Abstract
Purpose
To determine the effect of Cystus® tea (Naturprodukte Dr. Pandalis GmbH & Co. KG) as mouthwash compared to sage tea on oral mucositis in patients undergoing radio(chemo)therapy for head and neck cancer.
Methods
In this randomized, prospective phase III study, 60 head and neck cancer patients with primary or postoperative radio(chemo)therapy were included between 04/2012 and 06/2014. They received either sage or Cystus® tea for daily mouthwash under therapy. Mucositis was scored twice a week following the Radiation Therapy Oncology Group and the European Organization for Research and Treatment Cancer (RTOG/EORTC) scoring system. Dental parameters were also recorded. Statistical evaluation of the primary endpoint was performed using t‑test and log rank test.
Results
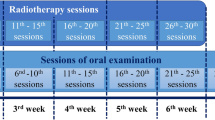
Data from 57 patients could be evaluated. Patient characteristics showed no significant difference between the two groups (n = 27 sage; n = 30 Cystus®). A total of 55 patients received the prescribed dose (60–66 Gy postoperative; 70–76.8 Gy primary). Mucositis grade 3 was observed in 23 patients (n = 11 sage; n = 12 Cystus®) and occurred between day 16 and 50 after start of therapy. There was no significant difference between the two groups in latency (p = 0.75) and frequency (p = 0.85) of the occurrence of mucositis grade 3. The self-assessment of the oral mucosa and the tolerability of the tea also showed no significant differences. Occurrence of dental pathologies appeared to increase over time after radiotherapy.
Conclusion
Cystus® and sage tea have a similar effect on the occurrence of radiation-induced mucositis regarding latency and incidence. Cystus® tea mouthwash solution is tolerated well and can be applied in addition to intensive oral care and hygiene along with the application of fluorides.



Similar content being viewed by others
References
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S, Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer, International Society of Oral Oncology (MASCC/ISOO) (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120(10):1453–1461. https://doi.org/10.1002/cncr.28592
Epstein JB, Beaumont JL, Gwede CK, Murphy B, Garden AS, Meredith R, Le QT, Brizel D, Isitt J, Cella D (2007) Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer 109(9):1914–1922. https://doi.org/10.1002/cncr.22620
Withers HR, Maciejewski B, Taylor JM, Hliniak A (1988) Accelerated repopulation in head and neck cancer. Front Radiat Ther Oncol 22:105–110
Bentzen SM, Thames HD (1991) Clinical evidence for tumor clonogen regeneration: interpretations of the data. Radiother Oncol 22(3):161–166
Hong CH, Napenas JJ, Hodgson BD, Stokman MA, Mathers-Stauffer V, Elting LS, Spijkervet FK, Brennan MT, Multi-national Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) (2010) A systematic review of dental disease in patients undergoing cancer therapy. Support Care Cancer 18(8):1007–1021. https://doi.org/10.1007/s00520-010-0873-2
Gupta N, Pal M, Rawat S, Grewal MS, Garg H, Chauhan D, Ahlawat P, Tandon S, Khurana R, Pahuja AK, Mayank M, Devnani B (2015) Radiation-induced dental caries, prevention and treatment—a systematic review. Natl J Maxillofac Surg 6(2):160–166. https://doi.org/10.4103/0975-5950.183870
Aguiar GP, Jham BC, Magalhaes CS, Sensi LG, Freire AR (2009) A review of the biological and clinical aspects of radiation caries. J Contemp Dent Pract 10(4):83–89
Kufta K, Forman M, Swisher-McClure S, Sollecito TP, Panchal N (2018) Pre-Radiation dental considerations and management for head and neck cancer patients. Oral Oncol 76:42–51. https://doi.org/10.1016/j.oraloncology.2017.11.023
Nagi R, Patil DJ, Rakesh N, Jain S, Sahu S (2018) Natural agents in the management of oral mucositis in cancer patients-systematic review. J Oral Biol Craniofac Res 8(3):245–254. https://doi.org/10.1016/j.jobcr.2017.12.003
Hannig C, Spitzmuller B, Al-Ahmad A, Hannig M (2008) Effects of cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J Dent 36(7):540–545. https://doi.org/10.1016/j.jdent.2008.04.002
Kalus U, Grigorov A, Kadecki O, Jansen JP, Kiesewetter H, Radtke H (2009) Cistus incanus (CYSTUS052) for treating patients with infection of the upper respiratory tract. A prospective, randomised, placebo-controlled clinical study. Antiviral Res 84(3):267–271. https://doi.org/10.1016/j.antiviral.2009.10.001
Attaguile G, Russo A, Campisi A, Savoca F, Acquaviva R, Ragusa N, Vanella A (2000) Antioxidant activity and protective effect on DNA cleavage of extracts from cistus incanus L. and cistus monspeliensis L. Cell Biol Toxicol 16(2):83–90
Hannig C, Sorg J, Spitzmuller B, Hannig M, Al-Ahmad A (2009) Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J Dent 37(7):560–566. https://doi.org/10.1016/j.jdent.2009.03.017
