Abstract
The treatment goals for metastatic breast cancer (MBC) are prolonging survival and improving the quality of life. Eribulin, a non-taxane tubulin inhibitor, demonstrated improved survival in previous studies and also showed mild toxicity when used in late-line therapy for MBC. We conducted a phase II study to investigate the efficacy of eribulin mesylate as the first-line chemotherapy for human epidermal growth factor receptor 2 (HER2)-negative MBC. This was a phase II, open-label, single-arm, multicenter trial conducted in Japan. Patients with HER2-negative MBC received intravenous eribulin (1.4 mg/m2 on days 1 and 8 of each 21-day cycle). The primary efficacy outcome was overall response rate (ORR). Secondary outcomes included time to treatment failure, progression-free survival (PFS), overall survival (OS), and safety. A total of 35 patients were enrolled and received a median of 8 (range 1–21) cycles of eribulin therapy. ORR and clinical benefit rate were 54.3 and 62.9 %, respectively. Median PFS was 5.8 months and median OS was 35.9 months. Grade 3 or 4 neutropenia was observed in 63 % of patients. The majority of non-hematological adverse events were mild in severity. The present trial demonstrated that eribulin has antitumor activity comparable with other key established cytotoxic agents with acceptable safety and tolerability. Thus, eribulin as first-line chemotherapy might be beneficial for patients with HER2-negative MBC.
Similar content being viewed by others
Background
The prognosis for patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) has improved significantly since anti-HER2 therapies became commercially available. However, the long-term survival of patients with HER2-negative breast cancer remains poor, with a 5-year survival rate of only 24.3 % for distant metastatic disease (Howlader et al. 2013). As MBC is currently incurable, the goals of therapy are to prolong survival, palliate symptoms, and optimize quality of life (QoL) (Partridge et al. 2014). Anthracycline- or taxane-based regimens have often been chosen as first-line therapy for HER2-negative MBC. The current guidelines suggest using a single agent to optimize both treatment length and QoL for first-line therapy, except in the case of immediately life-threatening disease (Partridge et al. 2014; Cardoso et al. 2014). Based on these guidelines, agents with reduced toxicity but comparable efficacy to anthracyclines and taxanes could be therapeutic options for first-line therapy in such patients. In fact, a recent clinical study conducted in Japan demonstrated non-inferiority of the oral 5-fluorouracil derivative S-1 in overall survival (OS) and superiority in QoL against taxane as first-line chemotherapy for MBC (Takashima et al. 2016).
Recently, eribulin, a non-taxane microtubule dynamics inhibitor belonging to the halichondrin class of antineoplastic agents, which has a mechanism of action distinct from currently available taxanes (Jordan et al. 2005; Smith et al. 2010), has become available for treatment of MBC. In a phase 3, open-label, randomized trial (EMBRACE study), eribulin showed a significant and clinically meaningful improvement in OS compared to treatment of the physician’s choice in patients with heavily pretreated MBC (Cortes et al. 2011). In a different trial, the survival benefit of eribulin was similar to that of capecitabine in patients with MBC who had previously been treated with anthracycline- and taxane-based regimens (Kaufman et al. 2015). Moreover, the pooled analysis of those two trials demonstrated that eribulin significantly prolonged the OS compared with controls (Twelves et al. 2014). In addition to OS benefit, the non-hematological toxicity reported with eribulin treatment is mostly mild. These two findings suggest that eribulin would be a suitable option for early-line treatment of MBC to minimize toxicity and maximize survival benefit.
Although eribulin has been approved in Japan for the treatment of patients with inoperable or recurrent breast cancer, and is not limited to those who have been previously treated with chemotherapy regimens, data on first-line use of eribulin for treatment of Japanese patients with MBC are still limited. To date, only one phase II trial conducted outside Japan has included a small number of Asian patients with MBC (McIntyre et al. 2014). Moreover, current guidelines do not specify a preferred regimen for HER2-negative MBC. Therefore, we conducted a phase II trial to investigate the efficacy and safety of eribulin for first-line treatment of Japanese patients with HER2-negative MBC.
