Abstract
Engineered T cells have been shown to be highly effective in cancer immunotherapy, although T cell exhaustion presents a challenge for their long-term function. Additional T-cell sources must be exploited to broaden the application of engineered T cells for immune defense and reconstitution. Unlimited sources of pluripotent stem cells (PSCs) have provided a potential opportunity to generate precise-engineered therapeutic induced T (iT) cells. Single-cell transcriptome analysis of PSC-derived induced hematopoietic stem and progenitor cells (iHSPC)/iT identified the developmental pathways and possibilities of generating functional T cell from PSCs. To date, the PSC-to-iT platforms encounter several problems, including low efficiency of conventional T subset specification, limited functional potential, and restrictions on large-scale application, because of the absence of a thymus-like organized microenvironment. The updated PSC-to-iT platforms, such as the three-dimensional (3D) artificial thymic organoid (ATO) co-culture system and Runx1/Hoxa9-enforced iT lymphopoiesis, provide fresh perspectives for coordinating culture conditions and transcription factors, which may greatly improve the efficiency of T-cell generation greatly. In addition, the improved PSC-to-iT platform coordinating gene editing technologies will provide various functional engineered unconventional or conventional T cells. Furthermore, the clinical applications of PSC-derived immune cells are accelerating from bench to bedside.
Similar content being viewed by others
Introduction
Embryonic stem cells (ESCs) isolated from blastocytes can be cultured in vitro and used to generate engineered cells and animal models. Furthermore, the technology of reprogramming somatic cells to induced pluripotent stem cells (iPSCs) [1, 2] provides a possible way to explore the applications of stem cells in regenerative medicine, without ethical and immune rejection concerns [3]. A recent study showed that the application of clinical-grade iPSC-derived functional retinal pigment epithelium is feasible and safe [4]. However, the biggest challenge is the inefficient reconstitution of iPSC-derived phenotypic cells in vivo.
Edited T cells are being studied for engineering chimeric antigen receptor (CAR) T cells which are a form of major cellular therapy for hematological malignancies [5,6,7]. However, the application of cell immunotherapy is limited by the availability of autologous T cells and associated complications and resistance [8,9,10]. Meanwhile, the tumor-killing ability of patient-derived engineered T cells is suppressed by the senescent and exhausted T compartments, or by increasing the Treg subset [11, 12]. Compared with rare HSPCs, PSCs have unique advantages, such as their efficient gene editing and long-term self-renewal properties in vitro. These advantages make them the best candidates for T-cell generation. However, the progress of PSC-to-iT technology is still facing hurdles because the thymic niche cannot be accurately simulated in vitro. With these challenges, another straightforward approach is to use the in vivo microenvironment to educate PSC-derived thymus-seeding progenitors (TSPs). In this review, we describe recent progress in understanding T cell development in the thymus, single-cell transcriptomes of PSC-iHSPC/iT, PSC-based T lymphocyte generation, and the potential applications of gene editing in the PSC-to-iT platform.
T lymphopoiesis in the embryo and adult
T lymphocytes play an essential role in adaptive immunity, including pathogen elimination [13], host homeostasis [14], and anti-tumor activity [15]. During hematopoiesis, fetal liver [16] and bone marrow-derived hematopoietic stem cells (HSCs) [17] differentiate into TSP, such as lymphoid-primed multipotent progenitors (LMPP) [18]. Current evidence demonstrates that non-HSC-derived TSP supports T lymphopoiesis before the emerge of HSCs [19,20,21,22,23] (Fig. 1a). Particularly, Flt3 signals induce CCR9 expression in TSP [24], which is then recruited into the thymus through CCL25 (TECK), secreted by thymic epithelial cells (TEC), and recruits the TSP into the thymus [25].
T lymphopoiesis in thymus. a HSC or embryonic non-HSC-derived TSP migrate into the CMJ, then differentiate into DN and DP cells, which are educated by different stromal cells (for example cTEC) in the cortex. DP cells mature into naïve conventional T cells and unconventional T cells in the presence of stromal cells (such as mTEC and DCs) in the medulla. b Different stages in T lymphopoiesis. A new cell atlas of human thymic development showed a unique pattern of T lymphopoiesis [31]: DN (early) → DN (P) → DN(Q) → DP(P) → DP(Q) → αβ T (entry), and new unconventional T subsets. Different TFs regulatory networks drive the formation of different stages and subsets. DN double negative T cells, DP double positive T cells, P proliferation, Q quiescent, HSC Hematopoietic stem cells, TSP thymus-seeding progenitors, CMJ cortico-medullary junction
Once the TSP cells seed into the cortico-medullary junction (CMJ) in the thymus [26], a new identity is acquired as early thymic progenitors (ETP) [27]. Published studies have shown that ETP have lineage pattern similar to that of LMPP with T cells, B cells, NK cells, and myeloid potential [28,29,30]. And ETP lack the megakaryocytic and erythroid potential. As a specialized organ for T lymphopoiesis, the thymus provides a complex, highly ordered, and unique niche. Although the microenvironment is complex, the thymus provides cascaded and conserved signaling pathways such as Notch signaling, morphogenic pathway, and protein tyrosine kinase signaling. The thymus mainly contains hematopoietic cells (T cells, B cells, NK cells, monocytes, dendritic cells [DCs], and macrophages) and non-hematopoietic stromal elements (TEC, fibroblasts, vascular smooth muscle cells, lymphatic endothelial cells, and endothelial cells) [31]. Nude mouse research has shown that TEC is the most pivotal element [32,33,34]. TEC and other stromal cells foster natural T lymphopoiesis by providing a microenvironment and expressing chemokines, Notch ligands (JAG1, JAG2, Delta-like ligand 1, and 4), Wnt ligands, Hh proteins, BMPs, SCF, and IL7 [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. Coordinating with stem/progenitor-cell gene network (Hoxa9, Runx1, Gata2, Meis1, Lmo2, Myb, Mycn, and others) (Fig. 1b), the thymic niche-provided Notch signal initiates the T-lineage-specific development program of TSP, as a pre-commitment phase [53]. Also, interfering with the function of Notch results in a complete block of T lymphopoiesis [54, 55].
During T lymphopoiesis in the thymus, ETP differentiates into immature CD4/CD8 double-negative thymocytes, (DN1, DN2, DN3, and DN4 cells), and immature CD4/CD8 double-positive thymocytes (DP cells) (Fig. 1a). After undergoing positive and negative selection, DP cells mature into naïve mature CD4 single-positive (CD4SP) thymocytes and CD8 single-positive (CD8SP) thymocytes. In addition to conventional T cell subpopulations (TCRαβ+ CD4SP T subset and TCRαβ+ CD8SP T subset), TSP also differentiate into unconventional T cell subpopulations (γδ T subset, Treg subset, CD8αα+ T cell subset, natural killer T-like [NKT-like] subset, and fetal TH17-like subset) [31]. The rapid deployment of single-cell sequencing technology has helped us to discriminate rare T precursors and unconventional T subsets from the thymus atlas [31], analyze the dynamics of thymocyte development, and simulate T-cell generation in vitro. New unconventional subsets were identified through single-cell RNA sequencing (sc-RNAseq), such as the CD8αα(I) subset (expressing PD-1, TNFRSF9, CD72, CREB3L3, GNG4, and XCL1 at mRNA level), CD8αα(II) subset (PD-1, ZNF683, and MME), T (agonist) subset (MIR155H), Treg (diff) subset (IKZF4, GNG8, and PTGIR), and Th17-like cell subset (CD40LG, RORC, KLRB1, and ZBTB16) (Fig. 1b). The marker genes CD34, ST18 and IGLL1 were used to identify cells at the double-negative (DN) early stage.
