Abstract
The Himalayan composting toilets (CTs) offer a sustainable solution for converting human faeces (HF) into compost, supplementing the low-fertile land of the region. However, CTs face challenges such as delayed composting processes (6–8 months), increased heavy metal content, and foul odour. Therefore, the current study evaluated biochar-amended psychrotrophic bacteria for HF degradation under low-temperature conditions (10 ± 2 °C). Out of 153 psychrotrophic bacteria isolated from HF compost, 17 bacterial strains were selected based on highest and two or more hydrolytic activities. Furthermore, considering the isolation source, bacterial strains were examined for haemolytic activity, biofilm formation, cytotoxicity and seed germination assay. In total, 14 potential strains belonging to Pseudomonas, Microbacterium, Arthrobacter, Streptomyces, Glutamicibacter, Rhodococcus, Serratia, Exiguobacterium, and Jeotgalicoccus genera were considered safe for both human handling and plants. The composting process was conducted in modified plastic drums at 10 ± 2 °C for 90 days through two treatments: Treatment 1 (T1) involving HF, non-immobilized biochar and cocopeat, and Treatment 2 (T2) involving HF, consortium-immobilized biochar and cocopeat. The consortium-immobilized biochar (T2) degraded HF within 90 days with hemicellulose and cellulose degradation ratios of 73.9% and 62.4%, respectively (p ≤ 0.05). The compost maturation indices like C/N ratio (16.5 ± 1.85), total nitrogen (2.66 ± 0.07), total phosphate (0.4 ± 0.005), total potassium (1.8 ± 0.05) also improved in T2 treatment (p ≤ 0.05). Additionally, T2 was more effective in achieving safe levels of faecal coliforms (< 1000 MPN g−1) and reducing heavy metal content compared to T1. 16S rRNA amplicon-based analysis demonstrated an enhancement of bacterial community diversity in T2, with the presence of Rhodococcus, Pseudomonas, Arthrobacter, and Streptomyces at the end of the composting period promoting HF degradation. Furthermore, T2-fertilized soil showed a germination index (121 ± 0.4, p ≤ 0.05) and stimulated root, shoot and yield by 110%, 45.2%, and 288%, respectively, in pea (Pisum sativum var. AS-10) compared to T1 (49.6%, 19%, and 5.8%, respectively) (p ≤ 0.05). In conclusion, the developed biochar-based formulation proved effective in degrading HF at low temperatures, mitigating foul odours, reducing heavy metals, and enhancing the agronomic value of the final compost. This study presents a promising approach for the sustainable management of HF that can supplement the non-nutritive soil of high-altitude regions.
Similar content being viewed by others
Introduction
The utilization of human faeces (HF) provides an opportunity to address the present-day sanitation challenges and soil nutrient depletion. Recycling HF nutrients back into the soil provides a sustainable way to improve soil health and better nutrient management [1]. However, significant challenges related to source separation, high volume, handling, and slow degradation of faeces pose difficulties in utilization. HF is presently disposed of via flush-based technologies through extensive sewer networks and septic tanks or directly discharged into water bodies [2, 3]. But, in areas where the construction of sewer networks is economically and topographically not feasible, opting for on-site sanitation systems using dry technologies is a more sustainable option [4]. Composting toilets (CTs) are among the dry sanitation technologies well-suited for arid and water-scarce regions lacking central water supply or sewerage infrastructure [5, 6]. CTs provide an opportunity for the separation and on-site treatment of HF at the source, and the obtained solids can be used as fertilizers or soil amendments for food and non-food crops [1, 7, 8]. The compost prepared in CTs is often characterized by high nutrient content and low contamination of domestic or industrial chemicals and heavy metals [9]. Furthermore, if adequate treatment methods are employed to minimize pathogenic risks and organic trace substances affecting environmental and human health, CTs can offer a sustainable solution for replacing flush-based toilets and addressing wastewater infrastructure issue [1].
In cold regions, composting process becomes more challenging due to lower microbial activity and lower temperatures, potentially leading to slower biological processes and limited breakdown of organic matter. [10,11,12]. In our previous studies on Himalayan CTs, a major issue associated with HF composting was the delayed degradation process due to low ambient temperatures [7, 10, 13]. Other limitations related to the final compost included a low C/N ratio and heavy metal accumulation due to the choice of co-composting materials in degrading faeces [7]. The use of psychrotrophic microorganisms at the onset of the decomposition process can accelerate the degradation rate [10, 11, 14,15,16]. Natural renewable resources, such as microorganisms and biochar in combination, have gained attention in many remediation studies [17,18,19,20]. However, there are few reports available on using microorganisms in HF degradation [8, 21, 22].
Based on the challenges arising in CTs, there was a need to improve the degradation process and address the issues of heavy metal content, foul odour, and agronomic value of the final compost. The ambient temperature in Himalaya typically ranges between 3 to 35 °C in summer, with an average minimum temperature of− 20 and − 35 °C during heavy snowfall in winter [12, 23]. Moreover, in our previous study, the temperature of the compost at the center remained around 10 °C (near ambient temperature) in the composting toilets of the Himalaya [7]. Therefore, indigenous psychrotrophic bacteria must be employed to efficiently degrade the HF under such extreme conditions. Indigenous bacteria can withstand harsh climatic conditions, survive frigid temperatures, and produce a range of cold-active hydrolytic enzymes during composting. However, most degrading bacteria have a limited lifespan in liquid media as they face intensive competition from opportunistic microorganisms in the environment [24]. Also, the growth medium cannot be liquid because the prolonged winters in the Himalaya (snow-covered for almost six months) would freeze the media, jeopardizing the survival of beneficial bacteria [7]. Therefore, exploring a reliable carrier material capable of absorbing bacteria and providing a microhabitat to maintain bacterial viability during prolonged winters was essential. Biochar can offer a better option for carbon sequestration and nutrient recovery by promoting HF degradation. Biochar is known for improving aeration, enhancing microbial activities, improving humification, reducing greenhouse gas emissions, immobilizing organic pollutants and heavy metals during composting [18, 25,26,27], as well as suppressing foul odour from HF [27, 28]. Additionally, biochar derived from bamboo prepared using conventional pyrolysis technologies is significantly economical due to its low price, enhanced growth rate, and high biomass yield [29, 30].
Further, centralized sanitation systems are not feasible due to topographical challenges in the Himalayan regions [7, 12]. Flush-based technologies also cannot function properly in the Himalaya during winter conditions due to the freezing of sewer networks. As a result, CTs are prominent in the Himalayan region, particularly in the northwestern Himalaya and Ladakh areas of India [7]. Based on the limitations of existing CTs, our experimental design was set up in modified plastic drums with minimal alterations to evaluate the impact of low temperatures on the composting process. Composting is one of the techniques that can help in nutrient recovery and pathogen inactivation in HF before it can be used as a soil conditioner in agriculture [31]. So, it is further suggested that the final compost utilised for crop applications must be non-pathogenic and have high fertilizing efficiency. Additionally, previous studies have shown that composting faecal sludge for 84 days in drum composters minimized parasitic nematodes and faecal coliforms [27, 32].
Therefore, in this study, we focused on providing a sustainable solution to degrade HF at low temperatures through psychrotrophic bacteria-amended biochar treatment. The objectives of this study were as follows: (1) to develop an effective formulation using an indigenous psychrotrophic bacterial consortium immobilized on bamboo biochar; (2) to evaluate the effect of bamboo biochar-immobilized bacterial consortium on degrading human faeces in modified plastic drums; (3) to explore the effect of bamboo biochar-immobilized consortium on the bacterial community; and (4) to elucidate the stability, safety and agronomic value of the final compost. The results of this study are likely to provide a sustainable approach for HF composting in high-altitude regions and help promote traditional CTs.
Materials and methods
Isolation, enumeration, and molecular characterization of bacteria
The human faeces (HF) compost utilized for isolating psychrotrophic bacteria was previously evaluated for its physiochemical properties by Borker et al. [7]. The enumeration of psychrotrophic bacteria from matured HF compost was carried out using serial dilution and spread plate methods on nutrient agar (HiMedia, India), Antarctic bacterial medium [33], and soil extract agar (HiMedia) plates at 10 °C. The viable bacteria were counted as colony-forming units, and unique morphotypes were purified and maintained at 4 °C. The bacterial isolates were preserved in 25% glycerol at − 80 °C for further use. The genomic DNA of selected isolates was extracted using the cetyltrimethylammonium bromide (CTAB) method [10], and the identification of isolates was carried out by partial 16S rRNA gene sequencing as described earlier by Kumar et al. [34].
Evaluation of hydrolytic and plant growth promoting activities
The hydrolytic potential of bacterial strains was initially determined using a plate assay method at 10 °C. Each exponentially grown bacterial isolate was spot inoculated for protease on skim milk agar (HiMedia, India), cellulase on carboxymethylcellulose agar [35], amylase on starch agar (HiMedia, India), xylanase on xylan agar [36], and pectinase on MP-5 medium (HiMedia, India). The hydrolysis capacity was calculated following Borker et al. [10]. The quantitative estimation of polysaccharide degrading enzymes, i.e., cellulase, xylanase, amylase, and pectinase, was performed using the 3, 5—dinitrosalicylic acid colorimetry method [37,38,39]. The protease activity was determined using Folin and Ciocalteu reagent-based assay [40]. The plant growth promoting (PGP) potential of each bacterial strain was determined following Borker et al. [10].
Safety assessment
The pathogenic potential of selected bacterial strains was determined by assessing their haemolysin activity and biofilm formation [10]. The selected bacterial strains were also evaluated for antagonism by the cross-streaking method [41]. Further, the cytotoxicity assay of psychrotrophic hydrolytic bacteria (PHB) consortium with three different concoctions (bacterial consortium, extracellular supernatant, and intracellular lysate) was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay [42] against human keratinocyte (HaCaT) cell line. In 96-well plates, 103 HaCaT cells in a 100 µl volume were plated and treated with different concentrations (log10 dilutions up to 5) of intact bacterial consortium (OD600 was ~ 0.6), extracellular supernatant (filtered extracellular media of grown bacterial consortia at OD600 of 0.6) and intracellular lysate (filtered centrifuged lysate of sonicated bacterial consortia), respectively, in quadruplicates. Further, HaCaT cells were treated with 100 µg/ml lipopolysaccharides (LPS), phosphate-buffered saline solution with 0.05% tween 20 (PBST) as a positive control, phosphate-buffered saline (vehicle control) and Luria broth was used for bacterial culturing (media control) as a negative control. After 2 h of incubation, the medium was removed from the wells, and 100 µl of Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum (FBS) containing MTT solution (0.5 mg/ml) was added and incubated in a CO2 incubator for 4 h at 37 °C. The formazan product was solubilized using 50 µl dimethyl sulfoxide (DMSO), and the absorbance was taken at 570 nm in a multi-mode microplate reader (SpectraMax iD3). To visualize cell viability and integrity, 0 h and 24 h time points were considered, and undiluted bacterial consortium fractions used for the MTT assay were captured using a Carl Zeiss Axio Vert. A1 microscope (Carl Zeiss, India).
Biochar preparation and immobilization of bacterial consortium
Biochar was prepared from bamboo (Bambusa bambos L. Voss) via. slow pyrolysis at 400–550 °C for 24–48 h under oxygen stress conditions. Before use, bamboo biochar (BB) was pulverized and sieved using a 0.25mm sieve. The surface morphology of the BB was observed using scanning electron microscopy (SEM) (S-3400 N SEM Hitachi, Japan) at accelerating voltages of 15kV. The BET surface area, mean pore diameter and total pore volume of BB samples were evaluated by nitrogen adsorption–desorption at 77 K using a Quantachrome BET surface analyzer (St 1 on NOVA touch 4LX Instrument, Boynton Beach, FL, USA).
