Abstract
The fragility and weakness of historical, archaeological, and heritage leather artifacts in museums, and libraries due to unsuitable environmental conditions are significant challenges. This study aims to assess the effectiveness of Hydroxypropyl Beta Cyclodextrin, a novel material, in consolidating leather artifacts by examining their physical, chemical, and mechanical properties. Vegetable-tanned leather samples were treated with different concentrations of Hydroxypropyl Beta Cyclodextrin and subjected to artificial heat aging. Evaluation methods included digital and scanning electron microscopy, contact angle measurements, mechanical testing, color analysis, pH measurement, and Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy. Results showed that Hydroxypropyl Beta Cyclodextrin at 1% and 2% concentrations improved chemical stability, surface morphology, color retention, and mechanical properties of the leather samples. The third concentration yielded less favorable outcomes. This study recommends using Hydroxypropyl Beta Cyclodextrin at 1% and 2% concentrations for consolidating historical leathers.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Leather artifacts are a common part of tangible cultural heritage found in libraries and museums [1, 2]. Leather bookbinding, in particular, is highly valued for its ability to protect historical manuscripts and printed books [3].
Leather is made up of collagen, a protein that provides structure [4]. It consists of intertwined fibrils and fibers that are cross-linked during the tanning process. The material density of leather varies from the grain side to the reverse side, with the top layer made up of thin and tightly packed collagen fibers [5].
Leather artifacts in museums, libraries, and storehouses often show signs of deterioration such as warping, cracks, stains, and dust accumulation due to inappropriate conditions. The fragility of leather is a major concern, caused by physical, chemical, and biological factors. Fluctuations in humidity and temperature, unsuitable light exposure, air pollution, and microbial activity contribute to the degradation of leather artifacts [6,7,8,9,10,11].
Various factors, whether acting alone or in combination, significantly contribute to the fragility of archaeological leathers. As a result, numerous studies have been carried out to investigate leather fragility from the latter half of the twentieth century to the present day. Many researchers have employed consolidation materials to address this issue, aiming to prevent deterioration and enhance the mechanical properties of leather. The remarkable advancements in the field of materials science for conservation, coupled with the utilization of cutting-edge technologies for research and analysis, have shed light on the strengths and weaknesses of the consolidation materials utilized in the treatment of delicate historical and archaeological leathers [12].
Several authors have found that using specific consolidants can be beneficial for treating dry leather. For instance, Kronthal et al. [13] highlighted the advantages of BEVA 371 solution and film in adapting adhesive properties to suit various materials like skin and leather. They noted that BEVA 371 offers excellent resistance to deterioration factors. Koochakzaei et al. [14] demonstrated that treating dry leather with silicone oil enhances hydrothermal stability without altering color, pH, or physical properties significantly. Abdel-Maksoud et al. [15] reported that Polyacrylamide consolidation improved the resistance of fragile vegetable-tanned leather to heat aging, enhanced mechanical properties, stabilized chemical composition, and improved surface morphology.
Caruso et al. [16] found that specific materials have effectively consolidated leather. They recommended using materials commonly used in artworks to maintain the original chemical composition and aesthetic appeal. For instance, fibroin can be used to consolidate collagen in leather without altering its internal fat balance or appearance. Johnson [17] studied the effects of polymer treatment materials like Klucel G, SC6000, and Renaissance Wax on leather. In a related study, Koochakzaei et al. [18] investigated the effectiveness of a hydroxypropyl cellulose/zinc oxide nanocomposite for leather bookbinding consolidation.
Other authors have pointed out potential drawbacks of using certain consolidants on leather. Mahony [19] found that polyvinyl acetate emulsions like Vinamul 3252 can increase acidity and cause discoloration and instability over time, making them unsuitable for use. He also noted that while Paraloid B72 offers more flexibility, it can result in tackiness and migration. Koochakzaei et al. [14] demonstrated that treating dry leather with polyethylene glycol can reduce color stability, pH, and shrinkage temperature. Soriano and Moline [20] mentioned that natural polymers used for consolidation can become unstable and form a hard layer that cracks, peels, and detaches from the object's surface over time.
The field of heritage and archaeological materials conservation has seen significant advancements in materials science. As a result, there is a growing need to explore and assess new polymer materials for consolidating fragile leathers. This expansion of materials options will offer conservators a broader selection for consolidation, moving beyond reliance on a single or limited set of materials. Biopolymers, versatile substances used for various purposes such as cleaning, sterilization, and strengthening, present promising opportunities. Among these biopolymers, Hydroxypropyl-β-cyclodextrin (HP-β-CD) stands out as a material suitable for coating and consolidating processes in conservation work.
Hydroxypropyl-β-cyclodextrin (C63H112O42) is a cyclodextrin derivative commonly used in the pharmaceutical and food industries. This biopolymer has excellent properties that make it suitable for use as a coating and consolidant for dry vegetable-tanned leathers. By applying this compound, leathers can be protected from fragility, improved in chemical stability, and enhanced in mechanical properties. Hydroxypropyl-β-cyclodextrin is a truncated cone-shaped oligosaccharide with hydroxypropyl groups, featuring a hydrophilic external surface and a hydrophobic central cavity [21]. This structure gives the polymer a high melting point of 278 °C [22], increasing the hydrothermal stability of leather and providing resistance to temperature changes in environments like museums and libraries. Additionally, its clear and colorless solution makes it a practical choice for leather treatment [23].
Analyses and investigations are crucial in the conservation field and play a significant role in evaluating the characteristics of leather treated with the studied polymer. This will help the authors determine the appropriate concentration to apply for treating fragile vegetable-tanned leathers.
