Abstract
Background
Alterations in the structure and physiology of interneurons in the prefrontal cortex (PFC) are important factors in the etiopathology of different psychiatric disorders. Among the interneuronal subpopulations, parvalbumin (PV) expressing cells appear to be specially affected. Interestingly, during development and adulthood the connectivity of these interneurons is regulated by the presence of perineuronal nets (PNNs), specialized regions of the extracellular matrix, which are frequently surrounding PV expressing neurons. Previous reports have found anomalies in the density of PNNs in the PFC of schizophrenic patients. However, although some studies have described alterations in PNNs in some extracortical regions of bipolar disorder patients, there are no studies focusing on the prefrontocortical PNNs of bipolar or major depression patients. For this reason, we have analyzed the density of PNNs in post-mortem sections of the dorsolateral PFC (DLPFC) from the Stanley Neuropathology Consortium, which includes controls, schizophrenia, bipolar and major depression patients.
Results
We have not observed differences in the distribution of PV+ cells or PNNs, or in the percentage of PV+ interneurons surrounded by PNNs. The density of PV+ interneurons was similar in all the experimental groups, but there was a significantly lower density of PNNs in the DLPFC of bipolar disorder patients and a tendency towards a decrease in schizophrenic patients. No differences were found when evaluating the density of PV+ cells surrounded by PNNs. Interestingly, when assessing the influence of demographic data, we found an inverse correlation between the density of PNNs and the presence of psychosis.
Conclusions
The present results point to prefrontocortical PNNs and their role in the regulation of neuronal plasticity as putative players in the etiopathology of bipolar disorder and schizophrenia. Our findings also suggest a link between these specialized regions of the extracellular matrix and the presence of psychosis.
Similar content being viewed by others
Background
Psychiatric disorders affect 1 out of 4 people at some point during their lives and represent a huge economic and social burden (World Health Organization 2001). However, the neuronal mechanisms underlying the development of these diseases still remain unclear, highlighting the need for further research in basic neuroscience. Bipolar disorder, major depression or schizophrenia are very different pathologies, but they share certain structural and functional abnormalities, including alterations in the inhibitory networks of the prefrontal cortex (PFC).
These alterations are especially pronounced in the PFC of schizophrenia and bipolar disorder patients, where reductions of GAD67, one of the enzymes responsible for GABA synthesis, have been described (Akbarian et al. 1995; Guidotti et al. 2000). There is also evidence of the involvement of the prefrontocortical GABAergic system in major depression: patients show reduced GABA levels (Hasler et al. 2007) and decreased expression of GABAergic genes (Zhao et al. 2018). Moreover, schizophrenic patients display altered expression of GABA-related genes in their PFC, such as transporters or receptors of this neurotransmitter (Benes et al. 1996; Hashimoto et al. 2008; Hoftman et al. 2015; Volk et al. 2001).
Parvalbumin (PV) expressing interneurons constitute one of the three families of cortical interneurons following a classification by their calcium-binding protein expression. PV expressing interneurons effectively synchronize large populations of neurons, regulating the synaptic excitatory tone in different cortical regions (Hu et al. 2014). Furthermore, alterations in this subpopulation of interneurons have been described in different psychiatric diseases, such as major depression, schizophrenia, autism or bipolar disorder (Marín 2012). In the PFC of bipolar disorder patients there is a reduction in the expression of PV mRNA (Sibille et al. 2011). Similarly, in schizophrenic patients there is a reduction in the expression of GAD67 mRNA, specifically in PV expressing cells (Hashimoto et al. 2003) and these alterations have been related to abnormalities in the synaptic input that PV expressing interneurons establish onto pyramidal cells (Lewis et al. 2012). Although some studies on major depression patients have not found changes in PV expression or in PV+ cell density in the PFC (Beasley et al. 2002; Rajkowska et al. 2007; Sibille et al. 2011), at least one found a decrease in PV gene expression (Tripp et al. 2012). Similarly, anomalies in PV expressing interneurons have been also reported in animal models of major depression (Perova et al. 2015; Pesarico et al. 2019; Sauer et al. 2015; Todorovic et al. 2019).
