Abstract
Background
Natural products, including those derived from higher plants have, over the years, contributed greatly to the development of modern therapeutic drugs. Due to the medicinal importance in traditional practice and the diversified biology and chemistry of the constituents from Artemisia spp., the genus has been receiving growing attention. The aim of this study was to investigate the ability of the ethanol extract, four fractions (F1–F4) and five compounds namely artemisinin (1), scopoletin (2), chrysosplenetin (3), eupatin (4) and 3-O-β-d-glucopyranoside of sitosterol (5) isolated from A. annua to modulate the activity of anticholinesterase (AchE) and the production of nitric oxide (NO) in LPS-activated RAW 264.7 macrophages.
Results
At the lowest concentration tested (6.25 µg/mL), the crude extract and fraction F2 had the highest NO inhibitory activity (72.39 and 71.00 % inhibition respectively) without significant toxicity on the viability of macrophage cells (93.86 and 79.87 % of cell viability respectively). The crude extract inhibited AchE activity by 71.83 % (at 1 mg/mL) with an IC50 value of 87.43 µg/mL while F2 and F4 were the most active fractions (IC50 values of 36.75 and 28.82 µg/mL). Artemisinin (1) and chrysosplenetin (3) had the highest AChE activity with 71.67 and 80.00 % inhibition (at 0.1 mg/mL) and IC50 values of 29.34 and 27.14 µg/mL, respectively.
Conclusion
Our results validate the traditional use of A. annua and could help to support the usefulness of this plant in the treatment of inflammatory and neurological disorders especially where nitric oxide and a cholinesterase are involved.
Similar content being viewed by others
Background
Artemisia L. is a genus of small herbs and shrubs found in northern temperate regions. It belongs to the important family Compositae (Asteraceae), one of the largest plant families with about 1000 genera and more than 20,000 species. Within this family, Artemisia is included in the tribe Anthemideae and comprises over 500 species, which are mainly found in Asia, Europe and North America (Bora and Sharma 2011). Artemisia annua L., commonly known as sweet wormwood or Qinghao, is a large vigorous weedy annual shrub often reaching more than 2 m tall with aromatic leaves (Wright 2002)’ The leaves produce essential oils used in folk and modern medicine, and in the cosmetics and pharmaceutical industry (Teixeira da Silva 2004). It has traditionally been used in China for the treatment of fever and chills (Ferreira and Janick 1997). Some Artemisia species including A. annua have been traditionally used in pain, inflammation and febrile conditions (Habib and Waheed 2013; Huang et al. 1993). Though originally growing in Asia and Europe, the plant is cultivated in Africa and used as a tea for the treatment of malaria (Klayman 1993). The aerial parts of A. annua are source of artemisinin that has potent antimalarial activity (Bhakuni et al. 2001). Besides antimalarial activity, A. annua has biological activities such as antioxidant, antibacterial, antifungal, anti-inflammatory, angiotensin-converting enzyme inhibitory, cytokinin-like and antitumor effects (Bhakuni et al. 2001; Chougouo-Kengne et al. 2013; Chu et al. 2014; Woerdenbag et al. 1993; Kim et al. 2015). These activities relate to the presence of secondary metabolites such as sesquiterpenoids (Chu et al. 2014), flavonoids, terpenoids, steroids, aliphatic hydrocarbons, aromatic ketones, aromatic acids, phenylpropanoids (Brown 2010), alkaloids and coumarins (Bhakuni et al. 2001).
Natural products, including those derived from higher plants have, over the years contributed greatly to the development of modern therapeutic drugs. For instance, galantamine is an anticholinesterase (AchE) inhibitor drug used for the treatment of Alzheimer’s disease, originally isolated from several plants including Galanthus nivalus, bulbs and flowers of Galanthus caucasicus, Galanthus woronowii and related genera like Narcissus, Leucojum and Lycoris including Lycoris radiata (Olin and Schneider 2002).