Patients and methods
Patients
Key inclusion criteria included: female patients with histologically confirmed HER2-negative MBC (including patients with unresectable advanced disease); aged ≥20 and <75 years; no history of chemotherapy for MBC other than peri-operative therapy (patients who received hormone therapy, immunotherapy, or local radiotherapy for MBC could be included in this trial); at least 6 months since the last administration of neoadjuvant or adjuvant chemotherapy; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; having measurable lesion(s) based on the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 (New response evaluation criteria in solid tumours 2009); and adequate bone marrow, liver, renal, and lung functions. Key exclusion criteria included: hypersensitivity to eribulin; systemic infection; uncontrolled pleural effusion/ascites or pericardial effusion; symptomatic brain tumor; serious complications, active concomitant malignancy; pregnancy (including possible pregnancy) of premenopausal women. Patients who were considered ineligible by the investigator were also excluded.
Study design
This was a phase II, open-label, single-arm, multicenter trial conducted at eight sites in Japan. The study protocol and all amendments were approved by local ethics committees or the institutional review board at each study site. This trial was conducted in accordance with the Japanese Guidelines for Clinical Research of the Ministry of Health, Labor and Welfare and the Declaration of Helsinki, as well as other applicable regulatory requirements. All participants provided written informed consent prior to study entry. The present trial has been registered with the University Hospital Medical Information Network (UMIN) Center (ID: UMIN000006086). This was an investigator-initiated clinical trial that was not supported by any industry funding, nor requested by any organization.
Eribulin was administered intravenously, without any premedication, at a dose of 1.4 mg/m2 over 2–5 min on days 1 and 8 of a 21-day cycle (2-weeks-on, 1-week-off). For patients who were not eligible for administration of eribulin on day 8 (i.e., neutrophil count <1000/mm3, platelet count <75,000/mm3, ≤grade 2 non-hematological adverse events), the next cycle started on day 22. The dose was reduced to 1.1 mg/m2 if one of the following had occurred during the previous cycle: neutrophil count <500/mm3 for more than 7 days; presence of febrile neutropenia; grade 4 thrombocytopenia; and grade 3 or higher non-hematological toxicity. The dose was further reduced to 0.7 mg/m2 if there was a toxicity as described above despite dose reduction to 1.1 mg/m2. Patients who were refractory to eribulin were able to continue treatment based on the choice of the investigator. Concomitant use of other anticancer therapy (e.g., hormone therapy, targeted therapy, immune therapy, and chemotherapy other than eribulin) and any local therapy was prohibited. Concomitant use of bone modifying agents was permitted if the agents had been used since prior to the study entry. Use of granulocyte colony-stimulating factor was permitted, but not for prophylactic administration, by decision of the investigator based on the clinical practice guideline (Smith et al. 2006).
The primary efficacy outcome was overall response rate (ORR), defined as the proportion of patients who achieved a complete response (CR) plus those who achieved a partial response (PR). The secondary endpoints included progression-free survival (PFS), OS, time to treatment failure (TTF), and safety. Time to response and duration of response were also assessed.
Assessment
The information on patients’ characteristics at baseline was collected within 28 days prior to the initiation of eribulin administration. Baseline tumor assessments by radiographic evaluation (e.g., computerized tomography or magnetic resonance imaging scans) were also performed within 28 days prior to the initiation of eribulin administration, and tumor assessments were performed by the same methods every 2 cycles thereafter. Tumor assessments were analyzed based on the RECIST ver. 1.1 and classified as CR, PR, stable disease (SD), progressive disease, or not evaluable. Tumor response was confirmed at least 4 weeks after the criteria for response were met. PFS was defined as the time from initiation of eribulin to disease progression or death from any cause, OS was defined as the time from initiation of eribulin to death from any cause, and TTF was defined as the time from initiation of eribulin to treatment discontinuation for any reason (e.g., disease progression, treatment toxicity, patient preference, or death). Time to response was the time from initiation of eribulin to documentation of tumor response and duration of response was defined as the time from documentation of tumor response to disease progression, which was assessed among patients who reached ORR. For safety, adverse events, physical examination, vital signs, laboratory tests, and tumor markers (i.e., carcinoembryonic antigen and breast cancer antigen 15-3) were assessed during the study. All adverse events were graded according to the Common Terminology Criteria for Adverse Events ver. 4.0 (CTEP 2015).