Wittpahl G, Kolling-Speer I, Basche S, Herrmann E, Hannig M, Speer K, Hannig C (2015) The polyphenolic composition of cistus incanus herbal tea and its antibacterial and anti-adherent activity against streptococcus mutans. Planta Med 81(18):1727–1735. https://doi.org/10.1055/s-0035-1557822
Kalus U, Kiesewetter H, Radtke H (2010) Effect of CYSTUS052 and green tea on subjective symptoms in patients with infection of the upper respiratory tract. Phytother Res 24(1):96–100. https://doi.org/10.1002/ptr.2876
Ehrhardt C, Hrincius ER, Korte V, Mazur I, Droebner K, Poetter A, Dreschers S, Schmolke M, Planz O, Ludwig S (2007) A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res 76(1):38–47. https://doi.org/10.1016/j.antiviral.2007.05.002
Steinmann D, Eilers V, Beynenson D, Buhck H, Fink M (2012) Effect of Traumeel S on pain and discomfort in radiation-induced oral mucositis: a preliminary observational study. Altern Ther Health Med 18(4):12–18
Budach V, Stuschke M, Budach W, Baumann M, Geismar D, Grabenbauer G, Lammert I, Jahnke K, Stueben G, Herrmann T, Bamberg M, Wust P, Hinkelbein W, Wernecke KD (2005) Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German cancer society 95-06 prospective randomized trial. J Clin Oncol 23(6):1125–1135. https://doi.org/10.1200/JCO.2005.07.010
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346. https://doi.org/10.1016/0360-3016(95)00060-C
Pitts NB, Ekstrand KR, Foundation I (2013) International caries detection and assessment system (ICDAS) and its international caries classification and management system (ICCMS)—methods for staging of the caries process and enabling dentists to manage caries. Community Dent Oral Epidemiol 41(1):e41–52. https://doi.org/10.1111/cdoe.12025
Lieshout HF, Bots CP (2014) The effect of radiotherapy on dental hard tissue—a systematic review. Clin Oral Investig 18(1):17–24. https://doi.org/10.1007/s00784-013-1034-z
Lange DE, Plagmann HE, Eenboom A, Promesberger A (1977) Clinical methods for the objective evaluation of oral hygiene. Dtsch Zahnärztl Z 32(1):44–47
Doerr W, Groetz KA, Hartmann JT, Riesenbeck D (2007) Orale Mukositis. Onkologe 13(2):150–157
Beheshti-Rouy M, Azarsina M, Rezaie-Soufi L, Alikhani MY, Roshanaie G, Komaki S (2015) The antibacterial effect of sage extract (salvia officinalis) mouthwash against streptococcus mutans in dental plaque: a randomized clinical trial. Iran J Microbiol 7(3):173–177
Moricz AM, Szeremeta D, Knas M, Dlugosz E, Ott PG, Kowalska T, Sajewicz M (2018) Antibacterial potential of the cistus incanus L. phenolics as studied with use of thin-layer chromatography combined with direct bioautography and in situ hydrolysis. J Chromatogr A 1534:170–178. https://doi.org/10.1016/j.chroma.2017.12.056
Qnais EY, Abu-Dieyeh M, Abdulla FA, Abdalla SS (2010) The antinociceptive and anti-inflammatory effects of salvia officinalis leaf aqueous and butanol extracts. Pharm Biol 48(10):1149–1156. https://doi.org/10.3109/13880200903530763
Gericke S, Lubken T, Wolf D, Kaiser M, Hannig C, Speer K (2018) Identification of new compounds from sage flowers (salvia officinalis L.) as markers for quality control and the influence of the manufacturing technology on the chemical composition and antibacterial activity of sage flower extracts. J Agric Food Chem 66(8):1843–1853. https://doi.org/10.1021/acs.jafc.8b00581
Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L, Zilberberg MD (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66(3):253–262
Mallick S, Benson R, Rath GK (2016) Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol 273(9):2285–2293. https://doi.org/10.1007/s00405-015-3694-6
Frydrych AM, Slack-Smith LM, Parsons R (2017) Compliance of post-radiation therapy head and neck cancer patients with caries preventive protocols. Aust Dent J 62(2):192–199. https://doi.org/10.1111/adj.12491
Smullen J, Koutsou GA, Foster HA, Zumbe A, Storey DM (2007) The antibacterial activity of plant extracts containing polyphenols against streptococcus mutans. Caries Res 41(5):342–349. https://doi.org/10.1159/000104791
Carvalho JC, Schiffner U (2018) Dental caries in European adults and senior citizens 1996–2016: ORCA saturday afternoon symposium in Greifswald, Germany—part II. Caries Res 53(3):242–252. https://doi.org/10.1159/000492676
Acknowledgements
We would like to gratefully acknowledge financial support from Naturprodukte Dr. Pandalis GmbH & Co. KG. We thank our participating patients for their willingness to support this study. The personnel of the clinical trials office at OncoRay Dresden, nurses and study nurses in the patient care of the Department of Radiotherapy are gratefully acknowledged. We would like to dedicate this paper to our colleague Prof. Wolfgang Dörr who passed away in October 2019.