Most plant-derived secondary metabolites are known to interfere directly or indirectly with various inflammatory mediators including nitric oxide (NO). The NO radical is known to play a central role in inflammatory and immune reactions. However, excessive production of NO may cause tissue damage. It is well known that NO plays an important role in the pathogenesis of inflammatory diseases (Calixto et al. 2003). However, inflammatory processes are involved in the onset and maintenance of many severe disorders including neurodegenerative diseases such as Alzheimer’s disease (AD) (Scrivo et al. 2011). AD occurs as a result of decreased cholinergic transmission, increased oxidative stress and increased inflammatory condition (Perry et al. 1998). Due to the medicinal importance in traditional practice and the diversified biology and chemistry of the constituents from Artemisia spp., this genus has been receiving growing attention. The aim of this study was to investigate the ability of the ethanol extract, fractions (F1–F4) and purified compounds from A. annua to modulate the activity of AChE and the production of NO in LPS-activated RAW 264.7 macrophages.
Results
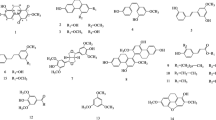
The ethanol crude extract of leaves and twigs of A. annua subjected to repeated silica gel column chromatography and Sephadex LH-20 yielded five compounds: artemisinin (1) (Rimada et al. 2009), scopoletin (2) (Darmawan et al. (2012), chrysosplenetin (3) (Calcagno-pissarelli et al. 2010), eupatin (4) (Jiang–Jiang et al. 2010) and 3-O-β-d-glucopyranoside of sitosterol (5) (Al-Oqail et al. (2012) (Fig. 1). The structures of these compounds were identified by analysis of their spectroscopic data and by comparison with those reported in the literature.
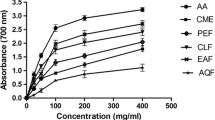
Compounds were assayed for their potential to inhibit the NO production in macrophage cells. For each sample, four concentrations were used 50, 25, 12.5 and 6.25 µg/mL for the crude extract and fractions (F1–F4) then 20, 5, 2 and 0.5 µg/mL for compounds and quercetin. All the samples tested reduced NO production to some extent. The percentage of NO inhibition calculated from the amount of NO produced revealed that, at the highest concentration, the crude extract, F2 and F3 as well as compounds 3 and 4 had the highest inhibitory activity with more than 100 % inhibition relatively to the control, with the respective cell viability values of 3.94, 33.39, 2.48, 15.89 and 7.49 %. At the same high concentrations, fraction F4, artemisinin (1) and scopoletin (2) inhibited the NO production 95.53, 99.15 and 81.30 %, respectively without obvious cytotoxic effect (Table 1). At the lowest concentration, scopoletin (2) still had significant NO inhibitory activity (65.48 % inhibition).
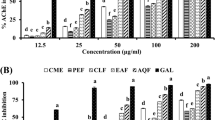
All the samples had AChE inhibitory activity, all had lower activity than galantamine. The crude extract showed 71.83 % inhibition with IC50 value of 87.43 µg/mL. Fractions F2 and F4 were the most actives (IC50 value of 36.75 and 28.82 µg/mL) (Table 2).
Discussion
Natural products continue to provide new and important leads for different pharmacological targets including inflammatory conditions. Therefore, in this study the extract, fractions and compounds were tested for their capacity to inhibit the production of NO in murine RAW 264.7 macrophages. Therefore, considering the high cytotoxicity (Table 1) The NO inhibition is more likely related to the cytotoxicity of the extracts and compounds on macrophages cells. However at the lowest concentration, the crude extract and fraction F2 remained active (72.39 and 71.00 % inhibition, respectively) without significant inhibition of the viability of macrophage cells (93.86 and 79.87 % of cell viability, respectively). These results are in accordance with the previous reported potential of extract from Artemisia species as inhibitors of nitric oxide production. Extracts of several species of Artemisia including A. stolonifera, A. selengensis, A. japonica, A. montana, A. capillaris, A. sylvatica, A. keiskeana and A. scoparia were significantly reduced the NO production at higher concentrations in the presence of LPS compared with that in control cells (Choi et al. 2013). The NO inhibitory effects observed in our study may therefore be related to the presence of artemisinin (1) and scopoletin (2) in the extract of A. annua. Artemisinin (1) and scopoletin (2) have been reported to exert anti-inflammatory activities (Zhu et al. 2013; Kang et al. 1999). Thus, these compounds could help to support the usefulness of the plant in the treatment of inflammatory diseases and to validate the traditional indication for this species.