This study evaluates the use of a new polymer, Hydroxypropyl-Β-Cyclodextrin, for the treatment and preservation of fragile historical leather artifacts in museums and libraries. The polymer was tested on both new and aged samples subjected to artificial accelerated heat aging. Various properties such as microscopy, contact angle, mechanical properties, color change, pH measurement, and ATR/FTIR analysis were used to assess the effectiveness of the treatment.
Materials and methods
Preparation of new vegetable-tanned leather samples
The authors prepared new vegetable-tanned leather from goat skin using mimosa extracted from the barks of Acacia mearnsii, following references [7, 26, 27]. The samples were 10 × 15 cm in size, with a total of 27 samples used in the study (three replicates for each treatment).
Accelerated aging
The new vegetable-tanned leather samples underwent accelerated heat aging at 105 °C in a dry oven for 2 weeks [7]. Subsequently, the treated samples underwent a second accelerated aging process at the same temperature for 1 week.
Preparation of polymer used
Hydroxypropyl-β-cyclodextrin (HP-β-CD) from Wacker, Germany, was dissolved in 70% ethanol to prepare solutions at concentrations of 1%, 2%, and 3%.
Application technique of the polymer
The aged untreated samples were soaked in polymer solutions of varying concentrations for 5 min. After immersion, the samples were taken out and allowed to air dry at room temperature for 24 h.
Analytical techniques
Digital light microscope
The Digital Light Microscope (Wireless Microscope, Model: USB, Magnification: 500, China) was utilized to examine the surface of the new untreated, aged untreated, treated, and aged treated samples. A single sample from each treatment was selected for examination.
Scanning electron microscope
Surface morphology changes were analyzed using a Quanta 3D 200i by FEI at 20.00 kV. Observations were conducted on untreated samples in low vacuum conditions. One sample from each treatment was selected for examination.
Contact angle measurement (wettability)
The contact angle of the samples was measured using a Compact Video Microscope (CVM) from SDL-UK, as described in studies by Arkas et al. [28] and Koochakzaei et al. [18]. A 2.5 μL water drop was applied to the surface of each sample, and the test was conducted at a temperature of 22 ± °C and relative humidity of 65 ± 5%. The measurement uncertainty was ± 1° (coverage factor, k = 2 for a confidence level of 95%). One sample from each treatment was examined.
Mechanical properties (tensile strength and elongation)
The mechanical properties were measured following ISO: 3376 standards [29]. Samples were taken from adjacent zones of the leather, measuring 90 mm in length with a free length between the testing machine jaws of 50 mm and a width of 10 mm. The stretching speed during testing was set at 100 ± 20 mm/min. The Tinius Olsen Model HSKT SDL—Atlas Tensile testing machine was used for the measurements. Tensile force and elongation (%) results were averaged from three measurements with an uncertainty of ± 2%.
Index method for the evaluation of mechanical properties: The index method, proposed by Abdel-Maksoud [7], compares the changes in tensile strength and elongation of vegetable-tanned leather samples before and after treatment. The index of the effectiveness of the conservation treatment and determination of accelerated heat aging rate is calculated using the following equation:
where TS = Tensile strength; E = Elongation; X1 = Tensile strength or elongation of the new sample (control); X2 = Tensile strength or elongation of aged untreated sample; X3 = Tensile strength or elongation of the treated sample with HP-β-CD before aging; X4 = Tensile strength or elongation of the treated sample with HP-β-CD after aging.
Color change measurement
Color changes of all samples studied were measured using the CIE*Lab system. The L* scale measures lightness from 0 (black) to 100 (perfect white). The a* parameter measures the red-green axis (+ a means redder, −a means greener), and the b* parameter measures the yellow-blue axis (+ b means more yellow, -b means bluer). The total color differences (ΔE) between the two specimens are determined using the following equation:
The measurement results were averaged from three readings using an Optimatch 3100® from the SDL Company [14, 30].
Measurement of pH value of treated leather samples
The pH values of new, aged untreated, treated, and aged treated leather samples with Hydroxypropyl-β-cyclodextrin (HP-β-CD) were measured following ISO: 4045 [31] guidelines with some adjustments. A sample weighing 0.50g was taken from each leather sample and placed in 10 ml of deionized water. The samples were soaked in deionized water for 6 h. The pH value was measured using the waterproof pH tester AD11 at a temperature of 20 ± 2 °C, calibrated with standard buffers of pH 4 and 7 (± 0.01 pH). The average of three measurements was taken for each sample.
The percentage change in pH values was calculated as follows [12]:
where pHt = \(\text{pH of the control sample or treated sample}\), pHa = \(\text{pH of the aged sample}.\)
Attenuated total reflectance Fourier transforms infrared spectroscopy (ATR-FTIR)
The chemical changes in the functional groups of the leather samples were studied using ATR-FTIR analysis. The FTIR spectra were obtained from the upper surface of each sample using a Bruker Optik Lumos OPUS 8.2 instrument from Germany. The measurements were conducted in the frequency range of 4000–400 cm−1, with one sample analyzed for each treatment.
Results and discussion
Digital light and scanning electron microscopes investigation of the surface of the studied samples
The results from digital light and scanning electron microscopes for the new vegetable leather sample (Figs. 1A and 2A) revealed a smooth, clear, flat surface with wide distances between follicles in the grain layer of the goat skin used. The grain surface pattern was easily identifiable. Additionally, the leather surface exhibited homogeneity in color (Fig. 1A), indicating that the new vegetable-tanned leather samples were well-prepared.
