Abstract
Background
A significant percentage of head and neck cancer (HNCs) patients receiving RT experience oral mucositis (OM). This study aimed to evaluate the effect of the polyherbal (containing chamomile, peppermint oil, Aloe vera, and honey) and zinc mouthwashes in comparison to the control (chlorhexidine) and placebo groups for prevention of radiation-induced OM.
Methods
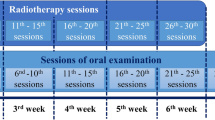
This study was a double-blinded randomized clinical trial, conducted on 67 patients with HNCs undergoing radiotherapy. The eligible participants were randomized to receive either one of the following; zinc sulfate, polyherbal, chlorhexidine (Vi-one 0.2% CHX), or placebo mouthwash for 6 weeks. Follow-up evaluation of oral hygiene and the checklists of OM and the intensity of pain were filled out according to WHO assessment tool, Oral Mucositis Assessment Scale (OMAS), and Visual Analog Scale (VAS) in all the participants weekly for seven consecutive weeks.
Results
The results of present clinical trial demonstrated that the use of either zinc sulfate or polyherbal mouthwash significantly reduced the scores of OM and the severity of pain during weeks 2 to 7 after consumption compared with the CHX or placebo mouthwashes (P < 0.05). According to the post hoc analysis and compared with the placebo, a significantly better result was reported for zinc sulfate and polyherbal mouthwashes at weeks 2 to 7, but not for the CHX mouthwash.
Conclusion
This study showed that the use of zinc sulfate or polyherbal mouthwashes is effective in prevention of both OM severity scores and pain related to OM intensity at weeks 2 to 7 following consumption in HNCs patients.
Trial registration IRCT20190123042475N1 and IRCT20190123042475N2. Registration date: 2019-06-09, 2019-07-26.
Similar content being viewed by others
Introduction
Cancer is the second leading cause of mortality in the developed countries after cardiovascular diseases. Head and neck cancers (HNCs) are the 6th most common malignancies in the world [1, 2]. Head and neck cancers are related to malignant tumor cells in airways and upper digestive tract, including oral and nasal cavity, nasopharynx, oropharynx, hypopharynx, pharynx, larynx, middle ear and salivary glands [3, 4]. Based on the type and the stage of tumor, the patient may require one or a combination of the following modalities for the management of cancer: surgery, chemotherapy, and radiotherapy (RT) [5, 6]. Chemotherapy and RT are both the mainstay of treatment of head and neck cancers [7]. Adverse effect of RT depends on the radiation dose, type of ionizing irradiation, volume and extent of irradiated tissue, fraction size and the patient’s clinical condition [8, 9]. A significant percentage of HNCs patients receiving RT experience oral mucositis (OM), especially those with cumulative radiation dose > 30 Gy, [10, 11]. The prevalence of OM increases with simultaneous chemotherapy and radiotherapy [12]. Furthermore, some factors like age, sex, oral hygiene, smoking, alcohol consumption, human papillomavirus and diet predispose an individual to experience OM [4, 10].
OM is characterized by disruption of epithelial integrity in the oral cavity, pharynx and gastrointestinal tract [13]. The most common symptoms associated with OM are pain, dysphagia, dysgeusia, dehydration, anorexia, and weight loss. MO may interfere with cancer treatment and its efficacy. Thereby, this adverse effect of radiotherapy may increase length of hospitalization, adjuvant supportive care and treatment failure which all have a negative economic impact [14, 15]. Notably, the underlying pathophysiology of radiation-induced MO is presumed to be inflammation and reactive oxygen species (ROS) which are generated by ionizing radiation [7]. Various compounds have been proposed for prevention and management of radiation-induced OM [16]. However, until now, no proven prophylactic or treatment strategies have been globally accepted for OM [16].