The immobilization of the consortium on BB was performed as previously described by Sun et al. [43] with slight modifications. All the PHB strains were cultured in nutrient broth (HiMedia, India) for 48 h to the logarithmic phase and then washed three times with 0.9% saline to remove any media traces. The cells were then re-suspended in 0.9% saline in equal proportion to set an OD600 at 1.0. The BB and PHBs cell suspension was mixed in 1:1, 1:2, 1:3, 1:4, and 1:5 (w/v) ratios and incubated for 24 h at 150 rpm to allow the bacterial consortium to immobilize on the BB. Immobilized BB (IBB) was obtained by centrifugation at 1000 rpm for 30 min, and OD600 of the supernatant was noted to determine the immobilization efficiency. The immobilization rate of PHB on BB was calculated using the formula given below:
where, OD0 is the initial OD600 of PHBs suspension, OD1 is OD600 of the supernatant.
The ratio of the BB and PHBs showing the highest immobilization rate was chosen, air-dried at room temperature, and stored at 4 °C for further use in analyzing IBB morphology using SEM [43].
Inoculum preparation
In this study, pure bacterial cultures were grown separately before inoculation. A 100 ml culture of each selected hydrolytic psychrotrophic bacterium (6 × 108 CFU/ml) was mixed in 10 L of nutrient broth (HiMedia, India) for inoculum preparation by fermentation at an optimum temperature of 10 ℃ and pH 7.0. The cell biomass (10 × 108 CFU/ml) was then separated through centrifugation at 1000 rpm and suspended in 3 L of phosphate-buffered saline (PBS) with a pH of 7.0. All the microbiological transfer was carried out inside the laminar air flow. Subsequently, bamboo biochar was initially blended with the consortium suspension in 1:3 ratio and allowed to stand overnight. Thereafter, it was combined with one part of cocopeat (CP). The resulting formulation, denoted as BCC, comprised bamboo biochar, coco peat, and the PHB consortium in a 1:1:3 ratio. In contrast, the formulation labelled as BCD included bamboo biochar, cocopeat, and sterile distilled water in a 1:1:3 ratio.
Composting process
Recycled high-density polyethylene plastic drums (capacity 35 L, height 0.41 m, and diameter 0.27 m) were used for HF composting. The drums were modified to facilitate passive air circulation by creating 5 mm diameter holes at 10 cm intervals from each other at the top, middle, and bottom sections (Additional file 1: Fig. S1). At the base of the drums, a hole of 15 mm diameter was made to insert a falcon (10 cm long and cut open at one end) for leachate collection (Additional file 1: Fig. S1). At the base of treatment 1 (T1) and treatment 2 (T2) drums, 300 g of BCD and BCC, respectively, was evenly spread so that faeces did not stick to the base at the time of compost turning (Additional file 1: Fig. S1). For the initial phase of the experiment, i.e., for 10 days, fresh faeces (~ 200 g) were added daily, and ~ 170 g of BCD and BCC were supplemented to cover the faeces in T1 and T2 drums, respectively. The drums were closed with a lid and kept at 10 ± 2 °C. After 10 days, ~ 4.2 kg of raw materials were present in T1 and ~ 4.1 kg in the T2 drums. The drums were kept under controlled conditions at a temperature of 10 ± 2 °C, and a relative humidity of 45–50% for 80 days. During the composting phase, raw materials were mixed after 10 day intervals, and the temperature was recorded using a handheld digital thermometer (MEXTECH, India). Approximately 50 g of samples were collected from both treatments (T1 and T2) in sterile containers and homogenised uniformly. From each treatment, ~ 30 g samples were oven-dried (at 37 °C for 48 h), powdered, and sieved using a 0.4 mm sieve. The remaining samples were stored at 4 °C for subsequent laboratory analysis.
Chemical analyses
The chemical analysis of raw materials (fresh HF, BB, coco peat, BCD, and BCC) and collected compost samples from the experiments was conducted following the standard methods provided by American Public Health Association (APHA) [7, 44]. The pH and electrical conductivity (EC) were recorded by a digital pH and EC meter (PC 450, Eutech, India), and moisture content (MC) was analyzed using a moisture analyser (MOC63u, Shimadzu, Japan). Bulk density (BD) was determined according to the protocol of Mandal et al. [45]. The organic matter (OM), total nitrogen (TN), total carbon (TC), total potash (TK), and total phosphates (TP) of dried samples were determined using standard methods provided by APHA [44]. Metal elements, i.e., calcium (Ca), magnesium (Mg), zinc (Zn), sodium (Na), copper (Cu), iron (Fe), lead (Pb), cadmium (Cd), chromium (Cr) and nickel (Ni) were determined using an Atomic Absorption Spectrometer (Shimadzu AA-6300) [44]. The cellulose, hemicellulose, and total protein content were also determined according to Borker et al. [7]. The lignocellulose degrading ratio was calculated using the formula:
where Rn is the degrading ratio for the nth day; m0 and mn are the lignocellulose content on the initial and nth days, respectively.
Determination of pathogenic bacteria
The estimation of pathogenic bacterial in the initial and final samples of T1 and T2 treatments was evaluated as previously described by López et al. and Soobhany et al. [46, 47]. The samples were further analyzed for the most probable number of faecal coliforms g−1, calculated using a 3-tube dilution series method [48]. Each test was performed in six replicates.
Microbial community analyses
The total genomic DNA from the initial (1st day) and final (90th day) compost samples of T1 and T2, respectively, was extracted in triplicates following the protocol described previously by Borker et al. [7]. The quality of the extracted DNA was checked using a Nano-Drop 2000 Spectrophotometer (Thermo Fisher Scientific, USA), and the DNA concentration was estimated using Qubit Fluorimeter v.3.0 (Thermo fisher Scientific, USA). The 16S rRNA gene amplification was performed using primers of the V3-V4 hyper-variable region (V3 Forward primer AGAGTTTGATGMTGGCTCAG3 and V4 Reverse primer TTACCGCGGCMGCSGGCAC3). The amplified product was used for library preparation using the NEBNext Ultra DNA library preparation kit. The quality estimation and quantification of the library were determined using the Qubit dsDNA High Sensitivity assay kit. The library was sequenced on the Illumina MiSeq platform using a 300 bp paired-end protocol..
The quality of the 16S rRNA paired-end reads was checked using FastQC v0.11.5, and adapter trimming was performed using Cutadapt v1.15. The trimmed sequences were processed using the QIIME 2 v.2022.2 platform [49]. The q2-DADA2 package was used for quality filtering, denoising, and chimera removal from the sequences [50]. The operational taxonomic units (OTUs) were built, and taxonomic assignments were based on the Greengenes v.13_8 database using the scikit-learn classifier of QIIME 2. The Unweighted UniFrac distances plotted on Principal Coordinate Analysis (PCoA) and α-diversity metrics (Shannon, Simpson, and Chao I) were calculated using the q2-diversity plugin. The circle plot for phylum levels was generated with CIRCOS (http://circos.ca/).
Phytotoxicity and pot experiment
The phytotoxicity of selected bacterial strains, raw materials, initial feed mixtures (BCD and BCC), and final compost samples of T1 and T2 was evaluated by measuring the germination index (GI) in Pisum sativum var. AS-10 (pea) seeds, following the method described by Siles-Castellano et al. [51].
The fertilizing efficiency of the final composted HF (T1 and T2) was evaluated by its application to Pisum sativum var. AS-10 plants. The pot experiment was conducted in controlled growth chambers in polyvinyl chloride pots filled with a 2 kg of autoclaved soil and sand mixture. Before the compost amendments, the pots were kept for 10 days at 25 ± 2 °C for preconditioning. The pots were supplemented with 100 g (5%) of final composted T1 and T2 samples. The unamended treatment containing the soil mixture served as a control for comparison purposes. A total of 15 replicates for control, T1 and T2, respectively, were prepared and kept at a 25 ± 2 °C, 100 µmol photon/m2/s fluorescent illumination, with a 16/8 h light/dark cycle, and 50–55% relative humidity. After 8 weeks, physiological plant growth was measured in terms of plant height, branch number, leaves number, fresh weight of foliage and root, root length, and pod number. The dry weight of foliage and root samples was measured after drying in the oven at 45 °C for 48 h.
Statistical analysis
The bacterial phytotoxicity and pot experiment data were subjected to one-way analysis of variance (ANOVA) and Tukey post hoc tests (p < 0.05) for measuring the statistical difference between different treatments was performed using IBM SPSS Statistics version 26 for Windows (SPSS, Chicago, IL). All the chemical analyses and phytotoxicity were performed in triplicates. The results represent their mean values and standard deviation.
Results and discussion
Development of bacterial consortium
A total of ~ 270 psychrotrophic bacterial strains were initially screened at 10 °C from human faeces (HF) compost collected from traditional dry/composting toilets (CTs) of the Himalaya [7]. Among these isolates, 153 distinct colonies were selected for identification and screened for potential psychrotrophic hydrolytic bacteria (PHB) (Additional file 1: Fig. S2; Additional file 1: Table S1). Based on 16S rRNA gene sequencing, the bacterial strains were distributed among four phyla (40.5% Actinobacteria, 33.3% Proteobacteria, 24.2% Firmicutes, and 1.96% Bacteroidetes) and 34 genera. As HF consists of 75% water and 25% solid matter comprising of protein, undigested lipids, polysaccharides, bacterial biomass, and undigested dietary leftovers [2, 52], the bacterial isolates were screened for protease, amylase, cellulase, pectinase, and xylanase activities (Fig. 1A; Additional file 1: Table S1). 75 psychrotrophic bacterial strains that showed hydrolytic potential qualitatively were further selected for quantitative enzyme assays. Out of the selected strains, 55 bacterial isolates exhibited one or more enzyme activities (30 showed protease, 43 cellulase, 05 amylase, 15 xylanase, and 08 pectinase activities at 10 °C) (Fig. 1B, C; Additional file 1: Table S2). The 16 bacterial strains belonging to Pseudomonas sp., Microbacterium sp., Arthrobacter sp., Streptomyces sp., Glutamicibacter sp., Rhodococcus sp., Serratia sp., Exiguobacterium sp., Bacillus sp., and Jeotgalicoccus sp., showing the highest, and two or more enzyme activities, were selected for developing the bacterial consortium (Additional file 1: Table S3). Many studies have shown that microbes producing a wide range of hydrolytic enzymes accelerate the decomposition and overcome the issue of delayed composting [53,54,55,56,57,58,59,60,61]. Psychrotrophic Pseudomonas sp., Exiguobacterium sp., and Bacillus sp. with multiple hydrolytic activities have been previously demonstrated to efficiently degrade organic domestic waste [11]. Zhai et al. [62] reported the role of protease producing Serratia sp. in swine carcass composting. The role of other selected bacterial strains in degradation of different substrates are provided in Additional file 1: Table S4. Additionally, the details of the physiological characterization of the selected strains are provided in Additional file 1: Table S5.
Selection of potential psychrotrophic bacterial strains to prepare novel consortium. A Qualitative estimation using plate assay for different hydrolytic enzymes at 10 °C. B Venn diagram depicting single and shared quantitative hydrolytic activities of psychrotrophic bacterial strains. C Heatmap representing the quantitative estimation of different hydrolytic enzymes. A total of 55 psychrotrophic strains showed one or more enzyme activities
Owing to the source of isolation, it was mandatory to ensure the human safety of each selected psychrotrophic hydrolytic bacteria (PHB) before forming the bacterial consortium. The 16 bacterial isolates were tested for pathogenicity by screening for haemolysin production on blood agar (Additional file 1: Fig. S3A). Typically, pathogenic bacteria employ a range of virulence factors to initiate pathogenesis, such as adhesion proteins and toxins like haemolysins [63]. The 14 bacterial strains showed no haemolysis, but Bacillus toyonensis LPH14 and Serratia nematodiphila LUS01 exhibited haemolytic activity and were not selected for the consortium development (Additional file 1: Table S6). Furthermore, the strains were tested for biofilm formation, and 14 bacterial isolates showed no biofilm formation (Additional file 1: Fig. S3B; Additional file 1: Table S7). Biofilm production is the initial step for pathogenic bacteria to adhere to host tissue cells, thereby inducing pathogenesis [63]. The 14 PHB strains that were safe, along with Glutamicibacter arilaitensis LJH19 [10], were assessed for their synergistic activity, and all the bacterial strains were found to grow synergistically (Additional file 1: Fig. S4). The details of hydrolytic capabilities and safety assessment for G. arilaitensis LJH19 have been published earlier [10].