Digital light microscope investigation of vegetable-tanned leather samples treated with Hydroxypropyl-β-cyclodextrin at different concentrations before and after accelerated aging: A New vegetable-tanned leather (control), B Aged untreated leather sample after 2 weeks, C Aged untreated sample after 3 weeks, D Treated sample (1%), E Treated sample (2%), F Treated sample (3%), G Aged treated sample (1%), H Aged treated sample (2%), I Aged treated sample (3%)
Scanning electron microscope micrographs of vegetable-tanned leather samples treated with Hydroxypropyl-β-cyclodextrin at different concentrations before and after accelerated aging: A New vegetable-tanned leather (control), B Aged untreated leather sample after 2 weeks, C Aged untreated sample after 3 weeks, D Treated sample (1%), E Treated sample (2%), F Treated sample (3%), G Aged treated sample (1%), H Aged treated sample (2%), I Aged treated sample (3%)
The digital light microscope investigation of the aged untreated leather sample after 2 and 3 weeks (Fig. 2B and C) revealed the impact of heat aging on the granular layer surface. The surface appeared coarse and dark compared to new un-aged vegetable-tanned leather. Witt [32] stated that leather is primarily composed of fibril-forming collagen, with collagen molecules forming a triple helical structure and further organized into a fibrillary superstructure. Heating causes leather to shrink as collagen deteriorates or denatures, leading to the disintegration of collagen molecules' triple helices into single strands and loss of the rod-like structure. Sebestyén et al. [33] and Abdel-Maksoud et al. [15] reported that thermal aging affects vegetable-tanned leather, resulting in a darkening of color. SEM investigation (Fig. 2B and C) showed shrinkage of the samples, with some deformation like cracks observed on the grain surface pattern, particularly in the aged sample after 3 weeks. The grain surface was visible in the aged untreated sample after 2 weeks but not after 3 weeks. Carol [34] said that accelerated heat aging is a significant factor in the deterioration of historical leather, along with other factors like humidity, light, and air pollutants. The initial signs of deterioration manifest as cracks on the leather surface, progressing to affect collagen fibers and eventually leading to the disintegration of the leather.
The results of the treated leather samples (Figs. 1D, F, and Fig. 2D–F) demonstrated that the surface was smooth, and the grain surface pattern was well recognized at 1% and 2% polymer concentration, and slightly at 3%. This indicates that the polymer showed good distribution and integration through the fiber structure at 1% and 2%, but at 3%, some polymer remained on the leather surface, indicating less effective bonding and integration. Under a digital light microscope (Fig. 1D–F), there was no change in color at 1% and 2%, but at 3%, there was a slight darkening compared to the aged untreated sample after 2 weeks.
The results of the aged treated samples using digital light at 1% (Fig. 1G) were similar to those of the treated sample at the same concentration. A slight change in color and appearance of the grain surface pattern occurred for the aged treated sample at 2% (Fig. 1H). Significant changes in color and smoothness were observed in the aged treated sample at 3% (Fig. 1I).
The SEM investigation of the aged treated samples at 1% (Fig. 2G) and 2% (Fig. 2H) yielded similar results to the treated samples before aging. The results of the aged treated sample at 3% (Fig. 2I) indicated that the surface was affected by accelerated heat aging, with a very thin crack observed; suggesting the polymer's permanence on the surface was impacted by heat aging.
Both microscopes' investigations showed that concentrations of 1% and 2% resulted in surface improvement, closely resembling the aged untreated sample after 2 weeks and outperforming the aged untreated sample after 3 weeks.
Contact angle (wettability)
The contact angle measurement is a crucial characteristic of leather, especially when exposed to water-containing solutions [35]. The data (Fig. 3) from the study indicated that the contact angle of new vegetable-tanned leather decreased from 79° at 0 s to 28° after 3 s. This decrease is typical for leather due to its porous nature. Previous research [35] has shown that the contact angle of untreated leather decreases steadily until water droplet absorption occurs, typically around 1 min. Aged untreated samples exhibited contact angles of 60° at 0 s and 52° after 3 s after 2 weeks of aging, and 70° after 3 weeks, indicating an increase in contact angle with aging time. Izquierdo et al. [36] reported that accelerated heat aging was found to increase the contact angle of vegetable-tanned leather, making it more hydrophobic and non-wettable. This change may be attributed to heat-induced chemical modifications of leather components, leading to increased hydrophobicity and rugosity at the nano- or micrometric scale. Heat exposure can also cause protein aggregation and rearrangement, contributing to the observed changes in contact angle.
Contact angle measurement of vegetable-tanned leather samples treated with Hydroxypropyl-β-cyclodextrin (HP-β-CD) at different concentrations before and after accelerated heat aging: a Control (0 time) = 79°, b Control (3 s) = 28°, c Aged untreated sample after 2 weeks (0 time) = 60°, d Aged untreated sample after 2 weeks (3 s) = 52°, e Aged untreated sample after 3 weeks = 70°, f Treated sample (1%) = 25°, g Aged treated sample (1%) = 67°, h Treated sample (2%) = 28°, i Aged treated sample (2%) = 67°, j Treated sample (3%) = 37°, k Aged treated sample (3%) = 54°
The contact angle of the treated samples was 25°, 28°, and 37° at concentrations of 1%, 2%, and 3%, respectively, which closely matched the results of the new vegetable-tanned leather (control). This suggests effective distribution and penetration of the polymer throughout the leather fiber structure. Additionally, the contact angle slightly increased with higher polymer concentrations.
After aging, the contact angle of the treated samples was 67° at 1% and 2%, and 54° at 3%. Aging increased the contact angle, but this increase was mitigated by higher polymer concentrations. The difference in contact angle between treated and aged samples was not significant. These findings are consistent with Resmerita et al. [37], who demonstrated that using Hydroxypropyl-β-cyclodextrin (HP-β-CD) treatment increased hydrophilicity, resulting in contact angles of 69° or less.