Single-cell transcriptome analysis of PSC-iHSPC/iT
Single-cell transcriptional profiling has been used for the analysis of adult/embryonic hematopoietic development and immune states monitoring. In most cases, it is difficult to accurately confirm whether the PSC-derived cells are the desired cell types. Indeed, the desired PSC-derived cells were mixed with undifferentiated PSC, mesodermal progenitors, endothelial cells, lineage-specific hematopoietic progenitors, and other unexpected cell types. Fortunately, the single-cell transcriptional sequencing technology has made it possible to reliably delineate the directed differentiation process of PSC to hematopoietic lineages (Table 1). Using such technology, Guo lab reported that PSCs are heterogenous cell populations themselves and thereby have variable efficiency of hematopoietic differentiation [56]. The PSC cell lines from different labs also showed obvious differences identified through sc-RNAseq analysis (Fig. 2a). Without mesodermal lineage differentiation-related cytokines, it is difficult for the PSCs to generate hemogenic endothelium cells (HECs) (Fig. 2a). The combination of glycogen synthase kinase (GSK) 3 inhibitor (CHIR99021) with BMP4 and Activin A helps PSCs efficiently differentiate into mesoderm progenitors (cytokine-driving differentiation pattern A). VEGF and bFGF further enforce these progenitors to differentiate into EC and HECs/hematopoietic cells (cytokine-driving differentiation pattern B) (Fig. 2b). There is strong evidence indicating that the heterogeneities of embryonic and PSC-derived HECs result in diverse lineage potentials as demonstrated at the sc-RNAseq levels (Fig. 2c)[57,58,59,60]. Based on the decision of the hematopoietic fate, HECs can be divided into two groups: primitive hematopoietic development-related HECs (pHECs) and definitive HECs (dHECs); dHECs are major populations producing T-lineage cells. Despite the comprehensive work of embryonic hematopoietic development at the sc-RNAseq level, mimicking hematopoietic development using PSC-derived hematopoietic cells remains a challenge. With the help of embryonic hematopoietic development and adult hematopoiesis at sc-RNAseq levels, the hematopoietic differentiation of PSC is moving closer and closer to physiological hematopoietic development by adding missing critical transcription factors and culture niche [60, 61]. Unlimited functional PSC-derived HSC or T/NK cells are one of the ultimate goals of PSC-based regenerative medicine, and several problems remain to be solved, such as Q1: how to efficiently get dHECs but not pHECs; Q2: how to enforce the differentiation of dHEC into bona fide HSCs and lymphoid-primed HPCs; and Q3: how to provide a suitable niche for T-lineage to mature and harvest both functional CD4+ T and CD8+ T cells robustly. Taken together, the single-cell transcriptional profiling of PSC-derived cells clearly shows the possibility of generating functional T cells in vitro, although some problems still remain.
scRNA-seq technology reveals the heterogeneity of PSCs and its derivatives and the complexity of the hematopoietic differentiation process of PSCs. a UMAP on the transcriptome of the PSCs and PSC-derived cells from Cheng’s lab (H1 ESC and H1 ESC-derived cells at day 2/4/6 during directed hematopoietic differentiation) and Guo’s lab (iPSCs and iPSC-derived EB cells at day 9/18/20 without adding any lineage-specific cytokines or conditions). b Schematic diagram of two types of PSC differentiation with or without lineage-specific cytokine combinations based on scRNA-seq datasets of Cheng’s lab and Guo’s lab. c A brief schematic overview of key differentiation steps (Q1, Q2, and Q3) from PSCs to T lymphocytes. pHEC primitive HEC, pErythro primitive erythroid cells, EMP erythromyeloid progenitor, dHEC definitive HEC, M-primed HPC myeloid-primed HPC, L-primed HPC lymphoid-primed HPC, MEP megakaryocyte-erythroid progenitor, Pro T progenitor T-cells
Generation of T lymphocytes from PSCs in vitro
Reprogramming of somatic cells to iPSCs [1] provides the possibility of solving the source problem arising from limited T-cell or HSC sources [69]. Early studies have illustrated the ability of ESCs differentiating to T lineage in vitro and in vivo [70,71,72,73,74,75]. Based on the understanding of T-lineage commitment in the thymus, researchers have established OP9-DLL1 as stromal cells to harvest T-lineage commitment cells from PSCs [76]. The OP9-DLL1/4-PSC co-culture system is widely applied to T cell development research in vitro as a stable and efficient culture method [77,78,79,80,81]. Interestingly, OP9-DLL1/4-PSC coculture exhibited unconventional T-subset bias in vitro, such as γδ T cells and NKT cells, compared with T lymphopoiesis in the thymus in vivo [77, 78].
The function of PSC-derived T lymphocytes was only partially defined, because of the random TCR rearrangements during T lineage differentiation in vitro. Meanwhile, complicated and unpredicted T-lineage commitment in vitro limits the knowledge about whether HLA restriction or positive/negative selection is normal [82]. The use of antigen-specific CD8+ T-derived iPSCs to regenerate specific T cells is a promising source of off-the-shelf immune cells [83]. However, endogenous expression of RAG1 and RAG2 may lead to an undesirable loss of antigen specificity with TCR rearrangement [69]. As a classic example of cellular immunotherapy [84], anti-CD19 CAR (CD19-CAR)‐modified T‐cell therapy provides new ideas for antigen-specific T-cell generation. One study showed the potential of anti-tumor therapeutic CAR-engineered PSCs [82]. Intriguingly, CD19-CAR engineered T cells from iPSCs were innate “γδ-like” CAR-T cells instead of conventional T subsets. Single-cell sequencing technology provides an opportunity to understand rare and unconventional cell subsets. Multiple-development-stage, large-scale, and high-throughput sc-RNAseq analysis of the human thymus revealed a rational framework for the generation of functional T lymphocytes [31]. The iPSC-derived “γδ-like” CD19-CAR-T cells conform the phenotype of TCRαβ+TCRγδ−CD8α+CD8β−/lowIL2RB−CCR7−CD62L (SELL)− (Fig. 3a). CD8αβ heterodimers, not CD8αα, provide co-receptor function for CD8-dependent TCR, as an effective co-receptor for TCR signaling [85] and binding to MHC-I molecules efficiently [86].
Schematic diagram of the differentiation strategies to generate T lineage subsets from PSCs. a iPSC-derived “γδ-like” CD19-CAR-T cell [82] is similar to the GNG4+CD8αα+ T(I) subset, which identified the phenotype of TCRαβ+TCRγδ−CD8α+CD8β−/lowIL2RB−CCR7−CD62L(SELL)− from the cell atlas of the human thymic development [31] (https://developmentcellatlas.ncl.ac.uk/datasets/HCA_thymus/fetal_thymus_Tcell_interactive_gene_expression_heatmap.html). b T-cell generation models in OP9-DLL1/PSC monolayer co-culture system. The ectopic-expressing Notch ligands on stromal cells enhanced the T-lineage commitment, the following immature T cells mature into CD8αα+ T cells or CD8αβ+ T cells under different culture conditions. c T-cell generation models in 3D co-culture system. 2-deoxyguanosine-treated thymus lobes, and MS5-DLL1/4-constructed ATO, can be used for 3D coculture system, which may provide a thymus-like microenvironment. MC, monolayer culture. d A scalable iPSC-to-iT platform under Ff condition
Following a previous OP9-DLL1/PSC monolayer co-culture protocol [87], Takuya Maeda and his colleagues harvested PSC-derived LMP2-specific CD8αα+ T cells, with low cytotoxic activity compared with primary CTLs [88] (Fig. 3b). Interestingly, purified iPSC-derived DP cells, but not DN cells, could differentiate into CD8αβ T cells after stimulation with CD3 Ab or agonist peptide (Fig. 3b). To avoid the loss of antigen-specificity caused by TCRα rearrangement, Shin Kaneko’s lab depleted RAG2 by CRISPR-Cas9 in antigen-specificT-derived iPSCs (T-iPSCs) [89]. Alternatively, myeloid cell-derived iPSCs carrying TCR expression cassettes have overcome the hurdle of mispaired TCRαβ. Song et al. also established a solid protocol for PSC-to-iT based on the OP9-DLL1/PSC monolayer co-culture system, which helped to harvest functional hepatitis B virus (HBV) Ag-specific T lymphocytes and target HBV Ag+ cells in a mouse model [90]. In summary, without a well-organized thymus-like microenvironment, the designed program of T lineage from PSCs is disrupted by unpredictable factors, such as PSC-derived unfavorable cells, abnormal TCR signal, or endogenous RAG gene expression.