The 15 PHB consortium was further assessed for their cytotoxic effect on the human keratinocyte (HaCaT) cell line. The skin, primarily composed of keratinocytes, is a highly exposed organ that serves as an efficient protective barrier separating the body from the external environment [64]. Thus, the evaluation of cytocompatibility of the human keratinocyte was critical to validate whether the developed bacterial formulation was appropriate for human handling. The in-vitro cytotoxicity of three different concoctions (intact bacterial consortia, extracellular supernatant, and intracellular lysate) against HaCaT cells was detected by MTT assay (Fig. 2A). The percentage of cell viability for all three concoctions was measured at different dilutions and ranged between 82.9% ± 5.43 and 117% ± 9.39 (Fig. 2A; Additional file 1: Table S8). The morphology of cells treated with different undiluted bacterial consortium fractions was compared to the untreated control (Luria broth used for bacterial culturing). The results showed cell viability and integrity at 0 and 24 h time points, respectively (Additional file 1: Fig. S5). Overall, the PHB consortium, extracellular supernatant, and intracellular lysate were not cytotoxic at various concentrations.
Safety assessment of potential psychrotrophic bacterial strains. A In-vitro cytotoxicity of bacterial consortium towards human keratinocyte (HaCaT) cell lines. The three concoctions of bacterial consortia (intact bacterial consortia, extracellular supernatant and intracellular lysate) were assessed for their cytotoxic effects against HaCaT cell lines and were non-cytotoxic at various concentrations. B In-vitro phytotoxic effect of selected psychrotrophic bacterial strains on Pisum sativum var AS10 seeds. All the selected strains were non-phytotoxic towards seed germination except for the LUR25 strain. The different letters in the figure describes the statistical difference (p < 0.05) calculated by Tukey post hoc tests among the samples. *Represent a significant difference
Although the bacterial consortium was non-pathogenic to humans, the phytotoxic effects of the selected 15 bacterial strains were measured under in-vitro conditions on Pisum sativum var. AS10 seeds to determine any toxicity towards plants (Fig. 2B). The germination rate (GR) of the seeds inoculated with strains increased by 16–58% compared to the control, except for Serratia bozhouensis LUR25, which showed a lower GR value (35.55%) with p < 0.05 (Additional file 1: Table S9). The bacterial strains also demonstrated a varied increase in root length (25–170%) of the seeds, but LUR25 showed a 23% decrease compared to the control. Similarly, enhanced shoot length (13–194%) was observed in the inoculated seeds, with LUR25 showing the least increase compared to the control (p < 0.05). The treated seeds with bacterial strains significantly influenced the germination index (GI) (392–161%; p < 0.05), except for the LUR25 strain (51%; p < 0.05), which showed a low GI value (Fig. 2B). The GI value of less than 60% is considered phytotoxic, while GI value > 100 have phytonutrient or phytostimulant effect [51]. Owing to the phytotoxic effects, strain LUR25 was not selected for the development of the bacterial consortium. The remaining 14 psychrotrophic bacterial strains were selected based on their hydrolytic capabilities and non-pathogenic nature for preparing a novel consortium (Table 1).
Biochar characterization and immobilisation of PHB consortium for final formulation development
Bamboo biochar (BB) was used as the carrier material for immobilizing the selected PHB consortium. Biochar is known for improving aeration, enhancing microbial activities, reducing nitrogen loss and greenhouse gas emissions, aiding in immobilizing organic pollutants and heavy metals during composting, [65] and suppressing foul odor from human faeces [28]. Moreover, biochar derived from bamboo is significantly economical due to its low price, enhanced growth rate, and high biomass yield [29]. The physicochemical characteristics of BB are listed in Table 2. The pH was in the alkaline range (8.7 ± 0.1) due to the high ash content (12.34 ± 0.07) in BB and the enrichment of non-degradable inorganic compounds [29]. The carbon content was significantly higher than that of the other nutrients (Na, P, K, Ca, Mn, and Mg) in BB (Table 2). Heavy metals were also detected in BB but were in the permissible range of fertilizer control order (FCO) standard (Table 2) [66].
The scanning electron microscope (SEM) images of BB revealed a rough surface with a porous structure, exhibiting a vast surface area (Additional file 1: Fig. S6A). BET analysis further determined that BB’s surface area, pore volume, and pore diameter Dv(d) as 9.53 m2 g−1, 0.019 cm3 g−1, and 3.37 nm, respectively. Sahoo et al. [29] also observed similar results for BB pyrolyzed at a similar temperature range (400–500 ℃). Based on the pore size, biochar was categorized as mesopores (2 nm to 50 nm) [67]. The morphological properties provide lodging for microorganisms, reduce density, and increase aeration of the compost pile [26, 65, 68, 69]. Considering the high porosity, strong adsorption properties, high contents of aromatic substances and oxidative functional groups, biochar has been widely used as an ideal amendment to composting [27, 68, 69]. Bolan et al. [68] also suggested biochar as an effective low-cost carrier for biofertilizers and other bacterial inoculants. Hence, the addition of biochar can stimulate the proliferation of microorganisms, accelerate the decomposition of organic matter, and ensure proper aerobic composting [26, 27, 67, 69].
The maximum immobilization of the BB to PHB consortium was observed at a 1:3 ratio (Additional file 1: Table S10). The consortium was immobilized in biochar, as evident by the SEM micrographs (Additional file 1: Fig. S6B). Schommer et al. [67] have shown that the initial attachment of microorganisms onto the biochar mainly occurs through physical adsorption and electrostatic attraction. Electrostatic attraction arises from weak interactions between oxygen-carrying functional groups on the biochar surface and organic molecules, while physical adsorption is induced by van der waals forces and hydrogen bonds between the microbe and the biochar [70, 71]. The hydrophobic properties of bacterial proteins, lipopolysaccharides, and biochar further enhance adsorption [67]. Further, Li et al. [72] reported that biochar also provides a physical carrier for enzyme immobilization and enzymes are key tools for microbial degradation of organic matter. The high surface area and functional groups of biochar offers positive site for high enzyme load and this extend the mass transfer channel through its porous structure [73]. The results suggest that BB serves as an excellent carrier material for immobilizing the PHB consortium. In accordance with the findings, two formulations were developed i.e., BCD comprising biochar, coco peat, and distilled water in a 1:1:3 ratio, and BCC comprising biochar, coco peat, and bacterial consortium in a 1:1:3 ratio.
Characterization of raw materials
The physiochemical properties of individual raw materials, as well as the initial mixtures (BCD and BCC) used for T1 and T2 treatments, are represented in Table 2. The moisture content (MC) of BCD and BCC slightly exceeded the optimum range of 40–60% [74], while the average bulk density fell within the desired range of 0.2–0.35 g/cm3 [75]. The average electrical conductivity (EC) of the raw materials ranged from 0.02 to 3.17 dS/m, and the C/N ratio was lowest for HF and highest for BB (Table 2). Macro and micro elements such as Ca, Mg, Na, Cu and Zn, were also detected in the raw materials (Table 2). The characteristics of BCD were similar to BCC, except for the GI, which was highest in the BCC due to the PHB amendment (Table 2). The lowest GI was recorded for HF, indicating its phytotoxic effects towards root growth (Table 2) [51].
In-vessel degradation of human faeces
Dynamics of physicochemical parameters during composting
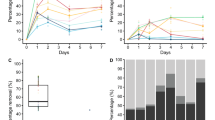
The initial temperature of T1 and T2 was slightly lower than the ambient temperature of 10℃ (Fig. 3A). The temperature of T2 began to rise on the 10th day of the composting process, attaining a maximum of 22°C by the 25th day (Fig. 3A). On the contrary, no increment in temperature was detected in T1 until the 20th day, and it reached a maximum of 20°C by the 40th day (Fig. 3A). However, no further increase in temperature was observed for both treatments (Fig. 3A). The observations were similar to Shi et al. [76] who reported no significant rise in temperature from the ambient during aerobic composting of human faeces. Despite the crucial role of temperature in composting, the smaller compost volume and continuous heat loss hinder in achieving high temperatures in a pile[76].
Changes in physicochemical properties of different treatments during in-vessel composting. A Temperature B Moisture content C pH D Electrical conductivity E Organic matter F C/N (carbon to nitrogen) ratio G Total phosphate H Total potash. The results are the mean of three replicates; The standard errors are indicated by error bars (n = 3); T1: Treatment 1 (Faeces + biochar + coco peat); T2: Treatment 2 (Faeces + biochar + coco peat + bacterial consortium)
MC at the beginning of the experiment was 84–85% due to the addition of human faeces (Fig. 3B). As faeces were regularly added until the 10th day, MC was maintained at 73–75% in T1 and T2, respectively (Fig. 3B). At the end of the experiment, a 20.6% decrease in MC was observed in T1, and T2 showed a 36.4% reduction (p < 0.05), respectively; however, it did not fall within the permissible range of FCO standard (Fig. 3B). The high moisture levels in T1 and T2 might be attributed to a lack of temperature rise in pile, resulting in reduced water loss, and a smaller compost volume, leading to heat loss [76]. Another reason could be the condensation of water vapours above compost bins, subsequently dripping back into the pile [77]. Castro-Herrera et al. [78] have also reported high MC during the thermophilic composting of human excreta. Borker et al. [7] and Nasri et al. [52] have also reported a similar moisture range from waterless toilets.
The results in Fig. 3C indicate a significant difference in the rise of pH value in both treatments (p < 0.05). The changes in the pH during the degradation process followed a similar trend; however, for T2, the maximum pH value of 8.3 was achieved around the 30th day, followed by a decrease to 7.2 at the end of the process. In contrast, for T1, the pH increased to 8.2 around the 50th day of composting, followed by a decrease to 6.5 by the end of the process (Fig. 3C). The pH of both treatment products complied with the required range of the FCO standard (6.5–7.5) [66]. The initial increase in pH may be attributed to the ammonification and mineralization of organic nitrogen to ammonium through bacterial activities [79]. Meanwhile, the decrease in pH could be due to ammonia volatilization or an increase in organic acid content resulting from OM degradation [80]. A similar trend of pH variation in compost piles resulting from the degradation of organic matter was reported by Xu et al. [77], Jia et al. [80], Zhou et al. [81], and Li et al. [82].
EC indicates the compost salinity and organic mineralization, signifying its end use as a fertilizer or soil conditioner [83]. Figure 3D illustrates variations in EC with p < 0.05 in both treatments. After the 10th day, a sharp increase in EC value was observed in T2 until the 30th day, attaining a maximum of 4.18 and then gradually decreasing to a value of 1.8. On the contrary, in T1, the EC value initially rose to 3.3 on the 40th day, then decreased to 2.3 on the 50th day, followed by a constant increase until the end of the composting process, reaching up to 4.26. The rise in EC correlates with organic matter mineralization, suggesting delayed or slower degradation in T1. Higher EC values represent high salinity and immaturity of the compost, potentially signify high phytotoxic effects on plant growth [51]. The higher salinity also interfere with plant nutrient uptake, thereby inhibiting growth and yield [83]. An EC value > 4 ds/m is considered a potential seed germination inhibitor [75, 83]. In T2, the EC values subsequently decreased until the 80th day and remained constant till the end of the experiment (Fig. 3D), falling within the permissible range of the FCO standard (< 4.0) [66].