Jia et al. [38] found that adding Hydroxypropyl-β-cyclodextrin to an object reduced the contact angle from 76.0° to 64.4°. They attributed this decrease to the abundance of hydroxyl groups in HP-β-CD.
Mechanical properties
The tensile strength test results (Table 1) indicated that the tensile strength of the new vegetable-tanned leather (control) was 35 N/mm2. However, this strength decreased in the aged untreated samples, with reductions of 14% and 22% after 2 and 3 weeks, respectively. The elongation of the control sample was 33%, but this value also decreased after accelerated heat aging at different times, with reductions of 18% and 33% after 2 and 3 weeks of aging. These findings are consistent with previous studies by Ӧrk et al. [39] and Abdel-Maksoud et al. [15], which reported that heat aging negatively impacted tensile strength and elongation, resulting in decreased values that worsened with longer aging periods.
The results from Table 1 show that the use of polymer at different concentrations (1%, 2%, and 3%) improved both tensile strength and elongation before and after accelerated heat aging. Before aging, the treated samples showed a percentage increase of 9%, 12%, and 14% at 1%, 2%, and 3%, respectively, compared to the aged untreated sample after 2 weeks. After 3 weeks of aging, the percentage increase was 18%, 20%, and 23% at 1%, 2%, and 3%, respectively. After aging, the percentage increase was 6%, 6%, and 3% at 1%, 2%, and 3%, respectively, compared to the aged untreated sample after 2 weeks. After 3 weeks of aging, the percentage increase was 17%, 17%, and 13% at 1%, 2%, and 3%, respectively.
The index method was used to measure the tensile strength of the samples. After 2 weeks, the treated and aged treated samples showed a 5%, 2.5%, and 1.25% improvement in tensile strength compared to the control and aged untreated samples. After 3 weeks, the improvement was 8%, 4%, and 2% for the treated samples at concentrations of 1%, 2%, and 3%, respectively, compared to the control and aged untreated samples.
The elongation results showed improvement in the treated and aged treated samples compared to the aged untreated samples after 2 and 3 weeks. The treated samples exhibited a percentage increase of 7%, 10%, and 7%, while the aged treated samples showed a percentage increase of 4%, 4%, and − 4% after 2 weeks of aging. After 3 weeks, the treated samples had a percentage increase of 24%, 27%, and 24%, and the aged treated samples had a percentage increase of 21%, 21%, and 15% at 1%, 2%, and 3% compared to the aged untreated sample.
The elongation index method showed that the treated and aged untreated samples had better results compared to the control and aged untreated samples at various time points. After 2 weeks of aging, the improvement in elongation for the treated and aged treated samples was 8%, 4%, and 3% compared to the control and aged untreated samples. After 3 weeks of aging, the improvement was 11%, 5.5%, and 4% for the treated and aged treated samples at 1%, 2%, and 3% concentrations, respectively, compared to the control and aged untreated samples.
The index method results for both tensile strength and elongation indicated that the best concentration was 1% polymer, followed by 2%, with the lowest results seen at the 3% concentration.
The treatment of vegetable-tanned leather samples with Hydroxypropyl-β-Cyclodextrin has been shown to improve their mechanical properties. The authors suggested that when vegetable-tanned leather samples are treated with Hydroxypropyl-β-Cyclodextrin, there is a noticeable enhancement in their mechanical properties. This improvement is attributed to the interaction between the cyclodextrin molecules and the leather fibers. The presence of hydroxypropyl groups in the cyclodextrin molecule allows for the formation of hydrogen bonds with the leather fibers, resulting in improved adhesion and cohesion within the material. As a result, the treated leather exhibits increased tensile strength and elongation compared to untreated aged leather samples. This novel treatment approach shows promise in enhancing the quality and durability of vegetable-tanned leather samples.
Change of color
Color change is a crucial factor in the evaluation of materials for conservation purposes. One of the key principles in conservation is that the materials used should not cause any color changes in historical objects. This criterion is considered essential when selecting materials for treatment. The results of color change are explained as follows:
Lightness (*L)
The results from Table 2 indicate that the Lightness (L*) of the new vegetable-tanned leather (control) was 75.79. However, this value decreased in the aged untreated sample to 60.68 and 56.67 after 2 and 3 weeks, representing a percentage loss of 20% and 25% compared to the control sample. This decrease was attributed to the impact of accelerated heat aging on the samples.
In contrast, the L* value of the treated samples also decreased, but the loss percentage was lower at 4%, 4%, and 5% for the treated samples. For the aged treated samples, the loss was 5%, 5%, and 6% at 1%, 2%, and 3% concentrations, respectively, compared to the aged untreated sample after 2 weeks.
Furthermore, the results revealed that the L value of the treated and aged treated samples at all concentrations was superior to the aged untreated sample after three weeks. The percentage loss of the aged untreated sample compared to the treated and aged treated samples ranged from 1% for the aged treated samples at 2% and 3% concentrations to 2% for the treated samples at all concentrations and the aged treated sample at 1%.
Overall, the study demonstrated minimal changes in all the samples studied, indicating that the polymer effectively withstood the accelerated aging process.
Red-green value (a*)
The results in Table 2 indicate that the control, aged untreated, treated, and aged treated samples turned red in color, with the intensity increasing as the aging time increased. This suggests that accelerated heat aging had an impact on the vegetable-tanned leather. The loss percentage of the aged untreated samples was 42% and 48% after 2 and 3 weeks of aging, respectively. Additionally, the a* value showed a slight increase for the treated and aged treated samples at different concentrations compared to the aged untreated sample after 2 weeks. However, after 3 weeks, the a* value decreased slightly for all samples except the aged treated sample at 3%.
