Abstract
Host defense peptides (HDPs) are small molecules with broad-spectrum antimicrobial activities against infectious bacteria, viruses, and fungi. Increasing evidence suggests that HDPs can also indirectly protect hosts by modulating their immune responses. Due to these dual roles, HDPs have been considered one of the most promising antibiotic substitutes to improve growth performance, intestinal health, and immunity in farm animals. This review describes the antimicrobial and immunomodulatory roles of host defense peptides and their recent applications in animal production.
Similar content being viewed by others
Introduction
Antibiotics have been used in animal production for more than 50 years [1]. However, the extensive use of antibiotics in animal production has resulted in widespread antimicrobial resistance by pathogens. Antibiotic residues in animal-derived foods transfer antibiotic resistant bacteria to humans, meaning that antimicrobial resistance has become a great threat to human health [2]. Novel and effective antibiotic substitutes are therefore urgently needed.
Host defense peptides (HDPs), also known as antimicrobial peptides (AMPs), are essential components of the innate immune system and exist in virtually all forms of life, ranging from single-celled bacteria to multi-cellular organisms [3]. In recent years, HDPs have been studied as antibiotic substitutes due to their potent antibacterial activities against Gram-positive, Gram-negative, and multidrug-resistant bacteria [4]. HDPs principally interact with the bacterial membrane and destroy membrane integrity, but they additionally act on intracellular targets to interfere with key cellular processes [5, 6]. HDPs modulate both innate and adaptive immune responses by regulating inflammation, recruiting leukocytes, activating immune cells, and modulating adaptive immunity, all of which help to protect the host against bacterial infection [7,8,9]. A key advantage of HDPs over conventional antibiotics is the ability of HDPs to modulate host immunity in response to microbial infection.
HDPs have received increasing attention for potential use in animal production due to their antimicrobial and immunomodulatory roles. Many HDPs can positively influence growth performance, nutrient digestibility, intestinal health, and immune function in animals. This review focuses on the antimicrobial and immunomodulatory roles of HDPs and their applications in livestock development.
HDPs
HDPs are evolutionarily conserved molecules, generally comprising 10–50 amino acids, that are found among nearly all forms of life that have defense functions [10]. More than 3000 HDPs have been identified to date and are hosted in the Antimicrobial Peptide Database (https://aps.unmc.edu/AP/main.php). Among the more than 3000 known HDPs, 2431 originate from animals, 361 from plants, 371 from bacteria, 5 from archaea, 8 from protists, 22 from fungi, and some from synthetic peptides.
HDPs are divided into three categories based on their origin: animal HDPs, plant HDPs, and bacterial HDPs. Based on structural differences and biological characteristics, animal HDPs can be further classified into two subfamilies: cathelicidins and defensins (including α-defensin and β-defensin). Among the known animal HDPs, human cathelicidin LL-37 is the most well-studied [11]. Plant HDPs are widely distributed; they are found in stems, flowers, leaves, roots, and seeds, and include thionins, defensins, hevein‑like peptides, knottin‑type peptides, lipid transfer proteins, and snakins. These plant HDPs defend against bacteria, fungi, and insects[12]. Bacterial HDPs, also called bacteriocins, play important roles in regulating competitive interactions in natural microbial systems. Some are narrow-spectrum, efficient antibacterial compounds, and these characteristics contribute to their potential to limit or prevent colonization by diarrheal pathogens [13, 14]. In addition to natural HDPs, an increasing number of synthetic HDPs have been reported in recent years. Compared with the natural HDPs, synthetic peptides have optimized sequences that confer low resistance to digestive enzymes and minimal cell cytotoxicity, circumventing the drawbacks of natural HDPs [15]. HDPs are highly diverse in sequence and structure but can be classified into four major structural groups: α-helical (e.g., melittin and cecropins), β-sheet (e.g., α- and β-defensins), β-hairpin (e.g., lactoferricin and tachyplesins), and extended HDPs (e.g., indolicidin and histatins) [5, 10]. Regardless of the natural source or structure, all HDPs can kill pathogens and modulate host immune responses.
Antimicrobial roles of HDPs
The unique ability of HDPs to kill pathogenic bacteria provides a platform for researchers to develop promising alternatives to antibiotics.
Broad-spectrum antimicrobial activities
Numerous researchers have shown that HDPs possess broad-spectrum antimicrobial activity against bacteria (Gram-negative, Gram-positive, and drug-resistant), fungi, and viruses [16, 17]. For example, human defensing (hBD)-3 was shown to have antimicrobial activity against several pathogenic bacteria, such as Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, Escherichia coli (E. coli), multidrug-resistant S. aureus, and vancomycin-resistant Enterococcus faecium, in addition to the fungal pathogen Candida albicans [18]. Wang et al. isolated 12 novel HDPs from frogs, and most showed potent antimicrobial activities against Gram-positive bacteria (E. coli, Enterococcus faecalis, and Enterobacter cloacae), Gram-negative bacteria (S. aureus, Klebsiella pneumonia, and Bacillus dysenteriae), and a fungus (C. albicans) [19]. Bovine myeloid HDPs, a group of α-helical cathelicidins, exhibited powerful inhibitory effects against antibiotic-resistant species such as methicillin-resistant S. aureus (MRSA), vancomycin-resistant E. faecalis (VREF), and multidrug-resistant strains of P. aeruginosa and Acinetobacter baumannii [20]. Subtilosin displays potent antiviral activity against herpes simplex virus type 1 and 2 [21, 22].
Antibacterial mechanisms
The antibacterial mechanisms of HDPs are diverse and dependent on the properties of individual HDPs and the microbial pathogens upon which they are acting. Based on their interacting sites, the antibacterial mechanisms of HDPs can be divided into membrane targeting and non-membrane targeting mechanisms.
Membrane targeting mechanism
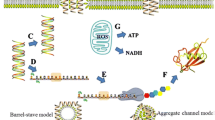
Cell membranes are widely considered to be the main site of antibacterial action for HDPs [5]. Bacterial membranes are negatively charged due to the presence of anionic phospholipids, namely lipopolysaccharides (LPS) in Gram-negative bacteria and teichoic acids (TA) in Gram-positive bacteria [7]. Most HDPs are rich in positively-charged residues such as lysine, arginine, and histidine [16, 23]. Therefore, the electrostatic interactions between bacterial membranes and HDPs lead to the initial targeting of cell membranes, although HDPs also later attract more peptides [24]. The efficiency of this process is strongly related to the cationic charge and concentration of HDPs that are bound to the membrane interface. Generally, increased binding activity results from more highly charged HDPs and higher HDP concentrations (within a physiological range) [25]. At higher concentrations of HDPs, interactions between the peptides and membranes tend to depend on hydrophobicity, which controls the extent to which peptides can penetrate the membrane layer [26, 27]. When the number of HDPs attached to the membrane reaches a critical concentration, self-association, multimerization, and conformational transformation occur. This change eventually causes membrane penetration and disruption; the specifics of this process have been described by various hypothetical models, such as the carpet, aggregate, toroidal pore, and barrel-stave models [28]. Membrane penetration eventually leads to leakage of intracellular ions and metabolites, ultimately causing cell death. In contrast to conventional antibiotics, which require days to be effective, HDPs cause bacterial death within minutes of exposure [29].
Non-membrane targeting mechanism
In addition to inducing perturbation of bacterial membranes, HDPs can kill bacteria by inhibiting metabolic and translational processes, such as protein synthesis, nucleic acid synthesis, and enzyme activity [30]. For example, proline-rich HDPs can bind to the ribosomal exit tunnel and subsequently prevent protein synthesis [31]. The histone-derived HDPs buforin II and desHDAP1 interact with the phosphate groups of DNA via hydrogen bonding, altering DNA conformation and function and thus inhibiting bacterial growth [32]. NP-6, an HDP derived from pepper seeds, targets E. coli by inhibiting β-galactosidase activity in a dose-dependent manner[33].
Immunomodulatory roles of HDPs
In animals, HDPs are often located at sites where environmental pathogen exposure is most likely to occur, such as the skin, ears, eyes, epithelial surfaces, lungs, and gut, but also in the bone marrow, testes, ovary and oviduct [34, 35]. Invasion by pathogens can induce expression of host HDP genes at early stages of infection, which facilitates elimination of bacteria by the host [36]. HDPs expression is likely a prophylactic response to infection. Immunomodulatory activities of HDPs are more extensive than the antimicrobial activities and seem to depend on the degree and phase of bacterial infection, the physiological status of host cells, and the HDP concentration[37, 38]. Understanding the mechanisms of HDPs immunomodulation will be helpful in determining practical applications of HDPs in animal production.