At the beginning of the composting process, AC in T1 and T2 was 15.2 ± 0.25% and 15.6 ± 0.4%, respectively. AC significantly increased in T2 to 25.2 ± 2.02%, compared to T1 (17.1 ± 0.44%) at the end of the composting period (p < 0.05). AC is an essential parameter in composting dynamics and serves as a degradation indicator, where higher accumulation signifies a high decomposition rate [84, 85]. The results indicate that biochar amended with PHBs exhibited a high ash content, representing decomposition.
The dynamics of organic matter (OM) are illustrated in Fig. 3E. The OM content decreased as composting progressed, with a rapid decline in T2, with maximum reduction (p < 0.05) occurring after 45 days. The easily degradable OM in a pile may favourably be utilized by microorganisms, leading to a decline in OM during the early stages of composting. In T2, OM decreased from an initial 90% to 75.6% at the end of the composting, but in T1, OM decreased from 90 to 81% (Fig. 3E). The difference in OM content was lower in T1 compared to T2, indicating a slower degradation rate (Fig. 3E). In biochar amended composting, the metabolic activity is influenced by the amorphous coupling between microbes, biochar and organic substrate [72]. Singh et al. [86] reported that organic metabolites are adsorbed on the surface of biochar through aromatic π, hydrogen bonding, covalent bonding, and strong dipole interactions with hydrogen donors. In a recent review, Pang et al. [87] reported that biochar also acts as an electron shuttle and has oxygen-containing functional groups like quinone groups on its surface, the redox and electron transfer involved in biochar further promotes the breakdown of OM. This suggests that the interaction of biochar with PHBs and substrate in T2 might have generated forces on its surface, affecting OM degradation and the metabolic pathway.A similar rapid degradation pattern was observed for total carbon (TC) in T2 (Additional file 1: Fig. S7A). At the end of the composting, TC contents decreased by 9.6% in T1 and 16% in T2 from the initial value (p < 0.05). The final TC content of T2 was lower than T1, indicating better decomposition efficiency of the inoculated PHB consortium (Additional file 1: Fig. S7A). A similar reduction in TC with the addition of microbial inoculants was reported by Li et al. [88] and Tuyen et al. [22], indicating the role of microorganisms in degrading organic carbon in the compost pile.
The total nitrogen (TN) exhibited a pattern of decrease and then increase for both T1 and T2 (Additional file 1: Fig. S7B). In T2, TN content increased from an initial 1.6 to 2.7, showing an increment of 67.3%, while in T1, TN rose from 1.42 to 1.98, indicating a 39.4% increase (Additional file 1: Fig. S7B). The upward trend in TN for both treatments was attributed to the addition of biochar, known to have the ability to reduce nitrogen loss from the compost [65, 89]. In addition, a significant TN increase in T2 with p < 0.05 may be attributed to nitrifying bacteria used in the consortium (Table 1). Zhao et al. [90] also demonstrated that inoculation with nitrifying bacteria reduces nitrogen loss and enhances maturity in sewage sludge composting.
Figure 3F represents the changes in the C/N ratio of each treatment group. The C/N ratio of each treatment showed a downward trend; however, the reduction was more significant (p < 0.05) in T2 than in T1 (Fig. 3F). After 40 days of composting, a sharp decrease in the C/N ratio was observed in T2, while T1 showed a slower downward trend (Fig. 3F). At the end of the 90 days, the T1 group had a higher C/N ratio (23.9 ± 3.6), whereas, T2 group supplemented with the PHB consortium had a lower C/N ratio (16.5 ± 1.85), complying with the recommended range of the FCO standard (20:1 or less) [66]. The rapid decline of the C/N ratio in T2 may have occurred due to the decomposition of organic carbon and nitrogen mineralization [91, 92]. The inclusion of microbial additives enhances carbon degradation and nitrogen maintenance, resulting in a reduced C/N ratio in the final product [91]. Compared to T1, the amendment of the PHB consortium in T2 presented a reduced C/N ratio, indicating that the compost attained maturity [93]. The observations were also similar to Gou et al. [94] and Tuyen et al. [22], who reported that the inoculation of psychrotrophic bacteria accelerated the onset of composting and promoted maturity by lowering the C/N ratio under cold-climatic conditions.
Initially, total phosphate (TP) content increased in both treatments until the 40th day, but in T1, it mostly remained constant and decreased by the end of the composting process (Fig. 3G). In T2, a rise in TP was observed on the 90th day but was within the permissible level of the FCO standard [66]. Zhang and Sun [95] have suggested that microorganisms consume dissolved P in large quantities to degrade lignocellulosic compounds. Similarly, Nasri et al. [52] have reported low P levels from different CTs. Nonetheless, after 90 days of composting, T2 has higher TP content than T1 with p < 0.05, suggesting that T2 compost can act as a soil amendment in improving the plants' efficiency for P uptake [96].
As shown in Fig. 3H, the total potash (TK) continuously increased after the 10th day in T2 and remained constant towards the end of the composting process. However, there was no significant difference (p < 0.05) in T1 until the 50th day, and then a slight increase was monitored before a TK loss was observed at the end of the experiment (Fig. 3H). The TK increase in T2 may be attributed to the K solubilizing properties of the inoculated PHBs [97] (Table 1) and within the permissible level of the FCO standard [66].
The changes in the lignocellulosic content in both treatments are shown in Table 3. The compositional analysis of substrates in both treatments revealed high cellulose and hemicellulosic content, followed by lignin and protein at the onset of the composting process (Table 3). The high lignocellulosic content may be derived from the addition of coco peat (CP) [98] and undigested plant material excreted in faeces [52, 99]. At the end of the composting process, hemicellulose and cellulose degradation ratios were 73.9% and 62.4% in T2, while in T1, the ratios observed were 41.5% and 13%, respectively. The hemicellulose and cellulose were degraded significantly in T2 than in T1 treatment (p < 0.05), perhaps due to the greater microbial activity of cellulase and xylanase-producing psychrotrophic bacteria [100]. On the contrary, lignin was observed to be more resistant to degradation in both treatments. The degrading ratios of lignin in T1 and T2 were 24.1% and 31.4%, respectively (p < 0.05), at the end of the composting. The results showed a significant difference in the degrading ratios of hemicellulose, cellulose, and lignin between PHB-inoculated T2 and non-inoculated T1 treatment groups. Similarly, protein content decreased drastically in T2 than in T1, with degrading ratios of 68.9% and 16%, further highlighting the role of protease-producing PHBs in the consortium. Recent studies on human faeces also suggested the potential role of biochar [27] and psychrotrophic bacteria [22] in improving the cellulose, hemicellulose and lignin degradation. Jia et al. [80], established greater degradation of lignocellulose during sewage sludge-reed straw composting with combined use of biochar and microbial agents. Wu et al. [101] also reported the role of composite microbial agents in degrading lignocellulose biomass of straw compost pile to improve the quality of compost product.
The changes in macro- and micronutrient concentrations of the initial and final composting treatments are illustrated in Table 3. At the onset of the composting experiment, high amounts of Ca, Fe, and Mg were detected in T1 and T2, which may have derived from HF and the initial feed mixtures (BCD and BCC) added in both treatments (Table 2). HF contain high Ca, Fe, and Mg levels; however, nutrient concentration mainly depends on the individual diet [102, 103]. After 90 days of composting, an increase in the total accumulation of Ca (61.7%), Mn (12.9%), Na (12.9%), Fe (39.8%) and Mg (1.2%) was observed in the T2 treatment, whereas in T1, the increase in Ca (2.5%), Mn (26.8%), Fe (9.7%) and Na (26.8%), and decrease in Mg (0.4%) were monitored (Table 3). The composting process tends to concentrate various metals during decomposition mainly due to the degradation of organic matter, release of CO2, and mineralization process [104, 105]. The mean concentration of elements was higher in T2 than in the T1 treatment (Table 3).
In T1 and T2, the heavy metal concentrations of Cd, Pb, and Ni were not detected throughout the composting process. However, Zn, Cu, and Cr were detected at the onset of the experiment (Table 3). The heavy metals Zn and Cu were present in BB, CP, and HF, which got accumulated into initial feed mixtures of the compost but were withinacceptable ranges as per the FCO standard (Table 2) [66]. A similar phenomenon of heavy metal accumulation was also observed at the completion of the in-vessel experiment (Table 3). The mean concentrations of Zn, Cu, and Cr increased in both T1 (45.7%, 30.1%, and 75%, respectively) and T2 treatments (23.5%, 27.3%, and 134%, respectively) from the initiation of the experiment (Table 3). The accumulation of heavy metal could result from the concentration effect during the decomposition of organic matter [7]. Similar results were reported earlier by Zhang et al. [105], wherein the concentration of Zn, Cu, and Cr increased at the end of the faeces composting. However, the overall heavy metal concentration in the final compost was within the permissible levels of the FCO standard for both the treatments (Table 3). This could also be due to the addition of biochar in both T1 and T2, as biochar is known for immobilizing potentially toxic elements by changing the chemical, physical and biological properties of its surrounding environment [106].
Bacterial community dynamics
The changes in the bacterial community composition in the initial and final samples of T1 and T2 were determined based on 16S rRNA gene amplicon sequencing. The 16S raw reads data were filtered, and 1,335,573 high-quality reads were retained from all the samples (Additional file 1: Table S11). A total of 495,367 operational taxonomic units (OTUs) were assigned to all the samples using 97% sequence identity (Additional file 1: Table S11). The number of OTUs was significantly raised in both the treatment groups at the end of the experiments (Additional file 1: Table S11), suggesting that biochar amendment may have provided favourable conditions for bacterial diversity [107], and the significant increase in T2 may be attributed to the addition of PHB consortium varying the microbial flora of the compost [14]. Recently, Zhou et al. [27] reported a similar alteration in bacterial diversity with the addition of biochar.
The community diversity indices (Shannon and Simpson) and microbial taxa abundance (Chao1) results showed that T2, amended with PHBs, exhibited higher Shannon indices than the T1 group (Additional file 1: Table S11). Initially, both T1 and T2 groups showed lower chao1 indices, but at the end of the experiment, higher Chao1 indices were observed, indicating richness in microbial abundance (Additional file 1: Table S11). Further, based on the unweighted UniFrac algorithm, principal coordinate analysis (PCoA) revealed low variability among the samples that occupied regions in terms of their PCoA1 (39.96%) and PCoA2 (19.48%), except for the initial sample of T1 (Additional file 1: Fig. S8A).
At the phylum level, both groups revealed differences in the bacterial community structure (Additional file 1: Fig. S8B). A total of 18 phyla were identified in the composting samples of T1 and T2 treatments (Additional file 1: Fig. S8B; Additional file 1: Table S12). In the initial samples of T1, the predominant phylum was Firmicutes (41.3%), followed by Proteobacteria (34%) and Bacteroidetes (16.5%), but in T2, Proteobacteria (29.6%) was the abundant taxon followed by Bacteroidetes (24.4%) and Firmicutes (21.1%) (Additional file 1: Fig. S8B; Additional file 1: Table S12). Proteobacteria have typically been reported as the most dominant (or second most abundant) phylum in aerobic composting [11, 108, 109] and were the predominant taxon in the compost collected from the dry toilets [7]. Moreover, Bacteroidetes are also dominant in faecal samples of healthy individuals [110]. The abundance of Firmicutes at the onset of the composting process is also crucial for organic matter degradation, particularly in low-temperature conditions [11]. Furthermore, Actinobacteria was the fourth dominant phylum in T1 and T2 (4.9% and 8.6%, respectively) at the start of the composting process (Additional file 1: Fig. S8B; Additional file 1: Table S12). The higher percentage in the T2 group can be explained, as nine of the 14 PHB consortium belonged to the Actinobacteria phylum (Additional file 1: Table S1; Additional file 1: Table S12).