Yellow-blue value (b*)
The results in Table 2 indicate that all b* values leaned towards yellow. Accelerated aging had an impact on the yellow color, causing a decrease in all samples compared to the control. The untreated aged samples experienced a 10% and 15% reduction in b* value after 2 and 3 weeks, respectively. Treated and aged treated samples showed a lower decrease in b* value compared to the aged untreated sample, with losses ranging from 5% for all treated samples at the concentrations used. Specifically, the b* value decreased by 7%, 9%, and 5% at 1%, 2%, and 3% concentrations, respectively, compared to the aged untreated sample after 2 weeks. After 3 weeks, the b* value of the treated samples at different concentrations showed a slight increase (1%) compared to the aged untreated sample, while the aged treated samples decreased by 2% and 3% at 1% and 2% concentrations, respectively. However, the aged treated sample at 3% concentration increased by 1%.
Cappa et al. [40] found that as the aging time and temperature increased, the untreated sample's color changed, with the brightness (L) showing an inverse relationship with aging temperature. They observed a shift towards yellow and red in the single-color coordinates (a and b) on both corium and grain sides with increasing aging temperature, indicating browning. This browning was attributed to denaturation and hydrolysis processes releasing amino acids that react to form brown pigments through a Maillard-type reaction.
The total color difference (ΔE)
The data from Table 2 showed minimal changes in the total color difference between the treated and aged treated samples compared to the aged untreated sample after 2 weeks. The difference ranged from 2.6 to 3.7, which is imperceptible to the human eye. This indicates that the polymer concentrations used provided excellent stability against aging and color alteration. Previous studies by Abdel-Maksoud and Khattab [41] and Abdel-Nasser et al. [10] have also shown that a total color difference of less than 3 is not visible to the naked eye.
pH measurement
The pH values measured in Table 3 revealed that the new untreated vegetable-tanned leather sample had a pH of 4.5. After two and three weeks of aging, the untreated samples showed a decrease in pH, with a loss of 29% and 36% respectively. This is consistent with findings from Carol [34], Lama et al. [42], and Abdel-Maksoud et al. [12], who observed that accelerated heat aging caused oxidation, hydrogen bond breakdown, and weakened collagen structure in leather.
The results showed that treating vegetable-tanned leather with Hydroxypropyl-β-cyclodextrin increased the pH values both before and after accelerated heat aging. The pH value of the treated samples rose with higher polymer concentrations. Specifically, the pH increase for the treated samples at 1%, 2%, and 3% concentrations was 32%, 33%, and 35%, respectively, compared to the aged untreated sample after 2 weeks. There was a slight pH change in the aged treated samples, with increases of 30%, 30%, and 29% compared to the aged untreated sample after 2 weeks. Overall, all concentrations led to an improvement in pH values, with the 1% and 2% concentrations showing better results than the 3% concentration after aging. The enhanced pH values in the treated and aged treated samples may be attributed to the high pH of the polymer solution, which had a pH of 6.5. Additionally, the effective penetration of the polymer at 1% and 2% concentrations into the fiber structure of the leather samples likely contributed to the improved resistance of the leather to accelerated heat aging.
The authors suggested that the pH increase in treated leather samples with Hydroxypropyl-β-Cyclodextrin compared to untreated aged leather can be attributed to the buffering capacity of Hydroxypropyl-β-Cyclodextrin. This compound has the ability to maintain a stable pH level by absorbing excess acidic or basic components in the leather, thereby preventing drastic pH changes. Additionally, the treatment with Hydroxypropyl-β-Cyclodextrin may also neutralize any acidic compounds present in the leather, leading to an overall increase in pH.
Attenuated total reflectance-Fourier transforms infrared spectroscopy (ATR-FTIR)
The ATR-FTIR analysis (Fig. 4) of the samples revealed the presence of collagen absorption bands. In the untreated vegetable-tanned leather sample, key bands such as Amide A (N–H), amide I (C=O), amide II (CN and NH), and amide III (in-plane NH and CH2) were observed at 3333.42 cm−1, 1643.88 cm−1, 1551.17 cm−1, and 1236.40 cm−1, with corresponding intensities. Additionally, CH bands were detected at 2927 cm−1 and 2850 cm−1 [43, 44].
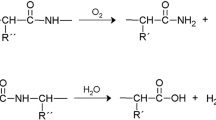
Upon subjecting the untreated sample to accelerated heat aging for 2 weeks, changes in the position and intensity of collagen bands were noted. The aged sample exhibited a shift to lower wavenumbers and reduced intensities in the Amide A, amide I, amide II, amide III, and CH bands. Notably, a widening of the absorption band at 1760–1810 cm−1 was observed in the aged sample, indicating oxidation and cleavage of peptide bonds in collagen. This suggests degradation of collagen in the aged untreated sample compared to the new vegetable leather.
The results showed that the wavenumbers decreased further and some intensities increased in the aged sample after 3 weeks compared to the untreated sample after 2 weeks. The amide A, amide I, amide II, amide III, and CH bands of the aged untreated sample after 3 weeks were observed at 3330.41 cm−1, 1634.23 cm−1, 1533.84 cm−1, 1233.12 cm−1, 2942.23 cm−1, and 2853.41 cm−1 with reduced intensities. Moreover, there was a broader range around 1699–1810 cm−1, indicating more collagen oxidation.
Cappa et al. [40] found that changes in band positions and intensities, such as peak height or peak position, are indicative of alterations in the molecular structure of collagen due to thermal aging. They observed a decrease in peak intensities of Amide I, Amide II, and Amide III, suggesting the gradual cleavage of peptide bonds in collagen molecules. Wu et al. [45] noted a decrease in the intensity of the broadband between 3500 and 3000 cm−1 in leather spectra after dry heat exposure, indicating the disruption of hydrogen bonds between water and collagen molecules. This suggests that water molecules were released from collagen fibers during dry heat treatment. Badea et al. [46] explained that oxidation of polypeptide chains leads to the formation of carbonyl compounds, which manifest as a band in the 1700–1750 cm−1 region.
