (according to Ad-Ba/PNS-Ba), and 13.9% (according to 1-Npaa/Npa). Children with AH presented greater antero-posterior jaw discrepancy (larger ANB, smaller SNB), greater mandibular divergence (larger MnP^SN), forward head posture (larger OPT^SN and C2ps-C4pi^SN), and anteriorly positioned hyoid bone (larger H-CV). Larger SNA (OR = 1.39–1.48), while smaller SNB (OR = 0.77–0.88) and Wits (OR = 0.85–0.87), were associated with greater likelihood of having AH, independently from the assessment method used.
Conclusions
The prevalence of children with AH ranged from 13.9 to 19.0% based on LCR. Greater antero-posterior maxillo–mandibular discrepancy and mandibular retrusion were independently associated with higher likelihood of having AH.
Similar content being viewed by others
Background
Adenoids are part of the lymphatic Waldeyer’s ring that is located in the superior nasopharynx. They grow after birth until the age of 5–7 years and shrink progressively thereafter [1, 2]. Adenoid hypertrophy (AH) can be physiological, secondary to infections, or a reaction to allergens [3] and, with a prevalence of about 34%, it is a common cause of upper airway obstruction in children [4]. AH is associated with increased nasal resistance to airflow and may lead to mouth-breathing [5], which may negatively impact the quality of life of children [6].
While nasopharyngeal endoscopy is the gold standard for diagnosing AH [7], lateral cephalometric radiograph (LCR) is a useful alternative tool to identify this condition because of its good diagnostic accuracy [7] and reliability [8]. Among numerous methods for assessing AH on LCR, the adenoid/nasopharyngeal ratio (At/Nd) [9], adenoid/retropalatal ratio (Ad-Ba/PNS-Ba) [10], and adenoid/nasopharyngeal area ratio (1-Npaa/Npa) [2] are based on relative measurements that account for individual size variations, leading to improved validity [11]. However, there is no consensus about which should be preferred, as authors reported inconsistent findings regarding the usefulness of both linear [12, 13] and area measurements [12, 14], and clinicians should consider the combination of multiple methods for assessing AH on LCR [15].
Since LCR also allows to assess craniofacial structures [16], it has been previously used for investigating the association between skeletal morphology and AH. However, conflicting results have been reported when children with AH were compared to controls [17, 18]. In fact, previous studies involved heterogeneous age groups that did not account for physiological changes in adenoids size [17, 18], and children were sampled from hospital departments, making them not representative of the general population [17, 18]. Overall, investigating the prevalence of AH and its associations with craniofacial structures requires studies on large samples selected via stratified random sampling from the general population. In addition, a cross-sectional design can reduce the bias related to physiological lymphoid tissue shrinkage [1, 2] and skeletal growth [19]. Twelve-year-old children represent a meaningful target population, as such age corresponds to an appropriate timing for the assessment of orthodontic treatment needs. Especially, given that AH may predispose children to sleep-disordered breathing [1], screening for this condition is advisable during treatment planning [20].
The objective of the present study was to estimate the prevalence of children with AH based on LCR, and to investigate the association between AH and craniofacial characteristics among 12-year-old children. It was hypothesised that children with increased antero-posterior jaw discrepancy, increased vertical facial height, forward craniocervical posture, and forward hyoid bone position had greater likelihood of having AH.
Methods
Participants
LCRs of 517 children were retrieved from a previous cross-sectional study that performed stratified sampling on 11 randomly selected schools in Hong Kong in the 1980s [21] and referred participants to Prince Philip Dental Hospital (Hong Kong SAR) for taking the X-ray. 12-year-old children, who had not received orthodontic treatment, and whose LCR was taken with similar methods were included. The study was approved by the Institutional Review Board of The University of Hong Kong / Hospital Authority Hong Kong West Cluster (UW12-405).
