Abstract
CD47, or integrin-associated protein, is a cell surface ligand expressed in low levels by nearly all cells of the body. It plays an integral role in various immune responses as well as autoimmunity, by sending a potent “don’t eat me” signal to prevent phagocytosis. A growing body of evidence demonstrates that CD47 is overexpressed in various hematological malignancies and its interaction with SIRPα on the phagocytic cells prevents phagocytosis of cancer cells. Additionally, it is expressed by different cell types in the tumor microenvironment and is required for establishing tumor metastasis. Overexpression of CD47 is thus often associated with poor clinical outcomes. CD47 has emerged as a potential therapeutic target and is being investigated in various preclinical studies as well as clinical trials to prove its safety and efficacy in treating hematological neoplasms. This review focuses on different therapeutic mechanisms to target CD47, either alone or in combination with other cell surface markers, and its pivotal role in impairing tumor growth and metastatic spread of various types of hematological malignancies.
Similar content being viewed by others
Background
Structure and expression of CD47
CD47 is a heavily glycosylated 50 Kd cell surface protein belonging to the immunoglobulin family, originally named integrin-associated protein (IAP) [1]. It has an extracellular N-terminal IgV domain, five transmembrane domains, and a short C-terminal cytoplasmic tail. The three domains are variable between humans and animals in terms of total amino acid composition, giving four alternative isoforms [2]. CD47 is expressed by virtually all cells in the body, including those that do not express integrins, such as erythrocytes. Therefore, it is now more appropriate to refer to it as CD47 rather than IAP [3]. CD47 can be found in a bigger, more complex form associated with heparin and chondroitin sulfate glycosaminoglycan. This form is expressed in both human and murine T-cells as well as endothelial cells and is responsible for signal inhibition after binding of T-cells to thrombospondins (TSPs) [4].
Mechanism of function and intracellular signalling
CD47 plays an essential role in various cellular functions including proliferation, apoptosis, adhesion, migration, and numerous immune responses. This occurs via cell-cell as well as cell-extracellular matrix interactions. Sick and colleagues published an exhaustive review of the biological functions of CD47 [5]. These functions are mediated through the binding of CD47 to its extracellular ligands such as signal regulatory proteins (SRPs) [6, 7] and thrombospondins (TSPs) [4, 5, 8]; membrane ligands such as integrins [9], vascular endothelial growth factor receptor-2 (VEGFR-2) [4], CD36 [8], and Fas (CD95) [10]; as well as intracellular ligands such as Gi proteins [11], BNIP3 [6], and Src and mitogen-activated protein kinases (MEK) [9] and protein 4.2 [12]. Figure 1 summarizes the CD47 structure and its ligands.
CD47 Structure and binding partners. CD47 is a transmembrane protein with 5 transmembrane domains, short intracytoplasmic C-terminal and N-terminal extracellular immunoglobulin variable (IgV) domain. Schematic diagram showing that CD47 can interact with αvβ3 integrin on the same cell or with SIRPα on the phagocytic cell through its IgV domain and activates “don’t eat me” signal. CD47 can also bind thrombospondin-1 (TSP) in the N-terminal, which promotes an interaction between CD47 and αvβ3 integrin and triggers αvβ3 integrin signaling
Interaction with extracellular ligands
SIRPα
is a transmembrane glycoprotein expressed on myeloid cells such as granulocytes, monocytes, macrophages, dendritic cells, and their precursors, including hematopoietic stem cells, as well as neuronal cells [13]. It is also known as CD172a, Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 (SHPS-1) or brain immunoglobulin-like molecule with tyrosine-based activation motifs (BIT). It consists of an extracellular N-terminal domain composed of three immunoglobulin-like and a cytoplasmic domain, which has two tyrosine phosphorylation sites and four immunoreceptor tyrosine inhibitory motifs (ITIMs) [14].
Binding of CD47 to N-terminal of SIRPα on the phagocytic cells induces a phosphorylation reaction of the ITIM. This activates protein tyrosine phosphatases (PTPases) Src homology region 2 (SH2) domain-containing phosphatase1(SHP-1) and 2(SHP-2) [15]. Subsequently, dephosphorylation of immunoreceptor tyrosine activation motifs (ITAM) prevents the contractile engulfment by the macrophages and gives a “don't eat me” signal to the innate immune system, a new signal that cancers seem to use to evade detection and destruction by the immune system [16].
TSPs
are extracellular matrix calcium-binding glycoproteins regulating cell motility, proliferation, and differentiation [17]. Five isoforms of TSPs are currently known; TSP-1 is the first endogenous ligand identified for CD47. It is secreted by vascular and inflammatory cells that regulate cellular functions. It is a major component of platelet α granules and is released upon activation [18]. Binding of TSP-1 through its C-terminal binding domain (CBD) peptide 4N1K, to extracellular IgV of CD47, is responsible for several biological processes such as inflammation, immune responses, cellular proliferation, apoptosis, adhesion, and migration [19]. This binding also has a role in thrombus formation through platelet activation and aggregation as well as homeostasis. The antiangiogenic properties of TSP-1 come from blocking the nitric oxide (NO) pro-survival responses in endothelial and vascular smooth muscle cells (VSMC) [20]. TSP1-CD47 has been reported to inhibit NO signaling in several cell types and consequently stimulate osteoclastogenesis [21]. Disruption of CD47-TSP-1 interaction by TSP-1-blocking antibodies or down-regulation of CD47 on tumor cells by RNA interference abrogates tumor-induced osteoclast formation in multiple myeloma [22]. It has also been shown that disruption of the TSP-1/CD47 interaction has positive outcomes in cancer therapy [23].
Integrins:
CD47 was initially found to interact with αvβ3 integrins [1]. However, it was then shown to also interact with other integrins such as α2β1—which has a role in migration and proliferation of smooth muscle cells [24], α4β1—which has a binding domain to N-terminal domain of TSP-1 and TSP-2 and a role in adhesion of reticulocytes [25], α5β1—which is involved in chondrocytes mechano-transduction [26], and α6β1—which has a role in fibrillar β-amyloid-mediated microglia activation and phagocytosis [27].
VEGFR-2:
Considering its ubiquitous expression, CD47 plays a central role in the tumor microenvironment (TME) [4]. Binding of CD47-TSP-1 inhibits VEGFR-2 phosphorylation and its downstream signaling, without affecting VEGF binding. Inhibition of tumor angiogenesis could be the rationale of the potential therapeutic effect of CD47 as an anti-cancer therapy [4].
CD36
is necessary for inhibition of some angiogenic responses of TSP-1 and is therefore assumed to be the receptor that mediates its anti-angiogenic activities after CD47 binding [8, 20].
Fas (CD95)
is expressed by various cell types and mediates apoptosis as part of the normal physiology and in response to various stimuli. The modulation of the level of expression of CD47 influences Fas-mediated apoptosis [28]. For example, Jurkat T cells lacking CD47 are resistant to apoptosis mediated by Fas but are killed by Fas-ligand upon expression of CD47 [10]. The association of Fas with the extracellular IgV domain of CD47 causes downstream activation of Fas-mediated apoptosis pathway. Subsequently, cytochrome c release from the mitochondria with loss of mitochondrial membrane potential and DNA cleavage. Thus, CD47 enhances downstream activation of caspase-dependent death pathways [10].
Interaction with intracellular ligands
Gi proteins
Many of the cellular effects of CD47 are mediated by Gi proteins. It is hypothesized that binding of CD47 (which holds five transmembrane spanning segments) to integrins that have two transmembrane domains forms a seven-transmembrane receptor complex. Ligation of this complex with an adhesive ligand or TSP could in turn activate G-protein signal transduction. Other evidence suggests that CD47 ligation, even without interaction with integrin, could activate G-protein signaling [29].
