Abstract
Confirmed since the twelth century, the Saxon community in Transylvania developed over the years in a rigorous powerful society, with its own lifestyle, social, economic, cultural and artistic standards. Together with research in historic documents, this society is now revealed by material studies of eighteenth– twentieth century objects in the Emil Sigerius collection, preserved in the ASTRA Museum, Sibiu, Romania. Embroideries made in Saxon households as well as representative Saxon coats manufactured in workshops, and dated between 1892 and 1908 (years embroidered on objects), were studied in terms of dye analysis, in order to understand if adoption of new materials goes together with the stylistic improvements. Dye analysis were performed by liquid chromatography with UV–vis and mass spectrometric detection (LC-DAD-MS), nowadays the most appropriate technique for the characterization and identification of colour components in heritage textiles. MS detection, with a triple quadrupole mass analyser, was used to achieve clear identification of each dye in a complex matrix. Construction of suitable spectral libraries through studies on standard dyes and dyed textiles definitely improved the ability to detect natural and early synthetic with greater certainty. Identification of fuchsine (1856), methyl violet (1861), orange II (1876) brilliant green (1879), quinoline yellow (1881) and rhodamine B (1887) in textiles dated about 20 years after the dyes first synthesis prove that transition from natural to synthetic dyes in the Saxon community was very fast, especially for textiles made in specialized workshops. The analytical configurations used prove their competence in textile studies, based on natural and early synthetic dyes research, and promotes the use of advanced analytical techniques in heritage science applications.
Similar content being viewed by others
Introduction
Traditional textiles are among the most representative material witnesses to document the nineteenth century and twentieth century rural life in the three historical provinces of modern Romania: Wallachia, Moldavia and Transylvania. Located in Transylvania, in the very centre of Romania, the Astra Museum in Sibiu preserves an exhaustive and eclectic textile collection characterized by diversity and multiculturalism. Representative for Romanians, Hungarians, Saxons, Roma and so on, the collection preserves a large variety of items, from raw materials to the most beautiful garments and decorative objects which adorn the traditional houses and churches. These skilfully manufactured objects illustrate the peasants creativity' and artistic taste' in the period before industrialization and until mid-twentieth century [1].
The Saxon textile collection preserved in the Astra Museum emerged from the care of the Saxon ethnologist Emil Sigerius, who gathered the first objects at the end of the nineteenth century, and has been enlarged by the museum specialists since then. The collection contains objects dated between the seventeenth and the twentieth century, most of them from the rural areas of Transylvania [2]. The Saxon community in Transylvania arrived from Germany in successive waves, starting from the twelth century, when they were brought by King Geza II of Hungary. They settled in different areas and developed over the years in a rigorous powerful society, with its own lifestyle, social, economic, cultural and artistic standards, strongly connected with Western Europe [3]. If one examined what the rural Saxon society appreciated in terms of materials, patterns, colour and fashion, the collection of objects in the ASTRA museum would be considered as a synthesis of the ethnic group that provided it. Most of the items (e.q. components of the traditional costume or home textiles) are handmade products, made in households by the community members, while others (such as the sheepskin coats) are supplied by workshops, or bought from specific shops as products worked in the nearby towns with local or imported traded goods (velvet, ribbons, hats/ head covers, fine fabrics or brocades) [4, 5]. Integrated in an approach aiming to better understand the ASTRA museum collection and document the social evolution of each ethnic group, objects belonging to the Saxon community were carefully studied, which also included material investigation and dye analysis.
Liquid chromatography with UV–vis (diode array) detection became the standard method for the identification of natural dyes in historical textiles since its first use in 1985 [6]. It combines high separation efficiency with the possibility of unambiguous attributions, especially when dedicated references libraries are used [7,8,9]. In order to achieve even more certain identifications by bringing new criteria for dyes recognition, mass spectrometers are added in the configuration [10, 11]. Due to the possibilities for tuning the selectivity as well as to increase sensitivity, through appropriately exploiting the functioning modes, ion trap or triple quadrupole mass spectrometers are preferred. They are usually equipped with electrospray ionization sources [12,13,14,15,16]. Further to the individual dyes recognition which is supported by experiments performed on standards, the interest is to evaluate the biological sources most likely used. This is facilitated by building dedicated databases to contain information accumulated on fibres dyed in the laboratory with well documented biological sources, mentioned in literature as being used for textile dyeing [17, 18]. In-depth studies have been developed to characterize certain biological sources [19,20,21,22] or to discriminate between related ones, in order to recognize them down to the specie level [23,24,25,26]. The biological sources for natural dyes were used only locally before the moment they became subject of trade. Nowadays, the commercial routes and related historical periods are recorded. Consequently, historical objects could be dated with respect to the composition of the biological sources and the documented moment when such a biological source became subject of trade [17]. Following their first discovery in 1856, a large number of synthetic dyes were produced and became commercially available [27,28,29]. They were adopted with enthusiasm, due to the bright hues and the variety of colours they could provide. The advantage of giving easily reproducible colours, which eliminated the dependence of natural sources, also contributed to their success.
Studies performed on traditional textiles in Romanian collections evidenced the use of various colour sources, natural or imported, as well as of 'early synthetic dyes' [30, 31], which is the name adopted for a selection of about 67 synthetic dyes, the most frequently used between 1856 and 1900 [32]. An in-depth survey on materials in traditional Romanian shirts decoration in a Transylvanian rural area less than 50 km long, not far from Sibiu, revealed some phases in the transition from natural to the early synthetic dyes: first, the three main colours red, yellow and blue were achieved by using natural dye sources such as madder, carminic-acid-based dyes, dyer’s broom, sawwort, tannins and indigo dyes, on wool; the colour palette was then enriched with orange or black hues by using mineral pigments lead chromate and Prussian blue; further on, natural dyes were applied on silk; finally, bright pink, violet, orange, blue or green colours were produced exclusively with synthetic dyes on silk [33].