Modulation of inflammation
Inflammation is a biological response of the innate immune system to defend against invading pathogens. However, overwhelming and uncontrolled inflammation can cause severe injury to the host [39]. HDPs exhibit both pro- and anti-inflammatory roles. For example, they can up-regulate inflammatory factors to activate the immune system, which helps to eliminate invading pathogens early in an infection. This is considered a pro-inflammatory response. Conversely, HDPs can suppress over-reactive inflammatory responses induced by bacteria or bacterial products. This is considered an anti-inflammatory response. Therefore, HDPs can modulate inflammation to maintain immune homeostasis.
Bactenecin-5 and epinecidin can induce transcription of interleukin (IL)-1β in the presence and absence of live Mycobacterium marinum in macrophage-like THP-1 cells. Bactenecin-5 was also found to significantly up-regulate tumor necrosis factor-α (TNF-α), but the pro-inflammatory activity of bactenecin-5 required co-stimulation with M. marinum [40]. Oral administration of sublancin, an HDP derived from Bacillus subtilis, restored expression of IL-2, IL-4, and IL-6 in immunosuppressed mice and accelerated recovery of phagocytic activity by macrophages [41]. These results indicate that HDPs can activate immune responses by inducing the release of pro-inflammatory cytokines.
LPS is a major component of Gram-negative bacterial outer membranes, and can be recognized by host toll like receptor-4 (TLR4), and activates production of pro-inflammatory cytokines by immune cells via TLR4 signaling [42]. The anti-inflammatory functions of HDPs are mainly due to LPS-neutralizing activity, which suppresses downstream TLR4 signaling pathways (such as mitogen-activated protein kinase [MAPK] and nuclear factor-κB [NF-κB] signaling) [43, 44]. For example, a frog-derived peptide, cathelicidin-MH, exerts LPS-neutralizing activity. This protects against LPS-induced sepsis in mice and significantly decreases production of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α by suppressing MAPK signaling [34]. Lactoferricin downregulated the secretion of the pro-inflammatory cytokines TNF-α, IL-6 in LPS-treated macrophages by targeting the MAPK and NF-κB pathways [45]. In addition to direct binding of HDPs to LPS, some HDPs (such as pigeon-derived cathelicidin Cl-CATH2 and 3, snake-derived cathelicidin Hc-CATH) bind to the opening region of the LPS-binding pocket on myeloid differentiation factor-2 (MD-2) of the TLR4-MD-2 complex in macrophages challenged by LPS. This direct binding inhibits activation of the TLR4 pathway induced by LPS, which in turn decreases expression of pro-inflammatory cytokines at the transcriptional level [46, 47].
Recruitment of leukocytes
HDPs exhibit direct chemotactic activity towards leukocytes (such as neutrophils, macrophages, mast cells, and T cells). The underlying mechanisms involve several cellular receptors, including chemokine receptors, formyl peptide receptors (FPRs), and G protein-coupled receptors (GPCRs) [42]. hBD-3 had been reported to utilize C–C chemokine receptor type 2 (CCR2) to induce monocyte/macrophage chemotaxis [48]. The HDPs scolopendrasin and LL-37 recruit neutrophils, monocytes, and T cells to sites of bacterial infection by interacting with FPR1 and formyl peptide receptor-like 1 (FPRL1), respectively [49, 50]. G-protein pathways are reportedly involved in mast cell chemotaxis induced by the synthetic cationic HDP IDR-1018, which is associated with increased intracellular Ca2+ mobilization [51].
HDPs also indirectly facilitate recruitment of leukocytes by inducing production of chemokines and chemokine receptors. Cathelicidins can induce chemokine receptor CCR2, CXCR2, IFNγ-R, MRC1, and LFA1 production in monocytes. Additionally, stimulated monocytes produce various chemokines like CCL2, CCL5, CCL7, CXCL10, and CXCL8 (IL-8) [52]. HDP-IBP5, derived from insulin-like growth factor-binding protein 5, induces production of cytokines and chemokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF), TNF-α, IL-8, MCP-1, MCP-3, macrophage inflammatory protein (MIP)-1α, and MIP-1β, which regulate migration of mast cells in a dose-dependent manner [53].
In vivo experiments have also demonstrated chemotaxis of HDPs. Inoculating mouse peritoneum with the scorpion-derived HDP ToAP2A increased levels of peritoneal macrophages and induced a greater chemotactic migration of neutrophils (and possibly eosinophils) [54]. Injection of PopuCATH (cathelicidin from tree frog) significantly elicited chemokines (CXCL1, CXCL2, and CXCL3)/cytokine (IL-1β and IL-6) production in macrophages through activating p38/ERK MAPKs and NF-κB p65 pathway, and rapidly drove neutrophil, monocyte/macrophage influx in mouse abdominal cavity [55].
Activation of immune cells
Activation of immune cells by HDPs directly increased the bactericidal activities of these peptides and promoted early clearance of infections. Two synthetic peptides, Pin2[G] and FA1, stimulate phagocytosis of Salmonella typhimurium by macrophages [56]. LL-37 and hBD-2 enhance the expression and induce translocation of NOD1, NOD2, and RIG-I innate immunity receptors, and also directly activate the pro-inflammatory and migratory responses of peritoneal mast cells in vitro [57]. PopuCATH significantly enhanced neutrophil phagocytosis via promoting the release of neutrophil extracellular traps [55].
Immune cell activation by HDPs may be mediated by some immune cell receptors. Human host defense peptides, such as LL-37, and IBP5, can activate mast cells via the MAS-related G protein-coupled receptor X2 (MRGPRX2), which is highly expressed on mast cells and responds to various exogenous and endogenous stimuli. Activation of mast cells leads to degranulation, release of eicosanoids, and multicellular signaling cascades [53, 58]. Murine β-defensin-2 (mDF2beta) can directly activate immature dendritic cells by acting as an endogenous ligand for TLR4, resulting in up-regulation of costimulatory molecules and maturation of dendritic cells [59].
Regulation of adaptive immunity
B and T cells are important participants in adaptive immunity and can influence the generation and polarization of lymphocyte responses. Stimulating resting porcine lymphocytes with nisin produced by Lactococcus lactis increased the percentage of CD4+CD8+ T cells. This effect may result from modulation of the stimulating potential of antigen-presenting cells [60]. Pathogenic stimulation significantly increased expression of cathelicidin genes in trout IgM+ and IgT+ B cells both in vitro and in vivo. Interestingly, these peptides increased the intracellular bactericidal, phagocytic, and reactive oxygen species (ROS) activities of trout IgM+ and IgT+ B cells [61]. Antigen-presenting cells (e.g., monocytes) can take up and process antigens and present them to T cells in concert with major histocompatibility complex (MHC) II molecules on the cell surface. Chicken cathelicidin-2 increases the antigen presentation capacity of chicken monocytes by up-regulating expression of the antigen presentation markers MHC-II and mannose receptor C-type 1 (MRC1) [62]. A similar effect on mouse macrophages was described in response to treatment with a chicken HDPs fowlicidin-1 [63]. Thus, HDPs improve antigen presentation capacity, which prepares antigen-presenting cells to function in an enhanced adaptive immune response against infection.
Applications of HDPs in animal production
HDPs are evolutionarily conserved components of the innate host defense system that are present in essentially all forms of life. Farm animals produce a variety of endogenous HDPs in gut, mainly cathelicidins and defensins [64,65,66,67]. Supplementing exogenous HDPs could mimic the physiological release of endogenous HDPs and thus improve the host immune response against bacterial infections [68,69,70]. In recent years, HDPs have been used as alternatives to antibiotics to improve animal growth performance, immunity, and intestinal health; they have also been used as novel therapeutic agents to reduce the frequency and severity of subclinical infections (Tables 1 and 2).
Swine
In swine, HDPs have been studied most extensively with weaned piglets. Post-weaning diarrhea is one of the most serious problems for swine producers worldwide. It is usually caused by proliferation of enterotoxigenic E. coli (ETEC) in the intestine and is characterized by reduced growth performance and increased mortality of piglets [87, 88]. Supplementation of feed with HDPs can effectively control post-weaning diarrhea and ameliorate the associated adverse effects on weaned piglets [89]. For example, Xiong et al. evaluated the effects of feeding composite HDPs (lactoferrin, cecropin, defensin, and plectasin) to weaned piglets from five different farms. Piglets were feed with dietary supplement of 3 g/kg composite HDPs (mixture of natural lactoferrin, cecropin, defensin, and plectasin) showed decreased incidence of diarrhea (from 9.42% to 5.22%) and increased survival rates (from 93.34% to 97.42%). Average daily gain (ADG) and feed efficiency (G/F) were also significantly improved [90].