BNIP3
(BCL-2 homology 3-only protein 19 kDa interacting protein-3) is a pro-apoptotic member of the BCL-2 family [6]. It mediates CD47-induced T-cell apoptosis triggered by TSP-1, which is independent of caspase activation and cytochrome c release and characterized by a plasma membrane and mitochondrial damage occurring prior to chromatin condensation and DNA fragmentation [6, 18].
Role of CD47 in hematological neoplasms
The following section will address the role of CD47 in hematological malignancies. The ubiquitous high level of CD47 gene expression in many hematological malignancies and its impact on the clinical outcomes and prognosis will be discussed. Diverse therapeutics, including monoclonal antibodies to CD47 or SIRPα, receptor decoys, and bispecific antibodies that block the CD47-SIRPα axis have demonstrated significant anti-tumor activity in preclinical models of various hematological neoplasms [30, 31]. This supported many preclinical and clinical trials that have investigated the safety and the efficacy of anti-CD47 in treating most of the hematological malignancies.
Role of CD47 in NHL
Many studies have demonstrated the important role of CD47 in various types of non-Hodgkin lymphomas.
B-cell lymphomas:
CD47 is variably expressed in many subsets of B-cell NHL including diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia (CLL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL) [32]. In cases of DLBCL, CD47 expression is significantly higher in activated B-cell like (ABC) compared with germinal center B-cell like (GCB) subtypes and does not carry independent prognostic value within GCB and ABC subtypes. However, CD47 expression remains an independent predictor of disease progression in multivariate analysis with the international prognostic index for DLBCL. In cases of CLL, higher levels are associated with unmutated Ig heavy chain variable regions, an adverse prognostic factor. In MCL, CD47 level is significantly correlated with the proliferative index [32].
Interestingly, the dissemination of tumor cells in a Burkitt lymphoma (BL) model was dependent on the level of CD47 expression. Also, CD47 expression was increased in disseminated tumoral cells in control DLBCL xenograft animals, as well as in peripheral blood lymphoma cells compared with lymph node tumoral cells from NHL patients (although note peripheral blood and lymph node cells were not harvested from the same patients), further supporting a role for CD47 in the dissemination of B-NHL tumor cells [33].
DLBCL and FL cancer cells express a high level of CD47 with an antiphagocytic signal that allows them to evade the immune response [34]. A recent phase 1b trial showed that the monoclonal antibody Hu5F9-G4 in combination with rituximab induced a high rate of tolerable and durable complete responses in heavily pretreated patients with rituximab-refractory DLBCL and FL. Blocking CD47-SIRPα interaction induced the phagocytic cells to recognize and attack cancer cells and this response was augmented by adding rituximab (please see “Targeting of CD47-SIRPα using antibodies” section for more details) [34].
T-cell lymphomas (TCL):
Jain et al. demonstrated in their study that CD47 is widely but variably expressed in human TCL cells lines and primary samples. In addition, major histocompatibility complex class 1 (MHC-1) was widespread among the lymphoma cell lines and suppressed the phagocytosis of tumor cells. Monoclonal antibodies targeting CD47-SIRPα interaction (SRF231, B6H12, MOPC-21) promoted phagocytosis of TCL cells and improved outcomes in patient-derived xenograft and immunocompetent TCL mouse models. There was a synergistic effect when combining with an antibody targeting MHC class I (W6/32) [35]. Flow cytometry performed on 25 patients with Sezary syndrome (SS) showed higher CD47 expression in tumor cells compared with benign T-cells from the same individual. Patients with high expression of CD47 had 6.5 times shorter overall survival (OS) than patients with low expression (median OS, 84.0 vs 12.9 months; P < .001) [36].
Primary effusion lymphoma (PEL):
Goto et al. showed that CD47 is highly expressed by flow cytometry in PEL cell lines. Furthermore, therapeutic knockdown of CD47 in PEL cell lines and anti-CD47 antibodies promoted the phagocytosis of the lymphoma cells in vitro. Treatment of a xenograft mouse model with anti-CD47 antibody (B6H12.2) inhibited ascites formation and organ invasion [37].
Role of CD47 in leukemia
Lymphoblastic lymphoma/Acute lymphoblastic leukemia (LBL/ALL:
CD47 was increased in B-ALL and T-ALL patient samples and the expression level correlated with worse outcomes. Higher CD47 expression also correlated with worse OS in a cohort of patients and in an independent gene expression dataset. In vitro, ex vivo, and in vivo experiments demonstrated the therapeutic effect of anti-CD47 antibody B6H12.2 and BRIC126 against human ALL cells [30].
CD47 expression by immunohistochemistry (IHC) and by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was significantly higher in lymph nodes involved by T-LBL/ALL compared with reactive lymph nodes. The levels were significantly higher in patients ≤25 years old. The overall 1-year survival rate was lower in patients with high levels of CD47 or programmed cell death ligand (PD-L1) proteins by IHC and mRNA by qRT-PCR. High expression of CD47 and PD-L1 proteins were independent prognostic factors by multivariate analysis. There was a positive correlation between CD47 and PD-L1 mRNA expression but not protein expression [38]. Another study in transgenic mouse models and MYC-induced T-ALL cell lines showed that MYC regulates CD47 and PD-L1 mRNA and its protein expression by binding to its promoter genes. Therefore, CD47 and PD-L1 upregulation could have a direct role in MYC-driven tumorigenesis, which has implications for other MYC-driven malignancies [39].
Acute myeloid leukemia (AML):
CD47 is highly expressed in AML leukemic stem cells and AML cells. Interestingly, CD47 mRNA had lower expression in cases harboring t (8;21), a favorable risk translocation, whereas higher expression is strongly correlated with FLT3-ITD mutations that confer worst survival in AML with normal cytogenetics. High CD47 expression was an independent prognostic factor for poor OS in two adult cohorts of AML patients. Monoclonal antibodies against CD47 (B6H12.2 and BRIC126) enabled phagocytosis of AML leukemic stem cells in vitro and inhibited their growth in mice models [40, 41].
Magrolimab, anti-CD47 antibody, has been shown to be effective and tolerated when combined with azacitidine in AML and myelodysblastic syndrome (MDS) patients [42].
Pietsch and colleges generated a panel of anti-CD47 antibodies using hybridoma and phage display technologies. They evaluated their activity and affinity to SIRPα of human and cynomolgus monkey CD47, as well as their capacity to induce hemagglutination and platelet aggregation. The majority of these mAbs were potent SIRPα blockers, only few did not induce hemagglutination or platelet aggregation. They found that 10 mg/kg of IgG1 C47B157, C47B161, and C47B222 suppressed leukemia growth in their xenograft human AML mice models, C47B222 showed the most consistent activity. However, a non-human study showed significant anemia after two doses of 1 mg/kg, raising concerns about potential toxicity [43].
Brierley et al. reported a decline in Hb levels in all patients (median Hb change, −1.0 g/dL; range, 0.4–1.6) and increased transfusion requirements with the administration of anti-CD47 Hu5F9-G4 in a phase 1 study of nineteen patients with relapsed/refractory AML (NCT02678338). Eighteen developed a newly positive direct antiglobulin test (DAT); however, there was no laboratory evidence of hemolysis [44]. The NCT02641002 trial, a phase 1 trial using the monoclonal anti-CD47 CC-90002 in treating relapsed and/or primary refractory AML and high-risk MDS patients was terminated because it did not offer a sufficiently encouraging profile for further dose escalation/expansion.
These early results suggest that perhaps different classes of anti-CD47 therapeutic drugs, or combinations with other types of anti-leukemia therapies, should be explored in future AML studies.