The present study aims to document the Saxon community in Transylvania as it results from the materials used and the interest to adopt new alternatives, such as artificial fibres and early synthetic dyes. Research hopes to bring scientific arguments for understanding the development of this particular ethnic group in relation and compared to the rest of the society, as well as to trace any possible differences between rural and urban areas, ethnic interferences, European links and connectivity. The approach is based on a survey which includes handmade textiles dated from the seventeenth to the twentieth century as well as a collection of sheepskin coats documented as manufactured in workshops, and having embroidered on them the year of fabrication, which is between 1876 and 1908.
Experimental
Sample description and preparation
Sixty samples from 18 objects belonging to ASTRA Museum were taken during the conservation procedures in the Textile Conservation Department. Two of them come from weavings while the rest are embroidery yarns used in decorations of sheepskin coats, head covers or home textiles. The museum archive mention the sheepskin coats as worked in dedicated workshops, between 1892 and 1908. The other objects are dated between the seventeenth century and the nineteenth century; some of them are handmade while the others are not documented (Table 1). Samples were first observed under the microscope at 10–80 × magnification and then non-destructively investigated by attenuated total reflectance infrared spectroscopy (FTIR-ATR), for fibres identification. If larger samples were available, only yarns about 3 mg (~ 0,5 cm long) were cut and 200 μL mixture 37% HCl/CH3OH/H2O 2:1:1 (v/v/v) were added for dyes extraction. Although the advantages of the mild extraction techniques in the case of (natural) flavonoid dyes were known, the use of acid hydrolysis was an assumed decision. The compromise was based on the existence of an in-house built database, with information collected on acid hydrolysed extracted natural and early synthetic dyes standard dyed fibres. The coloured yarns were kept in the solutions for 10 min, at 100 °C, and then extracts were evaporated to dryness in a vacuum desiccator. Each sample was re-dissolved in 100 μL solution CH3OH/H2O 1:1 (v/v) and centrifuged at 12,000 rpm for 10 min. The supernatants were transferred into chromatographic vials and injected into the chromatographic system. For the visual blue, green, violet and black samples, where the presence of indigo based dyes was suspected, a second extraction in 100 μL dimethyl sulfoxide (DMSO) was made, with the samples kept at 80 °C for 10 min. The two solutions were merged and analysed.
Integrated with the results obtained on the 18 objects mentioned above, data previously reported on another Saxon embroidery, dated 1841, will be discussed (Table 1) [34].
Fibre identification
Fibres documentation and image collection were made with a Nikon SMZ 1000 stereomicroscope coupled with a Nikon DSLR camera, model D3100 Kit AF-s 18–55 mm VR DX. Investigation was made by infrared spectroscopy (FTIR-ATR), where a Bruker Optics Alpha spectrometer equipped with a Platinum ATR single reflection diamond ATR module was used. Spectra were acquired in the 4000–400 cm−1 domain, with a resolution of 4 cm−1. Data collection and processing were made with a dedicated software, Opus 7.0.
Dye analysis
Samples were analysed in two different campaigns, part of them by liquid chromatography with UV–vis (diode-array) detection—LC-DAD on System 1, and the other by liquid chromatography with UV–vis and mass spectrometric detection, LC-DAD-MS on System 2 (Table 1).
System 1 (LC-DAD) is an Agilent 1260 Infinity II series liquid chromatograph (Agilent Technology, Santa Clara, CA, USA) consisting of a quaternary pump (G7129A), a standard autosampler (G7111B), a column thermostat (G7116A) and multi-channel diode-array detector (G7115A). OpenLAB CDS software was used for the chromatographic system control, data acquisition and processing. A Zorbax C18 column, 150 mm length, 4.6 mm i.d. and 5 μm particle size, was used, thermostated at 40 °C. The mobile phase consists of a mixture of aqueous 0.2% (v/v) formic acid (solvent A) and methanol/acetonitrile (1:1, v/v) plus 0.2% formic acid (as solvent B). Gradient elution was applied by using the following profile: at 0 min, 15% solvent B; from min 0 to 5, linear increase to 25% solvent B; from min 5 to 10, constant at 55% solvent B; from min 10 to 16, linear increase to 100% solvent B; from min 16 to 18, constant at 100% solvent B; and step jump at 15% solvent B, with 5 min re-equilibration period between runs (post-time). The flow rate was set at 0.8 mL/min and the injected volume of the samples was 10 μL. The UV–vis spectra were acquired from 200 to 900 nm, with a simultaneous monitoring at five wavelengths (255, 275, 295, 420 and 490 nm), with a frequency of 0.03 min and a 2 nm resolution.
System 2 (LC-DAD-MS) was an Agilent 1260 LC, composed of the following modules: quaternary pump (Model G1311C), automatic injector (G1367E) and column thermostat (G1316C). The diode array detector (G4212A) and the triple quadrupole mass spectrometer (G6410B) were serially connected. UV–Vis spectra were acquired with a DAD detector which was placed between the column and the MS ion source. Spectra were collected over the 190–640 nm range, with a resolution of 2 nm. The MS used an ESI ionization source (ESI, Model G1948B), operated under negative and positive ion monitoring modes. Chromatographic separation was the same as for System 1, except for Solvent B which was methanol/acetonitrile 1:1 (v/v) with no formic acid content. As for System 1, injection volume was 10 μL. For the MS detector, the following ESI operation parameters were used: drying gas temperature 350 °C; drying gas flow 8 L/min; pressure of the nebulising gas 40 psi; Vcap 2500 (−) in negative ion mode and 2500 ( +) in positive ion mode. The triple quadrupole used MS2 type scan when used as a single MS instrument; the data storage was set on profile and the peak width at 0.07; fragmentor 135 V; ΔEMV 400 V. The scanning interval for the mass to charge ratio (m/z) was between 100 and 600 a.m.u., and acceleration voltage on the collision cell: 7 V; Dwell Time 500 ms. Agilent MassHunter Quantitative Analysis B.06.00 software was used to control the chromatographic system, as well as for data acquisition and processing. The analytical procedure was described in detail in an earlier publication, where an ion trap mass spectrometer was used instead of the triple quadrupole [35]. Samples were analysed with single MS detection in the Full Scan mode and the resulting data was processed by extracting chromatograms, according to the molecular ions of the dyes in the database.