After 90 days of the composting process, Proteobacteria became the abundant taxon in T1 (24.9%), and in the T2 treatment, they remained the predominant phylum (30.7%) Additional file 1: Table S12). Moreover, the abundance of Proteobacteria and Bacteroidetes increased by 3.9% and 14.5%, respectively, in the T2 from the initial compost samples (Additional file 1: Table S12). The addition of biochar and coco peat may have provided large amounts of carbon sources, increasing the availability of suitable habitats for the bacterial phyla [107]. However, a significant decrease of 42% and 60% in Firmicutes was monitored in the final samples of both T1 and T2, respectively, from the initial samples (Additional file 1: Table S12). These results indicated that the decrease in Firmicutes was observed due to relatively low-temperature conditions, as higher abundances are mainly observed at the mesophilic and thermophilic levels of composting [11]. Similarly, a slight decrease in Actinobacteria was observed in T2 at the end of the experiment (Additional file 1: Table S12). Nonetheless, Proteobacteria, Bacteroidetes, and Actinobacteria are considered essential phyla for their composting efficacy in different types of composts [14, 108].
At the genus level, initial samples of the T2 treatment shoed higher abundances of Pseudomonas (26.6%), Pusillimonas (11.8%), and Sporosarcina (8%) (Additional file 1: Table S13). Previous studies have also shown these genera to dominate the initial composting process [111]. The high abundance of Pseudomonas may also be related to the addition of bacterial consortium. Pseudomonas can decompose complex polymers such as lignocellulose and are commonly distributed in the environment [112]. The other included PHBs, Streptomyces (1.1%), Rhodococcus (0.6%), and Arthrobacter (0.2%) were also detected in the initial composting samples of T2. Streptomyces is reported for its high degradation efficiency as it utilises lignocellulose as the sole carbon source and effectively depolymerizes cellulose, hemicellulose, and lignin [113]. Similarly, [114] have reported psychrotrophic Arthrobacter for effective degradation of lignocellulose agricultural residues. Rhodococcus are also considered to possess remarkable metabolic activities and can survive under harsh environmental conditions [115]. The initial samples of T2 exhibited a predominant gut microbiome, including Tissierella (8.5%), Clostridium (5.9%), Bifidobacterium (3.9%), Coprococcus (2%), and Bacteroides (2.5%) (Additional file 1: Table S13) [116, 117]. Similarly, in the non-amended T1, gut bacteria were also predominant genera (Tissierella (2.1%), Clostridium (6.5%), Bifidobacterium (3.5%), Coprococcus (0.6%), Bacteroides (0.34%)) (Additional file 1: Table S13). The high abundance of gut microflora in both treatments could be associated with the initial HF. However, at the end of composting, the abundance of anaerobic bacteria such as Alkaliphulis, Clostridium, and Opitutus suggested the prevalence of a hypoxia environment within the compost, as described by [118]. Consequently, oxygen-limiting conditions may have led to a decrease in the abundances of Pseudomonas (0.75%) and Streptomyces (0.2%) among other microflora in the compost. Although Rhodococcus increased significantly (from 0.6 to 6.64%), the genus Arthrobacter (0.1%) was almost similar in the final compost samples (Additional file 1: Table S13). Nonetheless, the lignocellulose content was decreased most in the T2 treatment (Table 3). These results may suggest that the addition of PHBs could depolymerize lignocellulose before a decrease in their abundance. Interestingly, the abundance of Bdellovibrio also increased in T1 (5.01%) and T2 (2.1%) treatments. Bdellovibrio are predatory bacteria that feed upon gram-negative bacteria and multidrug-resistant human pathogens [119]. The increased abundance of predatory bacteria like Bdellovibrio might contribute to pathogen control and could be valuable in enhancing the compost safety. Additionally, the abundance of Cellvibrio (3.5%) increased in T2 at the end of the composting time. The bacterium is known for degrading complex polysaccharides, including those in the plant cell [120]. Based on the findings, PHBs could significantly increase the available organic carbon by converting carbon from faeces, thereby enhancing the soil microbiota [14]. Moreover, biochar may also have provided a unique habitat for microorganisms (carbon source and pH) and indirectly enhanced the bacterial diversity in the compost [107, 121, 122].
Analysis of pathogenic bacteria
The pathogenic bacterial population during the composting process was assessed by examining total coliforms (Additional file 1: Fig. S9). At the onset of the experiment, both treatment groups (T1 and T2) showed a total coliform value of > 11,000 most probable number (MPN) faecal coliform g−1 (Table 3). However, the MPN values declined to 585 and 385 MPN faecal coliform g−1 for T1 and T2, respectively (Additional file 1: Fig. S9; Table 3). The observed values were lower than the recommended standard value of the US Environmental Protection Agency (< 1000 MPN faecal coliform g−1) [7]. The decline in total coliforms in both treatments can be attributed to the addition of biochar amendments that aid in pathogen reduction [27, 123]. However, the decline was more prominent in T2 than in T1. Furthermore, a reduction in E. coli (a faecal pathogenic indicator) was also observed in both treatments (Table 3). The elimination of E. coli during composting can also be related to high pH and the addition of biochar [124, 125]. Similarly, no traces of Salmonella. sp. were observed in the initial and final T1 and T2 treatment samples, respectively (Table 3).
Phytotoxicity and compost application
The phytotoxic effects of the final compost piles of T1 and T2 were measured on Pisum sativum var. AS10 seeds (Fig. 4A). GI is one of the most sensitive and economical tests to measure phytotoxicity levels and the inhibitory effect of compost on plant growth [7, 74]. The GI value of T1 was 58.2 ± 4.72, indicating toxicity and harmful effects on the plantlets (Fig. 4A) [7]. The greater EC value in T1 (> 4.0ds/cm) may have contributed to potential inhibitors, impeding the seed germination (Fig. 3D) [74]. The GI value of T1 was similar to that of HF (52.6 ± 5.95), signifying that the compost was premature, as the GI of BB and CP amendments in the compost pile were not toxic (Table 2). However, T2 samples amended with the bacterial consortium observed a GI value of 123 ± 2.24e, demonstrating its non-harmful effects on seed germination and compost maturity (Fig. 4A).
Assessment of in-vitro phytotoxicity and compost application of final composted samples of T1 and T2 treatments on Pisum sativum var AS10 seeds. A Phytotoxic effects of final compost samples of T1 and T2. (i) T1-treated seeds. (ii) T2-treated seeds. The germination index of T2 was higher (123 ± 2.24) than that of T1 (58.2 ± 4.72) treatment with p < 0.05, showing non-harmful effects on Pisum sativum var AS 10 seeds. B The fertilizing efficiency of final composted samples of T1 and T2 evaluated on Pisum sativum var. AS10 after eight weeks of growth. (i) T1 amended pea plantlets. (ii) T2 amended pea plantlets. (iii) Significant difference in the growth of pea plantlets with T1 and T2 amendments. The enhanced shoot and root stimulating effects and increased productivity on Pisum sativum var AS-10 were monitored with T2 amendment. T1: Treatment 1 (Faeces + biochar + coco peat); T2: Treatment 2 (Faeces + biochar + coco peat + bacterial consortium)
Further, the final composted samples of T1 and T2 fertilizing efficiency were evaluated after eight weeks of growth on Pisum sativum var. AS10 seeds (Fig. 4A; Additional file 1: Table S14). The T2 application showed an increase of 110% in root length and a 70.8% increase in root dry biomass of pea plants in the pots compared to the control (p < 0.05), indicating a stimulating effect on root growth (Additional file 1: Table S14). Although, in the T1 application, there was a 49.6% increase in root length from the control, the root dry biomass decreased by 25% compared to the control (p < 0.05) (Additional file 1: Table S14). Similarly, a decrease of 7% in branch number was also observed in T1; however, a significant increase of 228% was observed in the T2 amendment (p < 0.05) (Additional file 1: Table S14). Likewise, plant height, dry foliage weight, and leaf number were significantly higher (45.2%, 340%, and 337%, respectively; p < 0.05) in T2 amendments, and in T1, an increase of 19%, 6.7%, and 34.5%, respectively, was observed compared to the control pots (Additional file 1: Table S14). Pod yield is an important agronomy factor as variations in pod parameters directly influence productivity [126]. The pod number increased by 288% in T2 and 5.8% in T1 compared to the control pots (Additional file 1: Table S14). PHBs in the T2 amendment may be responsible for improving plant nutrient uptake as bacterial consortium also possess plant-beneficial properties (Table 1). Plant growth-promoting bacteria associated with compost have been reported earlier to promote nutrient uptake, growth, and yield [10, 127]. Kelova et al. [128] have also reported the use of treated HF obtained from composting dry toilets and observed higher barley yield than with mineral fertilizer. Similarly, the fermented residues of co-composted wheat stalks and HF exhibited higher germination rates and biomass in wheat growth [129]. Also, Hashemi and Han [130] have reported a higher yield of white radishes in soil fertilized with biologically treated HF and urine than with commercial fertilizer. The biochar addition may have also promoted soil characteristics and plant growth by easing drought and salinity stress, lowering ammonia emissions, immobilising heavy metals and organic pollutants, and increasing microbial activity [67, 68, 106]. This study showed enhanced shoot and root-stimulating effects on Pisum sativum var. AS-10 plantlets upon T2 amendment.
The current study determined that psychrotrophic hydrolytic bacteria (PHB), when amended in biochar and coco peat as a bulking agent, decomposed human faeces (HF) within 90 days and achieved the standard composting parameters. The results showed that the PHB consortium significantly improved composting efficiency by increasing the lignocellulose degradation ratios. The PHB-amended treatment also enhanced the bacterial community structure, and the presence of Rhodococcus, Pseudomonas, Arthrobacter, and Streptomyces, included in the PHBs, at the end of the composting period indicates their role in accelerating HF degradation. The study also demonstrated that the final compost comprised of acceptable pathogen levels, was free from phytotoxic effects, and considerably increased the growth and yield of Pisum sativum var. AS-10. Overall, the PHB amended treatment process has considerable potential for HF degradation at low temperatures, and the resultant compost has a high agronomic value with low environmental impact. However, a few limitations associated with the study were high moisture content in the compost, as high temperatures in the pile were not attained. Although low pathogen levels were reported, due to the possible role of biochar, drying compost before dumping it into agricultural fields is preferable to further minimize the risk of pathogenicity. Alternatively, toilet structure engineering with the perspective of raising the temperature in the composting chambers can further help minimize pathogenicity and solve the freezing issue during winters, so that the developed formulation can act more effectively yearlong in the HF degradation process. The composting strategy using a biochar-based formulation can benefit composting toilet users in ecologically fragile Himalayan regions, mountaineers travelling to remote locations, and users of composting toilets in other low-temperature regions.
Availability of data and materials
16S rRNA gene sequence data of different bacteria are available in US National Center for Biotechnology Information (NCBI) GenBank with accession no. MZ311603-MZ311754. The 16S amplicon-based data from this study have been deposited in NCBI and are available through BioProject PRJNA902103. BET analysis data of bamboo biochar have been deposited in figshare, which is publicly available at: https://figshare.com/articles/dataset/BET_Data_pdf/22121363.