Lateral cephalograms acquisition and analysis
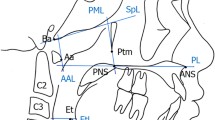
One X-ray machine (GE1000, General Electric, Milwaukee, WI, USA) was used to obtain all LCRs, which were acquired in natural head posture [21]. Cephalometric analysis was carried out with computer software (CASSOS, SoftEnable Technology, Hong Kong SAR), while the nasopharyngeal areas were measured with graphical software (ImageJ) [22]. Linear and area measurements were adjusted according to a magnification of 8.75% and 18.27%, respectively. Cephalometric points and lines were identified (Fig. 1 and Table 1), and variables were measured (Table 2). AH was defined according to three parameters: the adenoid/nasopharyngeal ratio (At/Nd) [9], adenoid/retropalatal ratio (Ad-Ba/PNS-Ba) [10], and adenoid/nasopharyngeal area ratio (1-Npaa/Npa) [2] (Fig. 2). Children were classified as either having AH or not having AH according to cut-off values of 0.62 for At/Nd (> 0.62 indicating AH) [9], 0.60 for Ad-Ba/PNS-Ba (> 0.60 indicating AH) [10], and 0.35 for 1-Npaa/Npa (< 0.35 indicating AH) [2]. Skeletal maturity was assessed using the cervical vertebral maturation (CVM) method and growth stages were defined as pre-pubertal (CS1-CS2), pubertal (CS3-CS4), and post-pubertal (CS5-CS6) [19].
Calibration and method error
The primary assessor (KLT) carried out all measurements after calibration with a secondary assessor (GM). The calibration consisted of tracing and discussing 10 LCR and repeating the procedure until an intraclass correlation coefficient (ICC) > 0.75 was achieved. For intra-assessor reliability, 20% of the LCRs (n = 104) were re-measured by the primary assessor after a wash-out period of about 2 weeks.
Sample size calculation
The sample size was calculated based on a binary logistic regression model with the presence of AH as dependent variable and 14 independent variables (2 covariates to adjust the model for confounding effects, and 12 factors to investigate their effect on the dependent variable). According to a reported prevalence of AH of 31.76% in children and adolescents [17], and requiring at least 10 subjects for each independent variable [23], the sample size was calculated as N = (14 × 10)/0.3176 = 441. Given the retrospective nature of the study, all the 517 records fulfilling the inclusion criteria were included.
Data analysis
The measurement error was calculated with Dahlberg’s formula [24]. ICC was used to calculate the intra-assessor agreement of continuous variables (< 0.50 “poor”, 0.50–0.74 “moderate”, 0.75–0.90 “good”, > 0.90 “excellent” [25]). Cohen’s kappa (K) was used to calculate the intra-assessor agreement of growth stages and the agreement among the three assessment methods of AH (≤ 0.20 “poor”, 0.21–0.40 “slight”, 0.41–0.60 “fair”, 0.61–0.80 “good”, 0.81–0.92 “very good”, ≥ 0.93 “excellent” [26]). Chi-square test was used to determine variations in the distribution of children by sex and growth stages with respect to the presence of AH, independently for each of the three diagnostic methods. Normal distribution of continuous data was assessed using Shapiro–Wilk test, and Mann–Whitney U test was used to compare the craniofacial variables between children with and without AH, independently for each of the three diagnostic methods. Binary logistic regression (with covariates adjustment for growth stage and sex) was used to assess the independent contribution of each craniofacial factor to the presence of AH. Three binary logistic regression models, one for each diagnostic method, were developed. Statistical analysis was performed using SPSS 27.0 (IBM, New York, USA), at significance α = 0.05.
Results
Sample characteristics
A total of 517 children, 286 males (55.3%) and 231 females (44.7%), were included. Regarding skeletal growth, 121 males (92.4%) and 10 females (7.6%) were in pre-pubertal stage, 157 males (55.7%) and 125 females (44.3%) were in pubertal stage, while 8 males (7.7%) and 96 females (92.3%) were in post-pubertal stage.