Dyes attribution, database
Dyes identification was based on their retention times, UV–Vis and MS data (where available), according to information collected on standards, dyes and dyed yarns. For natural dyes, attribution of the biological sources was based on data collected on yarns dyed in the laboratory, by following traditional dyeing recipes and was correlated with information available in literature. For the early synthetic dyes, information was acquired based on the analysis of yarns dyed in the laboratory with contemporary products available on the market (from various providers), as well as with analysis on the Schweppe collection of early synthetic dyed yarns. The collection, which contains a selection of 65 synthetic dyes, the most commonly used between 1850 and 1900 [32, 36], was offered by chemist Helmut Schweppe to European and worldwide museums in the 1960–1970, during a series of dyeing workshops with these well-documented products. In the present study, the collection prepared by Ronnee Barnett, textile restorer at the Metropolitan Museum of New York (MET) was used, offered as donation by Florica Zaharia, Muzeul Textilelor Băița, Romania (see Acknowledgements). Literature data on early synthetic dyes [28, 36,37,38,39,40] supplemented the analytical information from the in-house built database. Information regarding retention times, UV–Vis and MS data for the natural and early synthetic dyes discussed, is given in Table 2.
Results
Red, violet and orange
Seventeen samples were described as red, nine as violet and five as orange. In six samples, all characterized as red, alizarin was the main dye component detected. Its presence was evidenced by retention time correlated with UV–Vis data and was reinforced by the molecular ion (for alizarin [M-H]−, m/z 239), in cases where the mass spectrometric data was available (Table 3).
Detection of alizarin together with other natural anthraquinones suggest the use of madder (Rubia tinctorum L.), while the use of synthetic alizarin, a dye which became available in 1871, would be evidenced by alizarin and two marker compounds, anthrapurpurin and flavopurpurin [41]. According to the analysis performed, the presence of purpurin and/or munjistin (for purpurin [M-H]−, m/z = 255 and for munjistin [M-H]−, m/z = 283 respectively) in 5/6 samples (1958_P5, 2075_P5, 2491_P1, 1861_P1, 2940_P3) confirm the use of madder while in one case (2186_P2), where only UV–Vis data was available, no other dyes were detected to reveal a natural or synthetic source of alizarin. Madder was used on wool in a bed cover dated 1841, on silk in a nineteenth century towel and on cotton embroidery yarns in textiles dated 1802 or not dated. However, detection of madder and putting results in correlation with earlier data on traditional Romanian textiles [33] would be an argument to date the respective object (inv. 1861) in the nineteenth century or earlier.
Carminic acid was identified in six samples (3383_P1, 1936_P1, 2075_P6, 2954_P2, 6081_P3, 7515_P1). As was the case for alizarin, the presence of carminic acid was based on retention time and UV–Vis data and also supported by the presence of its molecular ion (for [M-H]−, m/z = 491), when mass spectrometric data was available. Carminic acid is the main dye component in a large number of insects, three of which played an important role in textile dyeing: Mexican cochineal (Dactylopius coccus C.), Armenian and Polish carmine scale insects (Porphyrophora hameli and Porphyrophora polonica). Identification down to the specie level is nowadays possible based on the calculation of relative ratios between minor compounds: flavokermesic acid C-glycoside (also called dcII, whose structure was recently elucidated [42]), kermesic and flavokermesic acids [23,24,25,26]. Unfortunately, for five of the samples mentioned above it was impossible to apply the methodologies cited before, as samples were too small for the minor compounds to be visible. For the one remaining sample (2075_P6), it was estimated that Mexican cochineal was used for dyeing. This supposition is based on studies performed in the same research team [43], where large amounts of flavokermesic acid C-glycoside relative to carminic acid were observed in the Mexican cochineal dyed samples. Carminic acid-based-dyes were used on wool in a towel dated 1832, on silk in a handkerchief dated 1779, a head cover dated 1869 and a nineteenth century pillow cover. It was also identified on rayon in a head cover not dated but could be considered as twentieth century due to the use of rayon (if the rayon yarn is not a later intervention). Mexican cochineal was used on wool in a bed cover dated 1841.
Synthetic dyes were detected in fourteen from the twenty-six samples described as red and violet. Fuchsine (1856) and methyl violet (1861), representatives of triarylmethane, the first group of synthetic dyes which became available, were identified in 8/14 samples. Fuchsine (also called magenta) was evidenced based on the detection of one or more of the four marker compounds described in literature: pararosaniline (λmax = 544 nm), rosaniline (λmax = 546 nm), methylrosaniline (λmax = 548 nm) and dimethylrosaniline (λmax = 550 nm) [28, 36, 38, 44] in seven samples, once on rayon and the others on silk. As stated in literature [38] and also discussed in a previous publication with reference to the use of early synthetic dyes in Romanian traditional textiles [33], two production processes were reported, which would result in the so called “early fuchsine”—characterized by a mixture of the four compounds mentioned above and “late fuchsine”—to give mainly methylrosaniline (also called magenta II) and dimethylrosaniline (new fuchsine). “Early fuchsine” is mentioned as obtained in the late nineteenth century by heating an oxidant with a coal tar distillate containing a mixture of aniline and toluidine in various ratios, with or without further inclusion of carbon tetrachloride, while “late fuchsine” would be the result of a later process that involved reaction of 4,4'methylene-di-o-toluidine and o-toluidine [37]. Consequently, samples 6081_P5 from a head cover dated 1869 and samples 5931_P7, 6787_P2, 6984_P1, 7230_P2 from decorations in sheepskin coats dated 1897, 1908, 1892 and 1903 respectively were dyed with “early fuchsine” and samples 9008_P4 (dated 1900) and 7515_P2 (on which fuchsine was used on rayon), with “late fuchsine” (Fig. 1).