Zinc has the potential to diminish oxidant injury caused by ROS [17]. It has a key role in collagen synthesis, fibroblast and keratinocyte proliferation [18]. Moreover, zinc is the cofactor of some cellular processes, such as synthesis of DNA, protein, RNA polymerase, and reverse transcriptase. Therefore, it has wound-healing properties while boosting the growth, immune and reproductive system [16, 19]. Chamomile (Matricaria recutita L.) is an herb used for its antibacterial, antifungal, inflammatory, angiogenesis, anti-carcinogenic, spasmolytic, antidiabetic and sedative properties for centuries [20, 21]. Peppermint oil is extracted from peppermint (Mentha piperita L.) plant. It has several active compounds such as terpenoids, flavonoids, polyphenol, alpha-tocopherol, betaine and choline [22]. Peppermint has multifunctional effects, better known for its analgesic and antimicrobial properties [23].
Aloe vera (Aloe barbadensis Miller) is an herbal medicine with valuable features. It is widely known for its anti-inflammatory, analgesic, immunoregulatory effects, and antimicrobial, wound healing, anti-proliferative and anti-tumor activities [24, 25]. There is a hypothesis stated that anti-inflammatory effect of A. vera is due to inhibition of cyclooxygenase, reduction of tumor necrosis factor alpha (TNF-α) levels and leukocyte adhesive molecule [26, 27]. Wound healing of A. vera arises from attenuation of vasoconstriction and platelet aggregation at wound site, increasing growth factors and collagen synthesis, enhancement of wound oxygenation, and neutralizing the free radicals [28, 29]. Honey has been used both as food and medicine from ancient times [30]. It has anti-inflammatory activity, antimicrobial, wound healing and reduction effect of free radicals [31, 32]. Moreover, honey contains an incredibly diverse range of nutrients to maintain a healthy weight in patient undergoing RT and/or chemotherapy [33, 34].
For prevention and management of OM, it is crucial to employ an efficient compound with likely safety profile which is easy to use. Currently, commercially therapeutic mouthwash on the market has high alcohol content, causing a burning sensation in the oral cavity [35]. They may also cause toxicity if swallowed or consumed in excess. In this clinical trial, we aimed to evaluate the effect of the polyherbal and zinc mouthwashes in comparison to control (chlorhexidine) and placebo groups for prevention of radiation-induced OM.
Materials and methods
Ethics considerations, setting, and patients
The study was approved by the Ethics and Research Committee of North Khorasan University of Medical Sciences Review Board. The trial was also registered and approved in the registry of clinical trials (IRCT20190123042475N1 and IRCT20190123042475N2). All the patients who took part in this study were provided informed consent prior to participation in the study. In this trial, we included patients over 18 years old undergoing RT with a minimum radiation dose of 30 Gy for the first time and when the oral cavity was in the range of radiation.
Preparation and formulation of mouthwash
For the formulation of 1% zinc sulfate mouthwash, zinc sulfate was mixed with wetting agents (glycerin and water), stabilizers (ascorbic acid and citric acid) and microbial protection (potassium sorbate). For preparation of polyherbal mouthwash, chamomile extract, peppermint oil, A. vera gel, honey, potassium sorbate (as microbial protection), ascorbic acid and citric acid (stabilizers), glycerin and water (as wetting agents) were intermixed together. For formulation of placebo mouthwash, the same schedule was performed without adding the active ingredient composition. The placebo mouthwash contained all the ingredients used in the mouthwash of the intervention groups, except for the active ingredients, so to prepare the placebo mouthwash, citric acid, ascorbic acid, and potassium sorbate were mixed with glycerin and distilled water. The final formulations were then assessed for possible microbial contamination according to the United States Pharmacopeia at a research center of natural products and medicinal plants affiliated to the university.
Sample size
The sample size was calculated considering a previous report by Mehdipour et al. [36]. We supposed to achieve a difference of 40% in obtaining the zero grade of OM in the intervention groups compared to the control and placebo groups after prescribing the intervention mouthwashes using the sample size equation (N = (z1 − α2/ − z1 − β) 2 (SD1 + SD2) 2/d2) for comparing the means. We also considered a confidence interval of 95%, statistically significant of 0.05, and statistical power of 80%. Therefore, the required sample size was calculated at least 15 patients per in each group, with a total sample size of 60 patients. The calculated sample size was increased to 67 to take into consideration the potential of loss to follow-up. A p-value of 0.05 or less was considered statistically significant.