A recent study compared the OP9-DLL1/PSC monolayer co-culture with 3D thymic co-culture and identified aberrant physiological developmental signals of T development in the OP9-DLL1 monolayer [91] (Fig. 3c). After agonist peptide and anti-CD3/CD28 stimulation, PSC-derived CD8β T cells with weak immunophenotype, converted characteristics as CD8αα+/DN cells in the OP9-DLL1/PSC co-culture system, and anti-TCR antibody stimulation leading to NKT-like cells separately. To generate an in vitro physiological thymic microenvironment, a fetal thymic organ culture (FTOC) system was used to facilitate the maturation of iPSC-derived immature T cells to CD8αβ T cells. As designed, 2-deoxyguanosine-treated fetal thymic lobes enforced the generation of functional CD62L+CD69−MHC-I+ CD8αβ T cells.
Although the 3D thymic co-culture system has unique advantages, the source of primary organs, production expansion, and quality control are irreconcilable challenges. The strategy of the 3D ATO co-culture system ensures positive selection and harvests conventional T cells from HSPCs in vitro, which provides a new method for conventional T-subset generation [92]. Crooks extended the ATO strategy to the PSC-to-iT field [93] (Fig. 3c). Purified PSC-derived CD326−CD56+ embryonic mesodermal progenitors (EMPs) were aggregated into 3D embryonic mesodermal organoids (EMO) with MS5-DLL1/4 in low-serum conditions. After two weeks, the T-lineage commitment medium was used for ATO culture where derivation of PSCs produced a dominant CD8αβ T subset with transit CD8αα T subset and a few CD4SP T cells. The pattern of CDR3 lengths and DNTT expression indicated that PSC-ATO could provide a fetal thymus-like microenvironment. By applying the same strategy, Shin Kaneko’s lab could also harvest CD4+ T helper (Th) cells with Th1 or Th2 function mediated by knocking out IL4 or TBX21, respectively [94]. Altogether, the FTOC system and ATO systems provide CD8αβ+TCRαβ+ T-cell and CD4+ Th cell generation platforms, which are closer to the thymic microenvironment. However, these approaches must be optimized to save time, reduce complex steps, and become operation friendly.
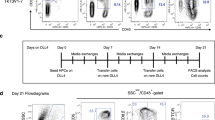
Shin Kaneko’s lab developed an efficient and scalable feeder-free (Ff) differentiation system that can regenerate cytotoxic T-cells from iPSCs[95]. This Ff system drives a well-defined T lineage commitment in vitro: iPSCs → CD235a−CD14−CD34+CD43+ iHPCs → CD7+CD5+ T-cell progenitors → CD4+CD8αβ+ DP cells → CD8αβ iT cells. The combination of several factors (CXCL12, SB203580, retronectin, IL-7, IL-15, IL-12, IL-18, IL-21, TL1A, and so on) in synergy helps to establish a novel strategy of large-scale production of CD8αβ+ T cells from iPSCs (Fig. 3d). Notably, CXCL12 and SB203580 can expand iT by approximately 3000-fold during T-cell differentiation. This culture system could avoid safety issues, such as replacing OP9-DLL4 stromal cells with DLL4 protein, FBS with BIT (BSA supplemented with insulin and transferrin) or serum-free medium. This is a credible and comprehensive culture system of PSC-to-iT; however, reducing the tedious technical process will be a serious challenge.
Reconstitution of T lymphopoiesis from PSCs in vivo
Reconstitution of T lymphopoiesis from PSC-derived TSP
Obtaining engraftable functional PSC-derived mature lineage cells is the most important challenge in the field of regenerative medicine, owing to the challenges of the recipient's immunological rejection, dysfunctional cell survival/ proliferation/differentiation signal, or inability of the cells to migrate to a suitable microenvironment [76, 80]. PSC-derived cells cannot effectively exert their physiological functions in vivo. However, under specific circumstances, PSC-derived T progenitors can produce CD4SP T cells and CD8SP T cells in subcutaneously implanted FTOCs, which indicates that these T progenitors lack thymus-seeding ability [76]. The latest platform of physiological conventional T-subset generation in vivo provides a novel idea for the practical application of PSC-to-T technology [60, 96].
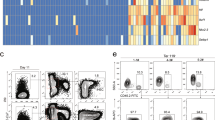
Transcription factors (TFs) are the core organizers of cell fate [97, 98]. Among them, Runx1 is the master regulator of embryonic hematopoietic development [99, 100], This factor helps the generation of T cells from PSCs. Transient expression of Runx1 during hematopoietic commitment, enforced the emergence of pre-HSC-like (CD31+CD41lowCD45−c-Kit+CD201high) inducible hemogenic endothelial cells (iHECs) and HPC-like cells, but not T cells in vitro or in vivo in the further differentiation, indicating that Runx1 alone is not sufficient to initiate the PSC-to-iT program. Further scRNA-seq analysis showed that inducible Runx1-mESC-derived iHEC has divergent gene expression patterns when compared with those from mouse E11 Type I pre-HSC (T1-pre-HSC), especially missing the expression of some important hematopoietic TFs, such as Hoxa family members, Hlf, Ikzf1, Setbp1, and Nkx2-3. Using the strategy of “Runx1 + 1”, the combination of Runx1 and Hoxa9 can enforce strong T lineage commitment markedly, but not other combinations (Fig. 4). The Hoxa family is essential for the proliferation of HSPC and lymphoid commitment, especially Hoxa9 [101]. The inducible Runx1-p2a-Hoxa9 mESC (iR9-ESC)-derived iHEC showed molecular features between E11 EC and T1-pre-HSC, and then differentiated to TSP-like (Lin−c-kit+CD127+/CD135+) progenitors. Also iR9-ESC-derived iHECs gave rise to T cells at the single-cell level efficiently, regardless of in vitro or in vivo conditions. After iR9-ESC-derived pre-thymic progenitors were transplanted into irradiated B-NDG mice, these progenitors generated inducible T (iT) cells, which showed features of abundant TCR diversity, multi-organ distribution, and conventional T development pattern. More importantly, different stages (DN1, DN2, DN3, DN4, DP, and conventional SP) of T lymphopoiesis were detected in the thymus. These PSC-derived iT cells have a physiological adaptive immune response, which has been identified by allogeneic skin transplantation. TCR-edited iPSC-derived iT cells efficiently eradicated E.G7-OVA tumor cells. Furthermore, these iPSC-derived functional iT cells can be further engineered with CD19-CAR T cells, which can robustly eliminate lymphoma cells both in vitro and in vivo [102]. Combining this strategy with those of Notarangelo’s lab or Mikkers’ lab reconstituted T lymphopoiesis in vivo and rescue severe combined immune deficiency (SCID) patients early in life [103, 104]. Altogether, regenerated bona fide TSP-like cells through transient expression of Runx1 and Hoxa9 are effective, allow normal conventional T development in the thymus, and avoid the generation of abnormal cells because of in vitro unfavorable factors.
