Abstract
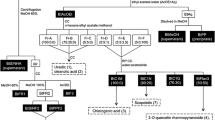
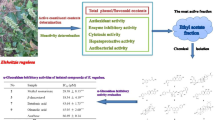
Alzheimer’s disease (AD) has the third highest health expenditures after heart disease and cancer. It has emerged as a serious global health issue. The discovery of new drugs to prevent and treat AD is of utmost importance. Pholidota cantonensis is an edible medicinal plant consumed in China. It is widely used in traditional Chinese medicine to treat various diseases. P. cantonensis has been reported to have antioxidant, anti-inflammatory, antitumor and antibacterial activities. Among these properties, its potent antioxidant activity has attracted our attention, since oxidative stress is one of the important pathological mechanisms involved in AD. This study aimed to isolate the compounds from the active extract and evaluate their bioactivities. Fifteen compounds, including one new compound, were obtained. The isolates were tested for 2,2′-diphenyl-1-picrylhydrazyl (DPPH)/2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activities, anti-acetylcholinesterase (anti-AChE) activities and inhibitory effects on nitrogen monoxide (NO) release in the BV-2 cells. Compounds 1, 2, 4, 6, 8, and 13–15 exhibited two kinds of AD-associated bioactivities. More importantly, compound 13 showed more potent NO inhibitory activity (IC50 = 0.72 ± 0.08 μM) than the positive control quercetin (IC50 = 12.94 ± 0.08 μM). Compound 13 also had a higher inhibitory rate (99.59 ± 0.43%) on AChE than that of the positive control galantamine (78.32 ± 1.16%) at the concentrate of 50 μg/mL. Our studies provide new insights into this plant in terms of its potential in the development of new multi-target anti-Alzheimer’s disease (anti-AD) drugs.


Similar content being viewed by others
References
Rong S, Xu R, Li W (2016) Phytosterols and dementia. Plant Foods Hum Nutr 71(4):347–354. https://doi.org/10.1007/s11130-016-0574-1
Alzheimer’s Disease International (2018) World Alzheimer report pp. 6–7. https://www.alz.co.uk/research/world-report-2018
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766. https://doi.org/10.1152/physrev.2001.81.2.741
Gong CX, Liu F, Iqbal K (2018) Multifactorial hypothesis and multi-targets for Alzheimer's disease. J Alzheimers Dis 64(s1):S107–S117. https://doi.org/10.3233/JAD-179921
Bolognesi ML (2013) Polypharmacology in a single drug: multitarget drugs. Curr Med Chem 20(13):1639–1645. https://doi.org/10.2174/0929867311320130004
Kulkarni A, Khan Y, Ray K (2017) Heterotrimeric kinesin-2, together with kinesin-1, steers vesicular acetylcholinesterase movements toward the synapse. FASEB J 31(3):965–974. https://doi.org/10.1096/fj.201600759RRR
Deb PK, Sharma A, Piplani P, Akkinepally RR (2012) Molecular docking and receptor-specific 3D-QSAR studies of acetylcholinesterase inhibitors. Mol Divers 16(4):803–823. https://doi.org/10.1007/s11030-012-9394-x
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (NY) 4(1):575–590. https://doi.org/10.1016/j.trci.2018.06.014
Ozben T, Ozben S (2019) Neuro-inflammation and anti-inflammatory treatment options for Alzheimer's disease. Clin Biochem 72:87–89. https://doi.org/10.1016/j.clinbiochem.2019.04.001