Randomization and blinding
Patients who met the inclusion criteria were randomized in a 1:1:1:1 ratio by a permuted block randomization method to one of the following groups: (1) zinc sulfate, (2) polyherbal, (3) CHX, or (4) placebo. So, blocks of four were applied and six-digit random numbers were generated. Patients were assigned to each of study group based on Random Number Table. To keep the concealment, the allocation list was given to a third person not involvedin the study.
To keep blinding, the mouthwashes were filled in identical bottles and coded by the principal investigator. Patients, radiation oncologist and investigator of clinical responses were all blind to the four arms of the study. When the weekly follow-up sessions were completed, the principal investigator decoded the random numbers of consumed bottles and assigned each to the proper arm.
Patients and study procedure
This study was a double-blinded randomized clinical trial in 67 patients with HNCs undergoing RT in a tertiary referral hospital affiliated to medical university. After inspecting the oral cavity of the patients for any oral lesions, patients over 18 years of age with the oral mucosa within the range of radiation, and the minimum amount of radiation of 30 Gy were included in the study. At the initiation of the study, we assessed 75 patients for eligibility criteria. Eight patients were excluded from the study due to having one of the exclusion criteria: known allergy to the ingredients of the mouthwashes, previous history of receiving RT, use of any other mouthwash, consumption of oral zinc dietary supplements, or receiving analgesics. Eventually, statistical analysis was performed on the remaining 67 patients.
At baseline, just the day before the first RT session, the demographical and baseline characteristics of each patient were recorded by the investigator of clinical responses. The eligible participants were randomly assigned into one of the following groups: zinc sulfate, polyherbal, chlorhexidine (Vi-one 0.2% CHX), or placebo mouthwash. Prior to the enrollment, demographic characteristics of the patients were recorded. Afterwards, the oral cavity of all patients was examined. Follow-up evaluation of oral hygiene was performed in all the participants weekly for seven consecutive weeks. The checklists of OM and the intensity of pain were filled out according to WHO assessment tool [37], Oral Mucositis Assessment Scale (OMAS) [38], and Visual Analog Scale (VAS) [39]. Based on WHO classification system, OM is categorized into four stages. Score 0 indicates no signs or symptoms of OM. In score 1, the oral mucosa appears erythematosus and painful while in score 2, the oral cavity is ulcerous and the patient is unable to eat normally. Score 3 is described when the ulcer has progressed to some extent that the patient can only drink fluid. In score 4, the patient cannot even drink fluid [40]. The OMAS criterion evaluates ulceration and erythema at nine sites of the oral cavity. In each site of this cavity, the presence of an ulceration is scored from 0 to 3 as follows: score 0 (no ulceration), score 1 (the cumulative surface area of the ulcer < 1 cm2), score 2 (1 cm2 ≤ the surface area ≤ 3 cm2). score 3 (the surface area > 3 cm2). Erythema is also scored from 0 to 2: score 0 (no redness), score 1 (mild to moderate redness), and score 2 (severe erythema with the color of fresh blood). Eventually, these scores in all nine parts of the oral cavity are then summed up and the average score is reported between 0 (absence of mucositis) and 5 (the worst severity of mucositis) [38]. The Visual Analog Scale is a means to measure the intensity of pain that is scored by the patient as a self-report. In this criterion, the intensity of pain is scored from 0 to 10, so that score 0 indicates the absence of pain and a score of 10 indicates the intensity of pain that is intolerable [41].
Each patient was instructed to rinse 5 ml of the mouthwash three times a day for 60 s from the first day of starting RT, then poured it out. The patients were informed to continue their treatment for the following 6 weeks. Patients were evaluated weekly during RT by radiation oncologist and investigator of clinical responses. In these weekly follow-up sessions, mouthwash was provided to the patients for one week. Compliance to the treatment was assessed by requesting the patients to take the rest of their bottles to the weekly appointments. The oral cavity was examined and the incidence and severity of OM and intensity of pain was evaluated on a weekly basis (for an overall of seven follow-up sessions).
Statistical analyses
Data analysis was performed using SPSS 23 software. We have used Chi-square test or Fisher exact test for comparing two qualitative variables. For comparing groups according to quantitative variables (as our quantitative variables did not have normal distribution), we have used Kruskal–Wallis test. Post hoc analysis was used to assess the difference between any pair of group means. In all cases, P-value < 0.05 was considered as statistically significant difference.