Statistical analysis
The following assumptions were made to determine target enrollment. In the EMBRACE study (Cortes et al. 2011), ORR for patients who received eribulin after a median of four previously administered regimens was 12 %. In addition, ORR of nanoparticle albumin-bound paclitaxel and paclitaxel for MBC was reported as 33 and 19 %, respectively, in all patients and 42 and 27 %, respectively, in the subgroup (40 % of the full cohort) who received those agents as first-line therapy in the phase III trial (Gradishar et al. 2005). Based on those results, we set threshold and expected values of ORR as 20 and 40 %, respectively. To meet the threshold and expected values of ORR with 80 % power and one-sided alpha error of 0.05, at least 32 patients were needed. Thus, we aimed to enroll 35 patients with the expectation of approximately 10 % ineligible patients.
Primary efficacy outcome (proportion of patients who achieved CR or PR for at least 4 weeks) was assessed in the full analysis set, which included all patients who received at least one dose of eribulin. In addition, clinical benefit rate (CBR) was defined as the proportion of patients who achieved CR, PR, or SD for at least 24 weeks. The median values with 95 % confidence interval (CI) for PFS, and OS curves were estimated with the Kaplan–Meier method. TTF, time to response, and duration of response were presented as median values with ranges. The safety analysis was also conducted in the full analysis set. All statistical analyses were one-sided, and probability values of <0.05 were considered to indicate a statistically significant difference.
Results
Patients
A total of 35 patients with HER2-negative MBC were enrolled between September 2011 and May 2014; none were excluded from our primary analysis. The characteristics of the patients at baseline are summarized in Table 1. The median age was 64 years (range 40–75), and the all patients had ECOG PS 0 or 1. Twenty-eight patients (80 %) were hormonal receptor-positive. Ten patients (29 %) received perioperative chemotherapy with anthracycline and/or taxane and five patients (14 %) received perioperative chemotherapy with other agents. The median number of cycles of eribulin administration was 8 (range 1–21), and the median relative dose intensity per week was 91.6 % (range 44.7–100 %). Dose modification was needed in four patients, and schedule modification in 19 patients. Patients were followed up for a median of 23.0 months (range 1.0–48.6) at data cut-off (October 15, 2015).
Efficacy analysis
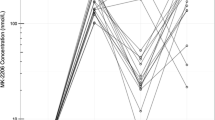
The ORR was 54.3 % (95 % CI 37.8–70.8) and CBR was 62.9 % (95 % CI 46.8–78.9) (Table 2; Fig. 1). Among eight patients with locally advanced disease, four discontinued eribulin therapy and were able to undergo surgery as a result of down-staging. In the subgroups stratified by estrogen receptor status of the tumor, ORR for patients with luminal-like disease and those with triple-negative disease was somewhat similar; 53.6 % (95 % CI 35.1–72.0) and 57.1 % (95 % CI 20.5–93.8), respectively. ORR for patients with a disease-free interval of <2 or ≥2 years was similar. On the other hand, ORR for patients who did not receive any neoadjuvant/adjuvant chemotherapy was higher at 70.0 % (95 % CI 49.9–90.1) compared to 33.3 % (95 % CI 9.5–57.2) in those who received neo/adjuvant chemotherapy. In addition, ORR for patients who received neo/adjuvant chemotherapy without anthracycline- or taxane-based regimens was higher at 64.0 % (95 % CI 45.2–82.8) compared to 30.0 % (95 % CI 1.6–58.4) in those who received anthracycline- or taxane-based neo/adjuvant chemotherapy. Moreover, patients without visceral metastasis had higher ORR at 66.7 % (95 % CI 40.0–93.3) compared to 47.8 % (95 % CI 27.4–68.2) in those with visceral metastasis.
The median PFS was 5.8 months (95 % CI 4.8–8.1) and median OS was 35.9 months. (Figs. 2, 3). The median TTF was 5.3 months (range 4.1–6.8), the median time to response was 1.4 months (range 1.2–3.7), and the median duration of response among patients who reached ORR was 4.6 months (range 0.6–22.1).
Safety analysis
Observed adverse events are shown in Table 3. Hematological adverse events of any grade were reported in all of the patients. The most commonly reported grade 3 or 4 hematologic adverse event was neutropenia (22 patients; 62.9 %), followed by leucopenia (9 patients; 25.7 %). Febrile neutropenia was reported in two patients (5.7 %). The most commonly reported any grade non-hematological adverse event was alopecia (26 patients; 74.3 %), followed by fatigue (22 patients; 62.9 %), sensory neuropathy (21 patients; 60.0 %), and fever (17 patients; 48.6 %). Grade 3 or higher non-hematologic adverse events were reported in three patients (8.6 %); sensory neuropathy, mucositis, and skin rash in one patient (2.8 %) each. Five patients (14.3 %) discontinued eribulin therapy due to adverse events.
