Abstract
Prostate cancer (PC) is a common tumor in men, and the incidence rate is high worldwide. Exosomes are nanosized vesicles released by all types of cells into multiple biological fluid types. These vesicles contribute to intercellular communication by delivering both nucleic acids and proteins to recipient cells. In recent years, many studies have explored the mechanisms by which exosomes mediate the epithelial-mesenchymal transition, angiogenesis, tumor microenvironment establishment, and drug resistance acquisition in PC, and the mechanisms that have been identified and the molecules involved have provided new perspectives for the possible discovery of novel diagnostic markers in PC. Furthermore, the excellent biophysical properties of exosomes, such as their high stability, high biocompatibility and ability to cross biological barriers, have made exosomes promising candidates for use in novel targeted drug delivery system development. In this review, we summarize the roles of exosomes in the growth and signal transmission in PC and show the promising future of exosome contributions to PC diagnostics and treatment.
Similar content being viewed by others
Background
As the leading cause of cancer deaths in men in 46 countries, prostate cancer (PC) is the most common tumor in men in 105 of 185 countries [1]. In recent years, the incidence of PC has increased worldwide [2].
According to the current clinical guidance [3], successful screening as well as early diagnosis and accurate risk assessment for PC are highly dependent on the detection of prostate-specific antigen (PSA), which remains the most commonly used PC biomarker [4]. Although the beneficial effects of PSA testing have been confirmed in a long-term multicenter follow-up study [5,6,7,8], the poor specificity of PSA tests has resulted in many misdiagnoses, and the negative effect of this insurmountable problem on treatment monitoring has continuously worn down the patience of physicians and patients [9,10,11]. Fortunately, the development of the PSA-based Prostate Health Index (PHI) [12] and the 4KScore [13], as well as the implementation of noninvasive PCA3 and TMPRSS2:ERG gene fusion screening, has had positive impacts on the accuracy of PC diagnostics. However, the true value of these discoveries has yet to be determined with large-scale prospective comparative studies [3]. Although significant advancements have been made in early PC management and in the use of radiation and chemotherapy in recent years, these clinical strategies are effective only for the local treatment of PC and show little impact on metastatic PC [3, 14].
As extracellular vesicles, exosomes range from 30 to 200 nm in diameter, with an average diameter of 100 nm [15, 16]. They are organelles with a single membrane and contain abundant proteins, lipids, nucleic acids, and glycoconjugates [17]. Exosomes are secreted by all cell types [18] and are involved in physiological and pathological processes by acting as carriers for intercellular substance exchange and/or signaling [19,20,21,22,23]. In recent years, the role played by exosome intercellular communication in PC development has gradually been demystified. Furthermore, excellent biophysical properties render exosomes promising for use as novel PC biomarkers and for developing new treatments [24, 25].
In this review, we summarize the potential mechanisms by which exosomes contribute to PC infiltration and metastasis, mediate drug resistance, and structure the tumor microenvironment. Furthermore, the biogenesis of exosomes and the regulatory factors of this biogenesis; the isolation and characterization of exosomes; the roles played by exosomes as biomarkers in PC screening, diagnosis, disease stratification, and treatment evaluation; and use of exosomes as therapeutic vectors in cutting-edge advancements for targeting PC are illustrated in this review.
Exosomes
Biogenesis of exosomes
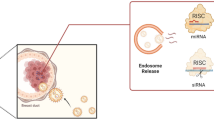
The best characterized pattern of exosome biogenesis is the endosomal pathway, which involves two separate instances of plasma membrane invagination and the generation of intracellular multivesicular bodies (MVBs) (Fig. 1). Although it is a continuous process, the endosomal pathway is generally divided into four main steps to enable better understanding: In step 1, various extracellular substances and fluids enter a cell via plasma membrane invagination or endocytosis, leading to the expression of cell surface proteins (e.g., CD9, CD63, CD81, and flotillin) in the inner layer of the exosomal phospholipid membrane, resulting in plasma membrane budding, and these budded vesicles either form early sorting endosomes (ESEs) or fuse with previously formed ESEs in a process regulated by endoplasmic reticulum (ER), trans-Golgi network (TGN), and/or mitochondrial components. In step 2, facilitated by the TGN, ESEs mature into late sorting endosomes (LSEs). In step 3, the plasma membrane invaginates at multiple LSE sites, leading to the formation of MVBs, which contain multiple small vesicles, ~ 40 nm in diameter, called intraluminal vesicles (ILVs). In step 4, MVBs either bind to autophagosomes or lysosomes or fuse with the plasma membrane, and their cargo is either degraded or released into the extracellular space. Specifically, ILV binding with either autophagosomes or lysosomes leads to the eventual degradation ILVs, which are then prone to extracellular disposal, and MVB fusion with the plasma membrane leads to extracellular secretion of MVB cargo and ILV development into true exosomes [15, 26, 27]. The endosomal pathway has been verified through many studies [28,29,30]. However, it has become increasingly clear that exosomes can directly bud from the plasma membrane. The original mechanism, in which ILVs become exosomes, was first challenged by the discovery of the plasma membrane as the primary site of HIV-1 outgrowth [31]. Subsequently, microvesicles (MVEs) germinated directly from the plasma membrane are the same size as exosomes but lack LSE markers, suggesting that these vesicles differ from exosomes derived from MVBs [32]. The application of atomic force microscopy in recent years has provided a direct view of this germination pathway [33].
Regulatory factors of exosome biogenesis and release
The biogenesis of exosomes is tightly controlled by multiple proteins and the networks in which these proteins are involved. Exploration into the molecular mechanisms regulating exosome biogenesis has provided a theoretical basis for the search for potential PC markers and therapeutic targets. Here, we review the regulatory factors involved in the four main steps of the endosomal pathway and exosome biogenesis (Fig. 1).
Step 1. Factors affecting ILV formation and MVB maturation. The endosomal sorting complex required for transport (ESCRT) is the main mechanism regulating ILV production and is critical for sorting ubiquitinated proteins into ILVs, indicating that exosome production in the endosomal pathway is dependent on functional ESCRT-related proteins [34, 35]. As an auxiliary component of the ESCRT machinery, Alix is a pivotal molecule for the Syntecan/Syntenin/Alix axis [36], the Ral/Arf6/PLD2/Syntenin/Alix axis [16, 37] to produce cross-linking with ESCRT and plays an important role in influencing exosomes [38]. Furthermore, ceramide plays a key role in the biogenesis of MVBs. For example, neutral sphingomyelinase 2 (NSMase2) hydrolysis of sphingolipids produces ceramides that enable ILVs to form MVBs; notably, exosome production was found to be decreased when NSMase2 activity was inhibited [39].
Step 2. Factors enhancing the destruction of MVBs. Interferon-stimulated gene conjugation (ISGylation) to TSG101 induces degradation of MVBs by aggregating and increasing MVB binding to lysosomes, decreasing the number of exosomes secreted [40]. Notably, when the expression of PIKfyve in PC-3 cells was blocked, the secreted exosomes were profoundly enriched with autophagy-related proteins [41]. Furthermore, autophagy inducers, such as starvation or rapamycin, promoted the fusion of MVBs with autophagic vesicles, leading to a decrease in exosome release [42].