Results
The current study is a double-blind, randomized clinical trial in 67 patients with HNCs. Among 75 patients assessed for eligibility, 67 were enrolled to receive either of interventions: zinc sulfate, polyherbal (chamomile, peppermint oil, A. vera, and Honey), CHX or placebo mouthwash. The baseline demographic and clinical characteristics of patients are presented in Table 1 and appeared to be comparable.
Outcomes
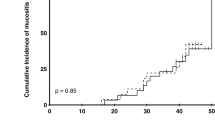
The details of the severity of OM are presented in Table 2 according to WHO scale over week 1 to 7 for CHX, polyherbal, zinc sulfate, and placebo groups. At study termination (week 7), most patients in the zinc sulfate group were free of symptoms (8 out of 17 patients, 47.1%). Then after, herbal group took the second place with 41.1% of patients free of symptoms (P-value = 0.001). Patients in the placebo group had the highest grade 3 experience of OM (56.25%). None of the patients in each of four groups experienced grade 4 of OM during the 7 weeks of the study period. The severity of mucositis increased significantly in placebo group as RT continued, compared with each of intervention groups (Additional file 1: Table S1).
The severity of oral mucositis according to OMAS over time is presented in Table 3. The results obtained from OMAS in the severity of OM were in line with the results of WHO scale. Any presented lesions were measured and scored in OMAS. As illustrated, both the size and redness of lesions were reduced significantly in each of interventional groups at weeks 2 to 7 compared to the placebo group.
The mean severity of pain in study groups were evaluated by using VAS score (Table 4). The mean (SD) severity of pain in polyherbal group in 2th, 3th, 4th, and 5th weeks were lower than the others groups while the mean severity of pain in zinc sulfate group were lower in 6th and 7th weeks.
Post hoc analysis, comparing the reduction in WHO, OMAS, and VAS scores at weeks 2 to 7 from baseline indicated no statistically significant in the zinc sulfate group vs polyherbal group and the CHX group vs placebo group (P-value > 0.05). Additional post hoc analysis showed a significant reduction in each of WHO, OMAS, and VAS scores in either zinc sulfate or polyherbal groups compared with the placebo group (P-value < 0.05). The results of the post hoc analysis of comparing zinc sulfate and polyherbal groups with the CHX group were varied. The reduction in VAS score were significant at weeks 5 to 7 for each of zinc sulfate or polyherbal groups vs CHX group, while the changes in either WHO or OMAS scores were significant at weeks 4 to 7 (Additional file 1: Table S1).
Discussion
The current double-blind, randomized clinical trial aimed to evaluate the effect of zinc sulfate and polyherbal (chamomile, peppermint oil, A. vera, and honey) mouthwashes compared with each of two control groups (CHX and placebo) in prevention of radiation-induced oral mucositis in patients with HNC. The results of this trial showed that the use of either zinc sulfate or polyherbal mouthwash significantly reduced the scores of OM and the severity of pain during weeks 2–7 after consumption compared with the CHX or placebo mouthwashes. According to the post hoc analysis and compared with the placebo, a significantly better result was reported for zinc sulfate and polyherbal mouthwashes at weeks 2–7, but not for the CHX mouthwash.
The polyherbal mouthwash formulated in this study contained chamomile extract, A. vera gel, peppermint oil, and honey, and the effectiveness of each component in this mouthwash has been evaluated in different clinical studies in OM. Honey, a natural product with wound-healing properties, prevents microbial growth due to high viscosity and osmolality along with the low PH. Natural honey stimulates the saliva secretion, and activates immune system responses [33]. Moreover, it has anti-inflammatory and antioxidant effects by decreasing the levels of prostaglandin and increasing nitric oxide levels in the lesional sites, so it accelerates the healing of acute and chronic wounds [42]. Favorable taste and lack of cytotoxic effect on host cells increase the compliance of using honey as a mouthwash in patients experiencing RT-induced OM [31]. The efficacy of topical honey has been shown in randomized clinical trials in preventing the development and severity of OM, the growth of aerobic bacteria and candida, attenuation the intensity of pain, and improving the quality of life [31, 43,44,45].