Weaned piglets are very susceptible to pathogens and stressors caused by changes in the intestinal flora due to the immature development of the immune system and compromised intestinal integrity. Consequently, effects of HDPs on immunity, intestinal barrier function, and composition of intestinal microbiota are important factors in attenuation of post-weaning diarrhea [91]. Dietary supplementation with microcin J25, an HDP isolated from a fecal strain of E. coli, can reduce systematic inflammation by decreasing concentrations of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α and increasing concentration of the anti-inflammatory cytokine, IL-10, in serum. Compared with the antibiotic colistin sulfate, microcin J25 also decreased the population of E. coli and increased the abundance of Lactobacillus and Bifidobacterium in the feces of weaned piglets [71]. Wu et al. [72] tested the effects of cecropin on piglets challenged with ETEC. They found that supplementation with cecropin significantly reduced the incidence of diarrhea and improved growth performance, similar to the effects observed with antibiotic supplementation (kitasamycin and colistin sulfate). In addition, both cecropin and antibiotics improved nitrogen retention, dietary energy digestibility, intestinal morphology, and intestinal microbiota of challenged piglets. Serum concentrations of IgA and IgG were higher in animals supplemented with cecropin than those treated with antibiotics. These responses indicate that cecropin may have superior performance compared to antibiotics because it can modulate host serum immune responses [72]. Effects of HDPs on immunoglobulin levels of weaned piglets were also reported by Yuan et al. They found that a mixed HDP (swine defensin and a fly HDP) increased serum concentrations of IgG, IgM, IgA, and the classical swine fever antibody CH50 in weaned piglets in a dose-dependent manner, suggesting that HDPs can improve humoral immunity of weaned piglets [73]. In addition to dietary inclusion, injection of the HDP WK3 (a linear trpzip-like β-hairpin HDP composed of 14 amino acids) alleviated diarrhea in piglets, increased villus height in the ileum, reduced cecal abundance of Enterobacterium spp., and attenuated intestinal oxidative damage caused by ETEC [74]. Intraperitoneal injection of cathelicidin-BF (isolated from Bungarus fasciatus in China) suppressed intestinal inflammation by inhibiting the NF-κB signaling pathway and enhancing immune cell phagocytosis via STAT-1 [88], improved the intestinal barrier function by increasing expression of tight junction proteins, including zonula occluden-1, occludin, and claudin-1 in the jejunum and colon of weanling piglets [75].
Deoxynivalenol (DON) is a mycotoxin produced by certain Fusarium species that often contaminates corn, wheat, oats, barley, rice, and other cereals in the field or during storage [92]. It is a serious threat to animal production worldwide, especially for pigs [93]. Ingestion of high concentrations of DON can cause intestinal injury, reduce feeding efficiency, and suppress growth; in serious cases, it causes emesis, rectal bleeding, and diarrhea [94]. HDPs can protect weaned piglets from the toxic effects of DON [76]. Composite HDP (primarily antibacterial lactoferrin peptides, plant defensins, and active yeast) supplementation has been shown to mitigate growth inhibition and oxidative damage and to repair DON-induced intestinal injury in weanling piglets. This improvement may be a result of the ability of HDPs to enhance immunity, intestinal morphology, epithelial cell proliferation, and intestinal protein synthesis of weanling piglets [76].
In a MRSA-challenged pig model, HDPs showed favorable therapeutic effects. Huang et al. reported that injection of 2.5 mg/kg body weight epinecidin-1 (derived from Epinephelus coioides) completely protected pigs against death caused by MRSA after one week, decreased pathogen counts in multiple organs, enhanced serum levels of the proinflammatory cytokines IL-6, IL-1β, and TNF-α [77].
Poultry
E. coli, Salmonella, and Clostridium perfringens are commonly found in poultry. These pathogens cause intestinal inflammation, diarrhea, epithelial damage, systemic sepsis, and death in severe cases. HDPs reportedly reduce bacterial pathogen load and increase the abundance of beneficial bacteria in the intestine, which alleviates negative effects on broilers [70]. Daneshmand et al. tested the effects of cLF36 (an HDP extracted from camel lactoferrin) on E. coli-challenged chickens. Dietary supplementation with cLF36 increased the abundance of Lactobacillus spp., decreased harmful bacteria in the ileum, and up-regulated genes associated with immune cells and tight junction proteins [78]. Supplementation with microcin J25 mitigated negative effects in broilers challenged with E. coli and Salmonella, mainly by decreasing populations of total anaerobic bacteria and E. coli in the feces, improving villus height in the duodenum and jejunum, and reducing concentrations of IL-6, IL-1β, and TNF-α [79]. Necrotic enteritis is a well-known enteric disease in broilers that is induced by Clostridium perfringens. Supplementation with the HDPs cLFchimera and sublancin ameliorated necrotic enteritis-related intestinal lesions and reduced growth inhibition in broilers challenged with Clostridium perfringens. These HDPs improved intestinal morphology and restored the balance of microbiota in the ileum and cecum by decreasing and increasing the abundance of Clostridium spp. and Lactobacillus spp., respectively [80, 81]. Moreover, cLFchimera also positively affected expression of cytokines, tight junction proteins, and mucin in the jejunum [80].
Heat stress causes a variety of physiological disturbances, such as systemic immune dysregulation, intestinal injury, endocrine disorders, and reduced antioxidant capacity [95]. Chickens are vulnerable to heat stress because their thick feathering and absence of sweat glands minimizes their capacity to reduce body heat [96]. Hu et al. evaluated the effects of swine gut host defense peptides on chickens under chronic heat stress. They found that supplying chickens under heat stress with SGAMPs improved ADG, G/F, villus height, gut mucosal thickness, the number of intestinal intraepithelial lymphocytes and goblet cells, and concentrations of IgA in the small intestine [82]. The protective effects of dietary plectasin on boilers were also observed by Ko et al. in tropical environmental conditions. Supplementation with plectasin increased goblet cell counts in the jejunum and ileum and increased the serum concentrations of IFN-γ and IgY [83]. These studies indicated that HDPs may alleviate the adverse effects of heat stress by improving intestinal health and influencing immunomodulatory responses of the intestinal mucous and the innate and humoral immune systems.
HDPs could also be supplemented as growth promoters or immunomodulators in the absence of bacterial challenge or heat stress. Choi et al. reported that dietary supplementation of HDP-A3 linearly improved ADG, retention of dry matter, dietary digestibility of energy and protein, and intestinal villus height compared with non-supplemented birds [84]. Compared to treatment with enramycin, plectasin improved ADG and G/F and enhanced levels of antibodies against Newcastle disease virus (NDV) and H9N2 avian influenza virus (AIV) in yellow-feathered chickens [85]. Compared with enramycin zinc bacitracin, treatment with combined HDPs (plectasin and cecropins) showed positive effects on growth performance and serum antibody levels of H9N2 AIV and improved development of the bursa and thymus [86].
Ruminants
The rumen is an essential organ in ruminants, which produces short chain fatty acids and essential amino acids and vitamins by microbial fermentation [97]. Methane is also a significant product of rumen fermentation, accounting for 2%–12% of gross energy lost from feeds [98]. Methane is a potent greenhouse gas, and methane emissions from agriculture represent 40% of total anthropogenic emissions, with the largest single contributor (25%) being enteric fermentation in ruminants [99, 100]. Some host defense peptides, especially bacteriocins, can inhibit Methanococcus vannielii, Methanobacterium, and Methanomassiliicoccus luminyensis through several different mechanisms [101, 102]. In an in vitro experiment, bovicin HC5 (a bacteriocin from Streptococcus bovis HC5) reduced methane production by 50%, even at low concentrations. Cultures gradually lost their ability to produce methane after treatment with bovicin HC5. Methane was not detected after four transfers, suggesting that ruminal methanogens could not quickly adapt and evolve resistance to bovicin HC5 [103]. Another in vitro study compared the effects of monensin and nisin on rumen fermentation and microbiota. Nisin showed greater effects in reducing methane production and acetate/propionate ratios than monensin did, indicating that nisin is a potential alternative to monensin for ruminants [104]. Although the roles of HDPs in suppressing methane production are documented in vitro, additional in vivo experiments are needed to determine the specific roles of HDPs in ruminant production.