Measurements error and agreement
The measurement error was < 1.0° for angular measurements, < 1.0 mm for linear measurements, and < 2.0% for ratios. Regarding the independent variables, the intra-assessor agreement was very good for skeletal growth (K = 0.823), and very good to excellent for craniofacial structures (ICC = 0.908–0.990). Regarding the methods used for identifying children with AH, the intra-assessor agreement of each method was excellent (ICC = 0.956–0.999), and the agreement among the methods was very good between At/Nd and Ad-Ba/PNS-Ba (K = 0.877), very good between At/Nd and 1-Npaa/Npa (K = 0.818), and good between Ad-Ba/PNS-Ba and 1-Npaa/Npa (K = 0.790) (Appendix Tables 6, 7).
Prevalence of adenoid hypertrophy
Ninety-one children (17.6%, 49 males and 42 females) were classified as having AH according to At/Nd, 98 (19.0%, 52 males and 46 females) according to Ad-Ba/PNS-Ba, and 72 (13.9%, 35 males and 37 females) based on 1-Npaa/Npa. There was no difference in sex distribution or skeletal growth stage between children with and without AH, independently from the assessment method used (Table 3).
Association between adenoid hypertrophy and craniofacial characteristics
Regarding horizontal measurements, ANB of children with AH was larger (for all methods) and SNB was smaller (for At/Nd and 1-Npaa/Npa). The logistic regression showed that ANB, SNB, and Wits were significant predictors in all models (for each degree of increase of ANB, children were 38.8–47.5% more likely of having AH; for each degree of decrease in SNB, they were 13.6–29.3% more likely to have AH; and for each millimetre of decrease in Wits, they were 15.6–18.0% more likely of having AH). Larger Cd-Gn was also significant (for At/Nd and 1-Npaa/Npa). For vertical measurements, MnP^SN was larger in children with AH (for At/Nd). However, no vertical parameter was significant in the logistic regression. Regarding craniocervical posture, both OPT^SN (for At/Nd) and C2ps-C4pi^SN (for At/Nd and Ad-Ba/PNS-Ba) were larger in children with AH. However, no craniocervical parameter was significant in the logistic regression. For the hyoid bone position, H-CV was larger among children with AH (for At/Nd). The logistic regression showed that children with more anteriorly positioned hyoid bone were more likely of having AH (for At/Nd) (Tables 4, 5).
Discussion
Adenoids have been assessed on LCR since 1946 [27], and numerous parameters have been proposed to identify children with AH. The present study adopted three methods, with the first based on the ratio between the maximum thicknesses of the adenoids (measured from the basilar part of the occipital bone) and the width of the nasopharynx (measured along the line connecting the posterior nasal spine with the spheno-occipital synchondrosis). This parameter, named At/Nd, was first described by Fujioka et al. [9]. An advantage of using ratio measurements is that the adenoidal size is considered with respect to the individual nasopharyngeal capacity. In children, At/Nd has excellent specificity (95%) and positive predictive value (94%), but low sensitivity (41%) and negative predictive value (39%) [28]. At/Nd has also been shown to correlate well with clinical symptoms and weight of surgically removed adenoids [29]. The second parameter consisted of the ratio between the thickness of the adenoids and the width of the nasopharynx (both measured along the line connecting the posterior nasal spine with Basion). This ratio, named Ad-Ba/PNS-Ba and proposed by Kemaloglu et al. [10], is similar to At/Nd but measured along a different line that is more representative of the retropalatal area. Among children, it has excellent specificity (97%) and positive predictive value (97%), but moderate sensitivity (71%) and negative predictive value (70%) [11]. The third parameter represented the proportion of the nasopharyngeal area that is not occupied by the adenoids (1-Npaa/Npa), as proposed by Handelman and Osborne [2]. It has excellent specificity (94%) and positive predictive value (95%), but moderate sensitivity (75%) and negative predictive value (72%) [11]. In general, the present study showed a prevalence of children with AH between 13.9% and 19.0%, with good agreement among the three measurements methods. Of note, the calculated prevalence was lower than the 34.46% reported by a previous meta-analysis of studies using nasoendoscopy in children [4]. The reason for this difference could be due to the lower sensitivity of LCR compared to nasoendoscopy, with a reported pooled sensitivity of 86% in children [7]. In particular, the only two identified studies using nasoendoscopy in randomly selected samples included children between 5- and 14-year-old [4], further justifying differences between previously reported values and the present cross-sectional study among 12-year-old children. No difference was present in the prevalence of AH between males and females, in agreement with a former investigation [30].