Another key component in polyherbal mouthwash was chamomile extract. The importance of flavonoids in the chamomile plant and the antibacterial, antifungal, and anti-inflammatory effects of this plant along with its low price and accessibility made it a distinctive herb in the pharmaceutical industry [46]. The use of chamomile liquid oral rinse in patients undergoing radiochemotherapy has the ability to delay the onset and severity of RT-induced mucositis compared with placebo [47].
Aloe vera was also applied in the polyherbal mouthwash formulated in the present study. A. vera is a medicinal plant, containing over 75 active constituents, which makes it a suitable choice for wound healing [24]. It appears that A. vera exerts its favorable effects in wound healing process through inhibition of cyclooxygenase, improvement of collagen synthesis and blood flow to the wound site, scavenging the free radicals, and antibacterial properties [28]. A. vera has shown to significantly reduce the incidence of sever RT-induced mucositis in patients with HNCs [48].
Peppermint has long been used as a cooling agent in topical pharmaceutical products. Peppermint oil has antioxidant and antimicrobial (bacteria, viruses and parasites) properties [49]. This compound has also been used in the prevention of mucositis in previous studies. The combination of Matricaria recutita and Mentha piperita in patients with hematopoietic stem cell transplantation significantly diminished the duration, the maximum and the average daily grade of OM compared with placebo. Moreover, the intensity of pain, dryness, and dysphagia were significantly lower in intervention group [50]. Therefore, it appears that a polyherbal product containing all of these components can potentially prevent this complication of radiotherapy. The results of this study showed that the severity of oral mucositis based on both WHO and OMAS scales, as well as pain based on VAS, was significantly lower in polyherbal group compared to the placebo group. In the present study, the patients in the polyherbal group did not experienced even grade 3 OM throughout the study. The overall OMAS score was lower in polyherbal group in comparison to the other groups.
The role of zinc sulfate has been shown previously in acceleration of wound healing process [17, 18]. This compound has been used both as an oral supplement and as a mouthwash for prevention of OM. The use of this agent has reduced the onset of this complication and the severity oral mucositis due to RT in HNC patients. Zinc sulfate could diminish the onset and severity of OM in HNC patients undergoing radiation, especially at weeks 4 to 6 [16, 51]. However, the results regarding the effectiveness of zinc sulfate are contradictory, since this component has not always been able to reduce the severity of mucositis and esophagitis in RT related OM [52]. In the current clinical trial, the recipient of zinc sulfate mouthwash showed a significant difference in the occurrence and severity of OM compared with both placebo and CHX groups.
Although the findings of the present RCT suggest that either zinc sulfate or polyherbal (containing chamomile, peppermint oil, A. vera, and honey) mouthwashes are potential preventive candidates for RT-induced OM in patients with HNCs, the limitations of the study should be considered and care should be taken in interpreting the results. The first limitation, due to the small sample size, is the generalizability of the results of the present study to all patients undergoing radiotherapy, which should be confirmed in a larger group of HNCs patients in future studies. Second, given that the effects of polyherbal mouthwash were evaluated in combination, it is not clear which component of the mouthwash was responsible for the beneficial reported effects. Furthermore, since the product was formulated as mouthwash, its efficacy was limited to only the oral cavity, while the radiotherapy-induced mucositis can also involve the mucous membranes in deeper areas. Although head and neck cancer was the main inclusion criteria for patients to enroll into the study, the type of cancer was unfortunately not recorded in the data collection form. Therefore, we were unable to perform subgroup analysis in this regard to figure out which type of HNCs caused the most scoring of oral mucositis.
Conclusion
The use of zinc sulfate or polyherbal (containing chamomile, peppermint oil, A. vera, and honey) mouthwashes in patients with HNCs showed a significant reduction in OM severity scores and the related pain at weeks 2–7. However, further evaluation with larger sample size is warranted to assess their safety and efficacy in this population of patients.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. The raw SPSS file of this study before analysis is available upon your request.