Step 3. Mature MVBs are induced to participate in autophagy, bind to lysosomes, or are transported along microtubules to the plasma membrane [27]. This transport is driven by kinesin [43] and regulated by several Rab proteins [28] and the small GTPase Arl8 [44]. The key roles of the Rab protein family in the biogenesis of exosomes have been extensively explored, and studies have shown, for example, that Rab11 promoted homotypic fusion of MVBs in a calcium-dependent manner [45], Rab27a and Rab27b play roles in MVB docking to the plasma membrane [28], and inhibition of Rab35 activity leads to increased accumulation of endosomal vesicles [46].
Step 4. Docking and fusion are the final steps in exosome biogenesis prior to their release. After migration to the plasma membrane, MVBs couple with SNARE localized on the intracellular membrane and fuse to the plasma membrane [47]. This process is regulated by Ras-related GTPase homolog (Ral-1) [37]. Both YKT6 [47] and Syntaxin 1A [48] are SNAKE proteins and play irreplaceable roles in MVB docking. Moreover, the binding of Rab27a to cortactin and coronin 1b plays a key role in maintaining the stability of the MVE docking site on cortical actin [49].
Exosome isolation and characterization
Exosomes are generally isolated from a complex variety of biological fluids, including blood, lymph, urine, cerebrospinal fluid, bile, saliva, milk, and amniotic fluid [50]. Many novel exosome isolation techniques have been developed, and they are more efficient than the original ultracentrifugation method. However, the complete purification of exosomes is still difficult, and each technical approach has advantages and limitations. Therefore, the term isolation is used to describe the process of harvesting as many exosomes as possible [51]. Generally, it is recommended that multiple types of technical approaches be used in combination to isolate exosomes (Table 1). After exosome isolation, identification techniques are promptly used to confirm the specific collection of exosomes (Table 1). Exosomes are commonly described on the basis of their size, shape, density, and markers [15], and exosome identification techniques are based on the biophysical properties of these characteristics [64]. Under cryo-electron microscopy, the diameter of only one-half of all types of vesicles fall in the range of 30-200 nm, which is generally considered the exosome diameter size. Although the shape of exosomes is usually regular, endosomes with multiple membranes and/or complex shapes are frequently observed [16]. Furthermore, the density of exosomes is affected by their protein content [71], and the characterization of surface markers reflects only the specific origins of the exosomes and are therefore not broadly representative [16]. It is clear that the characterization methods based on either exosome morphology and size lead to biased results, suggesting that characterization methods should be combined for the identification of exosomes.
Involvement of exosomes in prostate cancer progression
Exosome transport of cellular cargoes between cells is an intercellular communication mechanism in which the contents of a donor cell are transferred to a recipient cell to functionally regulate the recipient cell [72]. Increasing evidence has shown that the progression and metastasis of PC depends on intercellular communication based on exosomes [73, 74].
PC progresses through a combination of the following medical conditions: (1) The epithelial-mesenchymal transition (EMT) in PC leads to the loss of characteristic epithelial cell adhesion and thus PC cells acquire invasive capacity [73, 75]. (2) Angiogenesis creates suitable nutritional conditions for tumor invasion [76]. (3) The generation of a microenvironment suitable for tumor growth promotes the growth and spread of tumor cells [76]. As important extracellular matrix (ECM) components, both cancer-associated fibroblasts and tumor-associated macrophages enhance tumor cell invasion by secreting inflammatory factors and promoting the tumor stem cell proliferation [77]. (4) The acquisition of drug resistance leads to the rapid failure of antitumor drugs and enhances overall tumor drug resistance [78]. In recent years, studies have revealed the involvement of exosomes in the aforementioned mechanisms underlying PC progression (Table 2).
Exosomes mediate the EMT in PC
The EMT refers to the transformation of epithelial cells into a quasi-mesenchymal stem cell state in which invasive and metastatic capabilities are acquired while adhesiveness is reduced [103]. A considerable number of recent studies have focused on the role of exosomes in the EMT associated with PC. For example, exosomes containing the integrin α2 subunit enhance focal adhesion kinase (FAK) and ERK1/2 activity in recipient cells to induce the EMT, ultimately promoting the progression of PC into a more aggressive form [79], and exosomes carrying prostate-specific G protein-coupled receptors (PSGRs) have shown similar effects [80]. Furthermore, Lin et al. [81] demonstrated that Cav-1-containing tumor-derived exosomes (TDEs) promoted the EMT in neuroendocrine PC through the NF-κB signaling pathway.
In addition to the aforementioned protein-like content, microRNAs (miRNAs), important cargoes in exosomes, also significantly contribute to the EMT. Both miR-100-5p and miR-21-5p were abundant in exosomes derived from PC cells and promoted the EMT by enhancing the expression of MMP-2, MMP-9, MMP-13, and RANKL [82]. Similarly, miR-26a in exosomes significantly regulated the expression of EMT-related proteins and potently inhibited the proliferation, migration, and invasion of LNCAP and PC-3 cells [83]. Furthermore, the role of miR-217 and miR-23b-3p in upregulating EMT-associated PC aggressiveness has been illustrated [84]. Similar to PC cell-derived exosomes, exosomes derived from other types of cells have shown significant effects on mediating the EMT. For example, miR-146a-5p, the expression of which is decreased in the exosomes derived from cancer-associated fibroblasts, enhanced the EMT by activating the epidermal growth factor receptor (EGFR)/ERK pathway to accelerate cancer cell metastasis [85], while miR-95 was significantly upregulated in exosomes derived from tumor-associated macrophages (TAMs) and promoted the EMT by activating on the downstream gene JunB [86]. Constituting another important class of exosomal cargo, circular RNAs (circRNAs) regulated PC progression by sponging miRNAs; e.g., circ_0081234 has been shown to promote PC cell migration and invasion and the EMT via regulating miR-1 [87].
Exosome regulation of PC angiogenesis
TDEs harbor a variety of angiogenesis-stimulating factors, including vascular endothelial growth factor, fibroblast growth factor, platelet-derived growth factor, basic fibroblast growth factor, transforming growth factor β, tumor necrosis factors α and β, and interleukin 8 [104, 105], which may be involved in the development of PC. MiR-27a-3p in PC-3 cell-secreted exosomes is thought to be potentially involved in the angiogenic behavior of endothelial cells [88]. DeRita et al. [89] reported that exosomes secreted by PC cells are enriched with Src tyrosine kinase, insulin-like growth factor I receptor (IGF-IR), G protein-coupled receptor kinase (GRK), and FAK, all of which play important roles in angiogenesis. Furthermore, TDEs produced under hypoxic conditions have been shown to enhance matrix metalloproteinase activity in premetastatic niches, thereby promoting angiogenesis in PC [106,107,108].