References
Kelova ME, Eich-Greatorex S, Krogstad T (2021) Human excreta as a resource in agriculture—evaluating the fertilizer potential of different composting and fermentation-derived products. Resour Conserv Recycl 175:105748. https://doi.org/10.1016/j.resconrec.2021.105748
Harder R, Wielemaker R, Larsen TA, Zeeman G, Öberg G (2019) Recycling nutrients contained in human excreta to agriculture: pathways, processes, and products. Crit Rev Environ Sci Technol 49:695–743. https://doi.org/10.1080/10643389.2018.1558889
Kawa NC (2016) What happens when we flush? Anthropol Now 8:34–43. https://doi.org/10.1080/19428200.2016.1202580
Lourenço N, Nunes LM (2020) Review of dry and wet decentralized sanitation technologies for rural areas: applicability. Challenge Oppor Environ Manage 65:642–664. https://doi.org/10.1007/s00267-020-01268-7
Gao Y, Tan L, Zhang C, Li Q, Wei X, Yang B, Chen P, Zheng X, Xu Y (2022) Assessment of environmental and social effects of rural toilet retrofitting on a regional scale in China. Front Environ Sci 10:1–10. https://doi.org/10.3389/fenvs.2022.812727
Li J, Liu X, Li L, Zhu C, Luo L, Qi Y, Tian L, Chen Z, Qi J, Geng B (2022) Performance exploration and microbial dynamics of urine diverting composting toilets in rural China. J Environ Manage 321:115964. https://doi.org/10.1016/j.jenvman.2022.115964
Borker SS, Thakur A, Khatri A, Kumar R (2022) Quality assessment, safety evaluation, and microbiome analysis of night-soil compost from Lahaul valley of northwestern Himalaya. Waste Manag 149:42–52. https://doi.org/10.1016/j.wasman.2022.06.003
Hashemi S, Han M (2018) Optimizing source-separated faeces degradation and fertility using nitrifying microorganisms. J Environ Manage 206:540–546. https://doi.org/10.1016/j.jenvman.2017.10.074
Anand CK, Apul DS (2014) Composting toilets as a sustainable alternative to urban sanitation—a review. Waste Manag 34:329–343. https://doi.org/10.1016/j.wasman.2013.10.006
Borker SS, Thakur A, Kumar S, Kumari S, Kumar R, Kumar S (2021) Comparative genomics and physiological investigation supported safety, cold adaptation, efficient hydrolytic and plant growth-promoting potential of psychrotrophic Glutamicibacter arilaitensis LJH19, isolated from night-soil compost. BMC Genomics 22:1–17. https://doi.org/10.1186/s12864-021-07632-z
Hou N, Wen L, Cao H, Liu K, An X, Li D, Wang H, Du X, Li C (2017) Role of psychrotrophic bacteria in organic domestic waste composting in cold regions of China. Bioresour Technol 236:20–28. https://doi.org/10.1016/j.biortech.2017.03.166
Thakur A, Kumari S, Sinai Borker S, Prashant SP, Kumar A, Kumar R (2021) Solid waste management in indian himalayan region: current scenario, resource recovery, and way forward for sustainable development. Front Energy Res 9:1–18. https://doi.org/10.3389/fenrg.2021.609229
Singh, J., Prashant, S.P., Kumari, S., Borker, S.S., Kumar, R. (2020) .Conservation of the night soil composting and the importance of organic farming in high altitude regions: a review. http://nopr.niscair.res.in/handle/123456789/56371 (Accessed 24 Mar 2023).
Cheng Y, Huang M, Shen X, Jiang C (2022) Enhanced cornstalk decomposition by a psychrotrophic bacterial consortium comprising cellulose, hemicellulose, and lignin degraders with biochar as a carrier for carbon neutrality. Bioresour Technol 344:126259. https://doi.org/10.1016/j.biortech.2021.126259
Jia X, Qin X, Tian X, Zhao Y, Yang T, Huang J (2021) Inoculating with the microbial agents to start up the aerobic composting of mushroom residue and wood chips at low temperature. J Environ Chem Eng 9:105294. https://doi.org/10.1016/j.jece.2021.105294
Xie XY, Zhao Y, Sun QH, Wang XQ, Cui HY, Zhang X, Li YJ, Wei ZM (2017) A novel method for contributing to composting start-up at low temperature by inoculating cold-adapted microbial consortium. Bioresour Technol 238:39–47. https://doi.org/10.1016/j.biortech.2017.04.036
Waigi MG, Sun K, Gao Y (2017) Sphingomonads in microbe-assisted phytoremediation: tackling soil pollution. Trends Biotechnol 35:883–899. https://doi.org/10.1016/j.tibtech.2017.06.014
Guo S, Liu X, Tang J (2022) Enhanced degradation of petroleum hydrocarbons by immobilizing multiple bacteria on wheat bran biochar and its effect on greenhouse gas emission in saline-alkali soil. Chemosphere 286:131663. https://doi.org/10.1016/j.chemosphere.2021.131663
Tu Z, Ren X, Zhao J, Awasthi SK, Wang Q, Awasthi MK, Zhang Z, Li R (2019) Synergistic effects of biochar/microbial inoculation on the enhancement of pig manure composting. Biochar 1:127–137. https://doi.org/10.1007/s42773-019-00003-8
Xiang L, Harindintwali JD, Wang F, Redmile-Gordon M, Chang SX, Fu Y, He C, Muhoza B, Brahushi F, Bolan N, Jiang X, Ok YS, Rinklebe J, Schaeffer A, Zhu YG, Tiedje JM, Xing B (2022) Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ Sci Technol 56:16546–16566. https://doi.org/10.1021/acs.est.2c02976
Hashemi S, Han M (2018) Effect of nitrosomonas europaea bio-seed addition on the fate of carbon and nitrogen compounds in human feces. Waste Biomass Valor 9:715–723. https://doi.org/10.1007/s12649-017-9857-5
Tuyen DT, Phuong DTH, Thang LV, Thanh NTK, Hong DTT, Cuong NC, Hoai NT, Balakirev AE, Huan LD (2022) Effects of the psychrotrophic bacteria and thermophilic actinomycetes consortium inoculation on human faeces composting process under moderate cold climate conditions in Northern Vietnam. Biol Bull Russ Acad Sci 49(Suppl 1):S51–S59. https://doi.org/10.1134/S1062359022130210
Mujumdar M, Bhaskar P, Ramarao MVS, Uppara U, Goswami M, Borgaonkar H, Chakraborty S, Ram S (2020) Droughts and floods. Assess Clim Change Indian Region Rep Ministr Earth Sci Govern India. https://doi.org/10.1007/978-981-15-4327-2_6
Abdellah YAY, Li T, Chen X, Cheng Y, Sun S, Wang Y, Jiang C, Zang H, Li C (2021) Role of psychrotrophic fungal strains in accelerating and enhancing the maturity of pig manure composting under low-temperature conditions. Bioresour Technol 320:124402. https://doi.org/10.1016/j.biortech.2020.124402
Sun D, Hale L, Crowley D (2016) Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol Fertil Soils 52:515–522. https://doi.org/10.1007/s00374-016-1093-9
Wang Z, Xu Y, Yang T, Liu Y, Zheng T, Zheng C (2023) Effects of biochar carried microbial agent on compost quality, greenhouse gas emission and bacterial community during sheep manure composting. Biochar. https://doi.org/10.1007/s42773-022-00202-w
Zhou Y, Shen Y, Wang H, Jia Y, Ding J, Fan S, Li D, Zhang A, Zhou H, Xu Q, Li Q (2023) Biochar addition accelerates the humification process by affecting the microbial community during human excreta composting. Environ Technol. https://doi.org/10.1080/09593330.2023.2291418
Senanu BM, Boakye P, Oduro-Kwarteng S, Sewu DD, Awuah E, Obeng PA, Afful K (2021) Inhibition of ammonia and hydrogen sulphide as faecal sludge odour control in dry sanitation toilet facilities using plant waste materials. Sci Rep 11:1–13. https://doi.org/10.1038/s41598-021-97016-w
Sahoo SS, Vijay VK, Chandra R, Kumar H (2021) Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean Eng Technol 3:100101. https://doi.org/10.1016/j.clet.2021.100101
Sakhiya AK, Anand A, Kaushal P (2020) Production, activation, and applications of biochar in recent times. Biochar 2:253–285. https://doi.org/10.1007/s42773-020-00047-1
Manga M, Muoghalu CC, Acheng PO (2023) Inactivation of faecal pathogens during faecal sludge composting: a systematic review. Environ Technol Rev 12:150–174. https://doi.org/10.1080/21622515.2023.2182719
Appiah-Effah E, Nyarko KB, Awuah E, Antwi EO (2018) Rotary drum composter as a low cost method for the removal of Ascaris lumbricoides and Trichuris Trichiura in faecal sludge compost. Water Pract Technol 13:237–246. https://doi.org/10.2166/wpt.2018.018
Venkatachalam S, Gowdaman V, Prabagaran SR (2015) Culturable and culture-independent bacterial diversity and the prevalence of cold-adapted enzymes from the himalayan mountain ranges of India and Nepal. Microb Ecol 69:472–491. https://doi.org/10.1007/s00248-014-0476-4
Kumar R, Singh D, Swarnkar MK, Singh AK, Kumar S (2016) Complete genome sequence of Arthrobacter alpinus ERGS4:06, a yellow pigmented bacterium tolerant to cold and radiations isolated from Sikkim Himalaya. J Biotechnol 220:86–87. https://doi.org/10.1016/j.jbiotec.2016.01.016
Thakur V, Kumar V, Kumar V, Singh D (2021) Genomic insights driven statistical optimization for production of efficient cellulase by himalayan thermophilic Bacillus sp PCH94 using agricultural waste. Waste Biomass Valor 12:6917–6929. https://doi.org/10.1007/s12649-021-01491-1
Alves-Prado HF, Pavezzi FC, Leite RSR, De Oliveira VM, Sette LD, DaSilva R (2010) Screening and production study of microbial xylanase producers from Brazilian Cerrado. Appl Biochem Biotechnol 161:333–346. https://doi.org/10.1007/s12010-009-8823-5
Li D, Feng L, Liu K, Cheng Y, Hou N, Li C (2016) Optimization of cold-active CMCase production by psychrotrophic Sphingomonas sp FLX-7 from the cold region of China. Cellulose 23:1335–1347. https://doi.org/10.1007/s10570-016-0859-4
Daskaya-Dikmen C, Karbancioglu-Guler F, Ozcelik B (2018) Cold active pectinase, amylase and protease production by yeast isolates obtained from environmental samples. Extremophiles 22:599–606. https://doi.org/10.1007/s00792-018-1020-0
Xiao Z, Storms R, Tsang A (2005) Microplate-based carboxymethylcellulose assay for endoglucanase activity. Anal Biochem 342:176–178. https://doi.org/10.1016/j.ab.2005.01.052
Mageswari A, Subramanian P, Chandrasekaran S, Karthikeyan S, Gothandam KM (2017) Systematic functional analysis and application of a cold-active serine protease from a novel Chryseobacterium sp. Food Chem 217:18–27. https://doi.org/10.1016/j.foodchem.2016.08.064
Sarkar P, Chourasia R (2017) Bioconversion of organic solid wastes into biofortified compost using a microbial consortium. Int J Recycl Org Waste Agric 6:321–334. https://doi.org/10.1007/s40093-017-0180-8
Eivazzadeh-Keihan R, Radinekiyan F, Madanchi H, Aliabadi HAM, Maleki A (2020) Graphene oxide/alginate/silk fibroin composite as a novel bionanostructure with improved blood compatibility, less toxicity and enhanced mechanical properties. Carbohydr Polym 248:116802. https://doi.org/10.1016/j.carbpol.2020.116802
Sun T, Miao J, Saleem M, Zhang H, Yang Y, Zhang Q (2020) Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning. J Hazard Mater 398:122941. https://doi.org/10.1016/j.jhazmat.2020.122941
Rice, A., Baird, E.W., Eaton, R.B. (2017). APHA 2017 Standard Methods for Examination of Water and Wastewater (Washington: American Public Health Association, American Water Works Association, and Water Env. Federation ISBN). (Accessed 24 Mar 2023).
Mandal P, Chaturvedi MK, Bassin JK, Vaidya AN, Gupta RK (2014) Qualitative assessment of municipal solid waste compost by indexing method. Int J Recycl Org Waste Agric 3:133–139. https://doi.org/10.1007/s40093-014-0075-x
López A, Baguer B, Goñi P, Rubio E, Gómez J, Mosteo R, Ormad MP (2019) Assessment of the methodologies used in microbiological control of sewage sludge. Waste Manag 96:168–174. https://doi.org/10.1016/j.wasman.2019.07.024
Soobhany N (2018) Preliminary evaluation of pathogenic bacteria loading on organic municipal solid waste compost and vermicompost. J Environ Manage 206:763–767. https://doi.org/10.1016/j.jenvman.2017.11.029
Blodgett, R. BAM Appendix 2, https://www.fda.gov/food/laboratory-methods-food/bam-appendix-2-most-probable-number-serial-dilutions. (Accessed 24 Mar 2023).