Image to illustrate identification of “old” fuchsine in samples 6984_P1 and 7230_P2 and “new” fuchsine in sample 9008_P4, based on the two different production processes as reported in literature [38]
All the samples where fuchsine was used as single dye were violet while for the others they received the colour of the dyes used for combination: red in sample 6984_P1, when used with red dyes eosin A and traces of azo flavine 3R and violet in sample 7230_P2 when methyl-violet and rhodamine B were added. In what methyl-violet (1861), the other dye in the red and violet group which represents the triarylmethane class is concerned, apart from the sample mentioned above, with reference to a sheepskin coat dated 1903, it was also identified as individual dye in a violet sample from another sheepskin coat, dated 1892 (6984_P6).
Methyl-violet was also used in combinations with other early synthetic dyes to give dark green, brown or black hues in sheepskin coats embroidery yarns.
Rhodamine B (1887) and eosin A (1871) for the class of xanthenes, as well as chrysoin (also called tropaeolin, 1875), azo flavine 3R (1880) and orange II (1876) for the azo dye class were also detected, always on silk. Identification of rhodamine B was based on the detection of the two compounds (λmax = 556 nm and λmax = 558 nm) present in the standard dyed samples from the Schweppe collection and also mentioned in literature [28], while similar argumentation was used to reveal eosin A, based on its marker compounds (λmax = 472, 498 nm, λmax = 472, 500 nm, and eosin λmax = 530 nm). According to literature, the above-mentioned compounds are revealed as eosin without one bromine substituent and eosin methylester [45]. Rhodamine B was used twice as individual dye and twice in combinations with other synthetic dyes, always in the embroideries of sheepskin coats dated 1908 and 1903. It should be mentioned that this dye was detected four times in the same object (dated 1903), twice as individual dye and in two combinations to give different violet and red hues. Eosin A was identified only once, in a dyeing combination with fuchsine and traces of azo flavine 3R to achieve a red colour in a sheepskin coat dated 1892.
Chrysoin (Acid Orange 6), azo flavine 3R (Acid Orange 1), orange II (Acid Orange 7), and crocein orange G (Acid Orange 12) are all indexed as orange dyes, and became available between 1875 and 1878. Chrysoin and azo flavine 3R were identified only by UV–Vis spectrometric data and were used in dyeing combinations with other early synthetic dyes, such as orange II and rhodamine B for the first, and fuchsine and eosin A for the second. In both cases, they were used in sheepskin coats dated 1908 and 1892 respectively. The use of mixtures of three synthetic dyes in the sheepskin coats, documented as manufactured in workshops, evidence the dyers interest to provide a large variety of colours, the artistic taste of the craftsmen and their flexibility to answer the requests received from the community, in the early stages of the synthetic dyes. By opposite to the two aforementioned dyes, orange II was quite a popular dye. In perfect accordance with references from modern standards and the Schweppe collection, as well as with literature, in the present study Orange II was evidenced based on retention and UV–Vis (λmax = 482 nm) data. It was used as individual dye or with Rhodamine B, in 5 other samples, characterized as orange or orange-red, which means in all cases where an orange hue was intended. It was also detected in other traditional textiles from Romanian collections studied in the same research group [30].
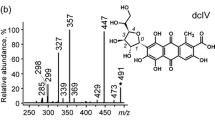
Crocein orange G was identified based on retention, UV–Vis (λmax = 485 nm) and mass spectrometric data ([M-Na]– 327), as compared with a standard dyed fibre from the Schweppe collection labelled Crocein orange G, Acid Orange 12, CI 15970 in two red silk samples (6050_P4 and 6081_P1) in embroidery heads dated 1876 and 1869 respectively (Fig. 2).
Comparative analytical data for crocein orange G dyed wool from Schweppe collection (black line), sample 6050_P4 (blue line) and 6081_P1 (red line) to support identification of crocein orange G in the two samples. From top to bottom: LC-DAD (490 nm); Single stage MS Full Scan; Extracted Ion Chromatograms (EIC) according to m/z = 327 [M-Na].−. EIC according to m/z = 357 (major ion) suggests the presence of another synthetic dye (see text)
Another dye component with the main ion m/z = 357 was also present in sample 6050_P4, probably to give the fibre a red hue. Identification of crocein organge G in silk fibres from two different head covers would suggest that the two objects were repaired, probably in the same context. This is very probable, as they are documented as acquired from the same village (Slimnic), in 1981. As crocein orange G is an early synthetic dye which was not in use in 1981, the interventions should be dated before the two head covers became part of the ASTRA collection.
Yellow
Eleven samples were described as yellow. Luteolin was identified in 5/11 cases, always by retention and UV–Vis data (λmax = 254, 266, 348 nm) and certified by the presence of the molecular ion (for [M-H]−, m/z = 285), when mass spectrometric data was available. Luteolin is the main dye component in several plants, three of which played an important role in textile dyeing in Romania. With reference to the acid hydrolysed extracts, weld (Reseda luteola L.) is characterized by the presence of luteolin, apigenin and chrysoeriol while luteolin, apigenin and 3-methyl-quercetin are expected for sawwort (Serratula tinctoria L.) samples. Dyer's broom (Genista tinctoria L.) acid hydrolysed extracts are defined by luteolin, genistein and apigenin as main compounds, and chrysoeriol and diosmetin as minor components. Unfortunately, in the present study, luteolin was the only dye component detected in 3 samples, probable due to the very small sample sizes, which made any attribution impossible. Apigenin and chrysoeriol were detected in one case (6050_P3) to confirm the use of weld (Fig. 3), while in one other sample (9008_P6) luteolin was accompanied by dyes (λmax = 338 nm; λmax = 350 nm and λmax = 340 nm) which match none of the existing references in the database.