Abbreviations
- HNCs:
-
Head and neck cancer
- OM:
-
Oral mucositis
- CHX:
-
Chlorhexidine
- OMAS:
-
Oral Mucositis Assessment Scale
- VAS:
-
Visual analog scale
- RT:
-
Radiotherapy
- ROS:
-
Reactive oxygen species
- A. vera:
-
Aloe vera
- TNF-α:
-
Tumor necrosis factor alpha
- N:
-
Number
- y:
-
Year
- BMI:
-
Body mass index
- BCC:
-
Basal cell carcinoma
- SCC:
-
Squamous cell carcinoma
- SD:
-
Standard deviation
- Gy:
-
Gray
References
Sudhakar A. History of cancer, ancient and modern treatment methods. J Cancer Sci Ther. 2009;1(2):1.
Bolouri AJ, Pakfetrat A, Tonkaboni A, Aledavood SA, Najafi MF, Delavarian Z, et al. Preventing and therapeutic effect of propolis in radiotherapy induced mucositis of head and neck cancers: a triple-blind, randomized, placebo-controlled trial. Iran J Cancer Prev. 2015. https://doi.org/10.17795/ijcp-4019.
Krishnatreya M, Rahman T, Kataki AC, Sharma JD, Nandy P, Baishya N. Pre-treatment performance status and stage at diagnosis in patients with head and neck cancers. Asian Pac J Cancer Prev. 2014;15(19):8479–82.
Döbróssy L. Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev. 2005;24(1):9–17.
Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers: the patient’s perspective. Cancer Nurs. 2002;25(6):461–7.
Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89.
Aghamohamamdi A, Hosseinimehr SJ. Natural products for management of oral mucositis induced by radiotherapy and chemotherapy. Integr Cancer Ther. 2016;15(1):60–8.
Vissink A, Burlage F, Spijkervet F, Jansma J, Coppes R. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):213–25.
Murad AM, Katz A. Oncologia: bases clínicas do tratamento. Oncologia: bases clínicas do tratamento1996. p. 435-.
Montserrat V, Gerry O, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106(2):329–36.
Huang C-J, Huang M-Y, Fang P-T, Chen F, Wang Y-T, Chen C-H, et al. Randomized double-blind, placebo-controlled trial evaluating oral glutamine on radiation-induced oral mucositis and dermatitis in head and neck cancer patients. Am J Clin Nutr. 2019;109(3):606–14.
Sutherland SE, Browman GP. Prophylaxis of oral mucositis in irradiated head-and-neck cancer patients: a proposed classification scheme of interventions and meta-analysis of randomized controlled trials. Int J Radiat Oncol Biol Phys. 2001;49(4):917–30.
Volpato LER, Silva TC, Oliveira TM, Sakai VT, Machado MAAM. Radiation therapy and chemotherapy-induced oral mucositis. Braz J Otorhinolaryngol. 2007;73(4):562–8.
Nonzee NJ, Dandade NA, Markossian T, Agulnik M, Argiris A, Patel JD, et al. Evaluating the supportive care costs of severe radiochemotherapy‐induced mucositis and pharyngitis: results from a northwestern university costs of cancer program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a veterans administration hospital, or a comprehensive cancer care center. Cancer. 2008;113(6):1446–52.
Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):1110–20.
Ertekin MV, Koç M, Karslioǧlu I, Sezen O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys. 2004;58(1):167–74.
Shuai T, Tian X, Shi B, Chen H, Liu X-L, Yi L-J, et al. Prophylaxis with oral zinc sulfate against radiation induced oral mucositis in patients with head and neck cancers: a systematic review and meta-analysis of four randomized controlled trials. Front Oncol. 2019;9:165.
Mansouri A, Hadjibabaie M, Iravani M, Shamshiri AR, Hayatshahi A, Javadi MR, et al. The effect of zinc sulfate in the prevention of high-dose chemotherapy-induced mucositis: a double-blind, randomized, placebo-controlled study. Hematol Oncol. 2012;30(1):22–6.
Oberleas D, Harland BF. Treatment of zinc deficiency without zinc fortification. J Zhejiang Univ Sci B. 2008;9(3):192–6.
Mazokopakis E, Vrentzos G, Papadakis J, Babalis D, Ganotakis E. Wild chamomile (Matricaria recutita L.) mouthwashes in methotrexate-induced oral mucositis. Phytomed. 2005;12(1–2):25–7.
Miraj S, Alesaeidi S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile). Electron Phys. 2016;8(9):3024.