Hypertrophic adenoids may obstruct the nasopharynx, forcing the child to breathe through the mouth, which may lead to the characteristic “adenoid facies” [31]. However, the relationship between AH and craniofacial morphology should be considered with caution, as patients may not show the expected “mouth-breathing dental stereotype” [18], and it is time for scientific studies to critically analyse confounders and statistical findings. In fact, multiple genetic and epigenetic factors may affect craniofacial growth [32]. In this complex scenario, despite the comparison of the median values of a variable (e.g. SNA) between two groups (e.g. children with AH versus without AH) provides information about the presence of an association, it does not quantify its strength and it does not account for confounders [33]. The present study showed that increased maxillo-mandibular sagittal discrepancy with retruded and hyperdivergent mandible was associated with AH, which was in partial agreement with the findings of a previous study in children with sleep-disordered breathing [34]. In addition, in the present work, forward head posture and forward position of the hyoid bone were present in children with AH. Although these findings are in contrast to previous works that found no significant evidence of such associations [17, 35], the former studies involved small samples (i.e. small statistical power), participants were recruited from hospitals (i.e. not representative of the general population), and they involved patients of a wide age-range (i.e. confounding factor due to changes in adenoids size). This said, a simple group comparison does not allow proper investigation of the association between AH and craniofacial characteristics. While “risk” is the probability of occurrence of an event (e.g. the chance of having AH), “odds” is the ratio of the probability of occurrence of an event to the probability of that event not occurring (e.g. the chance of having AH with respect to the chance of not having it). The “odds ratio” (OR) is the ratio of odds of an event (e.g. odds of having AH) in one group (e.g. children with retruded mandible) versus the odds of that event in another group (e.g. children without retruded mandible) [36]. Furthermore, multiple logistic regression calculates the “adjusted OR”, which provides the independent effect of a variable (e.g. SNA) while holding all other variables fixed (e.g. SNB, Wits) on a binomial outcome (e.g. having versus not having AH) [37]. The present study showed increased adjusted OR of having AH among children with skeletal Class II tendency characterised by retruded mandible and decreased Wits appraisal. Despite a former study performing a regression analysis found no significant relationship between craniofacial proportions and adenoid size, of note no horizontal skeletal parameter was included [38].
Overall, LCR accompanied by medical history examination may be useful for the screening of patients with suspected AH, providing indications of the need for referral to otorhinolaryngologist [39]. Given the excellent specificity but low/moderate sensitivity of the proposed methods (i.e. non-negligible chance to miss a patient truly having AH), orthodontists may not rely on a single parameter for identifying children with AH, and a combined assessment using linear and area measurements may be advisable. Since the onset of AH in children is multifactorial, with asthma, allergic rhinitis, and atopic dermatitis among the risk factors [30], orthodontic options should be integrated in a multidisciplinary treatment planning [40]. Appropriate timing for orthodontic intervention, with respect to medical and/or surgical options, should be discussed with other specialists for optimising clinical outcomes while minimising the burden to patients (Additional file 1).