Alterations of prefrontocortical PV expressing interneurons are likely to be mediated by the expression of molecules related to interneuronal plasticity. Known regulators of the structure and connectivity of PV expressing interneurons include the polysialylated form of the neural cell adhesion molecule (PSA-NCAM) (Nacher et al. 2013). In fact, we have described a reduced expression of PSA-NCAM in layers IV and V of the PFC of schizophrenic patients (Gilabert-Juan et al. 2012).
Other important regulators of interneuronal plasticity and particularly of PV expressing interneurons are the perineuronal nets (PNNs), specialized structures of the extracellular matrix, which predominantly surround the soma and proximal neurites of PV expressing interneurons (Sorg et al. 2016). PNNs are composed by hyaluronic acid, tenascin-R, link proteins and proteoglycans (mainly chondroitin sulphate proteoglycans) (Kwok et al. 2011). Although the functions of PNNs are not clear yet, it is known that they mediate the closure of the critical periods of cortical plasticity through the stabilization of recently formed synapses and the prevention of synaptogenesis (McRae and Porter 2012; Pizzorusso et al. 2002; Wang and Fawcett 2012). PNNs are also important to maintain the local homeostasis of ions (Morawski et al. 2015) and to protect neurons against oxidative stress (Cabungcal et al. 2013). Particularly, PNNs control the activity and excitability of PV expressing neurons (Balmer 2016; Dityatev et al. 2007; Favuzzi et al. 2017). In fact, the enzymatic attenuation of PNNs alters the excitatory input to fast-spiking PV expressing interneurons (Hayani et al. 2018). Moreover, alterations in the glycoprotein tenascin-R, a key component of the PNNs, which helps in the stabilization of these regions of the extracellular matrix, impairs the perisomatic inhibitory input to pyramidal neurons (Saghatelyan et al. 2000, 2001, 2003).
Interestingly, abnormalities of chondroitin sulfate proteoglycans have been implicated in the etiopathology of different psychiatric disorders (Berretta 2012). In this line, some studies have shown that subjects with schizophrenia had a reduced density of PNNs in the PFC (Enwright et al. 2016; Mauney et al. 2013) and similar reductions have been found in different animal models of this disorder (Castillo-Gómez et al. 2017; Matuszko et al. 2017; Paylor et al. 2016). Although there are some reports describing alterations in PNNs in certain extracortical regions of bipolar disorder patients (Pantazopoulos et al. 2010; Steullet et al. 2018), to our knowledge there are no studies to date focusing on the PFC of bipolar disorder or major depression patients.
In this study, we investigated whether the densities of PV expressing interneurons and PNNs were affected in the DLPFC of individuals suffering from major depression, schizophrenia or bipolar disorder. We have selected this prefrontocortical region because structural and functional alterations have already been found in these diseases (Phillips et al. 2003). Moreover, previous studies in the DLPFC, including our own (Gilabert-Juan et al. 2012), have found changes in molecules related to inhibitory neurotransmission and in PV+ cells and PNNs, specifically in Brodmann area 9 (BA9). We have performed histochemical analysis in brain sections from post-mortem samples of psychiatric patients and a control group and have measured the density of PV and PNN positive cells and their co-localization.
Methods
Samples and histological processing
Frozen 14 µm thick coronal sections containing the dorsolateral prefrontal cortex (DLPFC) of patients diagnosed with major depression, bipolar disorder or schizophrenia, and control subjects were obtained from the Stanley Medical Research Institute (Bethesda, MD, USA). All patient records were reviewed by one psychiatrist and summarized in narrative form, and the information was entered into a computerized database by identifying number only. When all the information was collected, a DSM-IV psychiatric diagnosis was made independently by two senior psychiatrists. If there was disagreement between them, the records were given to a third senior psychiatrist and a consensus diagnosis was established (Torrey et al. 2000). The cohort consists of 15 individuals in each group, although some samples were not analyzed due to the poor condition of the tissue. The demographic and recruitment data of patients have been described earlier (Gilabert-Juan et al. 2012) and are summarized in Table 1. All brains underwent clinical neuropathological examination by two neuropathologists, none demonstrated evidence of neurodegenerative changes or other pathological lesions.