HDPs have also been reported to show antibacterial activity against pathogens that induce bovine diseases such as bovine mastitis, bovine respiratory disease complex, and bovine viral diarrhea. Peptide H18R (H2) can be internalized into MAC-T cells and inhibit MRSA. H2 showed greater efficiency than vancomycin in controlling S. aureus, which causes mastitis. In a mouse model of S. aureus E48-induced mastitis, H2 reduced the bacterial load in the mammary glands and alleviated both histopathological damage in mammary tissues and polymorphonuclear neutrophil infiltration of alveoli, demonstrating that H2 can be used as a therapeutic agent to treat S. aureus-induced mastitis [105]. The cow mammary gland also secretes HDPs, such as psoriasin, cathelicidin and lactoferrin, which play different roles for local defense against bacterial infection in the mammary gland [106].
Mycoplasma bovis is an important contributor to the bovine respiratory disease complex. Bovine NK-lysin-derived peptides can damage the plasma membrane and kill M. bovis [107]. Małaczewska et al. compared the effects of nisin, lysozyme, lactoferrin, and combinations of these compounds against bovine viral diarrhea virus (BVDV) in vitro. All of the tested HDPs showed anti-BVDV effects. The combination of nisin and lactoferrin was the most potent in reducing extracellular viral titer and intracellular viral RNA levels [108]. These studies performed both in vitro and in animal models highlighted HDPs as promising new candidates for the treatment of bovine diseases.
Although many HDPs have been used as feed additives for pigs and chickens, only a few studies have evaluated their use in ruminants. Liu et al. and Ren et al. tested the effects of mixed HDPs (swine defensin and a fly antibacterial peptide) on goats. They found that mixed HDPs improved rumen microbial community structure by increasing Fibrobacter, Anaerovibrio, Succiniclasticum, and the ciliate genus Ophryoscolex, while simultaneously decreasing Selenomonas, Succinivibrio, Treponema, and the ciliate genera Polyplastron and Entodinium. Xylanase, pectinase, and lipase showed increased activity and acetic acid, propionic acid, and total volatile fatty acids were present at higher levels in the rumen after dietary treatment with HDPs [109, 110]. These results indicated that HDPs could be used as feed additives for goats to improve growth performance. However, additional feeding experiments should be conducted to evaluate the effects of HDPs supplementation on ruminants.
Challenges and prospects
In recent years, many HDPs have been used as antibiotic alternatives in animal feeding and shown beneficial effects on farm animals. However, the application of HDPs in animal production still faces some challenges.
Preparation of HDPs
The preparation of HDPs has limitations including low yield, high cost and conditions for activity maintenance, which limit their large-scale production and application in animal production. Currently, the methods for HDPs preparation mainly include: biological material extraction, chemical synthesis and gene engineering expression [111]. Although HDPs exist widely in organisms, their content in biological tissues is low and so the separation is difficult. Chemical synthesis can get a certain number of samples, however, the error rate and side reaction increase with the increase of the molecular weight of HDPs, and the cost is quite high. Gene engineering expression may be the most economical method to obtain large quantities of HDPs at present. However, exogenous expression of HDPs is more difficult than other peptides because they are easily attacked by proteases, and the more intractable problem for recombinant E. coli expression system is the toxicity for bacteria cells as well as bacterial LPS contamination. To overcome these problems, HDPs are often expressed by means of fusion proteins or hybrid peptides.
The common tags for fusion expression include thioredoxin (Trx), glutathione-stransferase (GST), maltose-binding protein (MBP) and small ubiquitin-like modifier (SUMO) et al. [112]. Meng et al. [113] expressed plantaricin as a fusion protein with Trx in E. coli BL21 (DE3) with a yield up to 9–11 mg/L, and purified plantaricin showed strong antimicrobial activity against Micrococcus luteus, Staphylococcus epidermidis, Lactococcus lactis, Lactobacillus paracasei and Listeria innocua. Cao et al. [114] successfully expressed broad spectrum of antibacterial peptide proSP-B (rat lung surfactant protein B precursor) by fusion with GST in E. coli pLySs, which showed low toxicity to E. coli. Lamer et al. [115] designed a His6-SUMO-peptide-intein system to express lactococcin A, leucocin A, faerocin MK, neopetrosiamide A in E. coli BL21(DE3), which protected these DHPs against degradation, and also improved yields (up to 17-fold) compared with standard expression and isolation procedures. However, fusion proteins are usually needed to be removed to release activated peptides, which increases the difficulty and cost of HDPs preparation.
Hybrid peptides refer to HDPs fused to other HDPs or functional proteins to provide bifunctional properties [116]. Sun et al. [117] combined bovine lactoferrin (LfcinB) and human lysozyme (hLY) in Pichia pastoris GS115 expression system, and the results showed that the antibacterial activity of hybrid peptides LfcinB-hLY against E. coli K88 was higher than that of hLY and LfcinB solely, and was not effected by trypsin and chymotrypsin digestion. Liu et al. [118] reported that hybrid peptide cecropinA-thanatin had broad-spectrum antimicrobial activity without hemolysis and good stability in vitro as well. However, the properties and application potential of hybrid HDPs need to been deeply investigated each.
In sum, developing appropriate expression system and perfecting the expression strategy would remain a challenge in this field.
Stability of HDPs
Many HDPs are susceptible to the digestion of endogenous proteases, such as trypsin and pepsase in digestive tract, which result in low efficiency at the site of action and limit their administration by oral or water. In addition, temperature, pH and salt concentration can also change the structure of HDPs and affect the interaction of HDPs with pathogens. Strategies such as amino acid substitution, peptide cyclization, peptide chain modification as well as encapsulation with nanoparticles have been used to improve metabolic stability, conformational stability and bioavailability of HDPs [119, 120].
Amino acid substitution is popular to improve peptide stability against protease digestion, including D- or unnatural amino acids residue substitution [121, 122]. For example, Lu et al. [123] synthesized derivatives of the cationic HDP Pep05 (the putative active domain of histatin 5) by substituting L-amino acid residues with D- and unnatural amino acids, such as D-lysine, 4-aminobutanoic acid, and the results showed that both improve the stabilities of the peptides toward proteases. Nonetheless, it should be noted that the cost of synthetic peptides containing D- and unnatural amino acids is higher than solely of L-amino acids. Cyclization enabled peptides to have a more rigid conformation and partly shield the potential protease-scissile sites at the free termini and backbone of peptides, therefore improved the protease stability. The type of peptide cyclization mainly includes four categories: head-to-tail, head-to-sidechain, sidechain-to-tail and sidechain-to-sidechain. But the outcome of peptide cyclization may depend on sequence diversity and the complicated structure of HDPs, which cannot be easily predicted [124, 125]. The common peptide chain modifications are amidation, acetylation, methylation, PEGylation, lipidation and glycosylation [120]. For example, C-terminal amidation of HDP-N6 (a variant of arenicin-3) enhanced its ability to penetrate the bacterial and stability toward trypsin, as well as reduced hemolysis [126]. C-terminal PEGylation of pig-derived HDPs protegrin-1 exhibited more efficient antibacterial activity and higher stability toward trypsin degradation [127]. Encapsulation with nanoparticles for HDPs delivery may provide another strategy to improve drug bioavailability and safety, avoid enzymatic degradation, enhance controlled release and prevent aggregation [128]. Lai et al. [129] reported a self-assembling peptide nanoparticles remained largely intact after 8 h of degradation by proteases, demonstrating the proteolytic stability of the self-assembling peptide nanoparticles. Nonetheless, the stability of HDPs encapsulated with nanoparticles should be eventually evaluated in vivo.
Safety of HDPs
The absorption and metabolism of most HDPs in vivo are rarely reported. Whether HDPs could be degraded or absorbed by intestinal tract? How are HDPs metabolized by the body? What are the effects of their metabolites on the body? These questions are still not been explained clearly. Therefore, potential toxicity of HDPs, such as immunogenicity and hemolysis in vivo should not be ignored. Additional efforts are required to explore the pharmacokinetics and pharmacodynamics of HDPs.
Conclusions
The broad-spectrum antibacterial activities of HDPs have been widely demonstrated, making them promising alternatives to antibiotics. The immunomodulatory properties of HDPs mean they likely have superior performance compared to antibiotics in production of livestock. It is reported that HDPs could improve growth performance, intestinal health, and immunity of farm animals. However, problems on preparation, stability and safety of HDPs still limit their large-scale application. With the in-depth study of HDPs and the development of biotechnology, these challenges to HDPs will be figured out for better application of HDPs in animal production.
Availability of data and materials
Not applicable.