Exosomes promote a microenvironment suitable for PC and regulate PC cell immune escape
Tumor cells promote tumor-favoring changes to the microenvironment in affected tissue to induce a series of biological functions in tumor cells, such as maintaining tumor cell characteristics, tumor cell proliferation, and resistance to immune responses and exerting antiapoptotic, and prometastatic effects [109]. The altered tissue microenvironment is called the tumor microenvironment, and it is composed of stromal cells (e.g., pericytes, fibroblasts, and neuroendocrine cells), immune cells (e.g., T and B lymphocytes, dendritic cells (DCs), and macrophages), and the ECM [107]. The interactions between the tumor microenvironment and tumor cells exert a profound effect on tumor development [110], and the intercellular communication function of exosomes and their cargoes in the tumor microenvironment play an important role in maintaining these interactions [107]. Cancer-associated fibroblasts (CAFs) derived from fibroblasts and mesenchymal stem cells have been shown to promote tumor growth [111], and the production and maintenance of CAFs depend on the transforming growth factor-β (TGF-β). In addition, PC cell lines secrete exosomes enriched with surface TGF-β that trigger the SMAD3-related signaling pathway and thus induce CAF phenotype acquisition [90, 112]. Exosomes in metastatic PC not only carry the proto-oncogenes KRAS and HRAS but also transfer their transcription products or RAB proteins to adipose-derived stem cells, transforming them into tumor cells [91]. Three miRNAs (i.e., miR-125, miR-130b, and miR-155) have been shown to induce adipose-derived stem cell tumor reprogramming in tumor-prone patients, and they play important roles in cloning PC cells [91, 113]. Li et al. [92] explored the effects of PC cell-derived exosomes on bone metastasis and found that miR-375 significantly promoted osteoblast activity after exosomes were delivered into osteoblasts. Similarly, lncAY927529 in TDEs is engaged in the establishment of a PC bone metastasis pre-microenvironment [96]. These results suggested that PC cells elicit stromal cell support by shaping stromal cell properties through the action of exosomes, thereby establishing a microenvironment suitable for PC cell survival. In addition to exosomes secreted by tumor cells, exosomes secreted by stromal cells into the microenvironment affect tumors. For example, cancer cells exhibit energy metabolism reprogramming, and therefore, the metabolic state of cancer cells differs from that of normal cells [114], and exosomes secreted by CAFs into the tumor microenvironment are involved in this PC cell metabolic reprogramming [115]. Specifically, these exosomes inhibit mitochondrial oxidative phosphorylation, enhance glycolysis, and even lower the pH of the tumor microenvironment, all outcomes consistent with the Warburg effect and conducive for maintaining the metabolic state of tumor cells and facilitating the survival of PC cells in a hypoxic environment [116]. Josson et al. [93] observed that CAFs transfer miR-409 via exosomes to adjacent epithelial cells, leading to tumorigenesis, the EMT, and epithelial cancer cell stemness. Three miRNAs (miR-209, miR-379, and miR-154) in the DLK1DIO3 region of chromosome 14 have been shown to regulate the EMT and PC bone metastasis [94]. In addition to those secreted by CAFs, exosomes secreted by other stromal cells play important roles in PC. For example, exosomes secreted by mesenchymal-derived osteoblasts exert a proliferative effect on PC-3 cells [95].
Immune cells constitute another important class of cells in the tumor microenvironment, and the immune system composed of them is a main obstacle preventing further development of cancer cells. However, tumors generally develop and progress even in the presence of strict immune system defenses due to tumor cell immune escape [117]. Exosomes mediate communication between tumor cells and immune cells and thus promote immune surveillance evasion. For example, exosomes derived from cancer cells containing FasL [118], TGF-β [119], NKG2D ligand [120], Galectin-9 [121], HSP72 [122] and/or other immunomodulatory factors support the immune escape of tumor cells and induce the differentiation of monocyte cell lines that support the tumor cell phenotype [118]. Studies have provided direct evidence supporting exosome involvement in PC cell immune escape. For example, PC-secreted exosomes expressing NKG2D ligands on the surface induced the downregulated expression of NKG2D on the surface of natural killer (NK) and CD8+ T cells, leading to a decrease in the cytotoxic effect of these killer cells and thus enabling PC cell immune escape [97], whereas a tumor antigen-specific T-cell response produced by DCs was significantly enhanced when Rab27a was added to cells to inhibit exosome release [98]. Furthermore, CD73 expression in DCs was induced by exosomes carrying prostaglandin E2 (PGE2), which inhibited the production of TNFα and IL-12 upon the addition of ATP, ultimately promoting communication between PC cell-derived exosomes, immune cell production and immunosuppression [98]. Kim et al. [99] indicated that the secretion of miR-125a-containing exosomes by PC cells inhibited AKT1 expression in monocytes and macrophages and suppressed their proliferation. Myeloid-derived suppressor cells (MDSCs) exert significant immunosuppressive effects and play key roles in tumor immune escape, and exosomes derived from PC cells promote the recruitment of MDSCs by upregulating chemokine receptor 4 (CXCR4) through the TLR2/NF-κB signaling pathway [100]. In recent years, programmed cell death-ligand 1 (PD-L1) has gained considerable attention. PD-L1 has been found in PC-derived exosomes, and the proliferation and activation of T cells are inhibited, while apoptosis is enhanced, when PD-L1 binds to programmed cell death protein-1 (PD-1) on T cells [123,124,125,126]. Furthermore, exosomes can carry PD-L1 to various parts of the body, creating positive conditions for immunosuppression and establishment of a premetastatic microenvironment [127].
Exosomes are involved in PC drug resistance
The emergence of drug resistance is an important obstacle in the fight against cancer. As important components in intercellular communication, exosomes are crucial to tumor drug resistance [78]. The molecular mechanisms underlying the mediation of tumor drug resistance by exosomes are usually classified into three categories: (1) tumor cells excreting chemotherapeutic drugs through exosomes [105], (2) exosomes carrying resistance cargo that communicates with drug-sensitive tumor cells; and (3) exosomes acting as decoy targets in immunotherapy [78]. In recent years, drug resistance in PC has been attributed to the second category of aforementioned mechanisms, as reported extensively in the literature. For example, PC cells acquire therapeutic resistance to docetaxel through castration-resistant PC (CRPC)-derived exosomes carrying caveolin-1, and many of these cells survive under radiation therapy [81]. Furthermore, exosome-derived miR-27a in PSC27 cells inhibits p53 gene expression and thus mediates the chemoresistance of PC-3 cells [101]. Moreover, CAF-derived exosomes carrying miR-423-5p inhibit GREM2 activity by the regulating the TGF-β pathway and thus increase PC resistance to taxane [102].