Summary of the reconstitution of T lymphopoiesis in vivo by transient Runx1 and Hoxa9 expression. Specific TFs combination of Runx1 and Hoxa9 were screened from several important TFs, which robustly drive the T-lineage specification. The iR9-PSCs differentiate into iHECs by mesoderm specification and HEC induction. And the OP9-DLL1 stomal cells promote T-lineage commitment with the transit expression of Runx1 and Hoxa9. PSC-derived iHPCs can be transplanted into B-NDG mice for T lymphopoiesis in the thymus, and differentiate into different functional T subsets, as the classical T-cell developmental pattern
Reconstitution of T lymphopoiesis from PSC-derived induced HSC
HSCs become the major source of thymopoiesis once these rare cells emerge. Reconstituting T lymphopoiesis by HSCs is an additional feasible way, but there is no robust culture method for expanding HSCs ex vivo. Generation of HSCs from pluripotent stem cells (PSCs) is a useful idea for cell therapy. Exogenous expression of hemogenic transcription factors to guide conversion of PSC-derived mesodermal cells to HSCs is a mainstream method reported by different research groups [105] (Fig. 5). Daley laboratory provides several options, such as Hoxb4 [106], Cdx4 [107], the combination of HOXA9/ERG/RORA/SOX4/MYB with shEZH1 targeting [108], and the combination of ERG/HOXA5/HOXA9/HOXA10/LCOR/RUNX1/SPI1 [61]. LIM-homeobox gene Lhx2 can drive the in vitro generation of HSC-like cells from mPSCs, but the inappropriate persistence of Lhx2 expression suppresses the developmental program at the DN stage in the thymus [109]. Terminating the Lhx2 expression can pave the way to mature T cells from the DN stage in vivo [110], which helps to reconstitute T lymphopoiesis from PSC-derived TSP-like cells. Tan et al. found that inducing MLL-AF4 expression promotes the generation of PSC-derived engraftable induced HSPCs (iHSPCs) with T lineage potential [111]. PSC-derived teratoma as a disorganized and spontaneous differentiation system may occasionally produce rare engraftable HSPC [112,113,114] (Fig. 5), but this must be optimized to control the risk of tumorigenesis. For example, large animal models, such as gene-edited immunodeficient pigs [115], can be used as containers to avoid the risk of teratoma formation or leukaemogenesis in patients, and produce sufficient engraftable iHSPCs from PSCs. Naturally, current strategies for PSC-derived iHSPC generation need to be modified by reducing the number of tumorigenesis-related TFs (such as MYB and MLL-AF4), or avoiding the formation of PSC-derived abnormal cells.
T-cell generation meets gene editing
Several forms of adoptive T-cell transfer (ACT), such as tumor-infiltrating lymphocytes (TILs), TCR-engineered T cells (TCR-T), CAR-T, and T cell antigen coupler-engineered T cells (TAC-T) [116], have been developed for antitumor therapy [6, 117], antivirus therapy [118], and targeting cardiac fibrosis [119]. CAR-T cells have unique advantages, such as MHC-independent recognition, which can kill tumor cells without MHC-associated antigens. Compared to CAR-T, MHC-dependent TCR-T cells have the advantages of intracellular targeting, lifelong persistence, robust ability to enter the solid tumor microenvironment, and reduced cytokine release syndrome. CAR-T and TCR-T cells can both effectively eliminate tumor cells and prolong patient survival. However, the efficiency of gene editing in patient-derived primary T cells remains an obstacle, which limits the purity of antigen-specific T cells and restrict the scope of gene editing at the genomic level.
The advent of the PSC-to-T technique provides a scalable system that can produce large doses of gene-edited T cells in one batch, reduces substantial economic burden, increases product consistency, and easily achieves predetermined genetic engineering. Theoretically, the existing gene-editing approaches in primary T cells can be applied to PSCs more efficiently (Fig. 6). To avoid graft-versus-host-disease (GVHD), Hiroki Torikai and his colleagues made universal CD19-CAR T cells by curbing the expression of endogenous αβ TCR [120]. Eliminating B2M in transplanted cells prevents the stimulation of allogeneic T cells, and expressing HLA-E can help avoid allogeneic rejection by preventing host NK-mediated lysis [121]. Therefore, we can introduce multiplex gene editing in the TCR/B2M locus and HLA-E expression in PSCs for universal engineered iT generation. Moreover, inactivation of HLA-A and HLA-B, but not HLA-C, is another ideal strategy which could cover a large population [122]. T-iPSCs with RAG2 knockout and non-T-iPSCs with transduced TCR can also help avoid unpredictable TCR generation [89]. It is notable that CAR, as an artificial fusion molecule, may disturb the normal pattern of T lymphopoiesis [123] and this can be overcome by constructing conditional expression cassettes at the stem cell level. Additionally, eliminating the expression of GM-CSF in CAR-T cells mitigates neurotoxicity and cytokine release syndrome (CRS) [124,125,126]. Notably, multi-target CAR-T cells are entering clinical trials [127, 128], which might help us to cope with more complex disease processes. Defects in CTLA-4, PD-1, or HPK1 in T cells enhance T cell function [129,130,131]. This notion prompts the disruption of these genes to generate function-enhanced T cells from PSCs. The safety concern for engineered T cells or regenerated cells relates to the off-tumor side effects and potential tumorigenicity. These risks can be solved by employing inducible suicide gene systems, such as HSVTK/GCV and iCasp9/AP1903 [132, 133]. The synNotch AND-gate circuit is another unique strategy for reducing the adverse effects on bystander tissues [134]. According to treatment purposes, we can perform precise gene editing mentioned above in PSCs to obtain multiplex engineered PSC-iT cells. However, the current PSC-to-T platforms are inefficient, limiting the development of immune cell-based regenerative therapies. When an efficient and stable PSC-to-T platform is established, diversified immunotherapy strategies through precise gene editing technologies will quickly translated to the clinic.
Clinical applications of PSC-derived immune cells
The PSC-derived immune cells are being quickly translated from bench to bedside. Many factors, such as safety issues and cell purity, that hinder the clinical applications of PSC-derived immune cells, are being addressed. Furthermore, various technologies have been developed to shorten the time to generate the patient-derived iPSC lines from somatic cells with adequate efficiency and safety (Fig. 7A)[135]. Unlimited sources and efficient gene-editing of iPSC show the high prospects for their clinical application and commercialization. Recently, a group reported a stromal cell/serum-free DL4-μbeads-based approach that supports the development of PSC-derived CD34+ cells to T lineage progenitors, which can eliminate the concerns over the safety of animal-derived substances (Fig. 7b). However, this study did not show the function or developmental progress of PSC-derived T lineage in vivo [68]. Many researchers and organizations are promoting the commercialization of iPSC-derived immune cells (Table 2). Interestingly, almost all iPSC-derived immune cell therapy products are NK cells (NK: 17/27, NK and/or T: 4/27; T: 4/27, Mac: 2/27). The reason for this may be that NK cell-mediated cytotoxicity does not require HLA-matching [136]. Several trials have demonstrated the safety of adoptive transfer of allogeneic NK cells [137]. These universal and “off-the-shelf” iPSC-derived NK cells can be produced easily. Furthermore, knocking out the HLA gene in iPSCs can help harvest universal iPSCs, which can subsequently be used for generation of universal iPSC- derived CAR-T cells.
Conclusions and future perspectives
The study of HSPC transplantation [138,139,140,141,142], as well as disorders of hematopoiesis, lymphatics, and immunity [143] has facilitated the understanding of the HSC differentiation cascade. T lineage commitment not only involves a precise transcription factor regulatory network, but also an organized thymus microenvironment [23]. Indeed, extensive research has demonstrated the feasibility of PSC-to-T [69]. To identify the T lymphopoiesis in the thymus, several single-cell transcriptional atlas of T lymphopoiesis and embryonic/adult thymus organogenesis have been established [23, 31, 144], which help us to identify the features of TSP, the interaction of thymocytes and stromal cells, and rare unconventional T subsets. Furthermore, several published scRNA-seq datasets of PSC-derived cells clearly showed the differentiation pathways and possibilities of generating physiological T-lineage cells (Table 2). More importantly, by deconstructing T lymphopoiesis in the thymus and eliminating unnecessary factors, an organized thymus-like microenvironment was reproduced in vitro for functional PSC-derived T-cell generation. The ATO co-culture system indicated the feasibility of conventional T-subset generation by constructing thymus-like niche in vitro. Defined TFs (Runx1 and Hoxa9) were used to generate transplantable PSC-derived TSP. Furthermore, the improved PSC-to-T platforms through gene editing technology will likely facilitate the clinical application of PSC-T, NK and macrophage cells for cancer immunotherapy [145,146,147,148,149,150,151,152].
Availability of data and materials
This is not applicable for this review.