Abbreviations
- HDPs:
-
Host defense peptides
- AMPs:
-
Antimicrobial peptides
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- VREF:
-
Vancomycin-resistant Enterococcus faecalis
- E. coli :
-
Escherichia coli
- LPS:
-
Lipopolysaccharides
- TA:
-
Teichoic acids
- GPCRs:
-
G protein-coupled receptors
- hDP:
-
Human beta-defensin
- FPRL1:
-
Formyl peptide receptor-like 1
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-α
- TLR4:
-
Toll like receptor-4
- MAPK:
-
Mitogen-activated protein kinase
- NF-κB:
-
Nuclear factor-κB
- MD-2:
-
Myeloid differentiation factor-2
- CCR2:
-
C–C chemokine receptor type 2
- MCP:
-
Monocyte chemoattractant protein
- CXCL1:
-
Chemokine ligand 1 protein
- MDC:
-
Macrophage-derived chemokine
- VEGF:
-
Vascular endothelial growth factor
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- MIP:
-
Macrophage inflammatory protein
- ETEC:
-
Enterotoxigenic E. coli
- ADG:
-
Average daily gain
- G/F:
-
Gain:feed
- DON:
-
Deoxynivalenol
- NDV:
-
Newcastle disease virus
- BVDV:
-
Bovine viral diarrhoea virus
- AIV:
-
Aavian influenza virus
- Trx:
-
Thioredoxin
- GST:
-
Glutathione-stransferase
- MBP:
-
Maltose-binding protein
- SUMO:
-
Small ubiquitin-like modifier
- LfcinB:
-
Bovine lactoferrin
- hLY:
-
Human lysozyme
References
Xiao H, Shao F, Wu M, Ren W, Xiong X, Tan B, et al. The application of antimicrobial peptides as growth and health promoters for swine. J Amin Sci Biotechnol. 2015;6:19. https://doi.org/10.1186/s40104-015-0018-z.
Bacanli M, Başaran N. Importance of antibiotic residues in animal food. Food Chem Toxicol. 2019;125:462–6. https://doi.org/10.1016/j.fct.2019.01.033.
Zhang Q, Yan Z, Meng Y, Hong X, Shao G, Ma J, et al. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Military Med Res. 2021;8:48. https://doi.org/10.1186/s40779-021-00343-2.
Lam SJ, O’Brien-Simpson NM, Pantarat N, Sulistio A, Wong EH, Chen Y, et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat microbiol. 2016;1:16162. https://doi.org/10.1038/nmicrobiol.2016.162.
Hollmann A, Martinez M, Maturana P, Semorile LC, Maffia PC. Antimicrobial peptides: Interaction with model and biological membranes and synergism with chemical antibiotics. Front Chem. 2018;6:204. https://doi.org/10.3389/fchem.2018.00204.
Koo HB, Seo J. Antimicrobial peptides under clinical investigation. Pept Sci. 2019;28:1176–89. https://doi.org/10.1111/exd.13979.
Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: Functions and clinical potential. Nat Rev Drug Discov. 2020;19:311–32. https://doi.org/10.1038/s41573-019-0058-8.
Mansour SC, Pena OM, Hancock RE. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014;35:443–50. https://doi.org/10.1016/j.it.2014.07.004.
Bowdish DM, Davidson DJ, Scott MG, Hancock RE. Immunomodulatory activities of small host defense peptides. Antimicrob Agents Ch. 2005;49:1727–32. https://doi.org/10.1128/AAC.49.5.1727-1732.2005.
Kumar R, Ali SA, Singh SK, Bhushan V, Mathur M, Jamwal S, et al. Antimicrobial peptides in farm animals: An updated review on its diversity, function, modes of action and therapeutic prospects. Vet Sci. 2020;7:206. https://doi.org/10.3390/vetsci7040206.
Lei J, Sun L, Huang S, Zhu C, Li P, He J, et al. The antimicrobial peptides and their potential clinical applications. Am J Transl Res. 2019;11:3919–31. https://doi.org/10.1007/s12010-022-03870-3.
Tang S, Prodhan ZH, Biswas SK, Le C, Sekaran SD. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry. 2018;154:94–105. https://doi.org/10.1016/j.phytochem.2018.07.002.
Benítez-Chao D, León-Buitimea A, Lerma-Escalera JA, Morones-Ramírez J. Bacteriocins: An overview of antimicrobial, toxicity, and biosafety assessment by in vivo models. Front Microbiol. 2021;12:630–95. https://doi.org/10.3389/fmicb.2021.630695.
Duquesne S, Destoumieux-Garzón D, Peduzzi J, Rebuffat S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep. 2007;24:708–34. https://doi.org/10.1039/b516237h.
Souza PF, Marques LS, Oliveira JT, Lima PG, Dias LP, Neto NA, et al. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie. 2020;175:132–45. https://doi.org/10.1016/j.biochi.2020.05.016.
Wang G. The antimicrobial peptide database provides a platform for decoding the design principles of naturally occurring antimicrobial peptides. Protein Sci. 2020;29:8–18. https://doi.org/10.1002/pro.3702.
Kočendová J, Vaňková E, Volejníková A, Nešuta O, Buděšínský M, Socha O, et al. Antifungal activity of analogues of antimicrobial peptides isolated from bee venoms against vulvovaginal Candida spp. Fems Yeast Res. 2019;19:z13. https://doi.org/10.1093/femsyr/foz013.
Harder J, Bartels J, Christophers E, Schröder J. Isolation and characterization of human μ-Defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. https://doi.org/10.1074/jbc.M008557200.
Wang Y, Zhang Y, Lee WH, Yang X, Zhang Y. Novel peptides from skins of amphibians showed broad-spectrum antimicrobial activities. Chem Biol Drug Des. 2016;87:419–24. https://doi.org/10.1111/cbdd.12672.
Benincasa M, Skerlavaj B, Gennaro R, Pellegrini A, Zanetti M. In vitro and in vivo antimicrobial activity of two α-helical cathelicidin peptides and of their synthetic analogs. Peptides. 2003;24:1723–31. https://doi.org/10.1016/j.peptides.2003.07.025.
Torres NI, Noll KS, Xu S, Li J, Huang Q, Sinko PJ. Safety, formulation and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus Type 1. Probiotics & Antimicro Prot. 2013;5:26–35. https://doi.org/10.1007/s12602-012-9123-x.
Greber KE, Dawgul M. Antimicrobial peptides under clinical trials. Curr Top Med Chem. 2017;17:620–28. https://doi.org/10.2174/1568026616666160713143331.
Di Somma A, Moretta A, Canè C, Cirillo A, Duilio A. Antimicrobial and antibiofilm peptides. Biomolecules. 2020;10(4):652. https://doi.org/10.3390/biom10040652.
Malanovic N, Lohner K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim Biophys Acta. 2016;1858:936–46. https://doi.org/10.1016/j.bbamem.2015.11.004.
Li J, Koh J, Liu S, Lakshminarayanan R, Verma CS, Beuerman RW. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front Neurosci. 2017;11:73. https://doi.org/10.3389/fnins.2017.00073.
Kuznetsov AS, Dubovskii PV, Vorontsova OV, Feofanov AV, Efremov RG. Interaction of linear cationic peptides with phospholipid membranes and polymers of sialic acid. Biochemistry. 2014;79:459–68. https://doi.org/10.1134/S0006297914050101.
Hollmann A, Martínez M, Noguera ME, Augusto MT, Disalvo A, Santos NC, et al. Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide–membrane interactions of three related antimicrobial peptides. Colloids Surf B Biointerfaces. 2016;141:528–36. https://doi.org/10.1016/j.colsurfb.2016.02.003.
Travkova OG, Moehwald H, Brezesinski G. The interaction of antimicrobial peptides with membranes. Adv Colloid Interfac. 2017;247:521–32. https://doi.org/10.1016/j.cis.2017.06.001.
Santo KP, Irudayam SJ, Berkowitz ML. Melittin creates transient pores in a lipid bilayer: Results from computer simulations. J Phys Chem B. 2013;117:5031–42. https://doi.org/10.1021/jp312328n.
Grein F, Schneider T, Sahl H. Docking on lipid II—a widespread mechanism for potent bactericidal activities of antibiotic peptides. J Mol Biol. 2019;431:3520–30. https://doi.org/10.1016/j.jmb.2019.05.014.
Graf M, Mardirossian M, Nguyen F, Seefeldt AC, Guichard G, Scocchi M, et al. Proline-rich antimicrobial peptides targeting protein synthesis. Nat Prod Rep. 2017;34:702–11. https://doi.org/10.1039/c7np00020k.