Sections were thawed and immediately fixed by immersion in a solution of paraformaldehyde 4% during 20 min. After fixation, sections were washed in phosphate buffer (PB: 0.1 M, pH 7.4) and processed immediately for immunohistochemistry. All the sections studied passed through the procedures simultaneously, to minimize any difference from the immunohistochemical protocols themselves. Experimenters were blind to the experimental condition until the end of the quantification.
Histochemistry
Tissue was processed for fluorescence histochemistry as follows. After PB washing, slices were incubated in 10% normal donkey serum (NDS), 0.2% Triton-X100 (Sigma) in phosphate buffered saline (PBS) for 1 h. Then, slices were washed again in PBS and incubated for 48 h at 4 °C with polyclonal guinea pig anti-PV antibody (1:2000, Synaptic Systems, Gottingen, Germany) and biotin-conjugated Wisteria floribunda agglutinin (WFA, 1:200, Sigma) diluted in PBS 0.2% Triton-X100. After washing with PBS, sections were incubated at room temperature for 2 h with the secondary antibody: A555-conjugated goat anti-guinea pig (1:400, LifeTechnologies, Carlsbad, CA, USA) and A647-conjugated streptavidin (1:400, LifeTechnologies, Carlsbad, CA, USA), both diluted in PBS 0.2% Triton-X100. Finally, sections were washed in PB, mounted on slides and coverslipped using fluorescence mounting medium (Dako, Glostrup, Denmark).
Confocal microscopy and image analyses
In order to analyze the densities of both PV positive interneurons and PNNs, single confocal planes were obtained using a laser scanning confocal microscope (Leica TCS SPE) and images were processed with FIJI (ImageJ, NIH). We analyzed three images of 275.17 μm2 from each patient, taken randomly in the deep layers of the DLPFC (layers III, IV, V and VI). In order to obtain these images, we delimited the total area containing the deep layers of the DLPFC and selected three squares of 275.17 μm2, which generated with random coordinates within this area. During this procedure we excluded the squares with areas that were partially outside the delimited region. This procedure was used for every patient and experimental group. PV expressing interneurons and PNNs were counted in all three images and their density was calculated as neurons/area.
Statistical analyses
Group differences were assessed using a one-way ANOVA and multiple comparisons p-value adjustment by Bonferroni post hoc analysis. We first performed a Bartlett’s test to assess the homoscedasticity of the data and Shapiro test for normality. No p-value was found to be statistically significant, thus, we were able to perform parametric tests such as ANOVA. Analyses were performed with R version 3.6.1 (R Core Team 2019). and graphs created using GraphPad Prism 6. All values are expressed as mean ± standard error of the mean (SEM). The cutoff for statistical significance was set as p = 0.05. Effect of post-mortem interval (PMI), brain pH, brain weight, age, gender, suicide, substance/alcohol abuse, presence of psychosis, side of the brain (right or left hemisphere), age of disease onset or lifetime neuroleptic use (in fluphenazine mg equivalents) was assessed by univariable and multivariable analyses. First, we performed a model for each variable in the data base: categorical variables were analyzed by one-way ANOVA (age, sex, suicide, alcohol abuse, presence of psychosis, side of the brain), continuous variables (age, brain pH, brain weight, PMI, age of disease onset or lifetime neuroleptic use) were analyzed by simple linear regression analysis. Second, we performed a multivariable analysis, searching for models that could integrate all the variables. For developing the models, we used the glmulti library (Calcagno 2018). The method for the selection of the best model has been based on the Akaike Information Criterion (Sakamoto et al. 1986). Since the model includes categorical and continuous variables and a continuous variable as dependent variable we used an analysis of covariance (ANCOVA).