Abbreviations
- PSCs:
-
Pluripotent stem cells
- iT:
-
Induced T
- 3D:
-
three-dimensional
- ATO:
-
Artificial thymic organoid
- ESCs:
-
Embryonic stem cells
- iPSCs:
-
Induced pluripotent stem cells
- CARs:
-
Chimeric antigen receptors
- HSPCs:
-
Hematopoietic stem/progenitor cells
- TSP:
-
Thymus-seeding progenitors
- HSCs:
-
Hematopoietic stem cells
- LMPP:
-
Lymphoid-primed multipotent progenitors
- CMJ:
-
Cortico-medullary junction
- ETP:
-
Early thymic progenitors
- thymic TEC:
-
Epithelial cells
- TFs:
-
Transcription factors
- FTOCs:
-
Fetal thymic organ cultures
- ATO:
-
Artificial thymic organoid
- EMP:
-
Embryonic mesodermal progenitors
- EMO:
-
Embryonic mesodermal organoids
- iHECs:
-
Inducible hemogenic endothelial cells
- scRNA-seq:
-
Single cell RNA-seq
- GVHD:
-
Graft-versus-host-disease
- ACT:
-
T-cell transfer
- TILs:
-
Tumor-infiltrating lymphocytes
- TCR-T:
-
TCR-engineered T cells
- TAC-T:
-
T cell antigen coupler-engineered T cells
References
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
Zhang L, Tian L, Dai X, Yu H, Wang J, Lei A, Zhu M, Xu J, Zhao W, Zhu Y, Sun Z, Zhang H, Hu Y, Wang Y, Xu Y, Church GM, Huang H, Weng Q, Zhang J. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13(1):153.
Sharma R, Khristov V, Rising A, Jha BS, Dejene R, Hotaling N, Li Y, Stoddard J, Stankewicz C, Wan Q, Zhang C, Campos MM, Miyagishima KJ, McGaughey D, Villasmil R, Mattapallil M, Stanzel B, Qian H, Wong W, Chase L, Charles S, McGill T, Miller S, Maminishkis A, Amaral J, Bharti K. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019;11(475):eaat5580.
Yin Z, Zhang Y, Wang X. Advances in chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Biomark Res. 2021;9(1):58.
Zhang H, Zhao P, Huang H. Engineering better chimeric antigen receptor T cells. Exp Hematol Oncol. 2020;9(1):34.
Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, Gao L, Wen Q, Zhong JF, Zhang C, Zhang X. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13(1):86.
Nie Y, Lu W, Chen D, Tu H, Guo Z, Zhou X, Li M, Tu S, Li Y. Mechanisms underlying CD19-positive ALL relapse after anti-CD19 CAR T cell therapy and associated strategies. Biomark Res. 2020;8(1):18.
Lemoine J, Ruella M, Houot R. Born to survive: how cancer cells resist CAR T cell therapy. J Hematol Oncol. 2021;14(1):199.
Hao Z, Li R, Meng L, Han Z, Hong Z. Macrophage, the potential key mediator in CAR-T related CRS. Exp Hematol Oncol. 2020;9(1):15.
Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol. 2018;11(1):91.
Guo R, Lü M, Cao F, Wu G, Gao F, Pang H, Li Y, Zhang Y, Xing H, Liang C, Lyu T, Du C, Li Y, Guo R, Xie X, Li W, Liu D, Song Y, Jiang Z. Single-cell map of diverse immune phenotypes in the acute myeloid leukemia microenvironment. Biomark Res. 2021;9(1):15.
Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12(4):295–305.
Belkaid Y, Blank RB, Suffia I. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol Rev. 2006;212(1):287–300.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48.
Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, Lipp M, Kondo S, Manley N, Takahama Y. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108(8):2531–9.
Adolfsson J, Månsson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge O-J, Thoren LAM, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SEW. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential: a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306.
Månsson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, Al-Hashmi S, Liuba K, Thorén L, Adolfsson J, Buza-Vidas N, Qian H, Soneji S, Enver T, Sigvardsson M, Jacobsen SEW. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26(4):407–19.
Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH, Yoder MC. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119(24):5706–14.
Yokota T, Huang J, Tavian M, Nagai Y, Hirose J, Zúñiga-Pflücker J-C, Péault B, Kincade PW. Tracing the first waves of lymphopoiesis in mice. Development. 2006;133(10):2041–51.
Tavian M, Robin C, Coulombel L, Péault B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15(3):487–95.
Ivanovs A, Rybtsov S, Welch L, Anderson RA, Turner ML, Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med. 2011;208(12):2417–27.
Zeng Y, Liu C, Gong Y, Bai Z, Hou S, He J, Bian Z, Li Z, Ni Y, Yan J, Huang T, Shi H, Ma C, Chen X, Wang J, Bian L, Lan Y, Liu B, Hu H. Single-Cell RNA sequencing resolves spatiotemporal development of pre-thymic lymphoid progenitors and thymus organogenesis in human embryos. Immunity. 2019;51(5):930-948.e936.
Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178(4):2008–17.
Williams KM, Lucas PJ, Bare CV, Wang J, Chu Y-W, Tayler E, Kapoor V, Gress RE. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008;112(8):3255–63.
Kohn LA, Hao Q-L, Sasidharan R, Parekh C, Ge S, Zhu Y, Mikkola HKA, Crooks GM. Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of L-selectin. Nat Immunol. 2012;13(10):963–71.
Ceredig R. Fates and potentials of thymus-seeding progenitors. Nat Immunol. 2012;13(4):309–10.
Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306.
Belluschi S, Calderbank EF, Ciaurro V, Pijuan-Sala B, Santoro A, Mende N, Diamanti E, Sham KYC, Wang X, Lau WWY, Jawaid W, Göttgens B, Laurenti E. Myelo-lymphoid lineage restriction occurs in the human haematopoietic stem cell compartment before lymphoid-primed multipotent progenitors. Nat Commun. 2018;9(1):4100–4100.
Luc S, Luis TC, Boukarabila H, Macaulay IC, Buza-Vidas N, Bouriez-Jones T, Lutteropp M, Woll PS, Loughran SJ, Mead AJ, Hultquist A, Brown J, Mizukami T, Matsuoka S, Ferry H, Anderson K, Duarte S, Atkinson D, Soneji S, Domanski A, Farley A, Sanjuan-Pla A, Carella C, Patient R, de Bruijn M, Enver T, Nerlov C, Blackburn C, Godin I, Jacobsen SEW. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat Immunol. 2012;13(4):412–9.
Park JE, Botting RA, Dominguez Conde C, Popescu DM, Lavaert M, Kunz DJ, Goh I, Stephenson E, Ragazzini R, Tuck E, Wilbrey-Clark A, Roberts K, Kedlian VR, Ferdinand JR, He X, Webb S, Maunder D, Vandamme N, Mahbubani KT, Polanski K, Mamanova L, Bolt L, Crossland D, de Rita F, Fuller A, Filby A, Reynolds G, Dixon D, Saeb-Parsy K, Lisgo S, et al. A cell atlas of human thymic development defines T cell repertoire formation. Science. 2020;367(6480):eaay3224.
Nehls M, Kyewski B, Messerle M, Waldschutz R, Schuddekopf K, Smith AJ, Boehm T. Two genetically separable steps in the differentiation of thymic epithelium. Science. 1996;272(5263):886–9.
Su DM, Navarre S, Oh WJ, Condie BG, Manley NR. A domain of Foxn1 required for crosstalk-dependent thymic epithelial cell differentiation. Nat Immunol. 2003;4(11):1128–35.
Bleul CC, Corbeaux T, Reuter A, Fisch P, Monting JS, Boehm T. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441(7096):992–6.
Rodewald HR, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3(3):313–9.
Di Santo JP, Rodewald HR. In vivo roles of receptor tyrosine kinases and cytokine receptors in early thymocyte development. Curr Opin Immunol. 1998;10(2):196–207.
Wang H, Pierce LJ, Spangrude GJ. Distinct roles of IL-7 and stem cell factor in the OP9-DL1 T-cell differentiation culture system. Exp Hematol. 2006;34(12):1730–40.
Ciofani M, Zuniga-Pflucker JC. The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol. 2007;23:463–93.
Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–60.
Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2(3):223–38.
von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–26.
Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7 -/- mice. J Immunol. 1996;157(6):2366–73.
Hagenbeek TJ, Naspetti M, Malergue F, Garcon F, Nunes JA, Cleutjens KB, Trapman J, Krimpenfort P, Spits H. The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling. J Exp Med. 2004;200(7):883–94.
Ciofani M, Zuniga-Pflucker JC. A survival guide to early T cell development. Immunol Res. 2006;34(2):117–32.