Limitations
Although the use of a retrospective archive from the 1980s allowed for analyses that would be impossible today for ethical reasons, the prevalence of AH may have changed over the past forty years. A recent meta-analysis [4] showed that only one 2005 study from Brazil [41] and another 2008 study from Turkey [42] included random samples from the general population. Even broadening the inclusion criteria, no study on AH prevalence was performed in Hong Kong and high heterogeneity was found between studies [4]. Furthermore, the incidence of adenotonsillectomy may be determined by factors other than the prevalence of AH [43] and local policies may influence environmental factors [44], limiting the use of adenotonsillectomy as a proxy for AH. Therefore, currently available data may not allow speculating on possible trends of changes in prevalence of AH over time in Hong Kong.
Further longitudinal studies are necessary to investigate possible causal relationships and the direction of such cause-effects between the development of altered craniofacial features and the onset of AH (e.g. whether a genetically small nasal cavity may lead to mouth-breathing and frequent upper respiratory tract infections causing AH [45, 46], or whether AH may lead to mouth-breathing and altered craniofacial development [5, 31]).
Conclusions
-
The prevalence of AH among 12-year-old children ranged between 13.9 and 19.0%, based on the method used for its assessment on LCR.
-
In this population, greater antero-posterior maxillo-mandibular discrepancy and mandibular retrusion were associated with higher likelihood of having AH, which should be considered during orthodontic treatment planning.
-
LCR is commonly used in orthodontic patients and, despite being a static 2D assessment with biohazard related to ionising radiations, it may help in the identification of those with AH for further consideration with other specialists.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files (Additional file 1: Raw dataset).
Abbreviations
- 1-Npaa/Npa:
-
Adenoid/nasopharyngeal area ratio
- 2D:
-
Two-dimensional
- Ad-Ba/PNS-Ba:
-
Adenoid/retropalatal ratio
- AH:
-
Adenoid hypertrophy
- At/Nd:
-
Adenoid/nasopharyngeal ratio
- CVM:
-
Cervical vertebral maturation
- ICC:
-
Intraclass correlation coefficient
- K:
-
Kohen’s Kappa coefficient
- LCR:
-
Lateral cephalometric radiograph
- OR:
-
Odds ratio
References
Papaioannou G, Kambas I, Tsaoussoglou M, Panaghiotopoulou-Gartagani P, Chrousos G, Kaditis AG. Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J Pediatr. 2013;162(2):269-74.e4.
Handelman CS, Osborne G. Growth of the nasopharynx and adenoid development from one to eighteen years. Angle Orthod. 1976;46(3):243–59.
Huang SW, Giannoni C. The risk of adenoid hypertrophy in children with allergic rhinitis. Ann Allergy Asthma Immunol. 2001;87(4):350–5.
Pereira L, Monyror J, Almeida FT, Almeida FR, Guerra E, Flores-Mir C, et al. Prevalence of adenoid hypertrophy: a systematic review and meta-analysis. Sleep Med Rev. 2018;38:101–12.
Dinis PB, Haider H, Gomes A. The effects of adenoid hypertrophy and subsequent adenoidectomy on pediatric nasal airway resistance. Am J Rhinol. 1999;13(5):363–9.
Jiang X, Ren X, Liu H, Tian J, Du C, Luo H, et al. Health-related quality of life among children with adenoid hypertrophy in Xi’an, China. Int J Pediatr Otorhinolaryngol. 2015;79(12):2321–6.
Duan H, Xia L, He W, Lin Y, Lu Z, Lan Q. Accuracy of lateral cephalogram for diagnosis of adenoid hypertrophy and posterior upper airway obstruction: a meta-analysis. Int J Pediatr Otorhinolaryngol. 2019;119:1–9.
Savoldi F, Xinyue G, McGrath CP, Yang Y, Chow SC, Tsoi JKH, et al. Reliability of lateral cephalometric radiographs in the assessment of the upper airway in children: a retrospective study. Angle Orthod. 2020;90(1):47–55.
Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol. 1979;133(3):401–4.