Role of CD47 in multiple myeloma
An analysis of patient samples with multiple myeloma (MM) (171 patients) and monoclonal gammopathy of undetermined significance (MGUS) (18 patients) showed that CD47 mRNA levels increased with progression from MGUS to MM [45]. Interestingly, comparison of samples in 6 patients with extramedullary lesions showed that extramedullary plasma cells show little to no expression of CD47 compared to bone marrow plasma cells, suggesting that the role of CD47 is less important once cells migrate outside the bone marrow environment. This particular study did not find an association between CD47 expression and OS [46]. However, a very recent study has reported that CD47 MM cells had remarkably higher CD47 expression than other cell populations in the bone marrow. These findings indicate that CD47 is specifically expressed on MM and can be used as a potential therapeutic target. They also showed that blocking of CD47 using an anti-CD47 antibody-induced immediate activation of macrophages and eliminated MM cells in the 3D-tissue engineered bone marrow model, as early as 4 h [47].
Kim et al. [48] demonstrated in their study of 37 patient samples that myeloma cells express higher levels of CD47 by flow cytometry compared with patient-matched normal bone marrow cells. CD47 was also consistently expressed in various myeloma cell lines. Blocking CD47 with B6H12 antibodies increased phagocytosis of myeloma cells in vitro. In their mice engrafted MM models, anti-CD47 Ab B6H12 inhibited the growth of myeloma cells and led to significant tumor regression and eradication, with a remission rate of 72% vs 19% in the control group at 6 weeks. Irradiation of mice before myeloma cell transplantation inhibited the efficacy of anti-CD47 antibodies delivered 2 weeks after radiation, and analysis of the bone marrow, spleen, and liver revealed that progeny of radiation-sensitive hematopoietic cells, and not only radiation-resistant resident macrophages, were necessary for CD47 antibody-mediated therapeutic effect [48].
Rastgoo and colleagues reported that high CD47 expression by IHC was associated with 17p (p53) deletions (p = 0.0407) and elevated beta-2 microglobulin level (p = 0.0323) in a cohort of 74 newly diagnosed MM patients [49]. High H-scores were associated with shorter median progression-free survival (PFS) and OS. This was also confirmed by analysis of CoMMpass database of 676 patients where high CD47 mRNA levels correlated with decreased PFS and OS. CD47 expression was also higher in drug-resistant cell lines, suggesting a potential role in drug response in MM [49].
Therapeutic approaches for targeting CD47 in hematological malignancies
As described in the previous sections, CD47 is overexpressed in a variety of hematological cancers and plays a significant role in tumor evasion of immune surveillance [50]. There are many efforts in trying to target CD47 at different points in its signaling pathway for the purpose of cancer treatment. We will focus on pre-clinical and clinical studies targeting the CD47 axis in hematological malignancies. Tables 1 and 2 summarize the data.
Targeting CD47-SIRPα
As described earlier, CD47 and SIRPα are both transmembrane proteins that interact with each other as well as with the TME. SIRPα is abundant in myeloid cells such as macrophages and dendritic cells (DCs). Murata et al. reviewed the CD47- SIRPα axis in depth and its potential applications for cancer therapy [50].
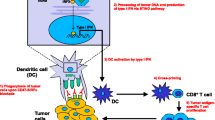
A growing evidence that the blockade of CD47-SIRPα interaction “don’t eat me” signal enhances the activity of phagocytes toward tumor cells in vitro as well as in a variety of xenograft models of cancer [55]. This blockade promotes the stimulation of tumor-specific cytotoxic T-cells, macrophages, or DCs. Biological agents targeting CD47-SIRPα, such as antibodies (Abs), recombinant peptide agonists, and miRNAs have been tested in preclinical and clinical trials. Such targeting modulates both innate and acquired immune responses and is considered a promising strategy for cancer treatment [68]. Combinations of molecules targeting CD47 and other hematological surface markers were also extensively studied and have shown synergistic effects [56]. Figure 2 summarizes different mechanisms of targeting CD47-SIRPα interaction.
Therapeutic approaches for targeting CD47 in hematological malignancies. Blockade of CD47-SIRPα interaction (blocking “don't eat me” signal) through different approaches including antibodies, recombinant peptide agonists, miRNAs, and QPCTL inhibitors enhances the activity of phagocytes toward tumor cells (activating “eat me” signal). Dual blockade of CD47 and other hematological surface markers by antibody therapies is another approach to synergistically target tumor cells
Targeting of CD47-SIRPα using antibodies
Hu5F9-G4
(Hereafter, 5F9), a macrophage immune checkpoint inhibitor, promotes tumor cell phagocytosis by the innate immune response through blocking of the CD47-SIRPα axis [34]. The most common adverse event (AE) of this humanized antibody is anemia due to its non-selective inhibition of CD47 on aging red cells. A phase1b study of 22 patients with heavily pre-treated rituximab-resistant DLBCL and FL were enrolled in their study. A total of 50% of the patients had an objective response, with 36% having a complete response. Most AEs during treatment were of grade 1 and 2, and the most common treatment-related AEs were chills in 9 patients, headaches in 9 patients, anemia in 9 patients, and infusion-related reactions in 8 patients. The treatment-related hemolytic anemia could be managed by giving a small priming dose of Hu5F9-G4 to eliminate the aging red cells and select for younger red cells (lacking prophagocytic signals), followed by a higher maintenance dose [34]. These results corroborate the beneficial effects of adding anti-CD47 antibodies to lymphoma treatments, especially when most of AEs were tolerated.
CC-90002
is another humanized IgG4 anti-CD47 antibody that inhibits CD47-SIRPα interaction and enabled phagocytosis in cancer cell lines, including hematological cancer cell lines, as well as in solid tumor xenografts [69]. In a current phase 1 multicenter study (NCT02367196), CC-90002 was combined with rituximab to treat CD20-positive relapsed/refractory NHL. Out of 28 enrolled subjects, 24 were treated with escalating doses of CC-90002 and rituximab. Anemia was common, but there was no evidence of hemolysis. The most frequent grade 3/4 AEs were neutropenia (38%) and thrombocytopenia (21%). Twenty subjects discontinued the study, mostly due to progressive disease or death. Seven deaths occurred; 6 from progressive diseases (PD) and 1 due to an AE. The overall response rate was 13%, with a median duration of response of 3.9 months [51].
SRF231
is a fully humanized monoclonal anti-CD47 antibody produced by phage technology. Data presented in an abstract showed it selectively blocks CD47- SIRP α, promoting phagocytosis of cancer cells and sparing T-cells and RBCs in vitro. Coadministration of anti-CD20 antibodies and SRF231 enhanced tumor phagocytosis in MM and lymphoma animal models [52]. A phase 1 study to evaluate the safety and tolerability of SRF231 as a monotherapy in solid and hematological malignancies is ongoing, but there are no clinical results available yet (NCT03512340).
B6H12.2
, a CD47 antibody, promoted phagocytosis of tumor cells in ALL cell lines. When ALL cells were coated with B6H12.2 ex-vivo, tumor cell engraftment in vivo was nearly abolished. Furthermore, it eliminated the mice engrafted ALL cells in peripheral blood, bone marrow, liver, and spleen. It could also induce long-term remissions in the treated mice [30]. Additionally, B6H12.2 was found to inhibit the extranodal dissemination of human BL Raji cells in mice models and a DLBCL xenograft model [33].