Image to support identification of weld in sample 6050_P3. From top to bottom: LC-DAD (255 nm) and UV–vis spectra of luteolin and apigenin; Single stage MS Full Scan; Extracted Ion Chromatograms (EIC) according to m/z = 285 [M-H]− (luteolin), m/z = 269 [M-H]− (apigenin), m/z = 299 [M-H].− (chrysoeriol)
Except for the last case discussed, luteolin- based-dye sources (including weld) were identified in textiles defined as handmade, dated 1802, 1832, nineteenth century and 1876. Weld and dyer's broom were also identified in visual green samples, also from objects dated 1876 and 1869 (which will be discussed in the section dedicated to green).
The presence of two early synthetic dyes was evidenced in the visual yellow samples: auramine O (1883), with a diphenylmethane structure and quinoline yellow (1882). The first was detected based on retention and UV–Vis data (λmax = 352 nm, λmax = 360 nm, λmax = 366, 486 nm and λmax = 368 nm), as individual dye. It was also detected together with other dyes in a visual brown sample. Quinoline yellow was detected as individual dye source in two yellow silk yarns as well as together with a blue dye in one other case, to achieve a green hue. Both auramine O and quinoline yellow were used for dyeing the decoration yarns in workshop manufactured sheepskin coats dated 1897, 1892, 1908 and 1903. A dye with λmax = 390 nm, which did not match the dyes in the database was detected in a yellow silk sample from a sheepskin coat dated 1892. Unfortunately, no mass spectrometric data was available for the respective sample.
Blue
From the eleven samples described as blue, indigotin was identified in eight cases, which suggests the use of indigo based dyes. Indigotin was evidenced in DMSO extracted samples by retention, UV–Vis (λmax = 285, 330, 610 nm) and mass spectrometric data (for [M-H]−, m/z = 261), when available. Although there are several biological sources which contain indigotin precursors, two of them may have been used in the traditional Saxon textiles: woad (Isatis tinctoria L.), available in Europe since Neolithic, and indigo (Indigofera tinctoria L.) accessible in the area since the seventeenth century [30, 33]. From the analytical point of view, it is not possible to distinguish between the two species neither to differentiate natural from synthetic indigo, which was first produced in 1882. However, considering that indigo based dyes were identified in handmade textiles dated seventeenth–nineteenth century, on wool (four cases), silk (three samples) and cotton (one sample), it is obvious that one of the natural sources was responsible for the colour. According to previous studies on textiles in Romanian collections [30, 31, 33], indigo-based-dyes were the only source of blue in traditional textiles, until synthetic dyes became available.
Two blue early synthetic dyes were also identified in visual blue samples from the present study: Alkali blue (1862), and Victoria Blue (1883), in one case each. They were evidenced based on retention correlated with UV–Vis data, according to information accumulated on standards and available in literature. The two dyes were all identified on silk, in objects dated 1876 and 1903. Victoria Blue seems quite a popular dye at the end on the 19-th beginning of the twentieth century, as it was also identified in Romanian traditional shirts decoration previously studied [33], in two other samples from this group, in combination with different synthetic dyes to achieve green and black hues. An un-identified blue synthetic dye (λmax = 270; 310; 600 nm) was present in head cover inv. 6081. However, this decoration should be considered as intervention at a later date, as the object is dated 1869, and the only synthetic dyes available at that time were indigo carmine and alkali blue, but they present different analytical features.
Green
Seven samples were characterised as green. In two cases, natural yellow dyes such as weld and dyer's broom (already discussed under the yellow section) were identified, which were probably used with iron mordants to obtain green hues. The two dye sources were detected in silk decorations in two head covers, dated 1876 and 1869. Dyer's broom was a very popular source of colour at the end of the nineteenth century, as results from its frequent identification in traditional textiles in Romanian collections as well as the numerous mentions in a collection of dyeing recipes edited by the Romanian Academy in 1914 [46].
Early synthetic dyes were identified in the other 5/7 green samples. In one case quinoline yellow and Victoria blue, two dyes discussed in the respective sections, were found together in a silk sample from a sheepskin coat dated 1897. Brilliant Green, also called Diamond Green G (1879), was revealed in two cases, both from the sheepskin coat dated 1908 and Malachite Green, also called Diamond Green B (1877), in the other two, in sheepskin coat dated 1892. Each of the above-mentioned green dyes was identified as individual dye in one case, while in the other in a dyeing combination with other dyes (methyl violet or an unknown dye) to give a darker green hue. The unknown dye components present in green sample 6984_P5 have the following profiles: λmax = 408, 578 (retention time 12.6 min) and λmax = 384 (14.2 min). The dyes Brilliant Green and Malachite Green were identified based on retention time and UV–Vis data, which were in perfect correlation with data collected on the respective standard dyed fibres in the Schweppe collection and with literature.
Brown and black
Two visual brown and two visual black samples were available in the study, all belonging to sheepskin coats decorations. In all cases these hues were achieved by combination of dyes. Methyl violet was detected in all but one samples, always on silk: once with malachite green and auramine O (7230_P8), in one other case with unknown dye having as characteristics λmax = 564 nm and λmax = 408 nm (9008_P8) and in the other with Victoria blue B. In the remaining (brown-black wool sample), Prussian blue was detected (by infrared spectroscopy) and unknown yellow dyes (λmax = 358 and λmax = 364 nm) were revealed by LC-DAD. Prussian blue was also detected, together with carminic acid-based-dyes, in a black silk yarn decoration from a Romanian shirt, from the same area, also preserved in the ASTRA Museum [30]. It could be supposed that the two colouring compounds identified were used on a darker shade of wool to give it a brown-black hue.