Prospective applications of exosomes in PC diagnostics and treatments
Exosomes as sources of biomarkers for PC diagnostics
Exosomes are highly stable, and the cell-like topology of their lipid bilayer protects their contents from enzymatic degradation [128]. Notably, exosome cargoes reflect the true primitive state of the parent cells, and the exosomal cargoes never come into contact with bodily fluids, which protects the pathological molecules derived from the secretory cells [129]. Furthermore, exosomes contain large amounts of target contents. For example, the greatest proportion of miRNAs isolated from urine have been shown to be in exosomes, and most of the miRNAs detected in serum and saliva are also found in exosomes [130]. Exosomes are commonly found in most biological fluids, such as plasma [131], urine [132], saliva [133], ascites [134], breast milk [135], and amniotic fluid [136], providing ample sources for investigating their functions, and these sources allow noninvasive or less invasive sampling of bodily fluids compared to solid biopsy sampling, which improves patient compliance and convenience. Due to their optimal properties, exosomes have been established as ideal biomarkers and extensively explored for use in the diagnosis, grading, prognostic assessment, and therapeutic monitoring of PC (Fig. 2; Table 3).
Both urine and blood samples have been extensively investigated because they are conveniently collected and readily available. Studies have revealed significant quantitative differences between urinary exosome contents obtained from PC patients and from benign prostatic hyperplasia (BPH) patients (or healthy individuals); for example, differences have been found in the quantities of lncRNA-p21 [137], miR-21, miR-451, miR-636 [138], miR-30b-3p, miR-126-3p [139], miR-574-3p, miR-141-5p, miR-21-5p [140], miRNA-375 [141], miR-196a-5p, miR-501-3p [142], miR-2909 [143]; proteins such as ITGA3, ITGB1 [144], flotillin 2, TMEM256, Rab3B, LAMTOR1, and Park7 [145]; and some lipid species such as phosphatidylserine and lactosylceramide [146]. Furthermore, the diagnostic potential of exosomes obtained from PC patient blood has been explored. For example, the levels of nucleic acid molecules, such as SAP30L-AS1, SChLAP1 [147], miR-125a-5p, miR-141-5p [148], miR375, miR21, and miR574 [149], and proteins, such as ephrinA2 [150], alpha-helical proteins and beta-folded proteins [151], gamma-glutamyltransferase [152], EpCAM [153], Claudin 3 [154], and other proteins, are significantly increased in PC patients, and variations in these levels in exosomes are expected to show diagnostic potential in PC. Furthermore, exosomal contents play important roles in PC staging, early diagnosis, progression tracking, prognostic assessment, and treatment monitoring. For example, a group of molecules, including PCGEM1, PC3 [155], miR-141 [156], miR-1246 [157], miR-375, miR423-3p [158], and FABP5 [159], and a series of small noncoding RNAs (sncRNAs) [160] have shown diagnostic potential in high-grade PC and even usefulness in PC staging. The nondifferential increase in the Gleason score of survivin in the plasma exosomes of PC patients suggested its potential for use in early PC diagnosis [161]. Furthermore, exosomal αvβ3 integrin in PC patients is a promising biomarker for use in tracking PC progression [162], and a group of predictors of prognostic status in patients with CRPC have been identified, including miR-1290, miR-375 [163] and AR-V7 [164]. Moreover, two miRNAs (i.e., miR-654-3p and miR-379-5p) in exosomes can be potentially used to predict the efficacy of carbon ion radiation therapy (CIRT) for the treatment of PC [165], and CD44v8-10 mRNA in exosomes can be used as a diagnostic marker for docetaxel-resistant CRPC [166].
In comparison to a single variable used as a diagnostic marker, a combination of exosomal cargoes can significantly improve diagnostic performance and create novel diagnostic opportunities. For example, the PCA3/PCGEM1 combination has significantly improved the accuracy of high-grade PC diagnoses, and the TM256/LAMTOR1 combination is highly sensitive for diagnosing PC in patients [167]. The combination of ADSV and TGM4 can be used to distinguish benign tumors from malignant prostate tumors. The ExoDx Prostate IntelliScore from Exosome Diagnostics, which is based on the detection of ERG, PCA3, and SPDEF RNA in urinary exosomes, can be used to distinguish high-grade PC from low-grade PC and benign disease, and the combination of five proteins (i.e., CD63, GLPK5, SPHM, PSA, and PAPP) able to serve a similar purpose [168, 170, 171]. Furthermore, the semen exosome-based PSA/miR-142-3p/miR-142-5p/miR-223-3p model has shown potential for improving PC diagnostic/prognostic efficiency [169].
Exosomes as potential therapeutic targets in PC treatment
To date, rapidly advancing drug delivery technologies have led to the development of many nanoscale drug delivery systems (DDSs), which show significant promise for improving targeted therapies. Unfortunately, induced toxicity and/or the mononuclear phagocyte system has prevented the clinical transformation of many DDSs [172]. However, exosomes may provide the ultimate solution to these problems (Fig. 2). In addition to their high permeability and capacity for prolonging drug half-lives due to their nanoscale structure [173], exosomes are hypotoxic compared to other DDSs [174]. The highly homologous nature of exosomes, particularly their parent cell-derived lipid membranes, endows them with immune privilege, improving the odds of exosomes evading phagocytosis by immune cells [175]. Moreover, the targeting ability conferred by modifications and innate biological barrier permeability significantly facilitate exosome translocation to desired target areas [172, 176,177,178]. Because of these optimal properties, exosomes have been extensively explored as potential therapeutic vehicles for PC treatment. For example, Saari et al. [179] introduced exosomes carrying paclitaxel into both LNCaP and PC-3 cell cultures and found that these exosomes exerted a cytotoxic effect after endocytosis by target cells. Although it has been shown that natural exosomes can be used as therapeutic carriers, various problems, such as insufficient targeting ability [180], have limited the clinical applications of these exosomes as therapeutic carriers. Therefore, it is important to improve the targeting properties of natural exosomes by enhancing their targeting ability. Pan et al. [181] developed a targeted therapeutic platform using urinary exosomes obtained from PC patients to develop PMA/Iron-HSA@DOX nanoparticles that target EGFR on tumor cells and downstream AKT/NF-kB/IkB signaling; notably, the high safety profile of this homologous infusion technique, which did not induce cellular inflammation, shows significant promise for clinical application. Similar to this is the modification of mesenchymal stem cell-associated exosomes (MSCs-Exo) by altanerova et al. Superparamagnetic iron oxide nanoparticles inside the piggybacked MSCs-Exo produced toxicity on PC3 cells after external alternating magnetic field-induced thermotherapy [182]. Furthermore, Vázquez-Ríos et al. [183] designed a nanoplatform loaded with oncology therapeutics that mimicked exosomes, and these nanoplatforms showed efficient targeting capability, similar to that of tumor-associated exosomes. Moreover, Severic et al. [184] added PSMA-targeting peptides to the surface of exosome mimics to target PSMA-positive PC cell lines (i.e., the LNCaP and C4-2B cell lines), and these mimics showed superior targeting ability both in vivo and in vitro.
