Abstract
Obesity is a health condition accompanied by life-threatening comorbidities; hence, there is an increasing need for anti-obesity agents. The anti-cancer effects of the leaves of Asimina triloba (pawpaw) has been reported. However, limited research has been conducted on the potential anti-obesity effects of A. triloba fruit. Therefore, this study aimed to explore the effects of A. triloba fruit extract on murine preadipocytes (3T3-L1). We specifically examined lipid droplet formation in these cells using Oil Red O solution and intracellular pro-adipogenic protein levels were examined using western blot analysis. The results revealed that treatment with A. triloba 70% ethanolic fruit extract effectively suppressed lipid droplet formation. Moreover, the expression of crucial proteins involved in adipogenesis, namely sterol regulatory element-binding protein 1, peroxisome proliferator-activated receptor γ, and fatty acid synthase, were significantly inhibited. These findings suggest that A. triloba fruit has the potential to prevent obesity by inhibiting fat synthesis and may serve as a natural source for anti-obesity functional agents.
.
Similar content being viewed by others
Introduction
Obesity has recently emerged as a pressing global health issue fueled by contemporary lifestyles. This phenomenon is witnessing a surge in prevalence that surpasses historical levels, and more than half of the world's population will be affected by obesity by 2050 [1, 2]. The repercussions of this escalating obesity epidemic extend beyond merely weight gain, as it is invariably accompanied by a multitude of associated comorbidities, including type 2 diabetes, cardiovascular disease, stroke, arthritis, and various forms of cancer [3]. Therefore, the need for anti-obesity agents is increasing [4].
The differentiation of preadipocytes and the accumulation of lipids in mature adipocytes are intricately regulated processes that involve complex interactions among signal transduction pathways, adipogenic transcription factors, and adipocyte-specific genes. Adipose tissues prominently express transcription factors such as peroxisome proliferator-activated receptors (PPARs), CCTTA/enhancer-binding proteins (C/EBPs), and sterol regulatory element-binding protein 1 (SREBP1), which play crucial roles in driving adipocyte differentiation. Notably, PPARγ and C/EBPα are key regulators of adipogenesis and are primarily expressed during the advanced stages of cellular differentiation. These molecules regulate the activation of adipocyte-specific target genes, including leptin, adiponectin, and fatty acid synthase (FAS), which collectively govern various aspects of triglyceride synthesis and subsequent accumulation in differentiated adipocytes. Moreover, PPARγ influences insulin resistance and glucose metabolism, with its expression being facilitated by C/EBPα. Prior investigations have consistently reported the essentiality of C/EBPα and PPARγ in adipogenesis, as their reduction or knockdown impedes this process and hampers lipid accumulation. Thus, modulating the expression of these adipogenic proteins represents a promising strategy for treating obesity [5,6,7,8,9,10].
Asimina triloba (Pawpaw) belongs to the Annonaceae family, a large family that encompasses numerous tropical and subtropical trees and shrubs. Pawpaws are known for their unique flavor, which is reminiscent of a blend of mangoes, bananas, and pineapple [11]. Furthermore, it is abundant in essential nutrients such as vitamins and minerals. In addition to North America, pawpaws are cultivated in several other countries, including Romania, Italy, Ukraine, Slovakia, Austria, Georgia, Russia, Japan, and Korea [12]. Since 2010, studies have focused on investigating the various health benefits and properties of different parts of pawpaw plants, including the roots, twigs, leaves, and seeds [13, 14]. Studies have explored their anticancer, antimalarial, anthelmintic, insecticidal, antiviral, and antimicrobial activities [15]. In Korea, a specific study focused on characterizing their properties [16]. This study involved conducting antioxidant activity tests and determining the total phenolic and flavonoid contents using 80% methanol and distilled water extracts of each plant part (roots, twigs, leaves, and fruit). Additionally, the phenolic compounds in each part were identified using high-performance liquid chromatography [17]. However, there is limited research on its inhibitory effects on adipocyte differentiation and the underlying mechanisms remain unexplored. Hence, the aim of this study was to assess the antiadipogenic potential of A. triloba fruits. In this study, the anti-adipogenic effects of A. triloba ethanolic fruit extract on murine preadipocytes (3T3-L1) were investigated. Intracellular protein levels were examined to elucidate the mechanism underlying the observed anti-adipogenic activity.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin solution, phosphate-buffered saline (PBS), and trypsin–EDTA solution were purchased from Gibco Co. (Grand Island, NY, USA). Oil Red O solution, dimethyl sulfoxide (DMSO), isopropanol, dexamethasone (DEX), 3-isobutyl-1-methylxanthine (IBMX), insulin, and-mercaptoethanol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cell counting kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto, Japan). Mini-PROTEAN® TGX™ stain-free gels, Trans-Blot® Turbo™ ready-to-assemble transfer polyvinylidene fluoride (PVDF) kits, and detergent compatible (DC) protein assay kits were purchased from Bio-Rad (Hercules, CA, USA). FAS, SREBP1, PPARγ, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Beverly, MA, USA). The 3T3-L1 cells were sourced from the American Type Culture Collection (Rockville, MD, USA). T-PER™ tissue protein extraction reagent and enhanced chemiluminescence (ECL) reagent was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Goat anti-rabbit IgG-heavy and -light chain horseradish peroxidase-conjugated secondary antibodies were purchased from Bethyl Laboratories, Inc. (Montgomery, TX, USA).