Discussion
Nineteen objects were available for study, eleven were characterized as handmade, three are not documented and five were made in dedicated workshops. All the handmade objects are dated seventeenth–nineteenth century and natural dyes were exclusively identified. Embroidery is made in the three main colours: red (including pink-violet hues), yellow and blue. The two samples from weavings are both blue. Red was obtained with madder or carminic acid-based-dyes, yellow with luteolin-dye sources and blue with indigo-based-dyes. In most of the samples available, these dyes were on wool, in some cases on cotton or on silk. The early objects where silk embroidery yarns were used are revealed as precious: embroidery inv. 3386 dated seventeenth century, handkerchief inv. 3383 dated 1779 and pillow cover inv. 2954 which is dated nineteenth century. It is very probable that towels inv. 1861 and inv. 2940, where only natural dyed embroidery yarns were used, mentioned in the museum archives as handmade but not dated, are also dated in the nineteenth century or earlier. It is also credible that handkerchief inv. 2186, also handmade and not dated, was made in the nineteenth century or earlier. This supposition is based on the fact that, according to previous analysis, synthetic alizarin was rarely used on silk (mostly on cotton), so it is more reasonable for madder to be responsible for dyeing. It is not documented if head covers inv. 6050 dated 1876, inv. 6081 dated 1869 and inv. 7515, undated in the museum archives, are handmade or produced in dedicated workshops. A possible explication could be that these objects were assembled in the households by using industrial produced components or that peasants bought the head covers readymade but unfinished, and decorated themselves with rich handmade embroidery. Both natural and early synthetic dyes were detected in the embroidery yarns which decorate the head covers. Crocein orange G (1878), as well as the un-identified red, and blue dyes, present in the dated objects, should be the result of interventions at a later date. Alkali blue (1862) for inv. 6050 and fuchsin (1856) for inv. 6081 could be seen as very early adoptions of the synthetic dyes. In the case of head cover 7515, identification of rayon in both of the samples available suggest that either the decoration yarns studied are later interventions or that the object dates from the twentieth century. According to the textile restorer, all the head covers underwent several conservation interventions which altered their original sewing and lining, and make impossible to trace the successive materials used.
Extremely valuable information could be achieved from the study of the five sheepskin coats dated between 1892 and 1908 and documented as manufactured in dedicated workshops (Fig. 4).
Image and detail of sheepskin coat 7230P dated 1903. Eight early synthetic dyes were identified, individual or in combinations, in the 8 samples available (Table 3)
Except for the detection of luteolin in one yellow sample, synthetic dyes, used individual or in combinations of two or three, were responsible for the colours. By contrary with the first objects discussed, where embroidery yarns were only in the three main colours, in the sheepskin coats decoration bright colours such as orange, violet, green as well as dark hues like brown and black were used. This coloration was obtained with the recent available synthetic dyes, discovered even only 15 years before, as in the case of malachite green (1887) in sheepskin coat inv. 6984P (dated 1892). These results help us to establish that the new synthetic dyes were quickly adopted by local Transylvanian workshops in their products. Moreover, the analytical evidence of the use of early synthetic dyes helps the textile conservator to take documented decisions for the objects preservation and specific treatments [47].
Conclusion
Natural and early synthetic dyes were successfully identified in traditional Saxon textiles and in sheepskin coats decoration. In the oldest objects studied, which are dated seventeenth–nineteenth century, natural dye sources such as madder, carminic acid, luteolin and indigo-based-dyes were revealed. Their use was limited to achieve the three main colours, red, yellow and blue, mostly on wool, in homemade textiles for domestic use. The use of silk was reserved to valuable textiles, as for example a handkerchief dated 1779 where carminic acid-based-dyes on silk were revealed. Coloured silk was also used to decorate head covers. Apart from the above-mentioned natural dyes, the first available synthetic alternatives were immediately adopted to obtain brighter hues, as proves the use of fuchsine (1856) in 1869 or alkali blue (1861) in 1876. The Saxons enthusiasm to rapid adopt the brilliant palette of the early synthetic dyes was evidenced by the large number of representatives, available for the first time between 1856 and 1887, and used in silk decorations of sheepskin coats dated from 1892 to 1908, manufactured in workshops.
Transition from the natural to the early synthetic dyes respects the same scenario observed for the Romanian traditional shirts decoration in objects from the area of Sibiu, also preserved in the ASTRA Museum collection. As the synthetic dyes identified in the two studies are almost the same, it may be supposed that they represent the offer of choice that existed at that time.
The analytical strategy elaborated succeeded to successfully identify twelve natural and fifteen synthetic dyes, with different structures: flavone, anthraquinone, indigoid, diphenylmethane, triarylmethane, xanthene, quinoline. Based on the use of liquid chromatography with UV–Vis (diode array) and mass spectrometric detection, the methodology was strongly supported by the in-house built databases, which included data collected on the Schweppe collection of fibres, dyed with a selection of the most frequently used early synthetic dyes.
Finally, the results provide an instrument to enrich the existing information on traditional textiles, based on the materials used, which will be helpful to characterize un-documented traditional objects. Further on, documented local textile collections would be helpful to better integrate the Romanian heritage in a European and worldwide context.
Availability of data and materials
Not applicable.
Change history
18 September 2023
A Correction to this paper has been published: https://doi.org/10.1186/s40494-023-01028-w
Abbreviations
- a.m.u.:
-
Atomic mass unit
- ATR:
-
Attenuated total reflectance
- DAD:
-
Diode array detection
- FS:
-
Full scan mode
- FTIR:
-
Fourier transform infra red spectrometry
- HPLC:
-
High performance liquid chromatography
- EIC:
-
Extracted ion chromatogram
- m/z:
-
Mass to charge ratio
- MS:
-
Mass spectrometry
References
Ștefan C. Textile decorative săsești (Saxon traditional textiles), Catalog de colecție (Collection catalogue). "Astra Museum". 2014.
Bucur IC. Muzeul civilizatiei populare traditionale “ASTRA” Dumbrava Sibiului (Museum of traditional civilisation “ASTRA” Dumbrava Sibiului), Catalog (Catalogue). Sibiu: Astra Museum; 2007. p. 322–67.