ALX148
antibody has been generated by fusing an inactivated human IgG1 Fc with a modified SIRPα D1 domain. It blocks the CD47-SIRPα interaction and stimulates both innate and acquired immune responses, promoting DCs, macrophage, and T-cell responses. Combination of ALX148 with obinutuzumab (anti-CD20 Ab) in a mouse subcutaneous xenograft model of MCL resulted in enhanced tumor growth inhibition compared with either drug alone (p < 0.01), whereas the combination of ALX148 with rituximab-enhanced tumor growth inhibition and survival compared with rituximab alone in Raji B-cell lymphoma tumors (p < 0.001 and p < 0.0001). ALX148 did not cause hemagglutination of human red blood cells and had a good safety profile in a non-human primate toxicity study [53].
KWAR23
, another blocking Ab to human SIRP α, was inert when administered on its own, but enhanced the effect of rituximab in a human BL xenograft model [31].
Targeting of CD47-SIRPα using CD47 peptide agonists
4N1K and 4 N1
are mimicry peptides of CD47. 4N1K is analogous to the C-terminal part of TSP-1. 4 N1 and 4N1K are non-selective and may bind to receptors independent from CD47 [70]. 4N1K was shown to induce apoptosis in monocytes; surviving cells could differentiate to DCs, but those had decreased capacity to secrete inflammatory cytokines IL-12 and TNFα [54]. The 4N1K peptide triggered caspase-independent apoptosis in Jurkat T cells through a G protein-mediated reduction of c-AMP levels and inhibition of protein kinase-A (PKA) [11].
SIRPα-IgG1 Fc
(TTI-621) is a checkpoint inhibitor that binds to human CD47 in tumor cells. As a decoy receptor protein, it is composed of N-terminal V domain of human SIRPα linked to the Fc region of human immunoglobulin G1 (IgG1). It prevents inhibitory signals to macrophages by binding to Fc gamma receptors (FcgR) and enhancing phagocytosis of tumor cells. It effectively controlled the growth of aggressive cancer cells and promoted macrophage phagocytosis of 77% (23/30) of hematological tumor cell lines. There was also enhanced phagocytosis in 97% (32/33) of primary samples from patients with hematological malignancies (AML, MDS, MM, B-ALL, and T-ALL). This effect appears to be tumor cell-specific. The antitumor activity of SIRPαFc (TTI-621) was detected in mouse AML and B-cell NHL xenografts models. There was an only minimal binding of human erythrocytes, which is of interest given the concerns for hemolytic anemia with CD47 mAbs [55].
The open-label phase 1a clinical trial (NCT02663518) used SIRPαFc (TTI-621) in five patients with SS. The patients were pretreated and had high leukemic counts. After single-dose infusion of TTI-621, 4 of 5 patients had a decrease in their count and a rapid decrease of lactate dehydrogenase (LDH). Additional experiments showed that SIRPαFc increased the phagocytosis of ex vivo Sezary cells extracted from patient blood, but not non-malignant lymphocytes [36].
SIRPα-IgG4 Fc
(TTI-622) is a soluble recombinant fusion protein created by directly linking the N-terminal CD47 binding domain of human SIRPα with the Fc domain of human immunoglobulin (IgG4). TTI-622 acts by binding human CD47 and preventing it from delivering an inhibitory “don’t eat me” signal to macrophages [56]. A clinical trial testing TTI-622 (NCT03530683) is currently recruiting patients. It will be conducted in two phases for patients with refractory lymphoma and MM. Phase 1a is a dose-escalation phase and phase 1b is a combination treatment phase. In phase 1b, TTI-622 will be given to subjects with CD20-positive NHL, classic Hodgkin lymphoma (cHL), and MM, in combination with other anti-cancer drugs to define their safety and efficacy. The combination treatments are TTI-622 + rituximab in DLBCL and indolent NHL patients, TTI-622 + PD-1 inhibitor nivolumab in cHL, and TTI-622 + Proteasome-inhibitor carfilzomib + dexamethasone in MM. The results are not available yet. Lin and colleges [71] demonstrated the efficacy of TTI-622 in their animal models of DLBCL, BL, and MM. TTI-622 monotherapy showed improved OS and partial tumor growth regression. Combination therapy of TTI-622 with daratumumab (anti-CD38 antibody) and cetuximab (anti-EGFR antibody) potentiates its therapeutic efficacy. TTI-622 associated risk of anemia is minimal because it does not induce hemagglutination and has minimal binding to human erythrocytes [71].
PKHB1
is a TSP1-derived CD47 agonist peptide. PKHB1 induced caspase-independent and calcium-dependent cell death in T-ALL cell lines. It was also tested in an immunocompetent leukemic mouse model, where it induced immunogenic cell death by promoting DC maturation and the release of several damage-associated molecular patterns (DAMPs) such as calreticulin (CRT), heat shock proteins 70, 90 (HSP70 and HSP90), ATP, and high-mobility group box-1 (HMGB1). This was further confirmed by giving mice a prophylactic vaccine of tumor cells previously treated with PKHB1, which prevented tumor engraftment. In tumor-bearing mice, PKHB1 treatment could induce complete tumor regression in most mice [57]. Treatment of tumor-bearing mice with the tumor cell lysate obtained from PKHB1-treated cells could similarly induce tumor regression, improve OS, and protect the mice from further relapse [58].
Targeting of CD47-SIRPα using microRNA-based approaches
Studies showed that microRNAs (miRNAs) encapsulated into liposome-protamine-hyaluronic acid nanoparticles could regulate CD47 expression levels, and results from animal models of solid tumors suggested that they could represent potential cancer strategies [72, 73]. In hematological malignancies, Huang et al. used Target Scan prediction and studied the microarray expression patterns of miRNA in cases of human ALL. They selected five miRNAs (miR-15a/b, miR-128, miR-143, and miR-708) with different expression patterns at diagnosis and relapse or remission of T-ALL. Among those five, miR-708 showed the most significant effects on luciferase activity of CD47, suggesting potent inhibition. This finding was also confirmed by miR-708 overexpression and knockdown experiments in various cell lines. Analysis of primary patient T-ALL samples confirmed the inverse correlation between miR-708 levels and CD47 mRNA expression. A combination of miR-708 and CD47 antibodies caused greater phagocytosis and apoptosis than either agent alone, suggesting a synergistic effect [60].
Our group recently used bioinformatics strategies to identify miR-155 as a potential regulator of CD47 [49]. We used luciferase assay, overexpression, and functional rescue assays to further demonstrate that miR-155 targets CD47 and that increased miR-155 expression could consequently induce phagocytosis of drug-resistant MM cells. Moreover, we showed that miR-155 also targets the tumor necrosis factor-alpha-induced protein 8 (TNFAIP8), which is a negative regulator of apoptosis. Downregulation of TNFAIP8 sensitized the MM cells to the protease inhibitor bortezomib (BTZ) and promoted the apoptosis of myeloma cells. Restoration of miR-155 levels in their xenograft mice model of resistant MM combined with Bortezomib suppressed tumorigenesis and extended overall survival. IHC showed an increase in the apoptotic index compared to Bortezomib or miR-155 mimics alone. These results support the fact that downregulated miR-155 plays an essential role in the pathogenesis of drug resistance in MM through upregulation of both CD47 and TNFAIP8, and that it represents a promising therapeutic strategy [49].
Targeting QPCTL
Glutaminyl peptide cyclotransferase-like protein (QPCTL) is a newly discovered major protein component of the CD47 signaling pathway. It is essential for forming pyroglutamate on the CD47-SIRPα binding site. Logtenberg and colleges showed in their study that SEN177, a glutaminyl cyclase inhibitor, enhanced antibody-dependent cellular phagocytosis of anti-CD20-treated BL (Raji) cells to the same or greater extent than combining rituximab with 12C4 (SIRPα-blocking agent) of B6H12. In a syngeneic peritoneal tumor model of HER2-expressing Ba/F3 pro-B cells, there was a significant increase of neutrophil-mediated selective killing of QPCTL- (and CD47-) deficient cells treated with anti-HER2. This anti-tumor activity was like a full genetic deficiency of CD47. QPCTL inhibitors represent a promising anti-cancer strategy that could potentially avoid the antigen sink of other anti-CD47 drugs due to CD47 expression on erythrocytes and other cells [59].