Increases in laboratory values were reported as follows: aspartate aminotransferase (29 patients; 82.9 %), alanine aminotransferase (29 patients; 82.9 %), gamma-glutamyl transpeptidase (13 patients; 37.1 %), alkaline phosphatase (9 patients; 25.7 %), bilirubin (9 patients; 25.7 %), and albumin (9 patients; 25.7 %). Grade 3 events of increased aspartate aminotransferase, alkaline phosphatase, and albumin were reported in one patient each (2.9 %), and Grade 3 increase of gamma-glutamyl transpeptidase was reported in two patients (5.7 %). No Grade 3 increase of alanine aminotransferase or Grade 4 non-hematologic toxicity was reported. The majority of changes in laboratory values and vital signs were not clinically significant. There were no serious adverse events reported.
Discussion
The current phase II study was to the first to investigate the efficacy and safety of eribulin as first-line chemotherapy for HER2-negative MBC in Japanese patients. The ORR and CBR were high, at 54.3 and 62.9 %, respectively. Interestingly, the ORR was higher in patients who had not received any neo/adjuvant chemotherapy, whose disease had luminal-like or triple negative features, or who had distant metastasis, than in those who had received neo/adjuvant chemotherapy. Hematological and non-hematological toxicities of any grade were reported in all of the patients; however, the majority of non-hematological adverse events were mild and tolerable. All reported adverse events were expected and no unexpected adverse events were reported.
The ORR and CBR (54.3 and 62.9 %, respectively) in this phase II trial were higher than those in a global phase II trial conducted by McIntyre et al. (28.6 and 51.8 %, respectively) (McIntyre et al. 2014), which might be due to differences in the proportion of patients who had received neo/adjuvant chemotherapy. In our trial, the ORR was higher in patients who had not received any neo/adjuvant chemotherapy than in those who had (70.0 vs. 33.3 %). Among patients who had received neo/adjuvant chemotherapy, patients who had not received anthracycline- or taxane-based regimens had higher ORR than those who had received anthracycline- or taxane-based neo/adjuvant chemotherapy (64.0 vs. 30.0 %). Our trial included a smaller proportion of patients who had received neo/adjuvant chemotherapy (43 %) than McIntyre’s trial (68 %). In addition, only 29 % of the patients included in our trial had received anthracycline- or taxane-based neo/adjuvant chemotherapy, while in McIntyre’s trial, 48 and 46 % of patients had received anthracycline- or taxane-based neo/adjuvant chemotherapy, respectively. Consequently, the lower proportion of patients in our trial who had received neo/adjuvant chemotherapy, especially anthracycline- or taxane-based neo/adjuvant chemotherapy, might have led to the higher ORR. Moreover, the ORRs were comparable to those with nanoparticle albumin-bound paclitaxel (42 %) and higher than those with paclitaxel (27 %) as first-line chemotherapy for MBC as shown in a phase III trial conducted outside Japan (Gradishar et al. 2005). The median time to response and duration of response (1.4 and 4.6 months, respectively) among patients who reached ORR in our trial were similar to those in McIntyre’s trial (1.4 and 5.8 months, respectively) (McIntyre et al. 2014).
The median PFS in this trial (5.8 months) was comparable to that reported in earlier clinical trials, including a study of first-line use of taxane (5.1 months) and anthracycline (7.2 months) for MBC (Piccart-Gebhart et al. 2008) and the phase II trial of eribulin conducted outside Japan (6.8 months) (McIntyre et al. 2014). Additionally, the median OS was 35.9 months in our trial, which seems to be comparable to taxane (37.2 months) and S-1 (35.0 months) for MBC in the Japanese population (Takashima et al. 2016). The survival benefit of eribulin has also been demonstrated in late-line therapy for MBC (Cortes et al. 2011; Kaufman et al. 2015; Twelves et al. 2014). One of the characteristics of eribulin is that it is associated with improvements in OS, but not PFS. This finding has also been noted with tumors other than breast cancer. A recent phase III trial of eribulin versus dacarbazine in patients with leiomyosarcoma and adipocytic sarcoma demonstrated that the median OS was significantly improved in patients treated with eribulin compared with those treated with dacarbazine, although median PFS was comparable between the patient groups (Schöffski et al. 2015). The survival benefit of eribulin might be due to improvement of the microenvironment of tumor cells, which was demonstrated by in vitro and in vivo preclinical studies (Funahashi et al. 2014; Yoshida et al. 2014; Terashima et al. 2014). Since one of the major goals of the therapy for MBC is to prolong survival, eribulin might be a suitable option to achieve this goal.