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Siles-Castellano AB, López MJ, López-González JA, Suárez-Estrella F, Jurado MM, Estrella-González MJ, Moreno J (2020) Comparative analysis of phytotoxicity and compost quality in industrial composting facilities processing different organic wastes. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.119820
Nasri B, Brun F, Fouché O (2019) Evaluation of the quality and quantity of compost and leachate from household waterless toilets in France. Environ Sci Pollut Res 26:2062–2078. https://doi.org/10.1007/s11356-017-0604-z
Borker SS, Prashant SP, Kumari A, Kumari S, Devi R, Kumar R (2021) Metabolic pathways in biodegrading psychrotrophic bacteria under cold environments. In: Pandey Anita, Sharma Avinash (eds) Extreme environments. CRC Press, Boca Raton, pp 217–233
Hemati A, Aliasgharzad N, Khakvar R, Khoshmanzar E, Asgari Lajayer B, van Hullebusch ED (2021) Role of lignin and thermophilic lignocellulolytic bacteria in the evolution of humification indices and enzymatic activities during compost production. Waste Manag 119:122–134. https://doi.org/10.1016/j.wasman.2020.09.042
Yang Y, Du W, Ren X, Cui Z, Zhou W, Lv J (2020) Effect of bean dregs amendment on the organic matter degradation, humification, maturity and stability of pig manure composting. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.134623
Padhan K, Patra RK, Sethi D, Mohanty S, Sahoo SK, Panda N, Pattanayak SK, Patra AK (2024) Exploitation of cellulose degrading bacteria in bioconversion of agro-wastes. Chemosphere 347:140654. https://doi.org/10.1016/j.chemosphere.2023.140654
Chang Y, Zhou K, Yang T, Zhao X, Li R, Li J, Xu S, Feng Z, Ding X, Zhang L, Shi X, Su J, Li J, Wei Y (2023) Bacillus licheniformis inoculation promoted humification process for kitchen waste composting: Organic components transformation and bacterial metabolic mechanism. Environ Res 237:117016. https://doi.org/10.1016/j.envres.2023.117016
Li T, Zhang X, Wang X, Yan Z, Peng C, Zhao S, Xu D, Liu D, Shen Q (2023) Effect of inoculating thermophilic bacterial consortia on compost efficiency and quality. Waste Manag 170:341–353. https://doi.org/10.1016/j.wasman.2023.09.023
Wang Q, Li N, Jiang S, Li G, Yuan J, Li Y, Chang R, Gong X (2024) Composting of post-consumption food waste enhanced by bioaugmentation with microbial consortium. Sci Total Environ 907:168107. https://doi.org/10.1016/j.scitotenv.2023.168107
Sun S, Abdellah YAY, Miao L, Wu B, Ma T, Wang Y, Zang H, Zhao X, Li C (2022) Impact of microbial inoculants combined with humic acid on the fate of estrogens during pig manure composting under low-temperature conditions. J Hazard Mater 424:127713. https://doi.org/10.1016/j.jhazmat.2021.127713
Detain J, Rémond C, Rodrigues CM, Harakat D, Besaury L (2022) Co-elicitation of lignocelluloytic enzymatic activities and metabolites production in an Aspergillus-Streptomyces co-culture during lignocellulose fractionation Curr. Res Microb Sci. https://doi.org/10.1016/j.crmicr.2022.100108
Zhai W, Li X, Duan X, Gou C, Wang L, Gao Y (2022) Development of a microbial protease for composting swine carcasses, optimization of its production and elucidation of its catalytic hydrolysis mechanism. BMC Biotechnol 22:1–15. https://doi.org/10.1186/s12896-022-00768-0
Zhao F, Yang H, Bi D, Khaledi A, Qiao M (2020) A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E coli isolated from urinary tract infections. Microb. Pathog. 144:104196. https://doi.org/10.1016/j.micpath.2020.104196
Shin MJ, Lee CS, Kim SH (2023) Screening for lactic acid bacterial strains as probiotics exhibiting anti-inflammatory and antioxidative characteristic via immune modulation in HaCaT cell. Probiotics Antimicro Prot. https://doi.org/10.1007/s12602-023-10048-8
Guo X, Liu H, Zhang J (2020) The role of biochar in organic waste composting and soil improvement: a review. Waste Manag 102:884–899. https://doi.org/10.1016/j.wasman.2019.12.003
SWM Rules: SWM Rules, (2016). Solid waste management. Gaz. India, extraordinary, https://cpcb.nic.in/uploads/MSW/SWM_2016.pdf. https://cpcb.nic.in/uploads/MSW/SWM_2016.pdf, (2016). (Accessed 24 Mar 2023).
Schommer VA, Nazari MT, Melara F, Braun JCA, Rempel A, dos Santos LF, Ferrari V, Colla LM, Dettmer A, Piccin JS (2024) Techniques and mechanisms of bacteria immobilization on biochar for further environmental and agricultural applications. Microbiol Res. https://doi.org/10.1016/j.micres.2023.127534
Bolan S, Hou D, Wang L, Hale L, Egamberdieva D, Tammeorg P, Li R, Wang B, Xu J, Wang T, Sun H, Padhye LP, Wang H, Siddique KHM, Rinklebe J, Kirkham MB, Bolan N (2023) The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci Total Environ 886:163968. https://doi.org/10.1016/j.scitotenv.2023.163968
Liu Q, He X, Wang K, Li D (2023) Biochar drives humus formation during composting by regulating the specialized metabolic features of microbiome. Chem Eng J 458:141380. https://doi.org/10.1016/j.cej.2023.141380
Ren H-Y, Wei Z-J, Wang Y, Deng Y-P, Li M-Y, Wang B (2020) Effects of biochar properties on the bioremediation of the petroleum-contaminated soil from a shale-gas field. Environ Sci Pollut Res 27:36427–36438. https://doi.org/10.1007/s11356-020-09715-y
Ha NTH, Toan NC, Kajitvichyanukul P (2021) Enhanced paraquat removal from contaminated water using cell-immobilized biochar. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-020-01996-8
Li Y, Kumar Awasthi M, Sindhu R, Binod P, Zhang Z, Taherzadeh MJ (2023) Biochar preparation and evaluation of its effect in composting mechanism: a review. Bioresour Technol 384:129329. https://doi.org/10.1016/j.biortech.2023.129329
Pandey D, Daverey A, Arunachalam K (2020) Biochar: production, properties and emerging role as a support for enzyme immobilization. J Clean Prod 255:120267. https://doi.org/10.1016/j.jclepro.2020.120267
Manu MK, Kumar R, Garg A (2019) Decentralized composting of household wet biodegradable waste in plastic drums: effect of waste turning, microbial inoculum and bulking agent on product quality. J Clean Prod 226:233–241. https://doi.org/10.1016/j.jclepro.2019.03.350
Siles-Castellano AB, López MJ, Jurado MM, Suárez-Estrella F, López-González JA, Estrella-González MJ, Moreno J (2020) Industrial composting of low carbon/nitrogen ratio mixtures of agri-food waste and impact on compost quality. Bioresour Technol 316:123946. https://doi.org/10.1016/j.biortech.2020.123946
Shi H, Wang XC, Li Q, Jiang S (2016) Degradation of typical antibiotics during human feces aerobic composting under different temperatures. Environ Sci Pollut Res 23:15076–15087. https://doi.org/10.1007/s11356-016-6664-7
Xu P, Shu L, Yang Y, Kumar S, Tripathi P, Mishra S (2024) Ecotoxicology and environmental safety microbial agents obtained from tomato straw composting effectively promote tomato straw compost maturation and improve compost quality. Ecotoxicol Environ Saf 270:115884. https://doi.org/10.1016/j.ecoenv.2023.115884
Castro-Herrera D, Prost K, Schäfer Y, Kim DG, Yimer F, Tadesse M, Gebrehiwot M, Brüggemann N (2022) Nutrient dynamics during composting of human excreta, cattle manure, and organic waste affected by biochar. J Environ Qual 51:19–32. https://doi.org/10.1002/jeq2.20312
Gong X, Zou L, Wang L, Zhang B, Jiang J (2023) Biochar improves compost humification, maturity and mitigates nitrogen loss during the vermicomposting of cattle manure-maize straw. J Environ Manage 325:116432. https://doi.org/10.1016/j.jenvman.2022.116432
Jia P, Wang X, Liu S, Hua Y, Zhou S, Jiang Z (2023) Combined use of biochar and microbial agent can promote lignocellulose degradation and humic acid formation during sewage sludge-reed straw composting. Bioresour Technol 370:128525. https://doi.org/10.1016/j.biortech.2022.128525
Zhou S, Li Y, Jia P, Wang X, Kong F, Jiang Z (2022) The co-addition of biochar and manganese ore promotes nitrous oxide reduction but favors methane emission in sewage sludge composting. J Clean Prod 339:130759. https://doi.org/10.1016/j.jclepro.2022.130759
Li YB, Liu TT, Song JL, Lv JH, Jiang JS (2020) Effects of chemical additives on emissions of ammonia and greenhouse gas during sewage sludge composting. Process Saf Environ Prot 143:129–137. https://doi.org/10.1016/j.psep.2020.05.056
Gondek M, Weindorf DC, Thiel C, Kleinheinz G (2020) Soluble salts in compost and their effects on soil and plants: a review. Compost Sci Util 28:59–75. https://doi.org/10.1080/1065657X.2020.1772906
Waqas M, Nizami AS, Aburiazaiza AS, Barakat MA, Ismail IMI, Rashid MI (2018) Optimization of food waste compost with the use of biochar. J Environ Manage 216:70–81. https://doi.org/10.1016/j.jenvman.2017.06.015
Jain MS, Jambhulkar R, Kalamdhad AS (2018) Biochar amendment for batch composting of nitrogen rich organic waste: Effect on degradation kinetics, composting physics and nutritional properties. Bioresour Technol 253:204–213. https://doi.org/10.1016/j.biortech.2018.01.038
Singh A, Sharma R, Pant D, Malaviya P (2021) Engineered algal biochar for contaminant remediation and electrochemical applications. Sci Total Environ 774:145676. https://doi.org/10.1016/j.scitotenv.2021.145676
Pang L, Huang Z, Yang P, Wu M, Zhang Y, Pang R, Jin B, Zhang R (2023) Effects of biochar on the degradation of organophosphate esters in sewage sludge aerobic composting. J Hazard Mater 442:130047. https://doi.org/10.1016/j.jhazmat.2022.130047
Li C, Li H, Yao T, Su M, Ran F, Han B, Li J, Lan X, Zhang Y, Yang X, Gun S (2019) Microbial inoculation influences bacterial community succession and physicochemical characteristics during pig manure composting with corn straw. Bioresour Technol 289:121653. https://doi.org/10.1016/j.biortech.2019.121653
Wang C, Lu H, Dong D, Deng H, Strong PJ, Wang H, Wu W (2013) Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ Sci Technol 47:7341–7349. https://doi.org/10.1021/es305293h
Zhao Y, Li W, Chen L, Meng L, Zheng Z (2020) Effect of enriched thermotolerant nitrifying bacteria inoculation on reducing nitrogen loss during sewage sludge composting. Bioresour Technol 311:123461. https://doi.org/10.1016/j.biortech.2020.123461
Greff B, Szigeti J, Nagy Á, Lakatos E, Varga L (2022) Influence of microbial inoculants on co-composting of lignocellulosic crop residues with farm animal manure: a review. J Environ Manage. https://doi.org/10.1016/j.jenvman.2021.114088
Awasthi MK, Duan Y, Awasthi SK, Liu T, Zhang Z, Kim SH, Pandey A (2020) Effect of biochar on emission, maturity and bacterial dynamics during sheep manure compositing. Renew Energy 152:421–429. https://doi.org/10.1016/j.renene.2020.01.065