Kemaloglu YK, Goksu N, Inal E, Akyildiz N. Radiographic evaluation of children with nasopharyngeal obstruction due to the adenoid. Ann Otol Rhinol Laryngol. 1999;108(1):67–72.
Souki MQ, Souki BQ, Franco LP, Becker HM, Araujo EA. Reliability of subjective, linear, ratio and area cephalometric measurements in assessing adenoid hypertrophy among different age groups. Angle Orthod. 2012;82(6):1001–7.
Hibbert J, Whitehouse GH. The assessment of adenoidal size by radiological means. Clin Otolaryngol Allied Sci. 1978;3(1):43–7.
Maw AR, Jeans WD, Fernando DC. Inter-observer variability in the clinical and radiological assessment of adenoid size, and the correlation with adenoid volume. Clin Otolaryngol Allied Sci. 1981;6(5):317–22.
Holmberg H, Linder-Aronson S. Cephalometric radiographs as a means of evaluating the capacity of the nasal and nasopharyngeal airway. Am J Orthod. 1979;76(5):479–90.
Major MP, Flores-Mir C, Major PW. Assessment of lateral cephalometric diagnosis of adenoid hypertrophy and posterior upper airway obstruction: a systematic review. Am J Orthod Dentofacial Orthop. 2006;130(6):700–8.
Gu M, McGrath CP, Wong RW, Hagg U, Yang Y. Cephalometric norms for the upper airway of 12-year-old Chinese children. Head Face Med. 2014;10:38.
Wysocki J, Krasny M, Skarzynski PH. Patency of nasopharynx and a cephalometric image in the children with orthodontic problems. Int J Pediatr Otorhinolaryngol. 2009;73(12):1803–9.
Souki BQ, Pimenta GB, Souki MQ, Franco LP, Becker HM, Pinto JA. Prevalence of malocclusion among mouth breathing children: do expectations meet reality? Int J Pediatr Otorhinolaryngol. 2009;73(5):767–73.
Baccetti T, Franchi L, McNamara JA. The cervical vertebral maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin Orthod. 2005;11(3):119–29.
Behrents RG, Shelgikar AV, Conley RS, Flores-Mir C, Hans M, Levine M, et al. Obstructive sleep apnea and orthodontics: an American Association of Orthodontists White Paper. Am J Orthod Dentofacial Orthop. 2019;156(1):13–28.
Michael Stephen C. Cephalometric analyses based on natural head posture of Chinese children in Hong Kong: 香港大學; 1986.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9.
Dahlberg G. Statistical methods for medical and biological students. London: George Allen and Unwin; 1940.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63.
Byrt T. How good is that agreement? Epidemiology. 1996;7(5):561.
Weitz HL. Roentgenography of adenoids. Radiology. 1946;47:66–70.
Wormald PJ, Prescott CA. Adenoids: comparison of radiological assessment methods with clinical and endoscopic findings. J Laryngol Otol. 1992;106(4):342–4.
Elwany S. The adenoidal-nasopharyngeal ratio (AN ratio). Its validity in selecting children for adenoidectomy. J Laryngol Otol. 1987;101(6):569–73.
Evcimik MF, Dogru M, Cirik AA, Nepesov MI. Adenoid hypertrophy in children with allergic disease and influential factors. Int J Pediatr Otorhinolaryngol. 2015;79(5):694–7.
Linder-Aronson S. Respiratory function in relation to facial morphology and the dentition. Br J Orthod. 1979;6(2):59–71.
Ho ACH, Savoldi F, Wong RWK, Fung SC, Li SKY, Yang Y, et al. Prevalence and risk factors for obstructive sleep apnea syndrome among children and adolescents with cleft lip and palate: a survey study in Hong Kong. Cleft Palate Craniofac J. 2023;60(4):421–9.
Altman N, Krzywinski M. Association, correlation and causation. Nat Methods. 2015;12(10):899–900.