Dual blockade of CD47 and other hematological malignancies markers
As presented in previous sections, dual blockade of CD47 and other hematological surface markers already targeted by antibody therapies was shown to have synergistic effects. This section will address bispecific antibodies that target two markers at the same time. It has been hypothesized that these antibodies could be more specific for neoplastic cells and avoid side effects such as hemolytic anemia or thrombocytopenia that result from the relatively high expression of CD47 on red blood cells and platelets [61].
Dual blockade of CD47 and PD-L1
As previously described, CD47 regulates the innate immunity and overexpression on tumor cells and TME promotes evasion of the immune surveillence [5]. On the other hand, PD-L1, is normally expressed on antigen-presenting cells (APCs) and pathologically expressed on diverse types of cancer cells and TME [74]. It interacts with the programmed cell death-1 (PD-1) receptor expressed on the surface of cytotoxic T-cells, thereby inhibiting T-cell-mediated immunity. Boussiotis extensively reviewed the PD-1/PD-L1 axis and its potential role in cancer therapy [74]. Previous studies showed enhanced anti-tumor effect with dual targeting of both CD47 and PD-L1 in melanoma and colon carcinoma [75, 76]. Lian and colleagues designed an Ep-CAM (epithelial cell adhesion molecule) liposome containing both CD47 and PD-L1 arms. It targets high-Ep-CAM cancer cells and effectively knocks down both CD47 and PD-L1 proteins. This therapeutic effect was proved in solid tumor cell lines and mice lung cancer models [77]. To our knowledge, this strategy has yet to be tested in hematological malignancies but given the known role of PD-L1 in numerous hematological malignancies, it likely represents a viable line of investigation [62,63,64,65].
Dual blockade of CD47 and CD20
A study in B-NHL xenograft mouse models of BL cell line, as well as primary human DLBCL and FL, showed a synergistic therapeutic effect when combining anti-CD47 antibodies (B6H12.2 and BRIC126) with rituximab and allowed long-term disease-free survival [32].
Piccione and colleagues developed a bispecific antibody (BsAb) that selectively binds to CD20 and CD47 by using the dual-variable-domain immunoglobulin (DVD-Ig) format. Two variants of CD20-CD47 DVD-Ig were formed either by short (SL) or long (LL) linker sequence between the variable domains. In human cell lines, the BsAb showed selective binding to tumor cells with dual expression of CD20 and CD47. The SL form binds poorly to erythrocytes compared to anti-CD47 (B6H12.2) alone, which is advantageous to avoid the potential issue of hemolytic anemia. In mice engrafted human NHL, the SL form showed potent antitumor activity and prolonged survival compared with anti-CD47 or rituximab alone, recapitulating the synergistic effect seen with a combination of anti-CD47 and rituximab [78]. They obtained similar results by grafting the SIRPα N-terminal Ig domain onto rituximab, who played the role of a tumor-specific scaffold [66].
Dual blockade of CD47 and CD19
Buatois et al. [61] designed a BsAb, NI-1701, that specifically and effectively targeted both CD19 and CD47. Flow cytometry confirmed strong binding of B-cells, without significant binding of T-cells or erythrocytes, and only limited binding of platelets. This antibody showed anti-tumor activity in vitro for cell lines of B-cell NHL (DLBCL, BL), ALL, and (CLL) and in vivo cell-derived xenograft BL model and patient-derived xenograft ALL model. It showed a 4.2 and 52-fold increase in the phagocytic activity against Raji cells when compared to anti-CD19 and anti-CD47 monovalent antibodies respectively. NI-1701 also showed a more potent anti-tumor effect than either monovalent antibodies alone in mice. NI-1701 was superior to rituximab monotherapy, but co-administration of NI-1701 and rituximab showed a synergistic effect, leading to significant tumor growth regression in mice models and increased survival compared with single agents and controls. NI-1701 also demonstrated a potent therapeutic effect in a variety of B-cell NHL and B-ALL primary patient samples and a patient-derived mouse model of B-ALL. It showed a favorable safety profile in cynomolgus monkeys [61].
Dual blockade of CD47 and CD70
CD70 shows only limited expression in normal cells but is expressed on most B-cell malignancies and in multiple myeloma, as well as other solid malignancies. It could represent a target in CD20-negative malignancies. Therefore, Ring and colleagues developed a bispecific anti-human CD70/KWAR23 antibody. It showed synergistic effect in renal carcinoma cell lines compared to anti-CD70 vorsetuzumab and anti-CD47 drugs alone or in combination, however its therapeutic effect did not outperform combination of the individual drugs when tested in a Burkitt lymphoma mouse model [31]. This suggests additional studies would be needed to better understand the function of this bispecific antibody.
Insights and challenges in targeting CD47 in hematological malignancies
CD47 is a target of interest and researchers are still trying to figure out what is the best way to target this pathway. Several anti-CD47 targeting agents have been developed and tested in many preclinical and clinical trials over a long-time scale. Such targeting successfully modulates both innate and acquired immune responses against tumor cells [79]. Despite promising results from most of these studies on the impact of anti-CD47 agents, challenges were found to be in selectivity, efficacy, and safety profile. Recent studies are trying to alleviate these challenges proving that CD47 targeting is a novel anti-cancer approach [80].
Specificity of anti-CD47 targeting agents
To minimize or avoid cross-reactivity and damage of normal cells due non-selectivity of anti-CD47 agents while exerting anti-cancer effects is a challenge that requires consideration while designing future anti-CD47 therapies [81]. Ho et al. produced high-affinity CD47-ectodomain antagonist to increase the antibody-dependent phagocytosis [82]. Moreover, Sim and colleges [83] discovered high-affinity pan-mammalian and pan-allelic antibodies against SIRPα. Given that older erythrocytes are more susceptible to phagocytosis, future studies of CD47-SIRPα targeting should consider patient age [84].
Fusion proteins such as SIRPα-IG1 Fc (TTI-621) was developed to avoid damage to normal cells by fusion of the N-terminal V domain of human SIRPα to the human IgG1 Fc region. At low therapeutic levels, it showed minimal binding affinity to human erythrocytes while exerting enough tumor binding [55].
Furthermore, combination therapies were developed to achieve increased tumor specificity and decreased toxicity to CD47-expressing non-malignant cells. For example, anti-CD47 antibodies (BRIC126 or B6H12) combined with anti-CD20 rituximab resulted in NHL ablation in xenograft models [32, 78]. Enhancement of the anti-tumor effects of high-affinity SIRPα-CD47 antibodies obtained when combined with tumor-specific antibodies [85]. Anti-SIRPα antagonists have also been combined with tumor-opsonizing antibodies such as rituximab showed anti-tumor efficacy in vitro [82] and in xenograft lymphoma and colon cancer models [86]. Similarly, targeting CD47 and PD-L1 [75, 76].
The development of BsAb improved the specificity and reduced the cytotoxicity of anti-CD47 agents. NI-1701, a BsAb that targets CD47 and CD19 was designed for B-cell lymphoma and refractory leukemia [61]. Other bispecific agents (LicMABs) have been produced by the binding domain of SIRPα to a tumor-targeting antibody such as anti-CD33 promoted elimination of AML tumor cells [67].