The O–H stretching vibration in the treated sample was observed between approximately 3401.78 to 3445 cm−1, attributed to the Hydroxypropyl-β-cyclodextrin polymer used. Other bands associated with the polymer were seen at 2930 cm−1 (C–H stretching vibrations), 1639 cm−1 (H–O–H bending), and 1033 cm−1 and 1080 cm−1 (C–H, C–O) stretching vibrations [47,48,49]. In the treated and aged treated samples, Amide A was detected between 3322–3340 cm−1, while Amide I appeared between 1634–1638 cm−1. The wavenumbers were similar to or slightly higher than the control sample. Amide A and amide I bands decreased compared to the control but increased compared to the aged untreated sample. Amide II was observed between 1526–1542 cm−1, and Amide III at 1234 cm−1 in all concentrations before and after accelerated heat aging. The region at 1797 cm−1 disappeared in the treated samples with 1% and 3%, possibly due to the absence of oxidized functional groups [50]. Treatment with 3% showed notable changes, especially after accelerated heat aging, with band positions and intensities higher than the aged untreated sample after 2 weeks and close to the control sample. This suggests that the polymer treatment at the specified concentrations enhanced the chemical stability of leather and protected it from accelerated heat aging.
Conclusion
The microscope analysis revealed that accelerated thermal aging caused changes in the fiber structures of untreated samples, resulting in color alteration, surface roughness, and fiber shrinkage. Treatment with Hydroxypropyl-β-cyclodextrin at different concentrations showed varying effects on leather fibers. The 1% and 2% treatments protected the fibers from heat aging, while the 3% treatment led to color darkening and loss of grain pattern clarity. Contact angle measurements confirmed the effective absorption and distribution of Hydroxypropyl-β-cyclodextrin in vegetable-tanned leather. Treated samples exhibited similar contact angles to new samples, with a consistent decrease as water droplets were absorbed. Aged treated samples maintained similar contact angles to aged untreated samples, indicating that the polymer did not block pores and preserved mechanical properties like elasticity. The polymer improved the mechanical properties of the leather at all concentrations, with the 1% concentration showing the most significant enhancement. However, the 3% concentration showed the least improvement, especially after accelerated heat aging. Color change measurements indicated positive results, with a total color difference below 3, which was not visible to the naked eye, highlighting the effectiveness of the polymer. The pH values of the treated samples increased compared to aged untreated samples after 2 and 3 weeks, suggesting an improvement in chemical stability. ATR-FTIR analysis confirmed that the polymer maintained chemical stability in the functional groups of the treated samples before and after exposure to accelerated heat aging. The consistent band positions and intensities closely resembled those of new vegetable-tanned leather samples and were superior to aged untreated samples after 2 weeks.
This study also recommends further research on additional properties like shrinkage temperature, porosity, water vapor permeability, etc., to provide a comprehensive understanding of the effectiveness of Hydroxypropyl-β-cyclodextrin in treating vegetable-tanned leather.
Data availability
The data presented in this manuscript is available upon request to the authors.
References
Abdel-Maksoud G, El-Nagar K, Kassem M. Evaluation of different coatings in protecting leather artifacts in museums and libraries from air pollution. Mapan - J Metrol Soc India. 2023;38(4):853–65. https://doi.org/10.1007/s12647-023-00658-6.
Thomson R. the role of leather science and technology in heritage conservation. Ph.D. Thesis. University of Northampton. 2011: 1–73.
Kouchakzaei A, Mehrabad M. Evaluation of iron on structural deterioration of chrome leather in buried conditions, based on degradation indices in FTIR spectra. Archaeometry. 2019;5(2):91–104. https://doi.org/10.29252/jra.5.2.91.
Vyskočilova G, Carşote C, Ševčik R, Badea E. Burial-induced deterioration in leather: a FTIR-ATR, DSC, TG/DTG, MHT and SEM study. Herit Sci. 2022;10(7):1–14. https://doi.org/10.1186/s40494-021-00638-6.
Meyer M, Dietrich S, Schulz H, Mondschein A. Comparison of the technical performance of leather. Artif Leather Trendy Altern Coat. 2021;11(2):226. https://doi.org/10.3390/coatings11020226.
Abdel-Maksoud G, Marcinkowska E. Effect of artificial heat ageing on the humidity sorption of parchment and leathers compared with archaeological samples. J Soc Leather Technol Chem. 2000;84(5):219–22.
Abdel-Maksoud G. Evaluation of wax or oil/fungicide formulations for preservation of vegetable-tanned leather artifacts. J Soc Leather Technol Chem. 2006;90(2):58–67.
Saada N, Abdel-Maksoud G, Abd El-Aziz M, Youssef A. Green synthesis of silver nanoparticles, characterization, and use for sustainable preservation of historical parchment against microbial biodegradation. Biocatal Agric Biotechnol. 2021;32: 101948. https://doi.org/10.1016/j.bcab.2021.101948.
Fouda A, Abdel-Nasser M, Khalil A, Hassan S, Abdel-Maksoud G. Investigate the role of fungal communities associated with a historical manuscript from the 17th century in biodegradation. NPJ Mater Degrad. 2022;88:1–13. https://doi.org/10.1038/s41529-022-00296-4.
Abdel-Nasser M, Abdel-Maksoud G, Abdel-Aziz M, Darwish S, Hamed A, Youssef A. Evaluation of the efficiency of nanoparticles for increasing α-amylase enzyme activity for removing starch stain from paper artifacts. J Cult Herit. 2022;53:14–23. https://doi.org/10.1016/j.culher.2021.11.004.