Asimina triloba fruit extract preparation
The A. triloba fruits used in this experiment were harvested in September 2022 from Geochang, Gyeongsangnam-do, South Korea. The A. triloba fruits were washed, remove the seeds, freeze-dried, and then added to solvent. The ratio of the dried product to solvent (0%, 30%, 50%, 70%, or 99% ethanol) was 1:10 and extraction was performed twice for 3 h each. The extracts were concentrated using a concentrator, lyophilized, and stored at − 80 °C for subsequent use.
3T3-L1 cell differentiation and treatment with A. triloba fruit extracts
The 3T3-L1 cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin and they were maintained at a temperature of 37 °C in a 5% CO2 incubator. To induce adipocyte differentiation, cells were seeded in 24-well plates at a density of 1 × 105 cells/well. After 24 h of culture, the cells were allowed to reach full confluence, and the medium was replaced with a differentiation medium composed of 10% FBS and an adipogenic cocktail (10 μM DEX, 1 μg/mL insulin, and 0.5 mM IBMX). The cells were subsequently cultured in DMEM supplemented with 10% FBS, 1% penicillin–streptomycin, and 1 μg/mL insulin for 2 days. The medium was replaced with fresh DMEM containing 10% FBS and 1% penicillin–streptomycin supplemented with insulin. This replacement step was repeated three times within a 2-day cycle. During adipocyte differentiation, the samples were treated with A. triloba ethanolic fruit extracts. The concentrations used for treatment were 1, 10, 25, 50, 100, and 200 μg/mL. The treatment of each sample coincided with the medium exchange for differentiation, which occurred every 2 days. Treatment of 3T3-L1 cells with A. triloba ethanolic fruit extracts is shown in Fig. 1.
Measurement of cell viability
To evaluate the effect of A. triloba ethanolic fruit extracts on the viability of 3T3-L1 cells, the cells were seeded in a 96-well plate at a density of 2 × 104 cells/well and cultured for 3 days until they reached confluence. Simultaneously, in a humidified atmosphere with 5% CO2 at 37 °C, the A. triloba fruit extracts were administered at varying concentrations of 1, 10, 25, 50, 100, and 200 μg/mL during the initiation of differentiation. On the 8th day after inducing differentiation, 10 μL of CCK-8 solution was added to each well and incubated in a 37 °C, 5% CO2 condition for 1 h. Subsequently, the CCK-8 solution was completely removed, and 100 μL of DMSO was gradually added to dissolve the resulting formazan crystals. The absorbance was measured at 570 nm using a microplate reader (SpectraMax; Molecular Devices, Sunnyvale, CA, USA).
Oil red O staining and lipid accumulation determination
To assess the effect of A. triloba ethanolic fruit extract on the differentiation of 3T3-L1 cells and fat accumulation, we performed Oil Red O staining. Cells were seeded in a 6-well plate at a density of 5 × 105 cells/well. Concurrent with the initiation of differentiation, the cells were treated with different concentrations (1, 10, 25, 50, 100, and 200 μg/mL) of the extract. On the 8th day after inducing differentiation, the culture medium was removed, and the cells were rinsed with PBS. Subsequently, cells were fixed with 10% formalin for 15 min. After fixation, cells were washed with PBS and stained with Oil Red O solution for 15 min. Following three rinses with distilled water, the stained cells were dissolved in 100% isopropanol and the absorbance was measured at 490 nm.