Due to the significant roles that exosomes play in promoting PC progression and mediating immunosuppression, exosome-based vaccines have been developed to block tumor progression signaling. For example, exosomes secreted by DCs have been used to develop an effective cell-free peptide-based vaccine that leverages MHC class I/peptide complex contributed by DCs to induce CD8+ T cells to eradicate tumor cells [185]. Furthermore, Shi et al. [186] developed an exosome vaccine by anchoring interferon-gamma fusion proteins to the surface of exosomes derived from PC cells, and subsequent validation experiments revealed that the number of M1 macrophages was increased and exosome phagocytosis was enhanced, while the concentration of antibodies against the exosomes was increased and the expression of vascular endothelial growth factor receptor 2 was downregulated.
Although the superior performance of exosomes compared to other nanocarriers has been shown, the disadvantageous properties of exosomes, e.g., low volume, high heterogeneity, complex cargo, and difficulty in characterization, has ultimately made it challenging to apply exosomes in clinical practice [178].
Conclusions
In recent years, as our understanding of exosomes and PC continued to rapidly grow, an increasing number of molecular mechanisms underlying exosomal involvement in PC progression was discovered. Both tumor cell-derived and stromal cell-derived exosomes were revealed to be important factors in PC progression. A significant amount of effort has been devoted to investigating the molecular mechanisms regulating the exosomal protein-like and nucleic acid-like content in inducing the EMT, tumor angiogenesis, tumor microenvironment establishment, and drug resistance, leading to tumor progression. The clarification of these mechanisms provides the precedence for further exploration into diagnostic markers and the development of effective drugs for PC prevention and treatment. As common nanocarriers, exosomes show the following inherent advantages compared to other nanocarriers: Exosomes are highly stable in biological fluids, exhibit excellent protection of their cargo, can encapsulate high concentrations of biomarkers, and can be used to differentiate tumor risk, subtype, recurrence likelihood, and progression based on their molecular contents, and they can be obtained through noninvasive procedures (i.e., in urine specimens). Undoubtedly biomarkers with these optimal properties offer significant promise for effective screening, diagnosis, prognosis, and treatment monitoring of PC in clinical applications. Fortunately, some of these markers have been tested in clinical trials. In addition to potential diagnostic markers of PC, exosomes have shown various advantages, including biological barrier permeability, prolonged circulation time, low toxicity, low immunogenicity, and modifiability, that endow them with significant advantages for use as therapeutics carriers. Substantial advancements have been made in using exosomes, including natural and engineered exosomes, as models to develop and improve targeted DDSs.
Although exosomes have a bright future in medical applications, many unsolved problems remain; e.g., the molecular mechanisms regulating the involvement of exosomes in PC remain unclear. Furthermore, current techniques for exosome isolation and identification require large samples and are expensive, ultimately hindering their clinical applications. Moreover, recent experiments have been mostly conducted at the cellular and molecular levels, and large-scale and multicentered in vivo experiments are needed to provide evidence for the safe and efficient applications of exosomes in clinical settings.
Availability of data and materials
Not applicable.
Abbreviations
- PC:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- PHI:
-
Prostate health index
- PCA3:
-
Prostate cancer antigen 3
- MVB:
-
Multivesicular body
- ESE:
-
Early sorting endosome
- ER:
-
Endoplasmic reticulum
- TGN:
-
Trans-Golgi network
- LSE:
-
Late sorting endosome
- ILV:
-
Intraluminal vesicle
- ESCRT:
-
Endosomal sorting complex required for transport
- ALIX:
-
Apoptosis-linked gene 2-interacting protein x
- Arf6:
-
ADP-ribosylation factor 6
- PLD2:
-
Phospholipase D2
- NSMase2:
-
Neutral sphingomyelinase 2
- TSG101:
-
Tumor susceptibility 101
- PIKfyve:
-
Phosphoinositide kinase
- YKT6:
-
YKT6 v-SNARE homolog
- SNARE:
-
Small NF90 (ILF3) associated RNA E
- EMT:
-
Epithelial-mesenchymal transition
- PSGR:
-
Prostate-specific G-protein coupled receptor
- TDE:
-
Tumor-derived exosome
- EGFR:
-
Epidermal growth factor receptor
- TAM:
-
Tumor-associated macrophage
- VEGF:
-
Vascular endothelial growth factor
- IGF-IR:
-
Insulin-like growth factor–I receptor
- GRK:
-
G protein-coupled receptor kinase
- FAK:
-
Focal adhesion kinase
- ECM:
-
Extracellular matrix
- CAF:
-
Cancer-associated fibroblast
- TGF-β:
-
Transforming growth factor-β
- SMAD3:
-
SMAD family member 3
- Fasl:
-
Fas ligand
- DCs:
-
Dendritic cells
- PGE2:
-
Prostaglandin E2
- TNFα:
-
Tumor necrosis factor α
- ATP:
-
Adenosine triphosphate
- PBMC:
-
Peripheral blood mononuclear cells
- AKT1:
-
AKT serine/threonine kinase 1
- MDSCs:
-
Myeloid-derived suppressor cells
- TLR2:
-
Toll-like receptor 2
- CXCR4:
-
C-X-C motif chemokine receptor 4
- PD-L1:
-
Programmed cell death 1 ligand 1
- PD-1:
-
Programmed cell death-1
- GREM2:
-
Gremlin 2
- miRNA:
-
MicroRNA
- lncRNA:
-
Long non-coding RNA
- ITGA3:
-
Integrin subunit alpha 3
- ITGB1:
-
Integrin subunit beta 1
- TMEM256:
-
Transmembrane protein 256
- LAMTOR1:
-
MAPK and MTOR activator 1
- Park7:
-
Parkinsonism associated deglycase
- SAP30L-AS1:
-
SAP30L antisense RNA 1
- SCHLAP1:
-
SWI/SNF complex antagonist associated with prostate cancer 1
- EpCAM:
-
Epithelial cell adhesion molecule
- PSMA:
-
Proteasome 20S subunit alpha
- PCGEM1:
-
PCGEM1 prostate-specific transcript
- FABP5:
-
Fatty acid binding protein 5
- sncRNA:
-
Small noncoding RNA
- CIRT:
-
Carbon ion radiation therapy
- CRPC:
-
Castration-resistant prostate cancer
- TM256:
-
Transmembrane protein 256
- TGM4:
-
Transglutaminase 4
- DDS:
-
Drug delivery system
- MHC:
-
Major histocompatibility complex
References
Carlsson SV, Vickers AJ. Screening for prostate cancer. Med Clin N Am. 2020;104:1051–62.
Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018;25:524–31.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62.
Dejous C, Krishnan U. Sensors for diagnosis of prostate cancer: looking beyond the prostate specific antigen. Biosens Bioelectron. 2020;173:112790.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35.
Hugosson J, Roobol MJ, Månsson M, Tammela TLJ, Zappa M, Nelen V, et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer. Eur Urol. 2019;76:43–51.
Moyer VA. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2012;157:120–34.
Bell N, Connor Gorber S, Shane A, Joffres M, Singh H, Dickinson J, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ. 2014;186:1225–34.