Kawashima S, Peltomaki T, Sakata H, Mori K, Happonen RP, Ronning O. Craniofacial morphology in preschool children with sleep-related breathing disorder and hypertrophy of tonsils. Acta Paediatr. 2002;91(1):71–7.
Baroni M, Ballanti F, Franchi L, Cozza P. Craniofacial features of subjects with adenoid, tonsillar, or adenotonsillar hypertrophy. Prog Orthod. 2011;12(1):38–44.
Bland JM, Altman DG. The odds ratio. BMJ. 2000;320:1468.
LaValley MP. Logistic regression. Circulation. 2008;117(18):2395–9.
Pawlowska-Seredynska K, Umlawska W, Resler K, Morawska-Kochman M, Pazdro-Zastawny K, Krecicki T. Craniofacial proportions in children with adenoid or adenotonsillar hypertrophy are related to disease duration and nasopharyngeal obstruction. Int J Pediatr Otorhinolaryngol. 2020;132: 109911.
Major MP, Saltaji H, El-Hakim H, Witmans M, Major P, Flores-Mir C. The accuracy of diagnostic tests for adenoid hypertrophy: a systematic review. J Am Dent Assoc. 2014;145(3):247–54.
Gu M, Savoldi F, Chan EYL, Tse CSK, Lau MTW, Wey MC, et al. Changes in the upper airway, hyoid bone and craniofacial morphology between patients treated with headgear activator and Herbst appliance: a retrospective study on lateral cephalometry. Orthod Craniofac Res. 2021;24(3):360–9.
Santos RS, Cipolotti R, D’Avila JS, Gurgel RQ. Schoolchildren submitted to video nasopharyngoscopy examination at school: findings and tolerance. J Pediatr (Rio J). 2005;81(6):443–6.
Aydin S, Sanli A, Celebi O, Tasdemir O, Paksoy M, Eken M, et al. Prevalence of adenoid hypertrophy and nocturnal enuresis in primary school children in Istanbul, Turkey. Int J Pediatr Otorhinolaryngol. 2008;72(5):665–8.
Rosenfeld RM, Green RP. Tonsillectomy and adenoidectomy: changing trends. Ann Otol Rhinol Laryngol. 1990;99(3 Pt 1):187–91.
Mason TG, Schooling CM, Chan KP, Tian L. An evaluation of the air quality health index program on respiratory diseases in Hong Kong: an interrupted time series analysis. Atmos Environ. 2019;211:151–8.
Kukwa W, Guilleminault C, Tomaszewska M, Kukwa A, Krzeski A, Migacz E. Prevalence of upper respiratory tract infections in habitually snoring and mouth breathing children. Int J Pediatr Otorhinolaryngol. 2018;107:37–41.
Warren DW, Hairfield WM, Seaton DL, Hinton VA. The relationship between nasal airway cross-sectional area and nasal resistance. Am J Orthod Dentofacial Orthop. 1987;92(5):390–5.
Acknowledgements
The present work was part of the thesis of Dr K.L.T. for the Master of Dental Surgery in Orthodontics and Dentofacial Orthopaedics (Faculty of Dentistry, the University of Hong Kong, Hong Kong SAR).
Funding
No.
Author information
Authors and Affiliations
Contributions
KLT, FS, and MG were involved in conception and design; KLT and MG helped in data acquisition; KLT, FS, and KYL contributed to data analysis; KLT and FS were involved in preparation of figures and tables; KLT, FS, and MG helped in data interpretation; KLT and FS contributed to drafting of the manuscript; FS was involved in writing of the final manuscript; FS, MG, CPM, and YY helped in critical revision. All authors approved the final version submitted for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW12-405)
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Complete raw dataset of the measurements used for the analysis presented in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tse, K.L., Savoldi, F., Li, K.Y. et al. Prevalence of adenoid hypertrophy among 12-year-old children and its association with craniofacial characteristics: a cross-sectional study. Prog Orthod. 24, 31 (2023). https://doi.org/10.1186/s40510-023-00481-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40510-023-00481-4