Results
Distribution of PNNs and PV expressing cells in the DLPFC
Although PNNs could be observed in all the extension of the DLPFC, we found a differential distribution among layers. In layer I we found virtually no PNNs. A low PNN density was found in layers II and IV. Layers V and VI had an intermediate density of PNNs and the highest PNN density was found in layer III.
The distribution of PV positive cells was, as expected, similar to that of PNNs (Fig. 1a). The highest density of PV positive cells was found in layers III and IV, followed by those in layers II and V. We found the lower density of cells in layers I and VI.
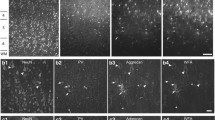
Alterations of PV and PNN densities in neuropsychiatric disorders. a Panoramic confocal microphotograph showing WFA-labeled PNNs (blue) surrounding PV positive somata (red) in the deep layers of the dorsolateral prefrontal cortex (DLPFC) of a control individual. b Images of single confocal planes showing PNNs surrounding PV+ somata in the DLPFC in patients suffering from major depression (MD), bipolar disorder (BD) and schizophrenia (SCHZ) and controls (NOR). White arrowheads point to PV+ somata surrounded by PNNs, yellow arrowheads point to PV+ cells lacking PNNs and white arrows point to PNNs surrounding PV− somata. c–g Histograms showing the density of PV expressing interneurons (c), PNNs (d) and the percentages of PV expressing somata surrounded by PNNs (e), of PNNs surrounding PV+ somata (f) and of PNNs not surrounding PV+ somata (g). There are significant differences in the density of PNN between control individuals and bipolar patients (*p = 0.012). Scale bar: 20 µm
We performed all our analyses in deep layers of the DLPFC, including layers III, IV, V and VI. The percentages of PNNs surrounding PV+ somata were around 75% in every group and no significant differences were found between them (controls: 69.3 ± 6.7%; schizophrenia: 78.6 ± 8.5%; bipolar: 76.64 ± 5.4%; major depression: 74.2 ± 5,4%; F(3, 35) = 0.3965, p = 0.7563). Interestingly, some of the PV negative somata surrounded by PNN had the typical morphology of pyramidal neurons.
Bipolar individuals show a lower PNN density in the DLPFC
We compared the density of both PNNs and PV immunoreactive neurons in the DLPFC of patient and control groups (Fig. 1b). One way-ANOVA analysis showed that there were no differences in the density of PV expressing interneurons between the four groups (F(3, 34) = 0.4334, p = 0.8099; Fig. 1c).
When analyzing the density of PNNs, we found significant differences in PNN density between the four groups (F(3, 38) = 3.581, p = 0.0225). Bonferroni’s post hoc analysis showed a significantly lower density of PNNs in patients suffering from bipolar disorder compared to the control group (F(3, 38) = 3.581, p = 0.012). We also found a tendency towards a lower density of PNNs in schizophrenic patients compared to the control group (F(3, 38) = 3.581, p = 0.0643; Fig. 1d).
Finally, we analyzed in the four groups the percentages of PV expressing interneurons surrounded by PNNs (F(3, 36) = 1.586, p = 0.1193; Fig. 1e), of PNNs surrounding PV expressing interneurons (F(3, 35) = 0.3965, p = 0.7563; Fig. 1f) and of PNNs not associated with PV+ somata (F(3, 35) = 0.3965, p = 0.7563; Fig. 1g). We did not find any significant difference between groups in any of these parameters.
Patients with psychosis show a lower density of PNNs
We performed univariable and multivariable analyses to assess the influence of demographic data (age, sex, PMI, weight of the brain…) on our experimental conditions (see “Methods” section). We only observed an effect of the presence of psychosis: patients who had suffered psychotic episodes had lower PNN density (Fig. 2). This significant difference was found both using univariable (Pr(> F) = 0.034) and multivariable analyses (Pr(> |t|) = 0.035).