Western blot analyses
The cells were washed with PBS and collected. After centrifugation, the cells were lysed using T-PER™ tissue protein extraction reagent. The protein concentration in each fraction was measured using the DC protein assay. Equal amounts of protein (10 μg) were electrophoresed on Mini-PROTEAN® TGX™ stain-free any kD gels and transferred onto PVDF membranes. Membranes were blocked with 5% skim milk and incubated with primary antibodies (1:1000 dilution). Subsequently, the membranes were incubated with goat anti-rabbit IgG-heavy and -light chain horseradish peroxidase-conjugated secondary antibodies. Antibody detection was performed using ECL reagent and visualized using a ChemiDoc imaging system (Bio-Rad). Band intensities were analyzed using Bio-Rad Image Lad Software.
Statistical analysis
The data was presented as mean ± standard deviation (SD), and statistical analysis was performed using GraphPad Prism software, version 7 (GraphPad Software, Boston, MA, USA). For comparisons involving three or more groups, data analysis was conducted using one-way analysis of variance (ANOVA), followed by Tukey's post hoc test. Statistical significance was set at p < 0.05.
Results
Extract yield and cytotoxicity
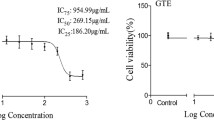
Asimina triloba fruits were extracted using solvent at various ethanol concentrations. The extraction yields were as follows: 28.20 ± 0.03% in water extraction, 46.50 ± 0.51% in 30% ethanol extraction, 49.19 ± 0.15% in 50% ethanol extraction, 75.00 ± 0.01% in 70% ethanol extraction, and 31.17 ± 0.18% in 99% ethanol extraction (Fig. 2a). To assess the cytotoxicity of A. triloba fruits, the CCK-8 method was employed to examine their effect on cell survival rate of both pre-adipocytes and adipocytes at various concentrations at the 8-day point. The results are presented in Fig. 2b. Compared with the control group, A. triloba fruit 70% ethanolic extracts (AT) ranging from 1 to 200 μg/mL exhibited no toxicity towards preadipocytes (Fig. 2b). The AT had the highest yield and was not toxic to 3T3-L1 cells; therefore, subsequent experiments were conducted using AT.
Extraction yield of Asimina triloba fruits and effects on cell viability. A Yield of AT ethanol extraction. B effects on cell viability of 70% ethanolic AT extract in 3T3-L1 cells. N: untreated group. C: differentiated adipocytes. 3T3-L1 cells were differentiated using the Asimina triloba fruit extracts. The data represents the mean ± S.E. of three experiments
Effect of AT on preadipocyte differentiation
We examined the effect of AT on lipid accumulation in 3T3-L1 cells. Figure 3a illustrates an increase in the quantity and size of lipid droplets in the control group compared with those in the normal group. Lipid droplet accumulation was not observed in untreated 3T3-L1 preadipocytes (N), whereas the control group (C) showed high lipid accumulation. Moreover, AT significantly inhibited lipid accumulation in a dose-dependent manner. In terms of relative lipid content, C (321.43 ± 1.59%) increased by approximately 3.21-fold compared with N (100.00 ± 3.17%). When treated with 1 µg/mL AT (156.24 ± 3.08%), the lipid content decreased by 2.06-fold compared with that in C, and at 200 µg/mL AT (129.44 ± 10.64%), it was similar to N. This physiological transformation is supported by the quantitative analysis shown in Fig. 3b.
Effect of AT on lipid accumulation in differentiated murine preadipocytes (3T3-L1). A Cells were fixed and stained with Oil Red O to visualize lipid droplets by light microscopy; Magnification, × 200. B Relative lipid content at different concentrations. N: untreated group. C: differentiated adipocytes. The data represents the mean ± S.E. of three experiments. ***p < 0.001 compared with normal group. ###p < 0.001 compared with control group
Effect of AT on protein expression levels
In the western blot assay, three high concentrations were selected and protein extraction was performed. Figure 4 shows the results of the protein expression levels of fat synthesis-related factors such as FAS, fat cell differentiation factors such as SREBP1, PPARγ and C/EBPα, energy metabolism regulators such as adiponectin, and phospho-AMP-activated protein kinase (p-AMPK) in lipid metabolism. As a result of inducing 3T3-L1 differentiation into adipocytes, FAS, SREBP1, PPARγ, C/EBPα, and adiponectin were all increased in the control group compared with those in the normal group. When treated with AT, the protein expression levels decreased in a concentration-dependent manner. In addition, p-AMPK protein expression, a representative factor of energy metabolism regulation, increased after AT treatment. Overall, treatment with AT inhibited the differentiation of 3T3-L1 preadipocytes and lipid synthesis by reducing the levels of proteins related to adipocyte differentiation and adipogenesis, and by regulating energy metabolism to inhibit lipid accumulation.