Sim S, Wang P, Beyer BN, Cutrona KJ, Radhakrishnan ML, Elmore DE. Investigating the nucleic acid interactions of histone-derived antimicrobial peptides. FEBS Lett. 2017;591:706–17. https://doi.org/10.1002/1873-3468.12574.
Hou X, Feng C, Li S, Luo Q, Shen G, Wu H, et al. Mechanism of antimicrobial peptide NP-6 from Sichuan pepper seeds against E. coli and effects of different environmental factors on its activity. Appl Microbiol Biot. 2019;103:6593–604. https://doi.org/10.1007/s00253-019-09981-y.
Chai J, Chen X, Ye T, Zeng B, Zeng Q, Wu J, et al. Characterization and functional analysis of cathelicidin-MH, a novel frog-derived peptide with anti-septicemic properties. Elife. 2021;20:10. https://doi.org/10.7554/eLife.64411.
Yoshimura Y. Avian β-defensins expression for the innate immune system in hen reproductive organs. Poult Sci. 2015;94:804–9. https://doi.org/10.3382/ps/pew379.
Lemaitre B, Reichhart J, Hoffmann JA. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–9. https://doi.org/10.1073/pnas.94.26.14614.
Haney EF, Straus SK, Hancock RE. Reassessing the host defense peptide landscape. Front Chem. 2019;4:7–43. https://doi.org/10.3389/fchem.2019.00043.
Hancock RE, Haney EF, Gill EE. The immunology of host defence peptides: Beyond antimicrobial activity. Nat Rev Immunol. 2016;16:321–34. https://doi.org/10.1038/nri.2016.29.
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204. https://doi.org/10.18632/oncotarget.23208.
Price RL, Bugeon L, Mostowy S, Makendi C, Wren BW, Williams HD, et al. In vitro and in vivo properties of the bovine antimicrobial peptide, Bactenecin 5. PLoS ONE. 2019;9:14. https://doi.org/10.1371/journal.pone.0210508.
Wang S, Huang S, Ye Q, Zeng X, Yu H, Qi D, et al. Prevention of cyclophosphamide-induced immunosuppression in mice with the antimicrobial peptide sublancin. J Immunol Res. 2018;7:4353580. https://doi.org/10.1155/2018/4353580.
Drayton M, Deisinger JP, Ludwig KC, Raheem N, Müller A, Schneider T, et al. Host defense peptides: Dual antimicrobial and immunomodulatory action. Int J Mol Sci. 2021;22:11172. https://doi.org/10.3390/ijms222011172.
Zhang J, Sun Y, Kang Y, Shang D. Antimicrobial peptide temporin-1CEa isolated from frog skin secretions inhibits the proinflammatory response in lipopolysaccharide-stimulated RAW264. 7 murine macrophages through the MyD88-dependent signaling pathway. Mol Immunol. 2021;132:227–35. https://doi.org/10.1016/j.molimm.2021.01.007.
Sun Y, Shang D. Inhibitory Effects of Antimicrobial Peptides on Lipopolysaccharide-Induced Inflammation. Mediators Inflamm. 2015;2015:167572. https://doi.org/10.1155/2015/167572.
Malone A, Clark RF, Hoskin DW, Power Coombs MR. Regulation of macrophage-associated inflammatory responses by species-specific lactoferricin peptides. Front Biosci (Landmark Ed). 2022;27:43. https://doi.org/10.31083/j.fbl2702043.
Yu H, Lu Y, Qiao X, Wei L, Fu T, Cai S, et al. Novel cathelicidins from pigeon highlights evolutionary convergence in avain cathelicidins and functions in modulation of innate immunity. Sci Rep. 2015;5:11082. https://doi.org/10.1038/srep11082.
Wei L, Gao J, Zhang S, Wu S, Xie Z, Ling G, et al. Identification and characterization of the first cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J Biol Chem. 2015;290:16633–52. https://doi.org/10.1074/jbc.
Jin G, Kawsar HI, Hirsch SA, Zeng C, Jia X, Feng Z, et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE. 2010;8:5. https://doi.org/10.1371/journal.pone.0010993.
Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. https://doi.org/10.1084/jem.192.7.1069.
Park YJ, Lee SK, Jung YS, Lee M, Lee HY, Lee HY, et al. Promotion of formyl peptide receptor 1-mediated neutrophil chemotactic migration by antimicrobial peptides isolated from the centipede Scolopendra subspinipes mutilans. BMB Rep. 2016;49:520. https://doi.org/10.5483/bmbrep.2016.49.9.098.
Yanashima K, Chieosilapatham P, Yoshimoto E, Okumura K, Ogawa H, Niyonsaba F. Innate defense regulator IDR-1018 activates human mast cells through G protein-, phospholipase C-, MAPK-and NF-ĸB-sensitive pathways. Immunol Res. 2017;65:920–31. https://doi.org/10.1007/s12026-017-8932-0.
Harten RV, Woudenbergh EV, Dijk AV, Haagsman H. Cathelicidins: Immunomodulatory antimicrobials. Vaccines (Basel). 2018;6:63. https://doi.org/10.3390/vaccines6030063.
Niyonsaba F, Song P, Yue H, Sutthammikorn N, Umehara Y, Okumura K, et al. Antimicrobial peptide derived from insulin-like growth factor-binding protein 5 activates mast cells via Mas-related G protein-coupled receptor X2. Allergy. 2020;75:203–7. https://doi.org/10.1111/all.13975.
Marques-Neto LM, Trentini MM, Das Neves RC, Resende DP, Procopio VO, Da Costa AC, et al. Antimicrobial and chemotactic activity of scorpion-derived peptide, ToAP2, against Mycobacterium massiliensis. Toxins. 2018;10:219. https://doi.org/10.3390/toxins10060219.
Yang Y, Wu J, Li Q, Wang J, Mu L, Hui L, et al. A non-bactericidal cathelicidin provides prophylactic efficacy against bacterial infection by driving phagocyte influx. Elife. 2022;11:e72849. https://doi.org/10.7554/eLife.72849.
Ibarra-Valencia MA, Espino-Solis GP, Estrada BE, Corzo G. Immunomodulatory responses of two synthetic peptides against salmonella typhimurium infection. Molecules. 2021;26:5573. https://doi.org/10.3390/molecules26185573.
Justyna A, Sylwia R, Magdalena W, Paulina E, Joanna P, Ewa BB. The RLR/NLR expression and pro-inflammatory activity of tissue mast cells are regulated by cathelicidin LL-37 and defensin hBD-2. Sci Rep. 2018;8:11750. https://doi.org/10.1038/s41598-018-30289-w.
Ogasawara H, Noguchi M. Therapeutic Potential of MRGPRX2 Inhibitors on Mast Cells. Cells. 2021;10:2906. https://doi.org/10.3390/cells10112906.
Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–9. https://doi.org/10.1126/science.1075565.
Małaczewska J, Kaczorek Łukowska E, Wójcik R, Rękawek W, Siwicki AK. In vitro immunomodulatory effect of nisin on porcine leucocytes. J Anim Physiol Anim Nutr. 2019;103:882–93. https://doi.org/10.1111/jpn.13085.
Zhang X, Wang P, Zhang N, Chen D, Nie P, Li J, et al. B cell functions can be modulated by antimicrobial peptides in rainbow trout Oncorhynchus mykiss: Novel insights into the innate nature of B cells in fish. Front Immunol. 2017;8:388. https://doi.org/10.3389/fimmu.2017.00388.
Kraaij MD, van Dijk A, Scheenstra MR, van Harten RM, Haagsman HP, Veldhuizen EJ. Chicken CATH-2 increases antigen presentation markers on chicken monocytes and macrophages. Protein Pept Lett. 2020;27:60–6. https://doi.org/10.2174/0929866526666190730125525.
Bommineni YR, Pham GH, Sunkara LT, Achanta M, Zhang G. Immune regulatory activities of fowlicidin-1, a cathelicidin host defense peptide. Mol Immunol. 2014;59:55–63. https://doi.org/10.1016/j.molimm.2014.01.004.
Yoshimura Y, Kondo H, Takamatsu K, Tsugami Y, Nii T, Isobe N. Modulation of the innate immune system by lipopolysaccharide in the proventriculus of chicks inoculated with or without Newcastle disease and infectious bronchitis vaccine. Poult Sci. 2022;101:101719. https://doi.org/10.1016/j.psj.2022.101719.