Fistos T, Fierascu I, Fierascu R. Recent developments in the application of inorganic nanomaterials and nanosystems for the protection of cultural heritage organic artifacts. J Nanomater. 2022;12(2):1–19. https://doi.org/10.3390/nano12020207.
Abdel-Maksoud G, Elnagar K, Ibrahim M, Mohamed O, Abdallah A, Youssef R, Elsayed D, Labib N, Mohamed W. comprehensive overview of the performance of polyamide 6 in the consolidation of vegetable-tanned leathers. J Cult Herit. 2023;64(1025):207–15. https://doi.org/10.1016/j.culher.2023.10.009.
Kronthal L, Levinson J, Dignard C, Chao E, Down J. BEVA 371 and its use as an adhesive for skin and leather repairs: background and a review of treatments. J Am Inst Conserv. 2003;42(2):341–62. https://doi.org/10.2307/3180075.
Koochakzaei A, Ahmadi H, Achachluei M. An experimental comparative study on silicone oil and polyethylene glycol as dry leather treatments. J Am Leather Chem Assoc. 2016;111(10):377–83.
Abdel-Maksoud G, Abdallah A, Youssef R, Elsayed D, Labib N, Mohamed W, Ibrahim M. Evaluation of the efficiency of polyacrylamide and poly(MMA-HEMA) for the consolidation of vegetable-tanned leather artifacts. Pigment Resin Technol. 2023. https://doi.org/10.1108/PRT-02-2022-0014.
Caruso M, D’Agostino G, Milioto S, Cavallaro G, Lazzara G. A review on biopolymer-based treatments for consolidation and surface protection of cultural heritage materials. J Mater Sci. 2023;58:12954–75. https://doi.org/10.1007/s10853-023-08833-5.
Johnson A. Evaluation of the use of SC6000 in conjunction with Klucel G as a conservation treatment for bookbinding leather: notes on a preliminary study. J Inst Conserv. 2013;36(2):125–44. https://doi.org/10.1080/19455224.2013.815646.
Koochakzaei A, Ghane Z, Achachluei MM. Zinc oxide nanoparticles in leather conservation: exploring the potential of hydroxypropyl cellulose/zinc oxide nanocomposite as a leather consolidation agent. Herit. 2023;6:7547–58. https://doi.org/10.3390/heritage6120396.
Mahoney C. Evaluation of consolidants for the treatment of red rot on vegetable tanned leather: the search for a natural material alternative. Master of Arts. University of California. 2014; 12:1–100.
Soriano A, Moline B. Consolidation of bone material: chromatic evolution of resins after UV accelerated aging. J Paleontol. 2016;15:46–67.
Li Z, Li K, Teng M, Li M, Sui X, Liu B, Tian B, Fu Q. Functionality-related characteristics of hydroxypropyl-β-cyclodextrin for the complexation. J Mol Liq. 2022;365: 120105. https://doi.org/10.1016/j.molliq.2022.120105.
Munir R, Hadi A, Khan S, Asghar S, Irfan M, Khan I, Hameed M, Inam S, Islam N, Hassan S, Memoona M, Shah P, Iqbal M, Syed H, Khames A, Abourehab M. Solubility and dissolution enhancement of dexibuprofen with hydroxypropylbetacyclodextrin (HPβCD) and Poloxamers (188/407) inclusion complexes: preparation and in vitro characterization. J Polym. 2022;14:579. https://doi.org/10.3390/polym14030579.
Al-Rawashdeh N, Al-Sadeh K, Al-Bitar M. Physicochemical study on microencapsulation of hydroxypropyl-beta-cyclodextrin in dermal preparations. Drug Dev Ind Pharm. 2010;36(6):688–97. https://doi.org/10.3109/03639040903449738.
Jiang L, Liu X, Xuan G. Preparation of pH-sensitive β-cyclodextrin derivatives and evaluation of their drug-loading properties. IOP Conf Ser Mater Sci Eng. 2020;774: 012009. https://doi.org/10.1088/1757-899X/774/1/012009.
Kırımlıoğlu G. Host-guest inclusion complex of desloratadine with 2-(hydroxy)propyl-β-cyclodextrin (HP-β-CD): preparation, binding behaviors and dissolution properties. J Res Pharm. 2020;24(5):693–707. https://doi.org/10.35333/jrp.2020.224.
Gustavson K. The chemistry of tanning processes. New York: Academic Press INC; 1956. p. 142–80.
Falcão L, Araújo MEM. Vegetable tannins used in the manufacture of historic leathers. Molecules. 2018;23(5):1081. https://doi.org/10.3390/molecules23051081.
Arkas M, Kythreoti G, Favvas EP, Giannakopoulos K, Mouti N, Arvanitopoulou M, Athanasiou A, Douloudi M, Nikoli E, Vardavoulias M, Dimitriou M, Karakasiliotis I, Ballén V, González SM. Hydrophilic antimicrobial coatings for medical leathers from silica-dendritic polymer-silver nanoparticle composite xerogels. Textiles. 2022;2:464–85. https://doi.org/10.3390/textiles2030026.
ISO 3376 IULTCS/IUP 6: Leather-Physical and mechanical tests-Determination of tensile strength and percentage elongation, 2020 (e): 1–9.
Koochakzaei A, Ahmadi H, Achachluei MM. Assessment of color stability of treated leathers with silicone oil and polyethylene glycol. J Afr leather leather prod adv. 2014;1(1):29–38.
ISO 4045 IULTCS/IUC 11: Leather-Chemical tests-Determination of pH and difference figure, Third edition 2018–05: 1–8.
Witt T, Mondschein A, Majschak J, Meyer M. Heat development at the knife roller during leather shaving. J Leather Sci Eng. 2021. https://doi.org/10.1186/s42825-021-00057-0.