Awasthi MK, Duan Y, Awasthi SK, Liu T, Zhang Z (2020) Effect of biochar and bacterial inoculum additions on cow dung composting. Bioresour Technol 297:122407. https://doi.org/10.1016/j.biortech.2019.122407
Gou C, Wang Y, Zhang X, Lou Y, Gao Y (2017) Inoculation with a psychrotrophic-thermophilic complex microbial agent accelerates onset and promotes maturity of dairy manure-rice straw composting under cold climate conditions. Bioresour Technol 243:339–346. https://doi.org/10.1016/j.biortech.2017.06.097
Zhang L, Sun X (2017) Addition of fish pond sediment and rock phosphate enhances the composting of green waste. Bioresour Technol 233:116–126. https://doi.org/10.1016/j.biortech.2017.02.073
Haouas A, El Modafar C, Douira A, Ibnsouda-Koraichi S, Filali-Maltouf A, Moukhli A, Amir S (2021) Evaluation of the nutrients cycle, humification process, and agronomic efficiency of organic wastes composting enriched with phosphate sludge. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.127051
Basak BB, Biswas DR (2010) Co-inoculation of potassium solubilizing and nitrogen fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop. Biol Fertil Soils 46:641–648. https://doi.org/10.1007/s00374-010-0456-x
Narendar R, Priya Dasan K (2014) Chemical treatments of coir pith: morphology, chemical composition, thermal and water retention behavior. Compos Part B Eng 56:770–779. https://doi.org/10.1016/j.compositesb.2013.09.028
Rose C, Parker A, Jefferson B, Cartmell E (2015) The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol 45:1827–1879. https://doi.org/10.1080/10643389.2014.1000761
Wang Y, Wu B, Ma T, Mi Y, Jiang H, Yan H, Zhao P, Zhang S, Wu L, Chen L, Zang H, Li C (2022) Efficient conversion of hemicellulose into 2, 3-butanediol by engineered psychrotrophic Raoultella terrigena mechanism and efficiency. Bioresour Technol 359:127453. https://doi.org/10.1016/j.biortech.2022.127453
Wu D, Wei Z, Gao X, Wu J, Chen X, Zhao Y, Jia L, Wen D (2020) Reconstruction of core microbes based on producing lignocellulolytic enzymes causing by bacterial inoculation during rice straw composting. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.123849
Krause A, Häfner F, Augustin F, Udert KM (2021) Qualitative risk analysis for contents of dry toilets used to produce novel recycling fertilizers. Circ Econ Sustain. https://doi.org/10.1007/s43615-021-00068-3
Wang Y, Ou YL, Liu YQ, Xie Q, Liu QF, Wu Q, Fan TQ, Yan LL, Wang JY (2012) Correlations of trace element levels in the diet, blood, urine, and feces in the Chinese male. Biol Trace Elem Res 145:127–135. https://doi.org/10.1007/s12011-011-9177-8
Ravindran B, Nguyen DD, Chaudhary DK, Chang SW, Kim J, Lee SR, Shin JDu, Jeon BH, Chung SJ, Lee JJ (2019) Influence of biochar on physico-chemical and microbial community during swine manure composting process. J Environ Manage 232:592–599. https://doi.org/10.1016/j.jenvman.2018.11.119
Zhang H, Sun X, Shan H, Xue C, Wang J (2020) Composting of night soil and horse manure with leaves as organic substrate. Compost Sci Util 28:158–168. https://doi.org/10.1080/1065657X.2021.1949409
Bandara T, Franks A, Xu J, Bolan N, Wang H, Tang C (2020) Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit Rev Environ Sci Technol 50:903–978. https://doi.org/10.1080/10643389.2019.1642832
Xu M, Xia H, Wu J, Yang G, Zhang X, Peng H, Yu X, Li L, Xiao H, Qi H (2017) Shifts in the relative abundance of bacteria after wine-lees-derived biochar intervention in multi metal-contaminated paddy soil. Sci Total Environ 599–600:1297–1307. https://doi.org/10.1016/j.scitotenv.2017.05.086
Liu T, Wang M, Awasthi MK, Chen H, Awasthi SK, Duan Y, Zhang Z (2020) Measurement of cow manure compost toxicity and maturity based on weed seed germination. J Clean Prod 245:118894. https://doi.org/10.1016/j.jclepro.2019.118894
Zhang L, Jia Y, Zhang X, Feng X, Wu J, Wang L, Chen G (2016) Wheat straw: an inefficient substrate for rapid natural lignocellulosic composting. Bioresour Technol 209:402–406. https://doi.org/10.1016/j.biortech.2016.03.004
Kaur K, Khatri I, Akhtar A, Subramanian S, Ramya TNC (2020) Metagenomics analysis reveals features unique to Indian distal gut microbiota. PLoS ONE 15:1–25. https://doi.org/10.1371/journal.pone.0231197
Niu J, Li X (2022) Effects of microbial inoculation with different indigenous Bacillus species on physicochemical characteristics and bacterial succession during short-term composting. Fermentation. https://doi.org/10.3390/fermentation8040152
Ma S, Fang C, Sun X, Han L, He X, Huang G (2018) Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour Technol 259:221–227. https://doi.org/10.1016/j.biortech.2018.03.054
Chi CP, Chu S, Wang B, Zhang D, Zhi Y, Yang X, Zhou P (2020) Dynamic bacterial assembly driven by Streptomyces griseorubens JSD-1 inoculants correspond to composting performance in swine manure and rice straw co-composting. Bioresour Technol 313:123692. https://doi.org/10.1016/j.biortech.2020.123692
Jiang C, Cheng Y, Zang H, Chen X, Wang Y, Zhang Y, Wang J, Shen X, Li C (2020) Biodegradation of lignin and the associated degradation pathway by psychrotrophic Arthrobacter sp. C2 from the cold region of China. Cellulose 27:1423–1440. https://doi.org/10.1007/s10570-019-02858-3
Kuyukina MS, Ivshina IB (2010) Application of Rhodococcus in bioremediation of contaminated environments. Biol Rhod. https://doi.org/10.1007/978-3-642-12937-7_9
Bien J, Palagani V, Bozko P (2013) The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Therap Adv Gastroenterol 6:53–68. https://doi.org/10.1177/1756283X12454590
Ilinskaya ON, Ulyanova VV, Yarullina DR, Gataullin IG (2017) Secretome of Intestinal bacilli: a natural guard against pathologies. Front Microbiol 8:1–15. https://doi.org/10.3389/fmicb.2017.01666
Liu N, Zhou J, Han L, Ma S, Sun X, Huang G (2017) Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour Technol 241:190–199. https://doi.org/10.1016/j.biortech.2017.03.144
Bratanis E, Andersson T, Lood R, Bukowska-Faniband E (2020) Biotechnological potential of Bdellovibrio and like organisms and their secreted enzymes. Front Microbiol 11:1–14. https://doi.org/10.3389/fmicb.2020.00662
Xie Z, Lin W, Luo J (2015) Genome sequence of Cellvibrio pealriver PR1, a xylanolytic and agarolytic bacterium isolated from freshwater. J Biotechnol 214:57–58. https://doi.org/10.1016/j.jbiotec.2015.07.021
Wang R, Wei S, Jia P, Liu T, Hou D, Xie R, Lin Z, Ge J, Qiao Y, Chang X, Lu L, Tian S (2019) Biochar significantly alters rhizobacterial communities and reduces Cd concentration in rice grains grown on Cd-contaminated soils. Sci Total Environ 676:627–638. https://doi.org/10.1016/j.scitotenv.2019.04.133
Singh H, Northup BK, Rice CW, Prasad PVV (2022) Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: a meta-analysis. Biochar 4:1–17. https://doi.org/10.1007/s42773-022-00138-1
Ezugworie FN, Okechukwu VC, Onwosi CO (2022) Biochar amendment aids in the reduction of antibiotic-resistant bacteria and heavy metals during composting of poultry litter. Bioremediat. J 0:1–18. https://doi.org/10.1080/10889868.2022.2079605
Chaudhary YK, Gough HL (2021) Identifying ranges of combined lime and heat treatments to achieve biosolids stabilization fecal coliform targets. J Environ Manage 282:111900. https://doi.org/10.1016/j.jenvman.2020.111900
Chung WJ, Chang SW, Chaudhary DK, Shin JDu, Kim H, Karmegam N, Govarthanan M, Chandrasekaran M, Ravindran B (2021) Effect of biochar amendment on compost quality, gaseous emissions and pathogen reduction during in-vessel composting of chicken manure. Chemosphere 283:131129. https://doi.org/10.1016/j.chemosphere.2021.131129
Ejaz S, Batool S, Anjum MA, Naz S, Qayyum MF, Naqqash T, Shah KH, Ali S (2020) Effects of inoculation of root-associative Azospirillum and Agrobacterium strains on growth, yield and quality of pea (Pisum sativum L.) grown under different nitrogen and phosphorus regimes. Sci Hortic. https://doi.org/10.1016/j.scienta.2020.109401
Tondello A, Fasolo A, Marcato S, Treu L, Bonato T, Zanardi W, Concheri G, Squartini A, Baldan B (2022) Characterization of bacterial communities isolated from municipal waste compost and screening of their plant-interactive phenotypes. Sci Total Environ 806:150592. https://doi.org/10.1016/j.scitotenv.2021.150592
Kelova ME, Ali AM, Eich-Greatorex S, Dörsch P, Kallenborn R, Jenssen PD (2021) Small-scale on-site treatment of fecal matter: comparison of treatments for resource recovery and sanitization. Environ Sci Pollut Res 28:63945–63964. https://doi.org/10.1007/s11356-021-12911-z
Liu D, Xie B, Dong C, Liu G, Hu D, Qin Y, Li H, Liu H (2018) Effect of fertilizer prepared from human feces and straw on germination, growth and development of wheat. Acta Astronaut 145:76–82. https://doi.org/10.1016/j.actaastro.2018.01.014
Hashemi S, Han M (2019) Field evaluation of the fertilizing potential of biologically treated sanitation products. Sci Total Environ 650:1591–1598. https://doi.org/10.1016/j.scitotenv.2018.09.009
Acknowledgements
SSB is thankful to UGC, Govt. Of India for the 'Research Fellowship' Grant [UGC-Ref. No.:461/ (CSIR-UGC NET DEC. 2016]. AT acknowledges the Indian Council of Medical Research (ICMR), Govt. of India, for the Senior Research Fellowship (SRF) award (No. 3/1/2/257/2021-Nut.). RK is grateful to NMHS project of MoEF&CC (sanction no. NMHS2022-23/MG11/01/282) for financial support. Authors duly acknowledge the technical support provided by K. K. Singh, Ramdeen Prasad and Om Prakash. The manuscript represents CSIR-IHBT communication no. 5219.
Funding
This work was financially supported by Council of Scientific and Industrial Research (CSIR) Fast Track Commercialization project (4th Tranche of E3OW) MLP-0182.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. SSB: Conceptualization, Data curation; Formal analysis; Software; Methodology; Roles/Writing—original draft; AT: Data curation; Formal analysis; Software; Roles/Writing—original draft; KKP: Methodology; PS: Methodology; MV: Methodology; AK: Software; RK: Conceptualization; Project administration; Funding acquisition; Writing—review and editing; Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary materials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borker, S.S., Thakur, A., Pandey, K.K. et al. Nutrient recycling of source-separated human faeces using biochar immobilized indigenous psychrotrophic bacteria for sustaining the agroecosystems of north-western Himalaya. Appl Biol Chem 67, 37 (2024). https://doi.org/10.1186/s13765-024-00887-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00887-6