Overall, the safety of eribulin was acceptable, although five patients (14.3 %) discontinued therapy due to adverse events. The majority of non-hematological adverse events were mild in severity. Grade 3 or 4 sensory neuropathy, which might lead to discontinuation of eribulin therapy, was reported in only one patient. Among hematological adverse events, grade 3 or 4 neutropenia was reported in 62.9 % of patients; thus eribulin should be administered with caution and patients should be monitored closely for severe neutropenia. However, since febrile neutropenia was reported in only two patients, the tolerability of eribulin was considered to be acceptable. Notably, all reported adverse events were those that might be anticipated with this treatment and no new adverse events were reported in the first-line use of eribulin in this Japanese population. The proportion of patients who experience severe adverse events after initiation of eribulin is relatively low compared to that after initiation of other key drugs for MBC (Cortes et al. 2011; Kaufman et al. 2015; McIntyre et al. 2014). Thus, many patients treated with eribulin might not experience deterioration of their QoL. The current guidelines suggest using a single agent to optimize both treatment length and QoL for first-line therapy, except in the case of immediately life-threatening disease (Partridge et al. 2014; Cardoso et al. 2014). Japanese guidelines (The Japanese Breast Cancer Society 2015) also support this statement and the oral 5-fluorouracil derivative S-1 has become a recommended first-line treatment for MBC, along with anthracycline and taxane based on a recent clinical trial conducted in Japan—this trial demonstrated non-inferiority of S-1 in OS and TTF over taxane; S-1 also demonstrated less toxicity and better QoL profile compared to taxane (Takashima et al. 2016). In this context, eribulin might also be a recommended first-line treatment for MBC in the Japanese population, though further investigation is warranted.
Although the present trial offers meaningful data to evaluate efficacy and safety of first-line eribulin for treatment of HER2-negative MBC in Japanese patients, some caution is needed in the interpretation of the results. The present phase II trial was an exploratory study and conducted without any comparator. In addition, since the number of patients included in this trial was small (N = 35), caution is required for interpretation of OS data, due to lack of statistical power.
Conclusion
In conclusion, the present phase II trial investigated the efficacy and safety of eribulin as first-line chemotherapy in Japanese women with HER2-negative MBC, and demonstrated that eribulin has antitumor activity comparable to that demonstrated by other key established cytotoxic agents. As eribulin has the potential to prolong survival in HER2-negative MBC patients, and has demonstrated acceptable safety and tolerability, it could be beneficial for such patients when used as a first-line therapy. Further research is necessary to confirm the results of the present phase II trial.
Abbreviations
- CBR:
-
clinical benefit rate
- CI:
-
confidence interval
- CR:
-
complete response
- HER2:
-
human epidermal growth factor receptor 2
- MBC:
-
metastatic breast cancer
- ORR:
-
overall response rate
- OS:
-
overall survival
- PFS:
-
progression-free survival
- PR:
-
partial response
- QoL:
-
quality of life
- SD:
-
stable disease
- TTF:
-
time to treatment failure
References
Cancer Therapy Evaluation Program (CTEP) (2015) Common terminology criteria for adverse events (CTCAE) v4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 8 Oct 2015
Cardoso F, Costa A, Norton L et al (2014) ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)†. Ann Oncol 25:1871–1888
Cortes J, O’Shaughnessy J, Loesch D et al (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377:914–923
Funahashi Y, Okamoto K, Adachi Y et al (2014) Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci 105:1334–1342
Gradishar WJ, Tjulandin S, Davidson N et al (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23:7794–7803
Howlader N, Noone AM, Krapcho M et al (eds) (2013) SEER cancer statistics review, 1975–2010. National Cancer Institute. Bethesda, MD, updated June 14, 2013. http://seer.cancer.gov/archive/csr/1975_2010/results_merged/sect_04_breast.pdf. Accessed 8 Oct 2015
Jordan MA, Kamath K, Manna T et al (2005) The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther 4:1086–1095
Kaufman PA, Awada A, Twelves C et al (2015) Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 33:594–601