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–6.
Weerkamp F, Baert MR, Naber BA, Koster EE, de Haas EF, Atkuri KR, van Dongen JJ, Herzenberg LA, Staal FJ. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci USA. 2006;103(9):3322–6.
Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32(4):967–71.
Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4(12):1177–82.
Famili F, Naber BA, Vloemans S, de Haas EF, Tiemessen MM, Staal FJ. Discrete roles of canonical and non-canonical Wnt signaling in hematopoiesis and lymphopoiesis. Cell Death Dis. 2015;6:e1981.
Outram SV, Varas A, Pepicelli CV, Crompton T. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 2000;13(2):187–97.
El Andaloussi A, Graves S, Meng F, Mandal M, Mashayekhi M, Aifantis I. Hedgehog signaling controls thymocyte progenitor homeostasis and differentiation in the thymus. Nat Immunol. 2006;7(4):418–26.
Shah DK, Hager-Theodorides AL, Outram SV, Ross SE, Varas A, Crompton T. Reduced thymocyte development in sonic hedgehog knockout embryos. J Immunol. 2004;172(4):2296–306.
Yui MA, Rothenberg EV. Developmental gene networks: a triathlon on the course to T cell identity. Nat Rev Immunol. 2014;14(8):529–45.
Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10(5):547–58.
Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14(6):637–45.
Han X, Chen H, Huang D, Chen H, Fei L, Cheng C, Huang H, Yuan G-C, Guo G. Mapping human pluripotent stem cell differentiation pathways using high throughput single-cell RNA-sequencing. Genome Biol. 2018;19(1):47.
Hou S, Li Z, Zheng X, Gao Y, Dong J, Ni Y, Wang X, Li Y, Ding X, Chang Z, Li S, Hu Y, Fan X, Hou Y, Wen L, Liu B, Tang F, Lan Y. Embryonic endothelial evolution towards first hematopoietic stem cells revealed by single-cell transcriptomic and functional analyses. Cell Res. 2020;30(5):376–92.
Li Y-Q, Gong Y, Hou S, Huang T, Wang H, Liu D, Ni Y, Wang C, Wang J, Hou J, Yang R, Yan J, Zhang G, Liu B, Lan Y. Spatiotemporal and functional heterogeneity of hematopoietic stem cell-competent hemogenic endothelial cells in mouse embryos. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.699263.
Zeng Y, He J, Bai Z, Li Z, Gong Y, Liu C, Ni Y, Du J, Ma C, Bian L, Lan Y, Liu B. Tracing the first hematopoietic stem cell generation in human embryo by single-cell RNA sequencing. Cell Res. 2019;29(11):881–94.
Guo R, Hu F, Weng Q, Lv C, Wu H, Liu L, Li Z, Zeng Y, Bai Z, Zhang M, Liu Y, Liu X, Xia C, Wang T, Zhou P, Wang K, Dong Y, Luo Y, Zhang X, Guan Y, Geng Y, Du J, Li Y, Lan Y, Chen J, Liu B, Wang J. Guiding T lymphopoiesis from pluripotent stem cells by defined transcription factors. Cell Res. 2020;30(1):21–33.
Sugimura R, Jha DK, Han A, Soria-Valles C, da Rocha EL, Lu YF, Goettel JA, Serrao E, Rowe RG, Malleshaiah M, Wong I, Sousa P, Zhu TN, Ditadi A, Keller G, Engelman AN, Snapper SB, Doulatov S, Daley GQ. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017;545(7655):432–8.
Angelos MG, Abrahante JE, Blum RH, Kaufman DS. Single cell resolution of human hematoendothelial cells defines transcriptional signatures of hemogenic endothelium. Stem Cells. 2018;36(2):206–17.
Canu G, Athanasiadis E, Grandy RA, Garcia-Bernardo J, Strzelecka PM, Vallier L, Ortmann D, Cvejic A. Analysis of endothelial-to-haematopoietic transition at the single cell level identifies cell cycle regulation as a driver of differentiation. Genome Biol. 2020;21(1):157.
Fidanza A, Stumpf PS, Ramachandran P, Tamagno S, Babtie A, Lopez-Yrigoyen M, Taylor AH, Easterbrook J, Henderson BEP, Axton R, Henderson NC, Medvinsky A, Ottersbach K, Romanò N, Forrester LM. Single-cell analyses and machine learning define hematopoietic progenitor and HSC-like cells derived from human PSCs. Blood. 2020;136(25):2893–904.
Han X, Zhou Z, Fei L, Sun H, Wang R, Chen Y, Chen H, Wang J, Tang H, Ge W, Zhou Y, Ye F, Jiang M, Wu J, Xiao Y, Jia X, Zhang T, Ma X, Zhang Q, Bai X, Lai S, Yu C, Zhu L, Lin R, Gao Y, Wang M, Wu Y, Zhang J, Zhan R, Zhu S, et al. Construction of a human cell landscape at single-cell level. Nature. 2020;581(7808):303–9.
Zhu Y, Wang T, Gu J, Huang K, Zhang T, Zhang Z, Liu H, Tang J, Mai Y, Zhang Y, Li Y, Feng Y, Kang B, Li J, Shan Y, Chen Q, Zhang J, Long B, Wang J, Gao M, Zhang D, Zhou M, Zhong X, Chen J, Pei D, Nie J, Liu B, Pan G. Characterization and generation of human definitive multipotent hematopoietic stem/progenitor cells. Cell Discov. 2020;6(1):89.
Shen J, Xu Y, Zhang S, Lyu S, Huo Y, Zhu Y, Tang K, Mou J, Li X, Hoyle DL, Wang M, Wang J, Li X, Wang ZZ, Cheng T. Single-cell transcriptome of early hematopoiesis guides arterial endothelial-enhanced functional T cell generation from human PSCs. Sci Adv. 2021;7(36):eabi978.
Trotman-Grant AC, Mohtashami M, De Sousa CJ, Martinez EC, Lee D, Teichman S, Brauer PM, Han J, Anderson MK, Zúñiga-Pflücker JC. DL4-μbeads induce T cell lineage differentiation from stem cells in a stromal cell-free system. Nat Commun. 2021;12(1):5023.
Guo R, Wu H, Du J, Wang J. T cell regeneration: an update on progress and challenges. Blood Sci. 2020;2(1):22–6.
Chen U, Kosco M, Staerz U. Establishment and characterization of lymphoid and myeloid mixed-cell populations from mouse late embryoid bodies, “embryonic-stem-cell fetuses.” Proc Natl Acad Sci USA. 1992;89(7):2541–5.
Gutierrez-Ramos JC, Palacios R. In vitro differentiation of embryonic stem cells into lymphocyte precursors able to generate T and B lymphocytes in vivo. Proc Natl Acad SciU SA. 1992;89(19):9171–5.
Muller AM, Dzierzak EA. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development. 1993;118(4):1343–51.
Potocnik AJ, Nielsen PJ, Eichmann K. In vitro generation of lymphoid precursors from embryonic stem cells. EMBO J. 1994;13(22):5274–83.
de Pooter RF, Cho SK, Carlyle JR, Zuniga-Pflucker JC. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood. 2003;102(5):1649–53.
Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, Zack JA. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103(31):11742–7.
Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5(4):410–7.
Timmermans F, Velghe I, Vanwalleghem L, De Smedt M, Van Coppernolle S, Taghon T, Moore HD, Leclercq G, Langerak AW, Kerre T, Plum J, Vandekerckhove B. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182(11):6879–88.
Chang CW, Lai YS, Lamb LS Jr, Townes TM. Broad T-cell receptor repertoire in T-lymphocytes derived from human induced pluripotent stem cells. PLoS ONE. 2014;9(5):e97335.
Zhou F, Li X, Wang W, Zhu P, Zhou J, He W, Ding M, Xiong F, Zheng X, Li Z, Ni Y, Mu X, Wen L, Cheng T, Lan Y, Yuan W, Tang F, Liu B. Tracing haematopoietic stem cell formation at single-cell resolution. Nature. 2016;533(7604):487–92.
Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zuniga-Pflucker JC, Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2(6):1722–35.