The ethanol extract, fractions and the five compounds obtained from A. annua were tested for their in vitro anticholinesterase (AChE) activity using galantamine as a positive control. To determine the inhibition percentage, samples were tested in a preliminary screening at a single concentration of 500 µg/mL for extract and fractions, 100 µg/mL for compounds and galantamine. The activity of samples with > 50 % inhibition was further tested at different concentrations to determine the IC50 values. Our findings of AChE activity corroborate with several previous studies. A variety of plant species have AChE activity and so may be relevant to the treatment of neurodegenerative disorders such as AD (Amoo et al. 2012; Mukherjee et al. 2007). The most active compounds were chrysosplenetin (3) and artemisinin (1) with 80.00 and 71.67 % inhibition and IC50 values of 27.14 and 29.34 µg/mL respectively. Many other authors have reported the AChE activity of flavonoids and sesquiterpenes (Ji and Zhang 2006; Ibrahim et al. 2013).
Conclusion
The current study presents evidence that the ethanol extract, fractions and compounds obtained from A. annua have AChE inhibitory activity and are able to prevent the production of NO. Artemisinin (1) and scopoletin (2) appeared to be responsible for the anti-inflammatory activity while chrysosplenetin (3) caused the antiAChE activity. The activities reported here validate the traditional use of this plant against inflammatory conditions and supports the use of A. annua in the treatment of inflammatory and neurological disorders where a cholinesterase and nitric oxide are involved.
Methods
General experimental procedures
Column chromatography was performed on silica gel Merck 60 F254 [(0.2–0.5 mm)] 70–230 and 230–400 mesh (Darmstadt, Germany). Pre-coated silica gel 60 F254 thin layer chromatography (TLC) plates (Merck; Germany) were used for monitoring fractions and spots were detected with UV light (254 and 365 nm) and then sprayed with 20 % sulphuric acid (H2SO4) or vanillin-H2SO4 reagent followed by heating to 100 °C.
Plant Materials
The aerial parts (leaves and twigs) of Artemisia annua were collected before the flowering period from the scholar plantation of the Notre Dame Catholic Primary School at Bangangte in the grassfield region of Cameroon in November 2013 between 9 a.m. and 3 p.m. The sample was authenticated by a botanist of the National Herbarium of Cameroon in Yaounde where our sample was compared to the deposited specimen having a voucher number 65642 HNC/Cam.
Extraction and isolation
Dried leaves and twigs of A. annua were ground to a fine powder (3 kg) which was macerated three times with 95 % ethanol (EtOH) (24, 48 and 72 h each time) at room temperature. After filtration and removal of the solvent using rotary evaporatoration, 114.9 g of crude extract were obtained.
Part of this extract (48 g) was subjected to silica gel column chromatography (CC) eluting with hexane (Hex), Hex/Ethyl Acetate (EtOAc), EtOAc–MeOH, and MeOH in increasing polarity to afford 37 fractions of 400 mL each. After comparative TLC, four combined fractions were finally obtained as follow: F 1 [Hex–EtOAc (100:0 and 90: 10); 17.2 g], F 2 [Hex–EtOAc (85:15, 75:25, 65:35, 55:45 and 45:55); 15.0 g], F 3 [Hex–EtOAc (35:65 and 0:100), EtOAc–MeOH (95:5, 90:10 and 80:20); 8.3 g], F 4 [EtOAc–MeOH (70:30, 50:50, 30:70 and 0: 100); 5.6 g]. Part of F2 (14 g) was subjected to a purification silica gel CC using a gradient mixture of Hex-acetone to afford four sub-fractions coded SF1, SF2, SF3 and SF4 after comparative TLC. Sub-fraction SF1 was subjected to Sephadex LH-20 CC using CH2Cl2–MeOH (4:1) to afford 1 (46 mg). Sub-fraction SF2 was subjected to silica gel CC eluted with CH2Cl2– acetone (49:1) to yield 2 (13 mg) and a mixture of two compounds that was further separated as 3 (20 mg) and 4 (8 mg) using repeated Sephadex LH-20 fractionation.