Effects of psychosis on the density of PNNs. a Confocal images, showing WFA-labeled PNNs (blue) surrounding PV positive somata (red) in the dorsolateral prefrontal cortex (DLPFC) of patients diagnosed with (a2) and without (a1) psychosis. b Histogram comparing the density of PNNs in individuals diagnosed with (black bar) or without (white bar) psychosis (Pr(> F) = 0.034; Pr(> |t|) = 0.035). Scale bar: 20 µm
Discussion
The present study compares the densities of PNNs and PV expressing neurons in the DLPFC of patients suffering from three different neuropsychiatric disorders (major depression, bipolar disorder and schizophrenia) and healthy individuals.
The distribution of PNNs and PV expressing cells in the DLPFC did not differ among the four groups of our study and it was very similar to that described before in this region (Brodmann area [BA] 9) (Beasley et al. 2002; Enwright et al. 2016; Hashimoto et al. 2003; Mauney et al. 2013; Sakai 2008). The study of Enwright et al. (2016) was focused in layer III, where, in accordance with our results, most of PV+ cells and PNNs are located, but in our present study we have also included in the analysis layers IV, V and VI. In consonance with this previous study on PV+ cells and PNNs in the DLPFC of schizophrenic patients, we have also observed that, although many PV+ cells were surrounded by PNNs, some of them (around a 25%) were devoid of these specialized extracellular matrix structures. Interestingly, no differences in this percentage were detected among the different groups analyzed. It is possible that we have failed to detect a subpopulation of PV+ cells with low levels of expression of this calcium binding protein. In fact, it has been described that, in the human DLPFC, PV+ cells surrounded by PNNs have lower immunoreactivity than those lacking these structures (Enwright et al. 2016). Some of the PNNs that were not surrounding PV+ interneurons had the typical morphology of pyramidal neurons, suggesting, as described before (Enwright et al. 2016), that a subpopulation of excitatory neurons is also enwrapped by PNNs. This is relevant for our study, since the differences in the density of PNNs that we have detected are not reflected in differences in the density of PNN specifically surrounding PV+ somata. Unfortunately, very little is known about the impact of PNN coverage on pyramidal neurons.
Regarding the density of PNNs, we have observed a trend towards a decrease in schizophrenic patients, which reaches significance if a less restrictive post hoc test is applied. This result is similar, although less dramatic, than that obtained by Mauney et al. (2013), who found a 70% reduction in this parameter. It is interesting to note that these authors improved substantially their PNN labeling when using sections from frozen samples and then postfixing them, as we have done in the present study. In a more recent study, Enwright et al. (2016) did not find changes in PNN density, but detected reduced intensities of fluorescence for PNNs markers. These results are in consonance with previous results, including our own, in mouse models of schizophrenia, which have found reductions in the number of PNNs in the medial PFC (Castillo-Gómez et al. 2017; Matuszko et al. 2017; Paylor et al. 2016).
To our knowledge only other report has studied the PNNs in the DLPFC of bipolar disorder patients and it did not find differences in their density (Mauney et al. 2013). This is apparently in contrast with our results, but this discrepancy may arise from the methodology employed to process the samples. Mauney et al. (2013) used fixed blocks of tissue to obtain their sections, while we have used sections obtained from frozen blocks and have postfixed them subsequently. This procedure improves dramatically the sensibility of the detection of PNNs, as described by the same authors (Mauney et al. 2013). Our results are in consonance with a recent study performed in the reticular thalamic nucleus, which has found decreases in the number of PNNs both in schizophrenic and bipolar patients (Steullet et al. 2018).
Our study is the first to have explored the presence of changes in the density of PNNs in the DLPFC of major depression patients, finding negative results. However, more studies need to be performed with different and larger samples of patients to discard an impact of major depression on PNNs. In fact, animals subjected to chronic stress, a model of depression, during the adolescence or during adulthood show alterations in the number of PNNs (Castillo-Gómez et al. 2017; de Araújo Costa Folha et al. 2017; Pesarico et al. 2019).