Kitayama S, Zhang R, Liu TY, Ueda N, Iriguchi S, Yasui Y, Kawai Y, Tatsumi M, Hirai N, Mizoro Y, Iwama T, Watanabe A, Nakanishi M, Kuzushima K, Uemura Y, Kaneko S. Cellular adjuvant properties, direct cytotoxicity of re-differentiated Valpha24 Invariant NKT-like Cells from human induced pluripotent stem cells. Stem Cell Rep. 2016;6(2):213–27.
Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31(10):928–33.
Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, Nakayama-Hosoya K, Iriguchi S, Uemura Y, Shimizu T, Takayama N, Yamada D, Nishimura K, Ohtaka M, Watanabe N, Takahashi S, Iwamoto A, Koseki H, Nakanishi M, Eto K, Nakauchi H. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12(1):114–26.
Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–23.
McNicol A-M, Bendle G, Holler A, Matjeka T, Dalton E, Rettig L, Zamoyska R, Uckert W, Xue S-A, Stauss HJ. CD8α/α homodimers fail to function as co-receptor for a CD8-dependent TCR. Eur J Immunol. 2007;37(6):1634–41.
Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 2008;28(2):149–59.
Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12(1):31–6.
Maeda T, Nagano S, Ichise H, Kataoka K, Yamada D, Ogawa S, Koseki H, Kitawaki T, Kadowaki N, Takaori-Kondo A, Masuda K, Kawamoto H. Regeneration of CD8alphabeta T cells from T-cell-derived iPSC imparts potent tumor antigen-specific cytotoxicity. Cancer Res. 2016;76(23):6839–50.
Minagawa A, Yoshikawa T, Yasukawa M, Hotta A, Kunitomo M, Iriguchi S, Takiguchi M, Kassai Y, Imai E, Yasui Y, Kawai Y, Zhang R, Uemura Y, Miyoshi H, Nakanishi M, Watanabe A, Hayashi A, Kawana K, Fujii T, Nakatsura T, Kaneko S. Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell Stem Cell. 2018;23(6):850-858.e854.
Haque M, Xiong X, Lei F, Das JK, Song J. An optimized protocol for the generation of HBV viral antigen-specific T lymphocytes from pluripotent stem cells. STAR Protoc. 2021;2(1):100264.
Vizcardo R, Klemen ND, Islam SMR, Gurusamy D, Tamaoki N, Yamada D, Koseki H, Kidder BL, Yu Z, Jia L, Henning AN, Good ML, Bosch-Marce M, Maeda T, Liu C, Abdullaev Z, Pack S, Palmer DC, Stroncek DF, Ito F, Flomerfelt FA, Kruhlak MJ, Restifo NP. Generation of tumor antigen-specific iPSC-derived thymic emigrants using a 3D thymic culture system. Cell Rep. 2018;22(12):3175–90.
Seet CS, He C, Bethune MT, Li S, Chick B, Gschweng EH, Zhu Y, Kim K, Kohn DB, Baltimore D, Crooks GM, Montel-Hagen A. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat Methods. 2017;14(5):521–30.
Montel-Hagen A, Seet CS, Li S, Chick B, Zhu Y, Chang P, Tsai S, Sun V, Lopez S, Chen HC, He C, Chin CJ, Casero D, Crooks GM. Organoid-induced differentiation of conventional T cells from human pluripotent stem cells. Cell Stem Cell. 2019;24(3):376-389.e378.
Yano H, Shinohara T, Koga K, Iriguchi S, Miyake Y, Song X, Tada M, Kassai Y, Kiyoi H, Kaneko S. Guided polarization of iPSC-derived CD4SP helper T Cells By CRISPR/Cas9-based genome-editing. Blood. 2019;134(Supplement_1):1937–1937.
Iriguchi S, Yasui Y, Kawai Y, Arima S, Kunitomo M, Sato T, Ueda T, Minagawa A, Mishima Y, Yanagawa N, Baba Y, Miyake Y, Nakayama K, Takiguchi M, Shinohara T, Nakatsura T, Yasukawa M, Kassai Y, Hayashi A, Kaneko S. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat Commun. 2021;12(1):430.
Guo R, Hu F, Weng Q, Lv C, Wu H, Liu B, Wang J. Reconstitution of T lymphopoiesis from pluripotent stem cells by defined transcription factors. Exp Hematol. 2019;76:S54.
Shu J, Deng H. Lineage specifiers: new players in the induction of pluripotency. Genomics Proteomics Bioinform. 2013;11(5):259–63.
Graf T. Transcription factor stoichiometry drives cell fate: single-cell proteomics to the rescue. Cell Stem Cell. 2019;24(5):673–4.
Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–91.
Freire AG, Butler JM. Blood making: learning what to put into the dish. Nature. 2020;9:1000.
Bhatlekar S, Fields JZ, Boman BM. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. 2018;2018:3569493.
Lv C, Chen S, Hu F, Huang D, Wang T, Du J, Wang J, Wu H. Pluripotent stem cell-derived CD19-CAR iT cells effectively eradicate B-cell lymphoma in vivo. Cell Mol Immunol. 2021;18(3):773–5.
Gardner CL, Pavel-Dinu M, Dobbs K, Bosticardo M, Reardon PK, Lack J, DeRavin SS, Le K, Bello E, Pala F, Delmonte OM, Malech H, Montel-Hagan A, Crooks G, Acuto O, Porteus MH, Notarangelo LD. Gene editing rescues in vitro t cell development of RAG2-Deficient induced pluripotent stem cells in an artificial thymic organoid system. J Clin Immunol. 2021. https://doi.org/10.1007/s10875-021-00989-6.
Themeli M, Chhatta A, Boersma H, Prins HJ, Cordes M, de Wilt E, Farahani AS, Vandekerckhove B, van der Burg M, Hoeben RC, Staal FJT, Mikkers HMM. iPSC-Based Modeling of <em>RAG2</em> Severe Combined Immunodeficiency Reveals Multiple T Cell Developmental Arrests. Stem Cell Reports. 2020;14(2):300–11.
Menegatti S, de Kruijf M, Garcia-Alegria E, Lacaud G, Kouskoff V. Transcriptional control of blood cell emergence. FEBS Lett. 2019;593(23):3304–15.
Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109(1):29–37.
Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102(52):19081–6.
Vo LT, Kinney MA, Liu X, Zhang Y, Barragan J, Sousa PM, Jha DK, Han A, Cesana M, Shao Z, North TE, Orkin SH, Doulatov S, Xu J, Daley GQ. Regulation of embryonic haematopoietic multipotency by EZH1. Nature. 2018;553(7689):506–10.
Kitajima K, Minehata K, Sakimura K, Nakano T, Hara T. In vitro generation of HSC-like cells from murine ESCs/iPSCs by enforced expression of LIM-homeobox transcription factor Lhx2. Blood. 2011;117(14):3748–58.
Kitajima K, Kawaguchi M, Miyashita K, Nakajima M, Kanokoda M, Hara T. Efficient production of T cells from mouse pluripotent stem cells by controlled expression of Lhx2. Genes Cells. 2015;20(9):720–38.
Tan Y-T, Ye L, Xie F, Beyer AI, Muench MO, Wang J, Chen Z, Liu H, Chen S-J, Kan YW. Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc Natl Acad Sci. 2018;115(9):2180–5.
Tsukada M, Ota Y, Wilkinson AC, Becker HJ, Osato M, Nakauchi H, Yamazaki S. In vivo generation of engraftable murine hematopoietic stem cells by Gfi1b, c-Fos, and Gata2 overexpression within Teratoma. Stem Cell Reports. 2017;9(4):1024–33.
Philipp F, Selich A, Rothe M, Hoffmann D, Rittinghausen S, Morgan MA, Klatt D, Glage S, Lienenklaus S, Neuhaus V, Sewald K, Braun A, Schambach A. Human teratoma-derived hematopoiesis is a highly polyclonal process supported by human umbilical vein endothelial cells. Stem Cell Reports. 2018;11(5):1051–60.