Sebestyén Z, Jakaba E, Badea E, Barta-Rajnai E, Şendrea C, Czégény Z. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J Anal Appl Pyrolysis. 2009;138:178–87. https://doi.org/10.1016/j.jaap.2018.12.022.
Carol M. Evaluation of consolidants for the treatment of red rot on vegetable tanned leather: the search for a natural material alternative. Master of Arts. University of California. 2014; 1-110.
Badea E, Carşote C, Hadîmbu E, Șendrea C, Lupaș M. The effect of halloysite nanotubes dispersions on vegetable-tanned leather thermal stability. Herit Sci. 2019;7(68):1–15. https://doi.org/10.1186/s40494-019-0310-x.
Izquierdo E, Robinet L, Boissiere M, Lavedrine B, Larreta-garde V. Characterization of the effect of heat on vegetable tanned leather and restoration trials through enzymatic processes. 5th International Conference on Advanced Materials and Systems; ICAMS. 2014; 1–6. https://hal.science/hal-01154036.
Resmerita A, Silion M, Cojocaru C, Farcas A. Structural and morphological characterization of a new semi-polyrotaxane architecture based on 2-hydroxypropyl-β-cyclodextrins and polyisoprene. React Funct Polym. 2022;181:1–8. https://doi.org/10.1016/j.reactfunctpolym.2022.105459.
Jia M, Liang Y, Liu Z, Liu Y, Zhanga X, Guo H. Hydroxypropyl-b-cyclodextrin-incorporated Pebax composite membrane for improved permselectivity in organic solvent nanofiltration. RSC Adv. 2022;12:16893–902. https://doi.org/10.1039/D2RA01491B.
Ӧrk N, Özgünay H, Mutlu M, Öndoğan Z. Comparative determination of physical and fastness properties of garment leathers tanned with various tanning materials for the leather skirt production. Tekstil Ve Konfeksiyon. 2014;24(4):413–8.
Cappa F, Paganoni I, Carsote C, Badea E, Schreiner M. Studies on the effects of mixed light-thermal ageing on parchment by vibrational spectroscopy and micro hot table method. Herit Sci. 2020;8(15):1–12. https://doi.org/10.1186/s40494-020-0353-z.
Abdel-Maksoud G, Khattab R. Evaluation of traditional, starch nanoparticle and its hybrid composite for the consolidation of tracing paper, Egypt. J Chem. 2021;64(11):6251–68. https://doi.org/10.21608/EJCHEM.2021.76272.3730.
Lama A, Antunes P, Covington A, Guthrie-Strachan J, Fletcher Y. Use of aluminium alkoxide and oxazolidine II to treat acid-deteriorated historic leather. J Inst Conserv. 2015;38:172–87. https://doi.org/10.1080/19455224.2015.1071713.
Șendrea C, Carșote C, Badea E, Adams A, Niculescu M, Iovu H. Non-invasive characterization of collagen-based materials by NMR-mouse and ATR-FTIR. UPB Sci Bull Series B. 2016;78(3):27–30.
Carsote C, Badea E, Caniola I, Paunescu S, Lupas M, Sendrea C, Miu L. The homiliary of Varlaam: scientific investigation of the leather bookbinding. Rev Chim. 2020;71(3):51–8. https://doi.org/10.37358/Rev.
Wu C, Zeng Y, Liao X, Zhang W, Shi B. Effect of retanning agents on dry heat resistance of leathers. J Am Leather Chem Assoc. 2013;108(8):294–9.
Badea E, Miu L, Budrugeac P, Giurginca M, Mašić A, Badea N, Gatta G. Study of deterioration of historical parchments by various thermal analysis techniques complemented by SEM, FTIR, UV–VIS–NIR and unilateral NMR investigations. J Therm Anal Calorim. 2008;91(1):17–27. https://doi.org/10.1007/s10973-007-8513-x.
Hamza M. development and evaluation of orodispersible films of lamotrigine: hydroxypropyl β cyclodextrin inclusion complex. Az J Pharm Sci. 2017;56:31–46. https://doi.org/10.21608/ajps.2017.28458.
George S, Vasudevan D. Studies on the preparation, characterization and solubility of 2- HP-B-cyclodextrin–meclizine HCL inclusion complexes. J Young Pharm. 2012;4(4):220–7. https://doi.org/10.4103/0975-1483.104365.
Wei N, Pei J, Jiang L, Xuan G. Preparation and property evaluation of vitamin A cyclodextrin inclusion complex. ITM Web Confer. 2022;45(1–9):01061. https://doi.org/10.1051/itmconf/20224501061.
Zghari B, Hajji L, Boukir A. Effect of moist and dry heat weathering conditions on cellulose degradation of historical manuscripts exposed to accelerated ageing: 13C NMR and FTIR spectroscopy as a non-invasive monitoring approach. J Mater Environ Sci. 2018;9(2):641–54. https://doi.org/10.26872/jmes.2018.9.2.71.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Gomaa Abdel-Maksoud: Conceptualization, manuscript administration, methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation, writing-review and editing. Hanaa Nasr: Methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation, review and editing, Samaha Sayed Hussein: Methodology, validation, formal analysis, investigation, data curation, writing-review and editing, Mahmoud Saad ELdeen Kassem: Methodology, validation, formal analysis, investigation, data curation, writing original draft preparation, writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abdel-Maksoud, G., Nasr, H.ES., Samaha, S.H. et al. Evaluation of the performance of Hydroxypropyl-β-cyclodextrin for the consolidation of vegetable-tanned leather artifacts. Herit Sci 12, 188 (2024). https://doi.org/10.1186/s40494-024-01294-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-024-01294-2