Alvariño R, Alonso E, Tribalat MA, Gegunde S, Thomas OP, Botana LM (2017) Evaluation of the protective effects of sarains on H2O2-induced mitochondrial dysfunction and oxidative stress in SH-SY5Y neuroblastoma cells. Neurotox Res 32(3):368–380. https://doi.org/10.1007/s12640-017-9748-3
Vladimir-Knežević S, Blažeković B, Kindl M, Vladić J, Lower-Nedza AD, Brantner AH (2014) Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 19(1):767–782. https://doi.org/10.3390/molecules19010767
Hayward NJ, Mcdougall GJ, Farag S, Allwood JW, Austin C, Campbell F, Horgan G, Ranawana V (2019) Cinnamon shows antidiabetic properties that are species-specific: effects on enzyme activity inhibition and starch digestion. Plant Foods Hum Nutr 74(4):544–552. https://doi.org/10.1007/s11130-019-00760-8
Editorial Committee of the Administration Bureau of Traditional Chinese Medicine (1999) Chinese Materia Medica (Vol 8). Shanghai Science & Technology Press, Shanghai
Luo X, Wang W, Liu L (2018) Comparisons of contents of total phenols and antioxidant activities of different extracts from Pholidota cantonensis. J Yangzhou Univ (Agric Life Sci Ed) 39(4):56–60, 99. https://doi.org/10.16872/j.cnki.1671-4652.2018.04.012
Liu L, Wang W, Zhao Z, Hu C, Tao L, Zhang X (2019) Pholidonone, an active stilbene derivative from Pholidota cantonensis, exhibits pro-apoptotic effect via induction of endoplasmic reticulum stress in human gastric cancer. Food Nutr Res 63:3553. https://doi.org/10.29219/fnr.v63.3553
Li B, Zhang X, Chen Y, Gao J, Wu C, Li S (2014) Study on antimicrobial activity of extracts from Pholidota cantonensis Rolfe. J Hunan Univ CM 34(6):9–12. https://doi.org/10.3969/j.issn.1674-070X.2014.06.004.009.04
Li B, Ali Z, Chan M, Li J, Wang M, Abe N, Wu CR, Khan IA, Wang W, Li SX (2017) Chemical constituents of Pholidota cantonensis. Phytochemistry 137:132–138. https://doi.org/10.1016/j.phytochem.2017.02.005
Du D, Zhang R, Xing Z, Liang Y, Li S, Jin T, Xia Q, Long D, Xin G, Wang G, Huang W (2016) 9,10-dihydrophenanthrene derivatives and one 1,4-anthraquinone firstly isolated from Dioscorea zingiberensis C. H. Wright and their biological activities. Fitoterapia 109:20–24. https://doi.org/10.1016/j.fitote.2015.11.022
Yu H, Dai B, Qian C, Ding Z, Jiang F, Jin B, Li M (2016) Antibacterial activity of chemical constituents isolated from fibrous roots of Bletilla striata. J Chin Med Mat 39(3):544–547. https://doi.org/10.13863/j.issn1001-4454.2016.03.019
Takagi S, Yamaki M, Inoue K (1983) Antimicrobial agents from Bletilla striata. Phytochemistry 22(4):1011–1015. https://doi.org/10.1016/0031-9422(83)85044-4
Guo X, Wang J, Wang N, Kitanaka S, Yao X (2006) Constituents from Pholidota yunnanensis and their inhibitory effects on nitric oxide production. Chin Tradit Herb Drugs 37(4):492–496
Majumder PL, Roychowdhury M, Chakraborty S (1998) Thunalbene, a stilbene derivative from the orchid Thunia alba. Phytochemistry 49(8):2375–2378. https://doi.org/10.1016/S0031-9422(98)00433-6
Garo E, Hu JF, Goering M, Hough G, O'Neil-Johnson M, Eldridge G (2007) Stilbenes from the orchid Phragmipedium sp. J Nat Prod 70(6):968–973. https://doi.org/10.1021/np070014j
Park JH, Yeon SW, Cho JG, Lee DY, Kim YS, Baek NI (2010) Lignans from silkworm droppings and their promotional activities on heme oxygenase-1 (HO-1). J Korean Soc Appl Biol Chem 53(6):734–739. https://doi.org/10.3839/jksabc.2010.111