Rahimi Y, Taleei A, Ranjbar M. Long-term water deficit modulates antioxidant capacity of peppermint (Mentha piperita L.). Sci Hortic. 2018;237:36–43.
McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother Res. 2006;20(8):619–33.
Sahebjamee M, Mansourian A, Hajimirzamohammad M, Zadeh MT, Bekhradi R, Kazemian A, et al. Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: a triple-blind, randomised, controlled clinical trial. Oral Health Prev Dent. 2015;13(4):309–15.
Mansouri P, Haghighi M, Beheshtipour N, Ramzi M. The effect of aloe vera solution on chemotherapy-induced stomatitis in clients with lymphoma and leukemia: a randomized controlled clinical trial. Int J Commun Based Nursing Midwifery. 2016;4(2):119.
Yagi A, Kabash A, Mizuno K, Moustafa S, Khalifa T, Tsuji H. Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from aloe vera gel. Planta Med. 2003;69(03):269–71.
Wei A, Shibamoto T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J Agric Food Chem. 2010;58(12):7218–25.
Ahmadi A. Potential prevention: Aloe vera mouthwash may reduce radiation-induced oral mucositis in head and neck cancer patients. Chin J Integr Med. 2012;18(8):635–40.
Farrugia C-JE, Burke ES, Haley ME, Bedi KT, Gandhi MA. The use of aloe vera in cancer radiation: An updated comprehensive review. Complement Ther Clin Pract. 2019;35:126–30.
Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2015. https://doi.org/10.1002/14651858.CD005083.pub4.
Howlader D, Singh V, Mohammad S, Gupta S, Pal U, Pal M. Effect of topical application of pure honey in chemo-radiation-induced mucositis and its clinical benefits in improving quality of life in patients of oral squamous cell carcinoma. J Maxillofacial Oral Surg. 2019;18(1):73–9.
Oryan A, Alemzadeh E, Moshiri A. Biological properties and therapeutic activities of honey in wound healing: a narrative review and meta-analysis. J Tissue Viability. 2016;25(2):98–118.
Khanjani Pour-Fard-Pachekenari A, Rahmani A, Ghahramanian A, Jafarabadi MA, Onyeka TC, Davoodi A. The effect of an oral care protocol and honey mouthwash on mucositis in acute myeloid leukemia patients undergoing chemotherapy: a single-blind clinical trial. Clin Oral Invest. 2019;23(4):1811–21.
Charalambous M, Raftopoulos V, Paikousis L, Katodritis N, Lambrinou E, Vomvas D, et al. The effect of the use of thyme honey in minimizing radiation-induced oral mucositis in head and neck cancer patients: a randomized controlled trial. Eur J Oncol Nurs. 2018;34:89–97.
Lemos-Júnior CA, Villoria GEM. Reviewed evidence about the safety of the daily use of alcohol-based mouthrinses. Braz Oral Res. 2008;22:24–31.
Mehdipour M, Zenoz AT, Kermani IA, Hosseinpour A. A comparison between zinc sulfate and chlorhexidine gluconate mouthwashes in the prevention of chemotherapy-induced oral mucositis. J Facul Pharm. 2011;19(1):71.
Tiemann P, Toelg M, Ramos FMH. Administration of Ratanhia-based herbal oral care products for the prophylaxis of oral mucositis in cancer chemotherapy patients: a clinical trial. Evid-Based Complement Altern Med. 2007;4(3):361–6.
Sung L, Tomlinson GA, Greenberg ML, Koren G, Judd P, Ota S, et al. Validation of the oral mucositis assessment scale in pediatric cancer. Pediatr Blood Cancer. 2007;49(2):149–53.
Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26.
Jham BC, da Silva Freire AR. Oral complications of radiotherapy in the head and neck. Braz J Otorhinolaryngol. 2006;72(5):704–8.
Ala S, Zamani N, Akbari J, Salehifar E, Janbabai G, Koulaeinejad N. Efficacy of gabapentin mouthwash in managing oral mucositis pain in patients undergoing chemotherapy: a prospective, randomised, double-blind, controlled clinical trial. Scott Med J. 2020;65(1):12–8.