Ionescu Ș, editor. Antique ottoman rugs in Transylvania. Rome: Verduci; 2005.
Klusch H. Siebenburgisch-sachsische Trachtenlandschaften. Honterus: Demokratisches Forum der Deutschen in Rumänien; 2014.
Vintilă C, Calvi G, Pakucs-Willcocks M, Roman N, Wasiucionek M, editors. Lux, modă și alte bagatele politicești în Europa de Sud Est sec XVI-XIX (Luxury, Fashion and other political bagatelles in Southeastern Europe, 16th-19th centuries). București: Humanitas; 2021.
Wouters J. HPLC of antraquinones: analysis of plant and insect extracts and dyed textiles. Stud Conserv. 1985;30:119–28.
Petroviciu I, VandenBerghe I, Cretu I, Albu F, Medvedovici A. Identification of natural dyes in historical textiles from Romanian collections by LC-DAD and LC-MS (single stage and tandem MS). J Cult Heritage. 2012;13:89–97.
Karapanagiotis I, Mantzouris D, Cooksey C, Mubarak MS, Tsiamyrtzis P. An improved HPLC method coupled to PCA for the identification of Tyrian purple in archaeological and historical samples. Microchem J. 2013;110:70–80.
Quye A, Wouters J. An application of HPLC to the identification of natural dyes. Dyes Hist Archaeol. 1991;10:17–21.
Szostek B, Orska-Gawrys J, Surowiec I, Trojanowicz M. Investigation of natural dyes occurring in historical Coptic textiles by high-performance liquid chromatography with UV–vis and mass spectrometric detection. J Chromatogr A. 2003;1012:179–92.
Manhita A, Balcaen L, Vanhaecke F, Ferreira T, Candeiasa A, Barrocas DC. Unveiling the colour palette of Arraiolos carpets: Material study of carpets from the seventeenth–nineteenth century period by HPLC-DAD-MS and ICP-MS. J Cult Her. 2014;15:292–9.
Lech K, Jarosz M. Identification of Polish cochineal (Porphyrophora polonica L.) in historical textiles by high-performance liquid chromatography coupled with spectrophotometric and tandem mass spectrometric detection. Anal Bioanal Chem. 2016;408:3349–58.
Rosenberg E. Characterisation of historical organic dyestuffs by liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2008;391(1):33–57.
Pauk V, Barták P, Lemr K. Characterization of natural organic colorants in historical and art objects by high-performance liquid chromatography. J Sep Sci. 2014;37(23):3393–410.
Han J, Wanrooij J, van Bommel M, Quye A. Characterisation of chemical components for identifying historical Chinese textile dyes by ultra high performance liquid chromatography – photodiode array – electrospray ionisation mass spectrometer. J Chromatogr A. 2017;1479:87–96.
Shahid M, Wertz J, Degano I, Aceto M, Ibrahim Khan M, Quye A. Analytical methods for determination of anthraquinone dyes in historical textiles: a review. Anal Chim Acta. 2019;1083:58–87.
Cardon D. Natural dyes—sources, tradition, technology, science. London: Archetype Publications; 2007.
Hofenk de Graaff JH. The colourful past. Origins, chemistry and identification of natural dyestuffs. London: Abegg Stiftung, Archetype Publications; 2004. p. 141–54.
Peggie DA, Hulme AN, McNab H, Quye A. Towards the identification of characteristic minor components from textiles dyed with weld (Reseda luteola L.) and those dyed with Mexican cochineal (Dactylopius coccus Costa). Microchemica Acta. 2008;162:371–80.
Trolen L, Phillips A, Peggie D, Barran P, Hulme A. Historical textile dyeing with Genista tinctoria L.: a comprehensive study by UPLC-MS/MS analysis. Anal Methods. 2014;6(22):8915–23.
Hulme AN, McNab H, Peggie DA, Quye A. Negative ion electrospray mass spectrometry of neoflavonoids. Phytochemistry. 2005;66:2766–70.
Sharif S, Nabais P, Melo MJ, Oliveira MC. Traditional yellow dyes used in the twenty-first Century in Central Iran: the knowledge of master dyers revealed by HPLC-DAD and UHPLC-HRMS/MS. Molecules. 2020;25:908.
Wouters J, Verhecken A. The coccid insect dyes: HPLC and computerized diode-array analysis of dyed yarns. Stud Conserv. 1989;34:189–200.
Wouters J, Verhecken A. The scale insect dyes (Homoptera: Coccoidea): species recognition by HPLC and diode-array analysis of the dyestuffs. Annales de la Société Entomologique de France. 1989;25(4):393–410.
Vanden BI. A special challenge in the field of dye analysis: the identification of cochineal species in Turkmen Weavings. In: Rageth J, editor. Turkmen carpets. A new perspective, Volume I. Abächerli Media AG: Sarnen; 2016. p. 303–10.
Serrano A, van den Doel A, van Bommel M, Hallett J, Joosten I, van den Berg KJ. Investigation of crimson-dyed fibres for a new approach on the characterization of cochineal and kermes dyes in historical textiles. Anal Chim Acta. 2015;897:116–27.
Welham RD. The early history of the synthetic dye industry. Colorantion Technol. 1963;79(3):98–105.
Tamburini D, Shimada CM, McCarthy B. The molecular characterization of early synthetic dyes in E. Knecht et al’s textile sample book “A Manual of Dyeing” (1893) by high performance liquid chromatography—diode array detector—mass spectrometry (HPLC-DAD-MS). Dyes Pigm. 2021;190:109286.
Tamburini D, Breitung E, Mori C, Kotajima T, Clarke ML, McCarthy B. Exploring the transition from natural to synthetic dyes in the production of nineteenth-century Central Asian ikat textiles. Herit Sci. 2020;8:114.
Petroviciu I, Wouters J. Analysis of natural dyes from Romanian nineteenth–twentieth century ethnographical textiles by DAD-HPLC. Dyes Hist Archaeol. 2002;18:57–62.