Novel methods for specific targeting CD47 and its ligands on tumor cells have been proved in recent studies. These included drug delivery vehicles such as CD47-conjugated nanoparticles [87] or quorum-sensing bacteria [88]. Such delivery resulted in T cell and macrophage induced phagocytosis of tumor cells by blocking CD47 and reduced tumor progression [87, 88]. Other nanoparticles as magnetic iron oxide have been developed as vehicles for selective simultaneous delivery of anti-CD47 antibodies and gemcitabine for the treatment of pancreatic cancer without cytotoxicity [89]. Mitomycin A-loaded nanoparticles showed downregulation of CD47 expression in xenografted mice [90]. Davis et al. combined anti-CD47 antibodies with nanoparticles to target ovarian cancer cells [91]. No update information about using such vehicles in targeting CD47 in hematological malignancies.
Efficacy of anti-CD47 targeting agents
The potency and the therapeutic effects of anti-CD47 agents varied between different studies according to the type of cancer, type of antibodies, stage of tumor, type of tumor model, drug pharmacokinetics, acquired drug resistanc,e and the state of the immune system [80]. Drug resistance and unsatisfactory results were in most cases related to tumor heterogeneity, tumor microenvironment changes, drug inactivation, decreased drug absorption, and epigenetic changes [92].
Toxicity and safety profile of anti-CD47 targeting agents
Given that CD47 is ubiquitously expressed by normal of the hematopoietic system [93] such as RBCs [94] and platelets [95], potential adverse events using anti-CD47 antibodies as cancer therapeutics include anemia and thrombocytopenia. Buatois et al. [61] showed that Hu47F9-G4 alone or in combination with other antibodies may cause accidental killing of normal hematopoietic cells. To alleviate this adverse effect, one study [34] proposed to give short priming low-dose of Hu5F9-G4 in combination with rituximab to selectively eliminate the aged red blood cells, followed by long-term treatment. The toxicity of anti-CD47 antibodies is proved to be Fc-dependent; SIRPα-Fc fusion proteins give this toxicity while high-affinity SIRPα generations don’t [85, 96, 97]. These high-affinity variants bind to CD47 with a greater potency compared with wild type SIRPα [85]. They showed regression of solid tumors and hematologic malignancies in preclinical trials but not yet in clinical trials [85, 98].
Conclusion and future perspectives
At the end of this review, we can conclude that CD47 is a novel promising target for cancer therapy. It is overexpressed in various hematological malignancies and plays a role in tumor dissemination. Strategies targeting the CD47-SIRPα axis, such as antibodies, inhibitory peptides, and miRNAs, demonstrated promising results for the treatment of hematological neoplasms. Combinations of CD47 inhibitory strategies and other anti-cancer therapies such as anti-CD20, anti-CD19, and others showed promising results suggesting a synergistic therapeutic effect. Early clinical phase 1/2 studies have shown encouraging therapeutic effects with tolerable AEs. Various strategies, such as priming with small doses to eliminate aging RBC, as well as many newly developed BsAbs, are being investigated trying to circumvent the hemolytic anemia and thrombocytopenia that can occur as a result of the expression of CD47 on red blood cells and platelets. Additional clinical trials are required to determine the clinical efficacy of these strategies. Moreover, certain aspects of targeting CD47 have not been fully investigated in hematological malignancies, such as the interaction between CD47 and TSP1, dual blockade of CD47 and PD-L1, targeting the QPCTL as proposed by Logtenberg and colleagues as well as the recently described nanoparticles delivery methods. This opens potential new avenues to target CD47 in hematological malignancies. Future advances in cancer screening would define which type and stage of cancer that could be treated with a specific type or types of anti-CD47 targeting agents.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ABC:
-
Activated B-cells
- Abs:
-
Antibodies
- AE:
-
Adverse Events
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- ATP:
-
Adenosine triphosphate
- BIT:
-
Brain immunoglobulin-like molecule with tyrosine-based activation motifs
- BL:
-
Burkitt Lymphoma
- BM:
-
Bone marrow
- BNIP3:
-
BCL2 Nineteen kD interacting protein 3
- BsAbs:
-
Bispecific antibodies
- c-AMP:
-
Cyclic adenosine monophosphate
- CBD:
-
C-terminal binding domain
- CD47:
-
Cluster designation or cluster of differentiation 47
- CLL:
-
Chronic lymphocytic leukemia
- CRT:
-
Calreticulin
- DAMPs:
-
Damage-associated molecular patterns
- DAT:
-
Direct Antiglobulin Test
- DC:
-
Dendritic cells
- DLBCL:
-
Diffuse large B-cell lymphoma
- DVD-Ig:
-
Dual-variable-domain immunoglobulin
- Ep-CAM:
-
Epithelial cell adhesion molecule
- FcgR:
-
Fc gamma receptors
- FL:
-
Follicular lymphoma
- GCB:
-
Germinal center B-cells
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- HMGB1:
-
High-mobility group box 1
- HSCs:
-
Hematopoietic stem cells
- HSP:
-
Heat shock protein
- IAP:
-
Integrin-associated protein
- ICD:
-
Immunogenic cell death
- IHC:
-
Immunohistochemical staining
- IL-12:
-
Interleukin 12
- IL-6:
-
Interleukin 6
- ITAM:
-
Immunoreceptor tyrosine activation motifs
- ITIM:
-
Immunoreceptor tyrosine inhibitory motifs
- KD:
-
Kilo-Dalton
- LBL:
-
Lymphoblastic Lymphoma
- LDH:
-
Lactate dehydrogenase
- LL:
-
Long linker sequence
- MCL:
-
Mantel cell lymphoma
- MDS:
-
Myelodysblastic Syndrome
- MEK:
-
Mitogen-activated protein kinase
- MGUS:
-
Monoclonal Gammopathy of Undetermind Significance
- Mi-RNA:
-
Micro-RNA
- MM:
-
Multiple myeloma
- MZL:
-
Marginal zone lymphoma
- NCT:
-
National Clinical Trial
- NHL:
-
Non-Hodgkin’s lymphoma
- NO:
-
Nitric oxide
- NTD:
-
Non-tolerated dose
- OS:
-
Overall survival
- PB:
-
Peripheral blood
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- PEL:
-
Primary effusion lymphoma
- PFS:
-
Progression-free survival
- PKA:
-
Protein kinase A
- PTPases:
-
Protein tyrosine phosphatases
- QPCTL:
-
Glutaminyl peptide cyclotransferase-like
- Q-RT-PCR:
-
Quantitative reverse transcriptase-polymerase chain reaction
- SH2:
-
Src Homology region 2
- SHP-1:
-
Src homology phosphatase-1
- SHP-2:
-
Src homology phosphatase-2
- Si-RNAs:
-
Silencing RNAs or small interfering RNAs
- SIRP:
-
Signal regulatory protein
- SL:
-
Short linker sequence
- SS:
-
Sezary syndrome
- T-LBL:
-
T-lymphoblastic leukemia
- TNFAIP8:
-
Tumor necrosis factor alpha-induced protein 8
- TNFα:
-
Tumor necrosis factor α
- TSP:
-
Thrombospondins
- VEGF:
-
Vascular endothelial growth factor
- VEGFR-2:
-
Vascular endothelial growth factor receptor-2
- VSMC:
-
Vascular smooth muscle cells
References
Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111:2785–94.
Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J Cell Sci. 1995;108:3419–25.
Oldenborg P-A. CD47: a cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol. 2013;2013:1–19.
Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–32.
Sick E, Jeanne A, Schneider C, Dedieu S, Takeda K, Martiny L. CD47 update: a multifaceted actor in the tumour microenvironment of potential therapeutic interest. Br J Pharmacol. 2012;167:1415–30.
Lamy L, Ticchioni M, Rouquette-Jazdanian AK, Samson M, Deckert M, Greenberg AH, et al. CD47 and the 19 kDA interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem. 2003;278:23915–21.
Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, et al. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009;284:1116–25.
Miller TW, Isenberg JS, Shih HB, Wang Y, Roberts DD. Amyloid-b inhibits no-cgmp signaling in a cd36- and cd47-dependent manner. PLoS One. 2010;5:1–10.
Shinohara M, Ohyama N, Murata Y, Okazawa H, Ohnishi H, Ishikawa O, et al. CD47 regulation of epithelial cell spreading and migration, and its signal transduction. Cancer Sci. 2006;97:889–95.
Manna PP, Dimitry J, Oldenborg PA, Frazier WA. CD47 augments fas/CD95-mediated apoptosis. J Biol Chem. 2005;280:29637–44.
Manna PP, Frazier WA. The mechanism of CD47-dependent killing of T cells: heterotrimeric Gi-dependent inhibition of protein kinase a. J Immunol. 2003;170:3544–53.
Bruce LJ, Ghosh S, King MJ, Layton DM, Mawby WJ, Stewart GW, et al. Absence of CD47 in protein 4.2– deficient hereditary spherocytosis in man: an interaction between the Rh complex and the band 3 complex. Blood. 2002;100:1878–85.
Van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175:7781–7.
Hatherley D, Graham SC, Turner J, Harlos K, Stuart DI, Barclay AN. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell. 2008;31:266–77.
Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003.
Murata Y, Kotani T, Ohnishi H, Matozaki T. The CD47-SIRPα signalling system: Its physiological roles and therapeutic application. J Biochem. 2014:335–44.
Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:1–29.
Broom OJ, Zhang Y, Oldenborg PA, Massoumi R, Sjölander A. CD47 regulates collagen I-induced cyclooxygenase-2 expression and intestinal epithelial cell migration. PLoS One. 2009.
Gao AG, Frazier WA. Identification of a receptor candidate for the carboxyl-terminal cell binding domain of thrombospondins. J Biol Chem. 1994;269:29650–7.
Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–80.
Lorenzo J, Choi Y, Horowitz M. Takayanagi H. Osteoimmunology: Osteoimmunology; 2011.
Kukreja A, Radfar S, Sun BH, Insogna K, Dhodapkar MV. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: implications for bone disease. Blood. 2009;114:3413–21.
Oaks J, Wang M, Zou H. Abstract 5623: Development of a CD47-blocking antibody as a cancer therapy. Cancer Res. 2018;78:5623 LP – 5623.
Wang XQ, Frazier WA. The thrombospondin receptor CD47 (IAP) modulates and associates with α2β1 integrin in vascular smooth muscle cells. Mol Biol Cell. 1998;9:865–74.
Brittain JE, Han J, Ataga KI, Orringer EP, Parise LV. Mechanism of CD47-induced α4β1 integrin activation and adhesion in sickle reticulocytes. J Biol Chem. 2004;279:42393–402.
Orazizadeh M, Lee HS, Groenendijk B, Sadler SJM, Wright MO, Lindberg FP, et al. CD47 associates with alpha 5 integrin and regulates responses of human articular chondrocytes to mechanical stimulation in an in vitro model. Arthritis Res Ther. 2008;10.
Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar β-amyloid through a β1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–46.
Strasser A, Connor LO, Dixit VM. APoptosis signaling. Annu Rev Biochem. 2000;69:217–45.
Brown E. Integrin-associated protein (CD47): an unusual activator of G protein signaling. J Clin Invest. 2001;107:1499–500.
Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–84.
Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114:E10578–85.
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713.
Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood. 2011;118:4890–901.
Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018;379:1711–21.
Jain S, Van Scoyk A, Morgan EA, Matthews A, Stevenson K, Newton G, et al. Targeted inhibition of CD47-SIRPa requires fc-FcgR interactions to maximize activity in T-cell lymphomas. Blood. 2019;134:1430–40.
Johnson LDS, Banerjee S, Kruglov O, Viller NN, Horwitz SM, Lesokhin A, et al. Targeting CD47 in Sézary syndrome with SIRPaFc. Blood Adv. 2019;3:1145–53.
Goto H, Kojima Y, Matsuda K, Kariya R, Taura M, Kuwahara K, et al. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer. 2014;50:1836–46.
Yang K, Xu J, Liu Q, Li J, Xi Y. Expression and significance of CD47, PD1 and PDL1 in T-cell acute lymphoblastic lymphoma/leukemia. Pathol Res Pract. 2019;215:265–71.
Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, et al. Erratum: MYC regulates the antitumor immune response through CD47 and PD-L1. Science (80). 2016;352.
Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–85.
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–99.
Chao MP, Takimoto CH, Feng DD, McKenna K, Gip P, Liu J, et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front Oncol. 2020;9.
Pietsch EC, Dong J, Cardoso R, Zhang X, Chin D, Hawkins R, et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 2017;7:e536–8.
Brierley CK, Staves J, Roberts C, Johnson H, Vyas P, Goodnough LT, et al. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion. 2019;59:2248–54.
Rendtlew Danielsen JM, Knudsen LM, Dahl IM, Lodahl M, Rasmussen T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol. 2007;138:756–60.
Muz B, Azab F, de la Puente P, Landesman Y, Azab AK. Selinexor overcomes hypoxia-induced drug resistance in multiple myeloma. Transl Oncol. 2017;10:632–40.
Sun J, Muz B, Alhallak K, Markovic M, Gurley S, Wang Z, et al. Targeting CD47 as a novel immunotherapy for multiple myeloma. Cancers. 2020;12.
Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–45.
Nasrin Rastgoo, Jian Wu, Mariah Liu, Maryam Pourabdollah, Eshetu G. Atenafu, Donna Reece, Weimin Chen, and Hong Chang. Targeting CD47 and TNFAIP8 By Mir-155 Overcomes drug resistance and inhibits tumor growth through induction of phagocytosis and apoptosis in multiple myeloma. Haematologica. 2019; 104:xxx doi:https://doi.org/10.3324/haematol.2019.227579.
Murata Y, Saito Y, Kotani T, Matozaki T. CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci. 2018;109:2349–57.
Abrisqueta P, Sancho J-M, Cordoba R, Persky DO, Andreadis C, Huntington SF, et al. Anti-CD47 antibody, CC-90002, in combination with rituximab in subjects with relapsed and/or refractory non-Hodgkin lymphoma (R/R NHL). Blood. 2019;134:4089.
Holland PM, Normant E, Adam A, Armet CM, O’Connor RW, Lake AC, et al. CD47 monoclonal antibody SRF231 is a potent inducer of macrophage-mediated tumor cell phagocytosis and reduces tumor burden in murine models of hematologic malignancies. Blood. 2016.
Kauder SE, Kuo TC, Harrabi O, Chen A, Sangalang E, Doyle L, et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS One. 2018;13:1–33.
Johansson U, Londei M. Ligation of CD47 during monocyte differentiation into dendritic cells results in reduced capacity for interleukin-12 production. Scand J Immunol. 2004;59:50–7.
Petrova PS, Viller NN, Wong M, Pang X, Lin GHY, Dodge K, et al. TTI-621 (SIRPαFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23:1068–79.
Hayat SMG, Bianconi V, Pirro M, Jaafari MR, Hatamipour M, Sahebkar A. CD47: role in the immune system and application to cancer therapy. Cell Oncol. 2020;43:19–30.
Uscanga-Palomeque AC, Calvillo-Rodríguez KM, Gómez-Morales L, Lardé E, Denèfle T, Caballero-Hernández D, et al. CD47 agonist peptide PKHB1 induces immunogenic cell death in T-cell acute lymphoblastic leukemia cells. Cancer Sci. 2019;110:256–68.