Konuk SD, Aydin M, Cangur S, Guven E. The effect of oral care with chlorhexidine, vitamin e and honey on mucositis in pediatric intensive care patients: a randomized controlled trial. J Pediatr Nurs. 2019;45:e95.
Khanal B, Baliga M, Uppal N. Effect of topical honey on limitation of radiation-induced oral mucositis: an intervention study. Int J Oral Maxillofac Surg. 2010;39(12):1181–5.
Rashad U, Al-Gezawy S, El-Gezawy E, Azzaz A. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J Laryngol Otol. 2009;123(2):223–8.
Motallebnejad M, Akram S, Moghadamnia A, Moulana Z, Omidi S. The effect of topical application of pure honey on radiation-induced mucositis: a randomized clinical trial. J Contemp Dent Pract. 2008;9(3):40–7.
Braga FT, Santos AC, Bueno PC, Silveira RC, Santos CB, Bastos JK, et al. Use of Chamomilla recutita in the prevention and treatment of oral mucositis in patients undergoing hematopoietic stem cell transplantation: a randomized, controlled, phase II clinical trial. Cancer Nurs. 2015;38(4):322–9.
Emrich S. Management of oral mucositis during local radiation and systemic chemotherapy: a study of 98 patients. J Prosthet Dent. 1991;66(3):361–9.
Puataweepong P, Dhanachai M, Dangprasert S, Sithatani C, Sawangsilp T, Narkwong L, et al. The efficacy of oral aloe vera juice for radiation induced mucositis in head and neck cancer patients: a double-blind placebo-controlled study. Asian Biomed. 2010;3(4):375–82.
Radivojac A, Bera O, Zeković Z, Teslić N, Mrkonjić Ž, Bursać Kovačević D, et al. Extraction of peppermint essential oils and lipophilic compounds: assessment of process kinetics and environmental impacts with multiple techniques. Molecules. 2021;26(10):2879.
Ardakani MT, Ghassemi S, Mehdizadeh M, Mojab F, Salamzadeh J, Ghassemi S, et al. Evaluating the effect of Matricaria recutita and Mentha piperita herbal mouthwash on management of oral mucositis in patients undergoing hematopoietic stem cell transplantation: a randomized, double blind, placebo controlled clinical trial. Complement Ther Med. 2016;29:29–34.
Mosalaei A, NASR EH, Shafizad A, Ahmadlou N, Ansari M, Mosleh SM, et al. Effect of oral zinc sulphate in prevention of radiation induced oropharyngeal mucositis during and after radiotherapy in patients with head and neck cancers. 2010.
Gorgu S, Ilknur A, Sercan O, Rahsan H, Nalan A. The effect of zinc sulphate in the prevention of radiation induced oral mucositis in patents with head and neck cancer. Int J Radiat Res. 2013;11(2):111.
Acknowledgements
The results of this trial are a part of two student thesis (Vahideh Aksi, Fatemeh Eslami).
Funding
This study was financially supported by a grant from Vice Chancellor of Research and Technology affairs of North Khorasan University of Medical Sciences, Bojnurd, Iran. This grant providing funding to prepare herbal formulation, data analysis and editorial assistance with the writing of the manuscript but had no role in study design and data collection.
Author information
Authors and Affiliations
Contributions
MS: conception and design of the study, supervising on data records, interpreting data. VA: collecting data. FE: collecting data. HL: analysis and interpreting data. JK: formulation of mouthwashes, GMP quality control tests. ShG: formulation of mouthwashes, GMP quality control tests. BP: head of treatment team, the physician providing clinical visit, consulting on the incidence of radiation mucositis. RN: drafting the manuscript. FS: drafting the manuscript. AS: the principal investigator and manager of the study, design and conduction the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics and Research Committee of North Khorasan University of Medical Sciences (NKUMS) Review Board. All the patients who took part in this study were provided informed consent prior to participation in the study.
Consent for publication
“Not applicable”.
Competing interests
The authors of present study declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Post hoc analysis on pairwise comparisons between the groups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sahebnasagh, M., Aksi, V., Eslami, F. et al. Prevention of radiotherapy-related oral mucositis with zinc and polyherbal mouthwash: a double-blind, randomized clinical trial. Eur J Med Res 28, 109 (2023). https://doi.org/10.1186/s40001-023-01015-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01015-8