Amabile G, Welner RS, Nombela-Arrieta C, D’Alise AM, Di Ruscio A, Ebralidze AK, Kraytsberg Y, Ye M, Kocher O, Neuberg DS, Khrapko K, Silberstein LE, Tenen DG. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood. 2013;121(8):1255–64.
Iqbal MA, Hong K, Kim JH, Choi Y. Severe combined immunodeficiency pig as an emerging animal model for human diseases and regenerative medicines. BMB Rep. 2019;52(11):625–34.
Helsen CW, Hammill JA, Lau VWC, Mwawasi KA, Afsahi A, Bezverbnaya K, Newhook L, Hayes DL, Aarts C, Bojovic B, Denisova GF, Kwiecien JM, Brain I, Derocher H, Milne K, Nelson BH, Bramson JL. The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity. Nat Commun. 2018;9(1):3049.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–5.
Bertoletti A, Tan AT. Challenges of CAR- and TCR-T cell–based therapy for chronic infections. J Exp Med. 2020;217(5):e20191663.
Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Pure E, Albelda SM, Epstein JA. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573(7774):430–3.
Torikai H, Reik A, Liu P-Q, Zhou Y, Zhang L, Maiti S, Huls H, Miller JC, Kebriaei P, Rabinovitch B, Lee DA, Champlin RE, Bonini C, Naldini L, Rebar EJ, Gregory PD, Holmes MC, Cooper LJN. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119(24):5697–705.
Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, Prunkard D, Colunga AG, Hanafi L-A, Clegg DO, Turtle C, Russell DW. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol. 2017;35(8):765–72.
Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27(4):523–31.
Albert S, Koristka S, Gerbaulet A, Cartellieri M, Arndt C, Feldmann A, Berndt N, Loureiro LR, von Bonin M, Ehninger G, Eugster A, Bonifacio E, Bornhauser M, Bachmann MP, Ehninger A. Tonic signaling and its effects on lymphopoiesis of CAR-armed hematopoietic stem and progenitor cells. J Immunol. 2019;202(6):1735–46.
Sachdeva M, Duchateau P, Depil S, Poirot L, Valton J. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J Biol Chem. 2019;294(14):5430–7.
Sterner RM, Cox MJ, Sakemura R, Kenderian SS. Using CRISPR/Cas9 to knock out GM-CSF in CAR-T cells. J Vis Exp. 2019. https://doi.org/10.3791/59629.
Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, Hansen MJ, Jin F, Ayasoufi K, Hefazi M, Schick KJ, Walters DK, Ahmed O, Chappell D, Sahmoud T, Durrant C, Nevala WK, Patnaik MM, Pease LR, Hedin KE, Kay NE, Johnson AJ, Kenderian SS. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709.
Liu B, Song Y, Liu D. Clinical trials of CAR-T cells in China. J Hematol Oncol. 2017;10(1):166.
Wei J, Han X, Bo J, Han W. Target selection for CAR-T therapy. J Hematol Oncol. 2019;12(1):62.
Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to pd1 inhibition. Clin Cancer Res. 2017;23(9):2255–66.
Hernandez S, Qing J, Thibodeau RH, Du X, Park S, Lee HM, Xu M, Oh S, Navarro A, Roose-Girma M, Newman RJ, Warming S, Nannini M, Sampath D, Kim JM, Grogan JL, Mellman I. The kinase activity of hematopoietic progenitor kinase 1 is essential for the regulation of t cell function. Cell Rep. 2018;25(1):80–94.
Simon B, Harrer DC, Schulerthurner B, Schaft N, Schuler G, Dorrie J, Uslu U. The siRNA-mediated downregulation of PD-1 alone or simultaneously with CTLA-4 shows enhanced in vitro CAR-T-cell functionality for further clinical development towards the potential use in immunotherapy of melanoma. Exp Dermatol. 2018;27(7):769–78.
Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol. 2014. https://doi.org/10.3389/fphar.2014.00235.
Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol. 2014. https://doi.org/10.3389/fphar.2014.00254.
Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision tumor recognition by t cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770–9.
Malik N, Rao MS. A review of the methods for human iPSC derivation. Methods Mol Biol. 2013;997:23–33.
Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19.
Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181-192.e185.
Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14(2):213–22.
Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125(17):2605–13.
Laurenti E, Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553(7689):418–26.
Chabannon C, Kuball J, Bondanza A, Dazzi F, Pedrazzoli P, Toubert A, Ruggeri A, Fleischhauer K, Bonini C. Hematopoietic stem cell transplantation in its 60s: a platform for cellular therapies. Sci Transl Med. 2018;10(436):eaap9630.
Dong F, Hao S, Zhang S, Zhu C, Cheng H, Yang Z, Hamey FK, Wang X, Gao A, Wang F, Gao Y, Dong J, Wang C, Wang J, Lan Y, Liu B, Ema H, Tang F, Göttgens B, Zhu P, Cheng T. Differentiation of transplanted haematopoietic stem cells tracked by single-cell transcriptomic analysis. Nat Cell Biol. 2020;22:630.
Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, Cowen EW, Freeman AF, Olivier KN, Uzel G, Zelazny AM, Daub JR, Spalding CD, Claypool RJ, Giri NK, Alter BP, Mace EM, Orange JS, Cuellar-Rodriguez J, Hickstein DD, Holland SM. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–21.
Kernfeld EM, Genga RMJ, Neherin K, Magaletta ME, Xu P, Maehr R. A single-cell transcriptomic atlas of thymus organogenesis resolves cell types and developmental maturation. Immunity. 2018;48(6):1258-1270.e1256.
Liu H, Pan C, Song W, Liu D, Li Z, Zheng L. Novel strategies for immuno-oncology breakthroughs with cell therapy. Biomark Research. 2021;9(1):62.
Qiu Y, Chen T, Hu R, Zhu R, Li C, Ruan Y, Xie X, Li Y. Next frontier in tumor immunotherapy: macrophage-mediated immune evasion. Biomarker Res. 2021;9(1):72.
Eladl E, Tremblay-LeMay R, Rastgoo N, Musani R, Chen W, Liu A, Chang H. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13(1):96.
Pan C, Liu H, Robins E, Song W, Liu D, Li Z, Zheng L. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol. 2020;13(1):29.
Tian Y, Li Y, Shao Y, Zhang Y. Gene modification strategies for next-generation CAR T cells against solid cancers. J Hematol Oncol. 2020;13(1):54.
Xu J, Niu T. Natural killer cell-based immunotherapy for acute myeloid leukemia. J Hematol Oncol. 2020;13(1):167.
Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol. 2020;13(1):168.
Jiang Z, Sun H, Yu J, Tian W, Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol. 2021;14(1):180.
Acknowledgements
We thank Linna Chen and Yimeng Du for assistance with thoughtful discussion. The authors thank Department of Translational Medicine Center (the First Affiliated Hospital of Zhengzhou University) for their support.
Funding
This work was supported by National Natural Science Foundation of China (No. 82100240, RQ. Guo), the China Postdoctoral Science Foundation (2021M692929, RQ. Guo), Key scientific research projects of colleges and universities in Henan Province (225320016, RQ. Guo), the Postdoctoral Research Start-up Funding of Henan Province (202001006, RQ. Guo), Joint Co-construction Project of Henan Medical Science and Technology Research Plan (LHGJ20200280, RQ. Guo), Provincial and Ministry Joint Co-construction Project of Henan Medical Science and Technology Research Plan (SBGJ202103045, RQ. Guo), Postdoctoral Research Start-up Funding of the First Affiliated Hospital of Zhengzhou University (RQ. Guo), Key Research and Development and Promotion Project of Henan province (RQ. Guo), Key scientific research projects of colleges and universities in Henan Province (No. 18B310026, ZX. Jiang), and The medical science and technology research project of Henan province (No. 201701004, ZX. Jiang).
Author information
Authors and Affiliations
Contributions
GR, SY, and JZ designed the study. GR drafted the manuscript. All authors were involved in manuscript preparation and revisions. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
No individual data were used in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, R., Li, W., Li, Y. et al. Generation and clinical potential of functional T lymphocytes from gene-edited pluripotent stem cells. Exp Hematol Oncol 11, 27 (2022). https://doi.org/10.1186/s40164-022-00285-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-022-00285-y