Petroviciu I, Teodorescu I, Albu F, Virgolici M, Nagoda E, Medvedovici A. Dyes and biological sources in nineteenth to twentieth century ethnographic textiles from Transylvania, Romania. Her Sci. 2019;7:1–15.
Ballard Mary W. (editor), Important early synthetic dyes, chemistry constitution date properties, 1991, Conservation Analytical Laboratory, Smithsonian Institution https://repository.si.edu/handle/10088/7054?show=full. Accessed 27 July 2023.
Petroviciu I, Teodorescu I, Vasilca S, Albu F. Transition from natural to early synthetic dyes in the Romanian traditional shirts decoration. Heritage. 2023;6:505–23.
Vasilca S, Petroviciu I, Negut D, Virgolici M, Albu F, Medvedovici A. Development of a new mild extraction method for the analysis of natural dyes in Cultural Heritage textiles by LC-DAD-MS. Paper presented at Dyes in History and Archaeology 40, unpublished.
Petroviciu I, Albu F, Medvedovici A. LC/MS and LC/MS/MS based protocol for identification of dyes in historic textiles. Microchem J. 2010;95:247–54.
Souto CS. analysis of early synthetic dyes with HPLC-DAD-MS—An important database for analysis of colorants used in cultural heritage. In Faculdade de Ciencias e Tecnologias; Universidade de Lisbon: Lisbon, Portugal, 2010.
Van Bommel M, Vanden Berghe I, Wallert A, Boitelle R, Wouters J. High-performance liquid chromatography and nondestructive three-dimensional fluorescence analysis of early synthetic dyes. J Chromatogr A. 2007;1157:260–72.
Chen VJ, Smith GD, Holden A, Paydar N, Kiefer K. Chemical analysis of dyes on an Uzbek ceremonial coat: Objective evidence for artifact dating and the chemistry of early synthetic dyes. Dye Pigment. 2016;131:320–32.
Pirok BW, den Uijl MJ, Moro G, Berbers SV, Croes CJ, van Bommel MR, Schoenmakers PJ. Characterization of dye extracts from historical cultural-heritage objects using state-of-the-art comprehensive two-dimensional liquid chromatography and mass spectrometry with active modulation and optimized shifting gradients. Anal Chem. 2019;91:3062–9.
Pirok BW, Moro G, Meekel N, Berbers SV, Schoenmakers PJ, Van Bommel MR. Mapping degradation pathways of natural and synthetic dyes with LC-MS: influence of solvent on degradation mechanisms. J Cult Herit. 2019;38:29–36.
Liu J, Zhou Y, Zhao F, Peng Z, Wang S. Identification of early synthetic dyes in historical Chinese textiles of the late nineteenth century by high-performance liquid chromatography coupled with diode array detection and mass spectrometry. Color Technol. 2016;132:177–85.
Stathopouloua K, Valianoub L, Skaltsounisa A, Karapanagiotis I, Magiatis P. Structure elucidation and chromatographic identification of anthraquinone components of cochineal (Dactylopius coccus) detected in historical objects. Anal Chim Acta. 2013;804:264–72.
Petroviciu I. Liquid chromatography as analytical technique in the characterisation of natural dyes and organic pigments in museum objects, PhD thesis, University of Bucharest. 2011.
Mantzouris D, Karapanagiotis I, Valianou L, Panayiotou C. HPLC–DAD–MS analysis of dyes identified in textiles from Mount Athos. Anal Bioanal Chem. 2011;399(9):3065–79.
Sabatini F, Degano I, Colombini MP. Development of a method based on high-performance liquid chromatography coupled with diode array, fluorescence, and mass spectrometric detectors for the analysis of eosin at trace levels. Sep Sci Plus. 2020. https://doi.org/10.1002/sscp.202000002.
Pamfile T, Lupescu M. Cromatica poporului român (The Romanian people chromatics). București: Socec & C. Sfetea; 1914.
Bruselius Scharff A. Synthetic dyestuff for textile and their fastness to washing in Brooks MM and Eastop D. (editors) Changing views of textile conservation of textile conservation art. 2011; 199–209
Acknowledgements
This article is based upon work from COST Action “Europe Through Textiles: Network for an integrated and interdisciplinary Humanities (EuroWeb), CA19131”, supported by COST (European Cooperation in Science and Technology). COST (European Cooperation in Science and Technology) is a funding agency for research and innovation networks. Our Actions help connect research initiatives across Europe and enable scientists to grow their ideas by sharing them with their peers. This boosts their research, career and innovation. www.cost.eu. The authors also express their gratitude to Ronnee Barnett, textile restorer at the Metropolitan Museum of New York (MET) who built the Helmut Schweppe collection of early synthetic dyes used as reference database and to Florica Zaharia, Muzeul Textilelor Baita, Romania who offered it for research. They are thankful to Agilrom Scientific SRL Romania and IRASM Department in “Horia Hulubei” National Research Institute for Physics and Nuclear Engineering, Romania, who offered access to the analytical instrumentation and sample preparation facilities.
Funding
This article/publication is based upon work from COST Action “Europe Through Textiles: Network for an integrated and interdisciplinary Humanities” - EuroWeb, CA19131 supported by COST (European Cooperation in Science and Technology).
Author information
Authors and Affiliations
Contributions
IP and IT designed the research and wrote the main manuscript; IT provided and documented the samples; SV and IP settled the LC–DAD analytical method, analysed the samples and processed the data; FA and AM set up, together with IP, the LC–DAD–MS analytical protocol and provided all the support for LC and MS data acquisition and procession. All authors reviewed and accepted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: “Acknowledgements and Funding information sections have been updated”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Petroviciu, I., Teodorescu, I., Vasilca, S. et al. Liquid chromatography as analytical tool for the study of natural and early synthetic dyes in traditional Saxon textiles. Herit Sci 11, 164 (2023). https://doi.org/10.1186/s40494-023-00969-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-023-00969-6