Artemisinin ( 1 )
White crystal; m.p. 155–156 °C; molecular formula: C15H22O5; 1H NMR (300 MHz) in CDCl3 δ H: 1.31 (m, H-1), 2.00/1.44 (m, H-2a/H-2b), 2.43/2.06 (ddd, H-3a/H-3b), 5.86 (s, H-5), 1.75 (m, H-7), 1.85/1.05 (m, H-8a/H-8b), 1.75/1.05 (m, H-9a/H-9b), 3.39 (dq, H–H), 1.18 (d, 7.0 Hz, H-13), 0.98 (d, 6.0 Hz, H-14) and 1.42 (s, H-15); 13CNMR (75 MHz) in CDCl3, δ C: 172.1 (C-1), 105.4 (C-2), 93.7 (C-3), 79.5 (C-4), 50.1 (C-5), 44.9 (C-6), 37.5 (C-7), 35.9 (C-8), 33.6 (C-9), 32.9 (C-10), 25.2 (C-11), 24.8 (C-12), 23.4 (C-13), 19.8 (C-14), 12.5 (C-15).
Scopoletin ( 2 )
White powder, m.p. 203–205 °C, molecular formula: C10H8O4; 1H NMR (300 MHz) in CDCl3, δ H: 6.20 (d, 9.4 Hz, H-3) 7.51 (d, 9.4 Hz, H-4), 6.86 (s, H-5), 6.82 (s, H -8) and 3.82 (s, OCH3).
Chrysosplenetin ( 3 )
Yellow powder, m.p. 157–158 °C; molecular formula: C19H18O8; 1H NMR (300 MHz) in CDCl3, δ H: 6.50 (s, H-1) 7.66 (d, 2,1 Hz, H-2′), 7.05 (d, 8.6 Hz, H-5′), 7.71 (dd, 8.6 Hz, 2,1 Hz, H -6′), 12.61 (s, 5-OH), 5.74 (s, 4′-OH), 3. 86 (s, 3′-OCH3), 3.93 (s, 6-OCH3), 3.99 (s, 7-OCH3), 3.96 (s, 3′-OCH3); 13C NMR (75 MHz) in CDCl3 δ C: 155.9 (C-2), 105.40 (C-2), 138.7 (C-3), 178.9 (C-4), 152.8 (C-5), 132.3 (C-6), 158.7 (C-7), 90.3 (C-8), 152.3 (C-9), 106.6 (C-10), 122.4 (C-1′), 110.9 (C-2′), 146.3 (C-3′), 148.4 (C-4′), 114.6 (C-5′), 122.6 (C-6′), 60.1 (3-OCH3), 60.9 (6-OCH3), 56.1 (7-OCH3), 56.3 (3′-CH3).
Eupatin ( 4 )
Yellow crystal, m.p. 244–245 °C, molecular formula: C18H16O8; 1H NMR (300 MHz) in CDCl3 δ H: 12.75 (s, 5-OH), 7.74 (dd, 3.0 Hz, 12 Hz, H-6′), 7.61 (d, 3.0 Hz, H-2′), 7.01 (d, 12 Hz, H-5′), 6.81 (s, H-8), 4.00 (CH3O-6), 3.88 (CH3O-7), 3.81 (4′-OCH3); 13C NMR (75 MHz) in CDCl3 δ C : 148.2 (C-4′), 144.9 (C-3′), 121.2 (C-6′), 115.5 (C-2′), 115.3 (C-1′), 90.8 (C-8), 59.6 (6-OCH3), 59.3 (7-OCH3), 55.9 (4′-OCH3).
β-Sitosterol-3-O-β-d-glucopyranoside ( 5 )
White powder, m.p. 272–274 °C; molecular formula: C35H60O6; m/z 583.
Anti-inflammatory activity
Nitric oxide inhibitory activity and viability of LPS-activated RAW 264.7 macrophages
Cell culture
The RAW 264.7 macrophages cell line was purchased from the American Type Culture Collection (ATCC TIB-71, Rockville, MD, USA) and cultured in a plastic culture flask in DMEM containing l-glutamine supplemented with 10 % FCS and 1 % PSF solution at 37 °C with 5 % CO2. Cells were seeded (104 per well) in 96 well-microtitre plate and activated LPS alone (control) or with samples at different concentrations. Quercetin was used as a positive control (Mu et al. 2001).
Measurement of NO produced
The amount of nitric oxide released was determined by the Griess reagent as reported previously (Dzoyem et al. 2015).