Terada T, Nii T, Isobe N, Yoshimura Y. Effects of probiotics Lactobacillus reuteri and Clostridium butyricum on the expression of Toll-like receptors, pro- and anti-inflammatory cytokines, and antimicrobial peptides in broiler chick intestine. J Poult Sci. 2020;57:310–8. https://doi.org/10.2141/jpsa.0190098.
Terada T, Nii T, Isobe N, Yoshimura Y. Effect of antibiotic treatment on microbial composition and expression of antimicrobial peptides and cytokines in the chick cecum. Poult Sci. 2020;99:3385–92. https://doi.org/10.1016/j.psj.2020.03.016.
Valdez-Miramontes CE, De Haro-Acosta J, Aréchiga-Flores CF, Verdiguel-Fernández L, Rivas-Santiago B. Antimicrobial peptides in domestic animals and their applications in veterinary medicine. Peptides. 2021;142:170576. https://doi.org/10.1016/j.peptides.2021.170576.
Peng L, Scheenstra MR, van Harten RM, Haagsman HP, Veldhuizen EJ. The immunomodulatory effect of cathelicidin-B1 on chicken macrophages. Vet Res. 2020;51:1–12. https://doi.org/10.1186/s13567-020-00849-y.
Nazeer N, Uribe-Diaz S, Rodriguez-Lecompte JC, Ahmed M. Antimicrobial peptides as an alternative to relieve antimicrobial growth promoters in poultry. Br Poult Sci. 2021;62:672–768. https://doi.org/10.1080/00071668.2021.1919993.
Rodrigues G, Maximiano MR, Franco OL. Antimicrobial peptides used as growth promoters in livestock production. Appl Microbiol Biotechnol. 2021;105:7115–21. https://doi.org/10.1007/s00253-021-11540-3.
Yu HT, Ding XL, Li N, Zhang XY, Zeng XF, Wang S, et al. Dietary supplemented antimicrobial peptide microcin J25 improves the growth performance, apparent total tract digestibility, fecal microbiota, and intestinal barrier function of weaned pigs. J Anim Sci. 2017;95:5064–76. https://doi.org/10.2527/jas2017.1494.
Wu S, Zhang F, Huang Z, Liu H, Xie C, Zhang J, et al. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with E. coli. Peptides. 2012;35:225–30. https://doi.org/10.1016/j.peptides.2012.03.030.
Yuan W, Jin HT, Ren ZH, Deng JL, Zuo ZC, Wang Y, et al. Effects of antibacterial peptide on humoral immunity in weaned piglets. Food Agr Immunol. 2015;26:682–9. https://doi.org/10.2527/jas.2014-7933.
Zhang L, Guo T, Zhan N, Sun T, Shan A. The Effects of the antimicrobial peptide WK3 on diarrhea, growth performance and intestinal health of weaned piglets challenged with enterotoxigenic E. coli K88. Food Nutr Res. 2021;11:65. https://doi.org/10.29219/fnr.v65.3448.
Feng J, Wang L, Xie Y, Chen Y, Yi H, He D. Effects of antimicrobial peptide cathelicidin-BF on diarrhea controlling, immune responses, intestinal inflammation and intestinal barrier function in piglets with postweaning diarrhea. Int Immunopharmacol. 2020;85:106658. https://doi.org/10.1016/j.intimp.2020.106658.
Xiao H, Wu MM, Tan BE, Yin YL, Li TJ, Xiao DF, et al. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J Anim Sci. 2013;91:4772–80. https://doi.org/10.2527/jas.2013-6426.
Huang H, Pan C, Su B, Wu H, Chen J. Epinecidin-1 protects against methicillin resistant staphylococcus aureus infection and sepsis in pyemia pigs. Mar Drugs. 2019;17:693. https://doi.org/10.3390/md17120693.
Daneshmand A, Kermanshahi H, Sekhavati MH, Javadmanesh A, Ahmadian M. Antimicrobial peptide, cLF36, affects performance and intestinal morphology, microflora, junctional proteins, and immune cells in broilers challenged with E. coli. Sci Rep. 2019;9:14176. https://doi.org/10.1038/s41598-019-50511-7.
Wang G, Song Q, Huang S, Wang Y, Cai S, Yu H, et al. Effect of antimicrobial peptide microcin J25 on growth performance, immune regulation, and intestinal microbiota in broiler chickens challenged with E. coli and Salmonella. Animals. 2020;10:345. https://doi.org/10.3390/ani10020345.
Daneshmand A, Kermanshahi H, Sekhavati MH, Javadmanesh A, Ahmadian M, Alizadeh M, et al. Effects of cLFchimera peptide on intestinal morphology, integrity, microbiota, and immune cells in broiler chickens challenged with necrotic enteritis. Sci Rep. 2020;10:17704. https://doi.org/10.1038/s41598-020-74754-x.
Wang S, Zeng XF, Wang QW, Zhu JL, Peng Q, Hou CL, et al. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers. J Anim Sci. 2015;93:4750–60. https://doi.org/10.2527/jas.2015-9284.
Hu F, Gao X, She R, Chen J, Mao J, Xiao P, et al. Effects of antimicrobial peptides on growth performance and small intestinal function in broilers under chronic heat stress. Poult Sci. 2017;96:798–806. https://doi.org/10.3382/ps/pew379.
Ko SKK, Paraso MGV, Pajas AMGA, Cruz JFD. Immunomodulatory responses in plectasin-supplemented broilers under tropical environmental conditions. Trop Anim Health Prod. 2021;53:1–7. https://doi.org/10.1007/s11250-021-02691-6.
Choi SC, Ingale SL, Kim JS, Park YK, Kwon IK, Chae BJ. An antimicrobial peptide-A3: Effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Br Poult Sci. 2013;54:738–46. https://doi.org/10.1080/00071668.2013.838746.
Zhang X, Zhao Q, Wen L, Wu C, Yao Z, Yan Z, et al. The effect of the antimicrobial peptide plectasin on the growth performance, intestinal health, and immune function of Yellow-Feathered chickens. Front Vet Sci. 2021;8:688611. https://doi.org/10.3389/fvets.2021.688611.
Xie Z, Zhao Q, Wang H, Wen L, Li W, Zhang X, et al. Effects of antibacterial peptide combinations on growth performance, intestinal health, and immune function of broiler chickens. Poult Sci. 2020;99:6481–92. https://doi.org/10.1016/j.psj.2020.08.068.
Rhouma M, Fairbrother JM, Beaudry F, Letellier A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet Scand. 2017;59:31. https://doi.org/10.1186/s13028-017-0299-7.
Yi H, Yu C, Zhang H, Song D, Jiang D, Du H, et al. Cathelicidin-BF suppresses intestinal inflammation by inhibiting the nuclear factor-κB signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Int Immunopharmacol. 2015;28:61–9. https://doi.org/10.1016/j.intimp.2015.05.034.
Shuai W, Xiangfang Z, Qing Y, Shiyan Q. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci. 2016;17(5):603. https://doi.org/10.3390/ijms17050603.
Xiong X, Yang HS, Li L, Wang YF, Huang RL, Li FN, et al. Effects of antimicrobial peptides in nursery diets on growth performance of pigs reared on five different farms. Livest Sci. 2014;167:206–10. https://doi.org/10.1016/j.livsci.2014.04.024.
Kim IH. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets—A review. Animals (Basel). 2021;11(8):2418. https://doi.org/10.3390/ani11082418.
Suzuki T, Iwahashi Y. Deoxynivalenol and its toxicity. Toxins. 2015;7:187–200. https://doi.org/10.2478/v10102-010-0019-x.
Pierron A, Alassane-Kpembi I, Oswald IP. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016;2:21. https://doi.org/10.1186/s40813-016-0041-2.
Bryden WL. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim Feed Sci Tech. 2012;173:134–58. https://doi.org/10.1016/j.anifeedsci.2011.12.014.
Liu L, Ren M, Ren K, Jin Y, Yan M. Heat stress impacts on broiler performance: A systematic review and meta-analysis. Poult Sci. 2020;99:6205–11. https://doi.org/10.1016/j.psj.2020.08.019.
Lara LJ, Rostagno MH. Impact of heat stress on poultry production. Animals. 2013;3:356–69. https://doi.org/10.3390/ani3020356.
Zened A, Forano E, Delbes C, Verdier-Metz I, Morgavi D, Popova M, et al. Ruminant microbiota: Research status and impacts of microbiota on animal performance and health. Inra Prod Anim. 2020;33:249–60 (https://doi.org/10.20870).
Hook SE, Wright AG, McBride BW. Methanogens: Methane producers of the rumen and mitigation strategies. Archaea. 2010;30:945785. https://doi.org/10.1155/2010/945785.