Guo XY, Wang J, Wang NL, Kitanaka S, Yao XS (2007) 9,10-dihydrophenanthrene derivatives from Pholidota yunnanensis and scavenging activity on DPPH free radical. J Asian Nat Prod Res 9(2):165–174. https://doi.org/10.1080/10286020500480522
Boonyaratavej S, Tantayanontha S, Kitchanachai P, Chaichantipyuth C, Chittawong V, Miles DH (1992) Trans-triacontyl-4-hydroxy-3-methoxycinnamate, a new compound from the Thai plant Bridelia ovata. J Nat Prod 55(12):1761–1763. https://doi.org/10.1021/np50090a007
Peng W, Han T, Wang Y, Xin WB, Zheng CJ, Qin LP (2011) Chemical constituents of the aerial part of Atractylodes macrocephala. Chem Nat Compd 46:959–960. https://doi.org/10.1007/s10600-011-9795-6
Zaabat N, Hay AE, Michalet S, Skandrani I, Chekir-Ghedirac L, Dijoux-Francaa MG, Akkal S (2020) Chemical composition, antioxidant, genotoxique and antigenotoxic potentials of Phlomis bovei de Noé aerial parts. Iran J Pharm Res 19(1):282–291. https://doi.org/10.22037/IJPR.2019.15197.12938
Liu L, Zhang W, Yin Q, Zhang Y, Ji N, Zhang Y, Hu R (2015) Chemical constituents from Goodyera schlechtendaliana. J Chin Med Mat 38(12):2547–2549. https://doi.org/10.13863/j.issn1001-4454.2015.12.023
Chauthe SK, Sharma RJ, Aqil F, Gupta RC, Singh IP (2012) Quantitative NMR: an applicable method for quantitative analysis of medicinal plant extracts and herbal products. Phytochem Anal 23(6):689–696. https://doi.org/10.1002/pca.2375
Xu JJ, Chen YP, Dong WX, Teng Q, Lyu LC, Li YP (2020) Bioactive chemical constituents of Pleione yunnanensis. Chem Nat Compd 56:518–520. https://doi.org/10.1007/s10600-020-03075-2
Mazouz W, Haouli NEH, Gali L, Vezza T, Bensouici C, Mebrek S, Hamel T, Galvez J, Djeddi S (2020) Antioxidant, anti-alzheimer, anti-diabetic, and anti-inflammatory activities of the endemic halophyte Limonium spathulatum (Desf.) kuntze on LPS-stimulated RAW264 macrophages. S Afr J Bot 135:101–108. https://doi.org/10.1016/j.sajb.2020.08.021
Seebaluck-Sandoram R, Lall N, Fibrich B, Staden AB, Mahomoodally F (2017) Antibiotic-potentiation, antioxidant, cytotoxic, anti-inflammatoryand anti-acetylcholinesterase potential of Antidesma madagascariense Lam. (Euphorbiaceae). S Afr J Bot 111:194–201. https://doi.org/10.1016/j.sajb.2017.03.034
Ahn CB, Park PJ, Je JY (2012) Preparation and biological evaluation of enzyme-assisted extracts from edible seaweed (Enteromorpha prolifera) as antioxidant, anti-acetylcholinesterase and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Int J Food Sci Nutr 63(2):187–193. https://doi.org/10.3109/09637486.2011.616486
Acknowledgements
This research was supported by the Qinglan Project of University of Jiangsu Province, High-end Talents Supporting Project of Yangzhou University, Qinglan Project of Yangzhou University (20180210), Key talents of Kejiaoqiangwei of the 13th five year plan of Yangzhou (ZDRC20187), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX20_1385).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 0.99 mb)
Rights and permissions
About this article
Cite this article
Liu, L., Zou, M., Zeng, K. et al. Chemical Constituents and their Antioxidant, Anti-Inflammatory and Anti-Acetylcholinesterase Activities from Pholidota cantonensis. Plant Foods Hum Nutr 76, 105–110 (2021). https://doi.org/10.1007/s11130-020-00874-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00874-4