Livingston CJ, Freeman RJ, Mohammad A, Costales VC, Titus TM, Harvey BJ, et al. Choosing wisely® in preventive medicine: the American college of preventive medicine's top 5 list of recommendations. Am J Prev Med. 2016;51:141–9.
Barisiene M, Bakavicius A, Stanciute D, Jurkeviciene J, Zelvys A, Ulys A, et al. Prostate health index and prostate health index density as diagnostic tools for improved prostate cancer detection. Biomed Res Int. 2020;2020:9872146.
Kretschmer A, Tilki D. Biomarkers in prostate cancer - current clinical utility and future perspectives. Crit Rev Oncol Hematol. 2017;120:180–93.
Duffy MJ. Biomarkers for prostate cancer: prostate-specific antigen and beyond. Clin Chem Lab Med. 2020;58:326–39.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79.
Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70.
Arienti G, Carlini E, Verdacchi R, Cosmi EV, Palmerini CA. Prostasome to sperm transfer of CD13/aminopeptidase N (EC 3.4.11.2). Biochim Biophys Acta. 1997;1336:533–8.
Stegmayr B, Brody I, Ronquist G. A biochemical and ultrastructural study on the endogenous protein kinase activity of secretory granule membranes of prostatic origin in human seminal plasma. J Ultrastruct Res. 1982;78:206–14.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72.
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600.
De Palma G, Di Lorenzo VF, Krol S, Paradiso AV. Urinary exosomal shuttle RNA: promising cancer diagnosis biomarkers of lower urinary tract. Int J Biol Markers. 2019;34:101–7.
Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9:358–67.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208.
van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30 sup pp 1-13.
Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J, et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol. 2018;217:1129–42.
Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164.
Welsch S, Keppler OT, Habermann A, Allespach I, Krijnse-Locker J, Kräusslich HG. HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007;3:e36.
Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109:4146–51.
Casado S, Lobo M, Paíno CL. Dynamics of plasma membrane surface related to the release of extracellular vesicles by mesenchymal stem cells in culture. Sci Rep. 2017;7:6767.
Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34:2398–407.
Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91.
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–85.
Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211:27–37.
Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7.
Villarroya-Beltri C, Baixauli F, Mittelbrunn M, Fernández-Delgado I, Torralba D, Moreno-Gonzalo O, et al. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat Commun. 2016;7:13588.
Hessvik NP, Øverbye A, Brech A, Torgersen ML, Jakobsen IS, Sandvig K, et al. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell Mol Life Sci. 2016;73:4717–37.
Fader CM, Sánchez D, Furlán M, Colombo MI. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic. 2008;9:230–50.
Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96.
Guardia CM, Farías GG, Jia R, Pu J, Bonifacino JS. BORC functions upstream of Kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 2016;17:1950–61.
Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–43.
Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–32.
Ruiz-Martinez M, Navarro A, Marrades RM, Viñolas N, Santasusagna S, Muñoz C, et al. YKT6 expression, exosome release, and survival in non-small cell lung cancer. Oncotarget. 2016;7:51515–24.
Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–34.
Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214:197–213.
Zhu L, Sun HT, Wang S, Huang SL, Zheng Y, Wang CQ, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13:152.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Iwai K, Minamisawa T, Suga K, Yajima Y, Shiba K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J Extracell Vesicles. 2016;5:30829.
Chen BY, Sung CW, Chen C, Cheng CM, Lin DP, Huang CT, et al. Advances in exosomes technology. Clin Chim Acta. 2019;493:14–9.
Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–67.
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347.
Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Ren Physiol. 2007;292:F1657–61.
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:1.
Amrollahi P, Rodrigues M, Lyon CJ, Goel A, Han H, Hu TY. Ultra-sensitive automated profiling of EpCAM expression on tumor-derived extracellular vesicles. Front Genet. 2019;10:1273.
Barnes B, Caws T, Thomas S, Shephard AP, Corteling R, Hole P, et al. Investigating heparin affinity chromatography for extracellular vesicle purification and fractionation. J Chromatogr A. 2022;1670:462987.
Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L, et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141:4640–6.
Martins TS, Catita J, Rosa IM, AB da Cruz e Silva O, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13:e0198820.
Salafi T, Zeming KK, Zhang Y. Advancements in microfluidics for nanoparticle separation. Lab Chip. 2016;17:11–33.
Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q, et al. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small. 2020;16:e1903916.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917–34.
Mørk M, Pedersen S, Botha J, Lund SM, Kristensen SR. Preanalytical, analytical, and biological variation of blood plasma submicron particle levels measured with nanoparticle tracking analysis and tunable resistive pulse sensing. Scand J Clin Lab Invest. 2016;76:349–60.
Maas SL, de Vrij J, van der Vlist EJ, Geragousian B, van Bloois L, Mastrobattista E, et al. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J Control Release. 2015;200:87–96.
Stetefeld J, McKenna SA, Patel TR. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys Rev. 2016;8:409–27.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727.
Wu Y, Deng W, Klinke DJ 2nd. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 2015;140:6631–42.
Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, et al. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles. 2015;4:25530.
Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5:e158.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–60.
Pan J, Ding M, Xu K, Yang C, Mao LJ. Exosomes in diagnosis and therapy of prostate cancer. Oncotarget. 2017;8:97693–700.
Asencio-Barría C, Defamie N, Sáez JC, Mesnil M, Godoy AS. Direct intercellular communications and cancer: a snapshot of the biological roles of connexins in prostate cancer. Cancers (Basel). 2019;11:1370.
Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11:28–39.
Su SA, Xie Y, Fu Z, Wang Y, Wang JA, Xiang M. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget. 2017;8:25700–12.
Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191.
Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist Updat. 2019;45:1–12.
Gaballa R, Ali HEA, Mahmoud MO, Rhim JS, Ali HI, Salem HF, et al. Exosomes-mediated transfer of Itga2 promotes migration and invasion of prostate cancer cells by inducing epithelial-mesenchymal transition. Cancers (Basel). 2020;12:2300.
Li Y, Li Q, Gu J, Qian D, Qin X, Li D. Exosomal prostate-specific G-protein-coupled receptor induces osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Transl Cancer Res. 2020;9:5857–67.
Lin CJ, Yun EJ, Lo UG, Tai YL, Deng S, Hernandez E, et al. The paracrine induction of prostate cancer progression by caveolin-1. Cell Death Dis. 2019;10:834.
Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7:3993–4008.
Wang X, Wang X, Zhu Z, Li W, Yu G, Jia Z, et al. Prostate carcinoma cell-derived exosomal MicroRNA-26a modulates the metastasis and tumor growth of prostate carcinoma. Biomed Pharmacother. 2019;117:109109.
Zhou C, Chen Y, He X, Zheng Z, Xue D. Functional implication of exosomal miR-217 and miR-23b-3p in the progression of prostate cancer. Onco Targets Ther. 2020;13:11595–606.
Zhang Y, Zhao J, Ding M, Su Y, Cui D, Jiang C, et al. Loss of exosomal miR-146a-5p from cancer-associated fibroblasts after androgen deprivation therapy contributes to prostate cancer metastasis. J Exp Clin Cancer Res. 2020;39:282.