Cell viability
The number of viable cells was determined as previously described by Mosmann (1983) on the macrophage cells with few modifications. Briefly, the cells were topped up with 200 µL DMEM after removal of media. 30 µL of 15 mg/mL MTT were added to each well and cells were incubated at 37 °C with 5 % CO2. The medium was aspirated after 2 h, and the formazan salt formed was dissolved using DMSO. The absorbance was read at 570 nm on a BioTek Synergy microplate reader. Cell viability percentage was then calculated with reference to the control (cells with LPS only considered as 100 % viable).
Acetylcholinesterase inhibition activity
Inhibition of acetylcholinesterase activity was determined using Ellman’s colorimetric method as previously described (Dzoyem and Eloff 2015) with a modification that galantamine (at 20 µg/mL) was used as positive control.
Statistical analysis
Experiments were performed three times and values expressed as mean ± standard deviation. Statistical analysis was performed with GraphPad InStat Software. The Fisher’s least significant difference (LSD) at 5 % significance level was used to assess the differences between values for significance. GraphPad Prism 7 Software was used for IC50 calculation.
Abbreviations
- AChE:
-
acetylcholinesterase
- NO:
-
nitric oxide
- LPS:
-
lipopolysaccharides
References
Amoo SO, Aremu AO, Moyo M, Van Staden J (2012) Antioxidant and acetylcholinesterase-inhibitory properties of long-term stored medicinal plants. BMC Complement Altern Med 7:87
Bhakuni RS, Jain DC, Sharma RP, Kumar S (2001) Secondary metabolites of Artemisia annua and their biological activity. Curr Sci 80:35–48
Bora KS, Sharma A (2011) The genus Artemisia: a comprehensive review. Pharm Biol 49:101–109
Brown GD (2010) The biosynthesis of artemisinin and the phytochemistry of Artemisia annua L. Molecules 15:7603–7698
Calcagno-pissarelli MP, Alonso-Amelot M, Mora R, Rodriguez D, Nùñez JLA (2010) Foliar exudates of Blakiella bartsiifolia (SF Blake) Cuatrec (Asteraceae) A preliminary study of the chemical composition. Av Quím 5:161–166
Calixto JB, Otuki MF, Santos AR (2003) Anti-inflammatory compounds of plant origin part I action on arachidonic acid pathway nitric oxide and nuclear factor kappa B (NF-kappaB). Planta Med 69:973–983
Choi E, Park H, Lee J, Kim G (2013) Anticancer antiobesity and anti-inflammatory activity of Artemisia species in vitro. J Trad Chin Med 33:92–97
Chougouo-Kengne RD, Fotsing-Kwetche PR, Kouamouo J, Domum-Tchouanche B, Somo-Moyo R, Kaptué L (2013) Antibacterial and antifungal activity of the essential oil extracted by hydro-distillation from Artemisia annua grown in West-Cameroon British. J Pharmacol Toxicol 4(3):89–94
Chu Y, Wang H, Chen J, Hou Y (2014) New sesquiterpene and polymethoxy-flavonoids from Artemisia annua L. Pharmacogn Mag. 10:213–216
Darmawan A, Kosela S, Kardono LBS, Syah YM (2012) Scopoletin a coumarin derivative compound isolated from Macaranga gigantifolia Merr. J Appl Pharm Sci 2175–2177
Dzoyem JP, Eloff JN (2015) Anti-inflammatory, anticholinesterase and antioxidant activity of leaf extracts of twelve plants used traditionally to alleviate pain and inflammation in South Africa. J Ethnopharmacol 160:194–201
Dzoyem JP, Tsamo TA, Melong R, Mkounga P, Nkengfack EA, McGaw LJ, Eloff JN (2015) Cytotoxicity, nitric oxide and acetylcholinesterase inhibitory activity of three limonoids isolated from Trichilia welwitschii (Meliaceae). Biol Res 48:57
Ferreira JFS, Janick J (1997) Artemisia annua: botany horticulture pharmacology. In: Janick J (ed) Horticultural reviews, vol 19. Wiley, New York, pp 319–371
Habib M, Waheed I (2013) Evaluation of anti-nociceptive anti-inflammatory and antipyretic activities of Artemisia scoparia hydromethanolic extract. J Ethnopharmacol 145:18–24
Huang L, Liu JF, Liu LX, Li DF, Zhang Y, Nui HZ, Song HY, Zhang CY (1993) Antipyretic and anti-inflammatory effects of Artemisia annua L. Zhongguo Zhong Yao Za Zhi 18(1):44–48