Funding
The funders Naturprodukte Dr. Pandalis GmbH & Co. KG of this study had no role in the design, data collection, data analyses, data evaluation, and preparation of the manuscript or decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Ebert, A. Kensche, S. Löck, W.W. Hadiwikarta, A. Hänsch, W. Dörr, M. Krause, C. Hannig and M. Baumann received financial support from Naturprodukte Dr. Pandalis GmbH & Co. KG for this study. The funding had no influence on collection, analyses, and data evaluation. N. Ebert was co-principal investigator for funded research projects to the University of Dresden by Merck KGaA (2014–open). N. Ebert confirms that the above funding source was not involved in the design of this study, the preparation of this paper, the materials used, or the collection, analysis, and interpretation of data. M. Krause declares that within the past 5 years she received funding for her research projects and for educational grants to the University of Dresden IBA (2016), Merck KGaA (2016–2030), Medipan GmbH (2014–2018). As chair of OncoRay (Dresden) she signed/s contracts for her institute(s) and for the staff for research funding and collaborations with different companies. In the past 5 years, M. Baumann attended an advisory board meeting of MERCK KGaA (Darmstadt), for which the University of Dresden received a travel grant. He further received funding for his research projects and for educational grants to the University of Dresden by Teutopharma GmbH (2011–2015), IBA (2016), Bayer AG (2016–2018), Merck KGaA (2014–open), Medipan GmbH (2014–2018). He is on the supervisory board of HI-STEM gGmbH (Heidelberg) for the German Cancer Research Center (DKFZ, Heidelberg) and also member of the supervisory body of the Charité University Hospital, Berlin. As former chair of OncoRay (Dresden) and present CEO and Scientific Chair of the German Cancer Research Center (DKFZ, Heidelberg), he has been or is still responsible for collaborations with a multitude of companies and institutions, worldwide. In this capacity, he discussed potential projects with and has signed/signs contracts for his institute(s) and for the staff for research funding and/or collaborations with industry and academia, worldwide, including but not limited to pharmaceutical corporations like Bayer, Boehringer Ingelheim, Bosch, Roche and other corporations like Siemens, IBA, Varian, Elekta, Bruker and others. In this role, he was/is further responsible for commercial technology transfer activities of his institute(s), including the DKFZ-PSMA617 related patent portfolio (WO2015055318 (A1), ANTIGEN (PSMA)) and similar intellectual propterty portfolios. Dr. Baumann confirms that to the best of his knowledge none of the above funding sources was involved in the preparation of this paper.
Ethical standards
The study was approved by the institutional ethics committee of the Technische Universität Dresden (EK 281082011). Only patients who met the inclusion criteria and signed a consent form were enrolled in the study and randomized to receive either Cystus® or sage tea.
Additional information
C. Hannig und M. Baumann share last co-authorship.
Availability of data and material
Data were stored at the Department of Radiation Oncology, except for the dental parameters that were stored at the Clinic of Operative Dentistry at the University Medical Center Carl Gustav Carus in Dresden. The data are not deposited in a repository.
Code availability
Statistical analysis and graph creations were performed using R version 3.5.0 (2018-04-23).
Rights and permissions
About this article
Cite this article
Ebert, N., Kensche, A., Löck, S. et al. Results of a randomized controlled phase III trial: efficacy of polyphenol-containing cystus® tea mouthwash solution for the reduction of mucositis in head and neck cancer patients undergoing external beam radiotherapy. Strahlenther Onkol 197, 63–73 (2021). https://doi.org/10.1007/s00066-020-01684-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01684-y