Guan H, Peng R, Fang F, Mao L, Chen Z, Yang S, et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J Cell Physiol. 2020;235:9729–42.
Zhang G, Liu Y, Yang J, Wang H, Xing Z. Inhibition of circ_0081234 reduces prostate cancer tumor growth and metastasis via miR-1/MAP3K1 axis. J Gene Med. 2021:e3376. https://doi.org/10.1002/jgm.3376.
Prigol AN, Rode MP, Silva AH, Cisilotto J, Creczynski-Pasa TB. Pro-angiogenic effect of PC-3 exosomes in endothelial cells in vitro. Cell Signal. 2021;87:110126.
DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, et al. C-Src, insulin-like growth factor I receptor, G-protein-coupled receptor kinases and focal adhesion kinase are enriched into prostate cancer cell exosomes. J Cell Biochem. 2017;118:66–73.
Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30.
Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32:983–97.
Li SL, An N, Liu B, Wang SY, Wang JJ, Ye Y. Exosomes from LNCaP cells promote osteoblast activity through miR-375 transfer. Oncol Lett. 2019;17:4463–73.
Josson S, Gururajan M, Sung SY, Hu P, Shao C, Zhau HE, et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34:2690–9.
Gururajan M, Josson S, Chu GC, Lu CL, Lu YT, Haga CL, et al. miR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin Cancer Res. 2014;20:6559–69.
Morhayim J, van de Peppel J, Demmers JA, Kocer G, Nigg AL, van Driel M, et al. Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J. 2015;29:274–85.
Li Q, Hu J, Shi Y, Xiao M, Bi T, Wang C, et al. Exosomal lncAY927529 enhances prostate cancer cell proliferation and invasion through regulating bone microenvironment. Cell Cycle. 2021;20:2531–46.
Lundholm M, Schröder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9:e108925.
Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, Tabi Z. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles. 2017;6:1368823.
Kim J, Morley S, Le M, Bedoret D, Umetsu DT, Di Vizio D, et al. Enhanced shedding of extracellular vesicles from amoeboid prostate cancer cells: potential effects on the tumor microenvironment. Cancer Biol Ther. 2014;15:409–18.
Li N, Wang Y, Xu H, Wang H, Gao Y, Zhang Y. Exosomes derived from RM-1 cells promote the recruitment of MDSCs into tumor microenvironment by upregulating CXCR4 via TLR2/NF-κB pathway. J Oncol. 2021;2021:5584406.
Cao Z, Xu L, Zhao S. Exosome-derived miR-27a produced by PSC-27 cells contributes to prostate cancer chemoresistance through p53. Biochem Biophys Res Commun. 2019;515:345–51.
Shan G, Gu J, Zhou D, Li L, Cheng W, Wang Y, et al. Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-β signaling pathway. Exp Mol Med. 2020;52:1809–22.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–26.
Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (review). Int J Mol Med. 2013;32:763–7.
Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75.
Deep G, Jain A, Kumar A, Agarwal C, Kim S, Leevy WM, et al. Exosomes secreted by prostate cancer cells under hypoxia promote matrix metalloproteinases activity at pre-metastatic niches. Mol Carcinog. 2020;59:323–32.
Akoto T, Saini S. Role of exosomes in prostate cancer metastasis. Int J Mol Sci. 2021;22:3528.
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7.
Shiao SL, Chu GC, Chung LW. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016;380:340–8.
Jin Y, Zhang P, Wang Y, Jin B, Zhou J, Zhang J, et al. Neddylation blockade diminishes hepatic metastasis by dampening cancer stem-like cells and angiogenesis in Uveal melanoma. Clin Cancer Res. 2018;24:3741–54.
Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–23.
Naito Y, Yoshioka Y, Yamamoto Y, Ochiya T. How cancer cells dictate their microenvironment: present roles of extracellular vesicles. Cell Mol Life Sci. 2017;74:697–713.
Bousquet M, Harris MH, Zhou B, Lodish HF. MicroRNA miR-125b causes leukemia. Proc Natl Acad Sci U S A. 2010;107:21558–63.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Jin B, Zhang P, Zou H, Ye H, Wang Y, Zhang J, et al. Verification of EZH2 as a druggable target in metastatic uveal melanoma. Mol Cancer. 2020;19:52.
de Vrij J, Maas SL, Kwappenberg KM, Schnoor R, Kleijn A, Dekker L, et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer. 2015;137:1630–42.
Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–66.
Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–58.
Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–66.
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9.
Liu J, He D, Cheng L, Huang C, Zhang Y, Rao X, et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene. 2020;39:3939–51.
Fan Y, Liu Y, Qu X. ASO author reflections: the prognostic role of exosomal PD-L1 in patients with gastric cancer. Ann Surg Oncol. 2019;26:851–2.
Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18:39.
Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules. 2014;19:1568–75.
Egea-Jimenez AL, Zimmermann P. Lipids in exosome biology. Handb Exp Pharmacol. 2020;259:309–36.
Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014;86:433–44.
Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–87.
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–73.
Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5:e8577.
Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107:563–71.
Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–78.
Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72:1095–102.
Işın M, Uysaler E, Özgür E, Köseoğlu H, Şanlı Ö, Yücel ÖB, et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168.
Shin S, Park YH, Jung SH, Jang SH, Kim MY, Lee JY, et al. Urinary exosome microRNA signatures as a noninvasive prognostic biomarker for prostate cancer. NPJ Genom Med. 2021;6:45.
Matsuzaki K, Fujita K, Tomiyama E, Hatano K, Hayashi Y, Wang C, et al. MiR-30b-3p and miR-126-3p of urinary extracellular vesicles could be new biomarkers for prostate cancer. Transl Androl Urol. 2021;10:1918–27.
Samsonov R, Shtam T, Burdakov V, Glotov A, Tsyrlina E, Berstein L, et al. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: application for prostate cancer diagnostic. Prostate. 2016;76:68–79.
Lee J, Kwon MH, Kim JA, Rhee WJ. Detection of exosome miRNAs using molecular beacons for diagnosing prostate cancer. Artif Cells Nanomed Biotechnol. 2018;46:S52–s63.
Rodríguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, et al. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer. 2017;16:156.
Wani S, Kaul D, Mavuduru RS, Kakkar N, Bhatia A. Urinary-exosomal miR-2909: a novel pathognomonic trait of prostate cancer severity. J Biotechnol. 2017;259:135–9.
Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJ, Jimenez CR. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles. 2013;2. https://doi.org/10.3402/jev.v2i0.22097.
Wang L, Skotland T, Berge V, Sandvig K, Llorente A. Exosomal proteins as prostate cancer biomarkers in urine: from mass spectrometry discovery to immunoassay-based validation. Eur J Pharm Sci. 2017;98:80–5.
Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–32.
Wang YH, Ji J, Wang BC, Chen H, Yang ZH, Wang K, et al. Tumor-derived exosomal long noncoding RNAs as promising diagnostic biomarkers for prostate cancer. Cell Physiol Biochem. 2018;46:532–45.