Ibrahim M, Farooq T, Hussain N, Hussain A, Gulzar T, Hussain I, Akash MS, Rehmani FS (2013) Acetyl and butyryl cholinesterase inhibitory sesquiterpene lactones from Amberboa ramose. Chem Cent J 7:116
Ji H-F, Zhang H-Y (2006) Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer’s disease. J Mol Struct THEOCHEM 767:3–9
Kang TH, Pae HO, Jeong SJ, Yoo JC, Choi BM, Jun CD, Chung HT, Miyamoto T, Higuchi R, Kim YC (1999) Scopoletin: an inducible nitric oxide synthesis inhibitory active constituent from Artemisia feddei. Planta Med 65:400–403
Kim WS, Choi WJ, Lee S, Kim WJ, Lee DC, Sohn UD, Shin HS, Kim W (2015) Anti-inflammatory antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. Korean J Physiol Pharmacol 19:21–27
Klayman DL (1993) Artemisia Annua: from weed to respectable antimalarial plant. In: Kinghorn AD, Balandrin MF (eds) Human medicinal agents from plants; ACS Symp Series, Washington, DC, USA, vol 534, pp 242–255
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mu MM, Chakravortty D, Sugiyama T, Koide N, Takahashi K, Mori I, Yoshida T, Yokochi T (2001) The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 2647 macrophage cells. J Endotoxin Res 7:431–438
Mukherjee PK, Kumar V, Mal M, Houghton PJ (2007) Acetylcholinesterase inhibitors from plants. Phytomedicine 14:289–300
Olin J, Schneider L (2002) Galantamine for Alzheimer’s disease. Cochrane Database Syst Rev 3:CD001747
Perry EK, Pickering AT, Wang WW, Houghton P, Perry NS (1998) Medicinal plants and Alzheimer’s disease: integrating ethnobotanical and contemporary scientific evidence. J Altern Complement Med 4:419–428
Rimada RS, Gatti WO, Jeandupeux R, Cafferata LFR (2009) Isolation Characterization and Quantification of Artemisinin by NMR from Argentinean Artemisia annua L. Bol Latinoam Caribe Plant Med Aromat 8:275–281
Scrivo R, Vasile M, Bartosiewicz I, Valesini G (2011) Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev 10(7):369–374
Teixeira da Silva JA (2004) Mining the essential oils of the Anthemideae. Afr J Biotechnol 3:706–720
Woerdenbag HJ, Bos R, Salomons MC, Hendriks H, Pras N, Malingré TM (1993) Volatile constituents of Artemisia annua L (Asteraceae). Flavour Fragr J 8:131–137
Zhu XX, Yang L, Li YJ, Zhang D, Chen Y, Kostecka P, Kmoniekova E, Zidek Z (2013) Effects of sesquiterpene flavonoid and coumarin types of compounds from Artemisia annua L on production of mediators of angiogenesis. Pharmacol Rep 65:410–420
Authors’ contributions
JPD performed experiments and wrote the first draft of manuscript. RDKC, YMMN, and MDA isolated the compounds. JK and PT supervised the chemistry part of the work. LJM and JNE sponsored and supervised the biological part of work. All authors read and approved the final manuscript.
Acknowledgements
The University of Pretoria provided a postdoctoral fellowship to JPD. The National Research Foundation (NRF) and Medical Research Council (MRC) provided funding to support this study. The Université des Montagnes provided financial support to phytochemical experimental part of this work.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The chemical structures and cell lines supporting the findings from this article are available in the http://pubchem.ncbi.nlm.nih.gov/; http://www.ncbi.nlm.nih.gov/pcassay and http://iclac.org/databases/cross-contaminations/repositories.
Ethics approval and consent to participate
This research did not involve data collected from humans or animals.
Informed consent
This manuscript does not contains any individual person’s data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chougouo, R.D.K., Nguekeu, Y.M.M., Dzoyem, J.P. et al. Anti-inflammatory and acetylcholinesterase activity of extract, fractions and five compounds isolated from the leaves and twigs of Artemisia annua growing in Cameroon. SpringerPlus 5, 1525 (2016). https://doi.org/10.1186/s40064-016-3199-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-3199-9