McIntyre K, O’Shaughnessy J, Schwartzberg L et al (2014) Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res Treat 146:321–328
New response evaluation criteria in solid tumours (2009) Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–2471
Partridge AH, Rumble RB, Carey LA et al (2014) Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 32:3307–3329
Piccart-Gebhart MJ, Burzykowski T, Buyse M et al (2008) Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol 26:1980–1986
Schöffski P, Maki RG, Italiano A et al (2015) Randomized, open-label, multicenter, phase III study of eribulin versus dacarbazine in patients (pts) with leiomyosarcoma (LMS) and adipocytic sarcoma (ADI). J Clin Oncol 33(suppl; abstr LBA10502)
Smith TJ, Khatcheressian J, Lyman GH et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Smith JA, Wilson L, Azarenko O et al (2010) Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 49:1331–1337
Takashima T, Mukai H, Hara F et al (2016) Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 17(1):90–98
Terashima M, Sakai K, Togashi Y et al (2014) Synergistic antitumor effects of S-1 with eribulin in vitro and in vivo for triple-negative breast cancer cell lines. SpringerPlus 3:417
The Japanese Breast Cancer Society (2015) Clinical guidelines for advanced breast cancer, 1. Treatment, ver 2015. Kanehara & Co., Ltd, Tokyo
Twelves C, Cortes J, Vahdat L et al (2014) Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat 148:553–561
Yoshida T, Ozawa Y, Kimura T et al (2014) Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer 110:1497–1505
Authors’ contributions
TT, ST, ST, SN, HK, SY, SN, YM, TS, KT, KI, YO, NO, TI, and KH contributed to conception and design. TT, ST, ST, SN, HK, SK, SY, SN, TN, YM, TS, KT, and TI contributed towards provision of patients. TT, HK, SN, and SK contributed towards collection and assembly of data. TT, ST, ST, SN, NO, TI, SK, and MT contributed towards data analysis and interpretation. All authors contributed to drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to express their deepest gratitude to the women who participated in this trial and their family members, in addition to the investigators and all staff members at the study sites for their contribution to the study. Medical writing service was provided by Koki Yamashita, PhD. Financial support was provided by Eisai Co., Ltd. (Tokyo, Japan) to CACTUS Communications for writing and editorial services.
Competing interests
Tsutomu Takashima reports receiving honoraria from Eisai Co., Ltd., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., and receiving lecture fees from Eisai Co., Ltd., Kyowa Hakko Kirin Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; Shinya Tokunaga reports receiving honoraria from Eisai Co., Ltd.; Seika Tei reports receiving lecture fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd. and Eisai Co., Ltd.; Shigehiko Nishimura reports receiving honoraria from Eisai Co., Ltd.; Hidemi Kawajiri reports receiving honoraria from Eisai Co., Ltd. and lecture fees from Eisai Co., Ltd. and Chugai Pharmaceutical Co., Ltd.; Shinichiro Kashiwagi declares no conflict of interest;; Shigehito Yamagata reports receiving honoraria from Chugai Pharmaceutical Co., Ltd. and Daiichi Sankyo Co., Ltd.; Satoru Noda reports receiving honoraria from Chugai Pharmaceutical Co., Ltd..; Takeo Nishimori reports receiving honoraria from Eisai Co., Ltd., Taiho Pharmaceutical Co., Ltd.; Yoko Mizuyama declares no conflict of interest; Takeshi Sunami declares no conflict of interest; Kenji Tezuka declares no conflict of interest; Katsumi Ikeda reports receiving honoraria from Tumura, Toshiba Medical Co; Yoshinari Ogawa declares no conflict of interest; Naoyoshi Onoda reports receiving honoraria from Eisai Co., Ltd., Bayer Yakuhin, Covidien Co., research grant from Eisai Co., Ltd., Bayer Yakuhin, research support from Novartis Parma KK. and Advisory board Eisai Co., Ltd. Bayer Yakuhin; Tetsuro Ishikawa declares no conflict of interest; Shinzoh Kudoh declares no conflict of interest; Minoru Takada reports receiving declares no conflict of interest;; Kosei Hirakawa reports receiving research grant from Taiho Pharmaceutical Co., Ltd.,Yakult Honsha Co., Ltd. and receiving honoraria from Taiho Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Takashima, T., Tokunaga, S., Tei, S. et al. A phase II, multicenter, single-arm trial of eribulin as first-line chemotherapy for HER2-negative locally advanced or metastatic breast cancer. SpringerPlus 5, 164 (2016). https://doi.org/10.1186/s40064-016-1833-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-1833-1