Li W, Dong Y, Wang KJ, Deng Z, Zhang W, Shen HF. Plasma exosomal miR-125a-5p and miR-141-5p as non-invasive biomarkers for prostate cancer. Neoplasma. 2020;67:1314–8.
Li M, Rai AJ, DeCastro GJ, Zeringer E, Barta T, Magdaleno S, et al. An optimized procedure for exosome isolation and analysis using serum samples: application to cancer biomarker discovery. Methods. 2015;87:26–30.
Li S, Zhao Y, Chen W, Yin L, Zhu J, Zhang H, et al. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J Cancer. 2018;9:2659–65.
Krafft C, Wilhelm K, Eremin A, Nestel S, von Bubnoff N, Schultze-Seemann W, et al. A specific spectral signature of serum and plasma-derived extracellular vesicles for cancer screening. Nanomedicine. 2017;13:835–41.
Kawakami K, Fujita Y, Matsuda Y, Arai T, Horie K, Kameyama K, et al. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer. 2017;17:316.
Wang Y, Li Q, Shi H, Tang K, Qiao L, Yu G, et al. Microfluidic Raman biochip detection of exosomes: a promising tool for prostate cancer diagnosis. Lab Chip. 2020;20:4632–7.
Worst TS, von Hardenberg J, Gross JC, Erben P, Schnölzer M, Hausser I, et al. Database-augmented mass spectrometry analysis of exosomes identifies claudin 3 as a putative prostate cancer biomarker. Mol Cell Proteomics. 2017;16:998–1008.
Kohaar I, Chen Y, Banerjee S, Borbiev T, Kuo HC, Ali A, et al. A urine exosome gene expression panel distinguishes between indolent and aggressive prostate cancers at biopsy. J Urol. 2021;205:420–5.
Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012;106:768–74.
Bhagirath D, Yang TL, Bucay N, Sekhon K, Majid S, Shahryari V, et al. microRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 2018;78:1833–44.
Guo T, Wang Y, Jia J, Mao X, Stankiewicz E, Scandura G, et al. The identification of plasma exosomal miR-423-3p as a potential predictive biomarker for prostate cancer castration-resistance development by plasma exosomal miRNA sequencing. Front Cell Dev Biol. 2020;8:602493.
Fujita K, Kume H, Matsuzaki K, Kawashima A, Ujike T, Nagahara A, et al. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci Rep. 2017;7:42961.
Wang WW, Sorokin I, Aleksic I, Fisher H, Kaufman RP Jr, Winer A, et al. Expression of small noncoding RNAs in urinary exosomes classifies prostate cancer into indolent and aggressive disease. J Urol. 2020;204:466–75.
Khan S, Jutzy JM, Valenzuela MM, Turay D, Aspe JR, Ashok A, et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One. 2012;7:e46737.
Krishn SR, Singh A, Bowler N, Duffy AN, Friedman A, Fedele C, et al. Prostate cancer sheds the αvβ3 integrin in vivo through exosomes. Matrix Biol. 2019;77:41–57.
Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33–41.
Joncas FH, Lucien F, Rouleau M, Morin F, Leong HS, Pouliot F, et al. Plasma extracellular vesicles as phenotypic biomarkers in prostate cancer patients. Prostate. 2019;79:1767–76.
Yu Q, Li P, Weng M, Wu S, Zhang Y, Chen X, et al. Nano-vesicles are a potential tool to monitor therapeutic efficacy of carbon ion radiotherapy in prostate cancer. J Biomed Nanotechnol. 2018;14:168–78.
Kato T, Mizutani K, Kawakami K, Fujita Y, Ehara H, Ito M. CD44v8-10 mRNA contained in serum exosomes as a diagnostic marker for docetaxel resistance in prostate cancer patients. Heliyon. 2020;6:e04138.
Øverbye A, Skotland T, Koehler CJ, Thiede B, Seierstad T, Berge V, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6:30357–76.
Sequeiros T, Rigau M, Chiva C, Montes M, Garcia-Grau I, Garcia M, et al. Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer. Oncotarget. 2017;8:4960–76.
Barceló M, Castells M, Bassas L, Vigués F, Larriba S. Semen miRNAs contained in Exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep. 2019;9:13772.
McKiernan J, Donovan MJ, O'Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2:882–9.
Kretschmer A, Kajau H, Margolis E, Tutrone R, Grimm T, Trottmann M, et al. Validation of a CE-IVD, urine exosomal RNA expression assay for risk assessment of prostate cancer prior to biopsy. Sci Rep. 2022;12:4777.
Peng Q, Zhang S, Yang Q, Zhang T, Wei XQ, Jiang L, et al. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials. 2013;34:8521–30.
Tran PH, Xiang D, Nguyen TN, Tran TT, Chen Q, Yin W, et al. Aptamer-guided extracellular vesicle theranostics in oncology. Theranostics. 2020;10:3849–66.
Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926–44.
Li MY, Liu LZ, Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer. 2021;20:22.
Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, Karamanos Y. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells. 2020;9:851.
Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6:1324730.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405.
Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–37.
Sadana A, Vo-Dinh T. Antibody-antigen binding kinetics. A model for multivalency antibodies for large antigen systems. Appl Biochem Biotechnol. 1997;67:1–22.
Pan S, Zhang Y, Huang M, Deng Z, Zhang A, Pei L, et al. Urinary exosomes-based engineered Nanovectors for Homologously targeted chemo-Chemodynamic prostate cancer therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials. 2021;275:120946.
Altanerova U, Babincova M, Babinec P, Benejova K, Jakubechova J, Altanerova V, et al. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int J Nanomedicine. 2017;12:7923–36.
Vázquez-Ríos AJ, Molina-Crespo Á, Bouzo BL, López-López R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnol. 2019;17:85.
Severic M, Ma G, Pereira SGT, Ruiz A, Cheung CCL, Al-Jamal WT. Genetically-engineered anti-PSMA exosome mimetics targeting advanced prostate cancer in vitro and in vivo. J Control Release. 2021;330:101–10.
André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–36.
Shi X, Sun J, Li H, Lin H, Xie W, Li J, et al. Antitumor efficacy of interferon-γ-modified exosomal vaccine in prostate cancer. Prostate. 2020;80:811–23.
Acknowledgements
Not applicable.
Funding
This work was supported by Special Funds for Taishan Scholar Project (tsqn202103180) and Key Research and Development Project of Shandong Province (2022CXGC010507).
Author information
Authors and Affiliations
Contributions
Xiaolin Cui and Qiang Fu collected the relevant literature and drafted the manuscript. Xiaohui Bai, Xueying Wang, Pengcheng Xia and Xianglun Cui participated in the conception and design of the manuscript. Zhiming Lu initiated the study and made the final version for approval. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, X., Fu, Q., Wang, X. et al. Molecular mechanisms and clinical applications of exosomes in prostate cancer. Biomark Res 10, 56 (2022). https://doi.org/10.1186/s40364-022-00398-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-022-00398-w