Thorpe A. Enteric fermentation and ruminant eructation: The role (and control?) of methane in the climate change debate. Clim Change. 2009;93:407–31. https://doi.org/10.1007/s10584-008-9506-x.
Key N, Tallard G. Mitigating methane emissions from livestock: A global analysis of sectoral policies. Clim Change. 2012;112:387–414. https://doi.org/10.1007/s10584-011-0206-6.
Varnava KG, Ronimus RS, Sarojini V. A review on comparative mechanistic studies of antimicrobial peptides against archaea. Biotechnol Bioeng. 2017;114:2457–73. https://doi.org/10.1002/bit.26387.
Garsa AK, Choudhury PK, Puniya AK, Dhewa T, Malik RK, Tomar SK. Bovicins: The bacteriocins of streptococci and their potential in methane mitigation. Probiotics Antimicro. 2019;11:1403–13. https://doi.org/10.1007/s12602-018-9502-z.
Lee SS, Hsu J, Mantovani HC, Russell JB. The effect of bovicin HC5, a bacteriocin from Streptococcus bovis HC5, on ruminal methane production in vitro. FEMS Microbiol Lett. 2002;217:51–5. https://doi.org/10.1111/j.1574-6968.2002.tb11455.x.
Shen J, Liu Z, Yu Z, Zhu W. Monensin and nisin affect rumen fermentation and microbiota differently in vitro. Front Microbiol. 2017;8:1111. https://doi.org/10.3389/fmicb.2017.01111.
Wang X, Teng D, Wang X, Hao Y, Chen H, Mao R, et al. Internalization, distribution, and activity of peptide H2 against the intracellular multidrug-resistant bovine mastitis-causing bacterium Staphylococcus aureus. Sci Rep. 2019;9:7968. https://doi.org/10.1038/s41598-019-44459-x.
Isobe N. Control mechanisms for producing antimicrobial factors in ruminant mammary gland. Anim Sci J. 2017;7:937–43. https://doi.org/10.1111/asj.12808.
Dassanayake RP, Falkenberg SM, Register KB, Samorodnitsky D, Nicholson EM, Reinhardt TA. Antimicrobial activity of bovine NK-lysin-derived peptides on Mycoplasma bovis. PLoS ONE. 2018;13:5. https://doi.org/10.1371/journal.pone.0197677.
Małaczewska J, Kaczorek-Łukowska E, Wójcik R, Siwicki AK. Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Vet Res. 2019;15:318. https://doi.org/10.1186/s12917-019-2067-6.
Liu Q, Yao S, Chen Y, Gao S, Yang Y, Deng J, et al. Use of antimicrobial peptides as a feed additive for juvenile goats. Sci Rep. 2017;7:12254. https://doi.org/10.1038/s41598-017-12394-4.
Ren Z, Yao R, Liu Q, Deng Y, Shen L, Deng H, et al. Effects of antibacterial peptides on rumen fermentation function and rumen microorganisms in goats. PLoS ONE. 2019;30:14. https://doi.org/10.1371/journal.pone.0221815.
Wibowo D, Zhao CX. Recent achievements and perspectives for large-scale recombinant production of antimicrobial peptides. Appl Microbiol Biot. 2019;103:659–71. https://doi.org/10.1007/s00253-018-9524-1.
Ki MR, Pack SP. Fusion tags to enhance heterologous protein expression. Appl Microbiol Biot. 2020;104:2411–25. https://doi.org/10.1007/s00253-020-10402-8.
Meng F, Zhao H, Zhang C, Lu F, Bie X, Lu Z. Expression of a novel bacteriocin—the plantaricin Pln1—in Escherichia coli and its functional analysis. Protein Expres Purif. 2016;119:85–93. https://doi.org/10.1016/j.pep.2015.11.008.
Cao W, Liu Q, Wang T, Zhang Q, Cheng F, Tang Y, et al. Recombinant expression of the precursor of rat lung surfactant protein B in Escherichia coli and its antibacterial mechanism. Protein Expres Purif. 2020;179:105801. https://doi.org/10.1016/j.pep.2020.105801.
Lamer T, van Belkum MJ, Wijewardane A, Chiorean S, Martin-Visscher LA, Vederas JC. SPI “sandwich”: Combined SUMO-Peptide-Intein expression system and isolation procedure for improved stability and yield of peptides. Protein Sci. 2022;31:e4316. https://doi.org/10.1002/pro.4316.
Zhang M, Shan Y, Gao H, Wang B, Liu X, Dong Y, et al. Expression of a recombinant hybrid antimicrobial peptide magainin II-cecropin B in the mycelium of the medicinal fungus Cordyceps militaris and its validation in mice. Microb Cell Fact. 2018;17:18. https://doi.org/10.1186/s12934-018-0865-3.
Sun J, Jiang J, Liu L, Wang Z, Wei C. Expression of the hybrid antimicrobial peptide lactoferrin-lysozyme in Pichia pastoris. Biotechnol Appl Bioc. 2019;66:202–8. https://doi.org/10.1002/bab.1705.
Liu Z, Zhu M, Chen X, Yang G, Yang T, Yu L, et al. Expression and antibacterial activity of hybrid antimicrobial peptide cecropinA-thanatin in Pichia pastoris. Front Lab Med. 2018;2:23–9. https://doi.org/10.1016/j.flm.2018.04.001.
Zhang C, Yang M. Antimicrobial peptides: From design to clinical application. Antibiotics (Basel). 2022;11:349. https://doi.org/10.3390/antibiotics11030349.
Lai Z, Yuan X, Chen H, Zhu Y, Dong N, Shan A. Strategies employed in the design of antimicrobial peptides with enhanced proteolytic stability. Biotechnol Adv. 2022;59:107962. https://doi.org/10.1016/j.biotechadv.2022.107962.
Wang X, Yang X, Wang Q, Meng D. Unnatural amino acids: promising implications for the development of new antimicrobial peptides. Crit Rev Microbiol. 2022;7:12254. https://doi.org/10.1080/1040841X.2022.2047008.
Zaet A, Dartevelle P, Daouad F, Ehlinger C, Quilès F, Francius G, et al. D-Cateslytin, a new antimicrobial peptide with therapeutic potential. Sci Rep. 2017;7:15199. https://doi.org/10.1038/s41598-017-15436-z.
Lu J, Xu H, Xia J, Ma J, Xu J, Li Y, et al. D- and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Front Microbiol. 2020;11:563030. https://doi.org/10.3389/fmicb.2020.563030.
He T, Qu R, Zhang J. Current synthetic chemistry towards cyclic antimicrobial peptides. J Pept Sci. 2022;28:e3387. https://doi.org/10.1002/psc.3387.
Bechtler C, Lamers C. Macrocyclization strategies for cyclic peptides and peptidomimetics. RSC Med Chem. 2021;12:1325–51. https://doi.org/10.1039/d1md00083g.
Han H, Li T, Na Y, Wang Z, Wang J. Improved stability and activity of a marine peptide-N6NH2 against Edwardsiella tarda and its preliminary application in fish. Mar Drugs. 2020;18:650. https://doi.org/10.3390/md18120650.
Yu W, Wang J, Wang Z, Li L, Shan A. PEGylation of the antimicrobial peptide PG-1: A link between propensity for nanostructuring and capacity of the antitrypsin hydrolytic ability. J Med Chem. 2021;64:10469–81. https://doi.org/10.1021/acs.jmedchem.1c00879.
Teixeira MC, Carbone C, Sousa MC, Espina M, Garcia ML, Sanchez-Lopez E, et al. Nanomedicines for the delivery of antimicrobial peptides (AMPs). Nanomaterials (Basel). 2020;10:560. https://doi.org/10.3390/nano10030560.
Lai Z, Jian Q, Li G, Shao C, Zhu Y, Yuan X, et al. Self-assembling peptide dendron nanoparticles with high stability and a multimodal antimicrobial mechanism of action. ACS Nano. 2021;15:15824–40. https://doi.org/10.1021/acsnano.1c03301.
Acknowledgements
Not applicable.
Funding
This research was funded by NSFC grand (32172754).
Author information
Authors and Affiliations
Contributions
DW, LF and WW designed and wrote the manuscript. DW and ND finalized the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, D., Fu, L., Wen, W. et al. The dual antimicrobial and immunomodulatory roles of host defense peptides and their applications in animal production. J Animal Sci Biotechnol 13, 141 (2022). https://doi.org/10.1186/s40104-022-00796-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-022-00796-y