In consonance with a previous study on the BA9 of the DLPFC, which used the same collection of samples that we have analyzed in the present study (Beasley et al. 2002), we have not found differences in the density of PV immunoreactive somata in any of the patient groups. Woo et al. (1997) also failed to find differences in the density of PV+ interneurons in the BA9 of schizophrenic patients. It has to be noted, however, that other study found this density reduced in BA10 (Beasley and Reynolds 1997). Sakai (2008) also found differences in PV+ somata density in BA9 of schizophrenic patients, but only in layer IV, where their density is relatively low. A more recent study (Enwright et al. 2016) has not found significant differences in the density of PV+ cells in the layer III of BA9 of schizophrenic patients, although they discovered that the intensity of PV immunolabeling was lower in these patients than in controls. This is also in accordance with the reduced expression of PV mRNA in layers III and IV in BA9 (Hashimoto et al. 2003).
Our negative results concerning the density of PV+ cells in the DLPFC of bipolar patients are confirmatory of those obtained before in BA9 using immunohistochemistry and in situ hybridization (Beasley et al. 2002; Bitanihirwe et al. 2010; Sakai 2008). However, a study in this area using real-time quantitative PCR found a decrease in PV mRNA (Sibille et al. 2011).
We have also failed to find significant differences in the density of PV+ cells in the DLPFC of major depression patients. Similar results were found in previous reports studying the density of PV+ somata (Beasley et al. 2002; Rajkowska et al. 2007) or the expression of PV mRNA (Sibille et al. 2011). Khundakar et al. (2011) reported a decrease in the density of PV+ cells in layer III of BA9, but this study was performed in a sample of elderly major depression patients; the mean age of the group studied in the present report is younger (48 years).
To assess the influence of the demographic data on our results, we performed univariable and multivariable analyses (see “Methods” section). We report here for the first time that patients who had suffered psychotic episodes had lower PNN density. Since all of our schizophrenic patients and most of bipolar disorder patients presented psychosis during their life, we think that the lower density of PNNs may be related to the presence of psychosis per se.
Since PNNs appear to have a protective role against oxidative stress in PV expressing interneurons (Cabungcal et al. 2013), the decrease in PNN density that we have reported in psychotic individuals could be related to an increase in oxidative stress. In fact, increases in oxidative stress have been described in the brain of both SCHZ and BD patients (Andreazza et al. 2008; Yao and Keshavan 2011). Moreover, similar to what we have observed with PNNs, a previous study on the expression of reelin, an extracellular matrix protein, reported a downregulation in psychotic bipolar and schizophrenic patients when compared to control individuals and bipolar patients without psychosis (Guidotti et al. 2000). Interestingly, the presence of psychosis has been linked to abnormalities in gamma band oscillations (McNally and McCarley 2016) and on PV+ interneuron function (Sohal et al. 2009).
Conclusion
The link between psychosis, interneurons and PNNs has to be explored in more detail, particularly using larger samples of individuals, because it may offer clues on the neurobiological bases of these symptoms in particular and on the etiopathogenesis of bipolar disorder and schizophrenia in general. In fact, several Genome Wide Association studies (GWAS) have found evidence for a common genetic basis for schizophrenia and bipolar disorder (Prata et al. 2019). Our results support this hypothesis of a common physiopathological pathway.
Availability of data and materials
The raw data from this study can be found online at: http://sncid.stanleyresearch.org/Default.aspx Neuropathology Consortium. Research material can be available from the authors after request.
References
Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258–66.
Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008;111(2–3):135–44.
Balmer TS. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro. 2016. https://doi.org/10.1523/ENEURO.0112-16.2016.
Beasley C, Reynolds G. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24(3):349–55.
Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52(7):708–15.
Benes FM, Vincent SL, Marie A, Khan Y. Up-regulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75(4):1021–31.
Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012;62(3):1584–97.
Bitanihirwe BK, Lim MP, Woo TUW. N-methyl-D-aspartate receptor expression in parvalbumin-containing inhibitory neurons in the prefrontal cortex in bipolar disorder. Bipolar Disord. 2010;12(1):95–101.
Cabungcal J-H, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci USA. 2013;110(22):9130–5.
Calcagno V. glmulti: Model selection and multimodel inference made easy 2013. R Package Version 2018, 1, 498.
Castillo-Gómez E, Pérez-Rando M, Bellés M, Gilabert-Juan J, Llorens JV, Carceller H, et al. Early social isolation stress and perinatal NMDA receptor antagonist treatment induce changes in the structure and neurochemistry of inhibitory neurons of the adult amygdala and prefrontal cortex. eNeuro. 2017. https://doi.org/10.1523/ENEURO.0034-17.2017.
de Araújo Costa Folha OA, Bahia CP, de Aguiar GPS, Herculano AM, Coelho NLG, de Sousa MBC, et al. Effect of chronic stress during adolescence in prefrontal cortex structure and function. Behav Brain Res. 2017;326:44–51.
Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67(5):570–88.
Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA. Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology. 2016;41(9):2206–14.
Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sánchez-Aguilera A, Mantoan L, et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron. 2017;95(3):639–55.e10.
Gilabert-Juan J, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Nácher J. Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci Lett. 2012;530(1):97–102.
Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–9.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23(15):6315–26.
Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13(2):147–61.
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200.
Hayani H, Song I, Dityatev A. Increased excitability and reduced excitatory synaptic input into fast-spiking CA2 interneurons after enzymatic attenuation of extracellular matrix. Front Cell Neurosci. 2018;30(12):149.
Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull. 2015;41(1):180–91.
Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin + GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345(6196):1255263.
Khundakar A, Morris C, Oakley A, Thomas AJ. Morphometric analysis of neuronal and glial cell pathology in the caudate nucleus in late-life depression. Am J Geriatr Psychiatry. 2011;19(2):132–41.
Kwok JCF, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71(11):1073–89.
Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67.
Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–20.
Matuszko G, Curreli S, Kaushik R, Becker A, Dityatev A. Extracellular matrix alterations in the ketamine model of schizophrenia. Neuroscience. 2017;14(350):13–22.
Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74(6):427–35.
McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry. 2016;29(3):202–10.
McRae PA, Porter BE. The perineuronal net component of the extracellular matrix in plasticity and epilepsy. Neurochem Int. 2012;61(7):963–72.
Morawski M, Reinert T, Meyer-Klaucke W, Wagner FE, Tröger W, Reinert A, et al. Ion exchanger in the brain: quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci Rep. 2015;1(5):16471.
Nacher J, Guirado R, Castillo-Gómez E. Structural plasticity of interneurons in the adult brain: role of PSA-NCAM and implications for psychiatric disorders. Neurochem Res. 2013;38(6):1122–33.
Pantazopoulos H, Woo TUW, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67(2):155–66.
Paylor JW, Lins BR, Greba Q, Moen N, de Moraes RS, Howland JG, et al. Developmental disruption of perineuronal nets in the medial prefrontal cortex after maternal immune activation. Sci Rep. 2016;23(6):37580.
Perova Z, Delevich K, Li B. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci. 2015;35(7):3201–6.
Pesarico AP, Bueno-Fernandez C, Guirado R, Gómez-Climent MÁ, Curto Y, Carceller H, et al. Chronic stress modulates interneuronal plasticity: effects on PSA-NCAM and perineuronal nets in cortical and extracortical regions. Front Cell Neurosci. 2019;7(13):197.
Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28.
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–51.
Prata DP, Costa-Neves B, Cosme G, Vassos EJ. Unravelling the genetic basis of schizophrenia and bipolar disorder with GWAS: A systematic review. Psychiatr Res. 2019;114:178–207.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.R-project.org/.
Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32(2):471–82.
Saghatelyan AK, Gorissen S, Albert M, Hertlein B, Schachner M, Dityatev A. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur J Neurosci. 2000;12(9):3331–42.
Saghatelyan AK, Dityatev A, Schmidt S, Schuster T, Bartsch U, Schachner M. Reduced perisomatic inhibition, increased excitatory transmission, and impaired long-term potentiation in mice deficient for the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci. 2001;17(1):226–40.
Saghatelyan AK, Snapyan M, Gorissen S, Meigel I, Mosbacher J, Kaupmann K, Bettler B, Kornilov AV, Nifantiev NE, Sakanyan V, Schachner M, Dityatev A. Recognition molecule associated carbohydrate inhibits postsynaptic GABA(B) receptors: a mechanism for homeostatic regulation of GABA release in perisomatic synapses. Mol Cell Neurosci. 2003;24(2):271–82.
Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2008;31:219–45.
Sakamoto Y, Ishiguro M, Kitagawa G. Akaike information criterion statistics. The Netherlands: Springer; 1986.
Sauer J-F, Strüber M, Bartos M. Impaired fast-spiking interneuron function in a genetic mouse model of depression. Elife. 2015. https://doi.org/10.7554/eLife.04979.
Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14(6):721–34.
Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702.
Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JCF, et al. Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci. 2016;36(45):11459–68.
Steullet P, Cabungcal J-H, Bukhari SA, Ardelt MI, Pantazopoulos H, Hamati F, et al. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry. 2018;23(10):2057–65.
Todorović N, Mićić B, Schwirtlich M, Stevanović M, Filipović D. Subregion-specific protective effects of fluoxetine and clozapine on parvalbumin expression in medial prefrontal cortex of chronically isolated rats. Neuroscience. 2019;1(396):24–35.
Torrey EF, Fuller Torrey E, Webster M, Knable M, Johnston N, Yolken RH. The stanley foundation brain collection and neuropathology Consortium. Schizophr Res. 2000;44(2):151–5.
Tripp A, Oh H, Guilloux J-P, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(11):1194–202.
Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158(2):256–65.
Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349(1):147–60.
Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154(7):1013–5.
World Health Organization. The world health report 2001: mental health: new understanding. New Hope: World Health Organization; 2001.
Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15(7):2011–35.
Zhao J, Verwer RWH, Gao S-F, Qi X-R, Lucassen PJ, Kessels HW, et al. Prefrontal alterations in GABAergic and glutamatergic gene expression in relation to depression and suicide. J Psychiatr Res. 2018;102:261–74.
Acknowledgements
Not applicable.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness to JN (SAF2015-68436-R). RG has a postdoctoral fellowship “Juan de la Cierva” from the Spanish Ministry of Science, Innovation and Universities (IJCI-2016-27758).
Author information
Authors and Affiliations
Contributions
All individuals included as authors of the paper have contributed substantially to the scientific process leading up to the writing of the paper. Such contribution includes the conception and design of the project, the performance of experiments and the analysis and interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The samples used in the present study come from the Stanley Foundation Neuropathology Consortium, a selection of matched specimens from the brain collection. It contains 15 cases each from individuals with schizophrenia, bipolar disorder, major depressive disorder without psychotic features, and normal controls. The patients were selected and recruited by the Stanley Foundation (Torrey et al. 2000).
Consent for publication
The work described has not been submitted for publication in whole or in part elsewhere and all the authors listed have approved the manuscript that is enclosed.
Competing interests
All authors disclose any actual or potential competing interest including any financial, personal or other relationships with other people or organizations within 3 years of beginning the submitted work that could inappropriately influence, or be perceived to influence, this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Alcaide, J., Guirado, R., Crespo, C. et al. Alterations of perineuronal nets in the dorsolateral prefrontal cortex of neuropsychiatric patients. Int J Bipolar Disord 7, 24 (2019). https://doi.org/10.1186/s40345-019-0161-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40345-019-0161-0