Martínez-Torres AC, Calvillo-Rodríguez KM, Uscanga-Palomeque AC, Gómez-Morales L, Mendoza-Reveles R, Caballero-Hernández D, et al. PKHB1 tumor cell lysate induces antitumor immune system stimulation and tumor regression in syngeneic mice with tumoral T lymphoblasts. J Oncol. 2019;2019.
Logtenberg MEW, Jansen JHM, Raaben M, Toebes M, Franke K, Brandsma AM, et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPα axis and a target for cancer immunotherapy. Nat Med. 2019;25:612–9.
Huang W, Wang WT, Fang K, Chen ZH, Sun YM, Han C, et al. MIR-708 promotes phagocytosis to eradicate T-ALL cells by targeting CD47. Mol Cancer. 2018;17:1–6.
Buatois V, Johnson Z, Salgado-Pires S, Papaioannou A, Hatterer E, Chauchet X, et al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol Cancer Ther. 2018;17:1739–51.
Tremblay-Lemay R, Rastgoo N, Chang H. Modulating PD-L1 expression in multiple myeloma: an alternative strategy to target the PD-1/PD-L1 pathway. J Hematol Oncol. 2018;11:1–16.
Gravelle P, Burroni B, Péricart S, Rossi C, Bezombes C, Tosolini M, et al. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies. Oncotarget. 2017;8:44960–75.
Hu L-Y, Xu X-L, Rao H-L, Chen J, Lai R-C, Huang H-Q, et al. Expression and clinical value of programmed cell death-ligand 1 (PD-L1) in diffuse large B cell lymphoma: a retrospective study. Chin J Cancer. 2017;36:1–11.
Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83.
Piccione EC, Juarez S, Tseng S, Liu J, Stafford M, Narayanan C, et al. SIRPα-antibody fusion proteins selectively bind and eliminate dual antigen-expressing tumor cells. Clin Cancer Res. 2016;22:5109–19.
Ponce LP, Fenn NC, Moritz N, Krupka C, Kozik JH, Lauber K, et al. SIRPα-antibody fusion proteins stimulate phagocytosis and promote elimination of acute myeloid leukemia cells. Oncotarget. 2017;8:11284–301.
Lin GHY, Chai V, Lee V, Dodge K, Truong T, Wong M, et al. TTI-621 (SIRPαFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS One. 2017;12:1–16.
Narla RK, Modi H, Wong L, Abassian M, Bauer D, Desai P, et al. Abstract 4694: The humanized anti-CD47 monclonal antibody, CC-90002, has antitumor activity in vitro and in vivo. Cancer Res. 2017;77:4694 LP – 4694.abstract.
Barazi HO, Li Z, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, et al. Regulation of integrin function by CD47 ligands: differential effects on αvβ3 and α4β1 integrin-mediated adhesion. J Biol Chem. 2002;277:42859–66.
Lin GHY, Viller NN, Chabonneau M, Brinen L, Mutukura T, Dodge K, et al. Abstract 2709: TTI-622 (SIRPα-IgG4 Fc), a CD47-blocking innate immune checkpoint inhibitor, suppresses tumor growth and demonstrates enhanced efficacy in combination with antitumor antibodies in both hematologic and solid tumor models. 2018;2709–2709.
Suzuki S, Yokobori T, Tanaka N, Sakai M, Sano A, Inose T, et al. CD47 expression regulated by the miR-133a tumor suppressor is a novel prognostic marker in esophageal squamous cell carcinoma. Oncol Rep. 2012;28:465–72.
Wang Y, Xu Z, Guo S, Zhang L, Sharma A, Robertson GP, et al. Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Mol Ther. 2013;21:1919–29.
Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–78.
Liu B, Guo H, Xu J, Qin T, Guo Q, Gu N, et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs. 2018;10:315–24.
Sockolosky JT, Dougan M, Ingram JR, Ho CCM, Kauke MJ, Almo SC, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113:E2646–54.
Lian S, Xie R, Ye Y, Xie X, Li S, Lu Y, et al. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine. 2019;42:281–95.
Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, et al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7:946–56.
Zhang X, Fan J, Ju D. Insights into CD47/SIRPα axis-targeting tumor immunotherapy. Antib Ther. 2018;1:27–32.
Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα Axis. Front Immunol. 2020;11:1–15.
Feliz-Mosquea YR, Christensen AA, Wilson AS, Westwood B, Varagic J, Meléndez GC, et al. Combination of anthracyclines and anti-CD47 therapy inhibit invasive breast cancer growth while preventing cardiac toxicity by regulation of autophagy. Breast Cancer Res Treat. 2018;172:69–82.
Ho CCM, Guo N, Sockolosky JT, Ring AM, Weiskopf K, Özkan E, et al. “Velcro” engineering of high affinity CD47 Ectodomain as signal regulatory protein α (SIRPα) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem. 2015;290:12650–63.
Sim J, Sockolosky JT, Sangalang E, Izquierdo S, Pedersen D, Harriman W, et al. Discovery of high affinity, pan-allelic, and pan-mammalian reactive antibodies against the myeloid checkpoint receptor SIRPα. MAbs. 2019;11:1036–52.
Anniss AM, Sparrow RL. Expression of CD47 (integrin-associated protein) decreases on red blood cells during storage. Transfus Apher Sci. 2002;27:233–8.
Weiskopf K, Ring AM, Ho CCM, Volkmer J, Levin M, Volkmer AK, et al. Engineered SIRPa variants as immunotherapeutic adjuvants to anticancer antibodies. Science AAAS. 2013;341:88–91.
Yanagita T, Murata Y, Tanaka D, Motegi SI, Arai E, Daniwijaya EW, et al. anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight. 2017;2.
Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2019;14:89–97.
Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 2019;25:1057–63.
Trabulo S, Aires A, Aicher A, Heeschen C, Cortajarena AL. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim Biophys Acta. 1861;2017:1597–605.
Liu XJ, Li L, Liu XJ, Li Y, Zhao CY, Wang RQ, et al. Mithramycin-loaded mPEG-PLGA nanoparticles exert potent antitumor efficacy against pancreatic carcinoma. Int J Nanomedicine. 2017;12:5255–69.
Davis RM, Campbell JL, Burkitt S, Qiu Z, Kang S, Mehraein M, et al. A raman imaging approach using CD47 antibody-labeled SERS nanoparticles for identifying breast cancer and its potential to guide surgical resection. Nanomaterials. 2018;8.
Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7:339–48.
Ishikawa-Sekigami T, Kaneko Y, Saito Y, Murata Y, Okazawa H, Ohnishi H, et al. Enhanced phagocytosis of CD47-deficient red blood cells by splenic macrophages requires SHPS-1. Biochem Biophys Res Commun. 2006;343:1197–200.
Oldenborg PA. Role of CD47 in erythroid cells and in autoimmunity. Leuk Lymphoma. 2004;45:1319–27.
Catani L, Sollazzo D, Ricci F, Polverelli N, Palandri F, Baccarani M, et al. Society for Hematology and Stem Cells. 2011;39:486–94.
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7.
Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015;10:1–23.
Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017;76:100–9.
Acknowledgements
Not applicable.
Funding
The study is funded in part from Cancer Research Society and Leukemia & Lymphoma Society of Canada.
Author information
Authors and Affiliations
Contributions
EE performed the study and drafted the manuscript; TLR, NR, RM, and WC performed the study and revised the manuscript. AL and HC supervised the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eladl, E., Tremblay-LeMay, R., Rastgoo, N. et al. Role of CD47 in Hematological Malignancies. J Hematol Oncol 13, 96 (2020). https://doi.org/10.1186/s13045-020-00930-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-020-00930-1