Effect of AT on signaling protein expression in 3T3-L1 cells. A Representative immunoblots. B Relative protein expression levels of fatty acid synthase (FAS)/glyceraldehyde 3-phosphate dehydrogenase (GAPDH), C sterol regulatory element-binding protein 1 (SREBP1)/GAPDH, D peroxisome proliferator-activated receptor (PPAR)γ/GAPDH, E CCTTA/enhancer-binding protein (C/EBP)α/GAPDH, F Adiponectin/GAPDH, and G phospho-AMP-activated protein kinase (p-AMPK)/AMP-activated protein kinase (AMPK). N: untreated group. C: Differentiated adipocytes. The data represents the mean ± S.E. of three experiments. ***p < 0.001 compared with normal group. ###p < 0.001 compared with control group
Discussion
Fat accumulation in the body occurs through enlargement and an increase in the number of adipocytes in the tissues [18]. Adipocyte hyperplasia, resulting from the proliferation and activation of adipocyte differentiation, contributes to an increase in adipocyte number and the accumulation of lipids through enhanced lipogenesis in differentiated cells [19]. The regulation of adipogenesis involves hyperplasia of adipocytes and proliferation and differentiation of preadipocytes. The balance between fat synthesis and lipolysis determines the hypertrophy of adipocytes [20].
To investigate the anti-obesity effect of A. triloba fruit, we examined the transcriptional and protein expression of key factors involved in adipogenesis, including PPARγ, C/EBPα, SREBP1, AMPK, and FAS in 3T3-L1 cells (Fig. 4). PPARγ and C/EBPα are critical transcription factors that regulate early adipocyte differentiation. Their expression influences the expression of adipogenic genes, leading to increased fatty acid synthesis and triglyceride production in adipose tissue [21]. Consequently, the suppression of PPARγ and C/EBPα expression reduces adipogenesis. We observed a significant decrease in the expression of PPARγ and C/EBPα in AT-treated cells compared with that in the control, indicating that AT inhibits adipogenesis and fat accumulation in adipocytes (Fig. 5).
Moreover, the activation of PPARγ and C/EBPα promotes the activity of SREBP1 and FAS, further promoting adipogenesis [22]. Thus, the notable reduction in SREBP1 and FAS during AT treatment can be attributed to the significant decrease in PPARγ and C/EBPα expression. Additionally, the transcription levels and phosphoprotein activity of AMPK was significantly increased in AT-treated cells compared with those in the control. AMPK, an enzyme crucial for cellular homeostasis [23], plays a vital role in enhancing glucose and fatty acid oxidation, inhibiting fat synthesis and gluconeogenesis in the liver, and regulating insulin secretion in the pancreas. When comparing the peel, seeds, and pulp of A. triloba fruit, the types and contents of phenolic compounds were the lowest in the pulp [24]. Nevertheless, the anti-obesity effect of the fruit was demonstrated in our results. According to the following research, A. triloba pulp contains phenolic acid (caffeic acid, chlorogeinc acid, coumaric acid, ferulic acid), flavonols (hyeroside, isoquercitrin, quercetin, quercitrin), benzoic acid (ellagic acid, gallic acid), and catechins (catechin, epicatechin) and vitamin C [13]. These polyphenols are known to have excellent antioxidant properties, and studies have shown that they can help with anti-obesity [25]. Additionally, according to a recent study, the seeds of the A. triloba fruit have the effect of inhibiting lipogenesis in 3T3-L1 cells [26]. In our study did not contain seeds, its potent anti-lipid production effect and no cell toxicity was confirmed, so it is believed that A. triloba fruit useful in anti-obesity. The study’s limitations include that there may be other compounds that have not been studied in the pulp and peel of A. triloba fruit used in the experiment and there may be anti-obesity effects due to metabolites. Therefore, animal experiments and substance analysis will be conducted in future studies.
Based on these findings, we established that the optimal ethanol concentration for A. triloba fruit extraction was 70%, which demonstrated both the highest extraction yield and non-cytotoxic properties. AT exhibited dose-dependent inhibition of lipid accumulation in 3T3-L1 cells, as measured by Oil Red O staining. Moreover, treatment with AT effectively suppressed the expression of key adipogenesis markers, including PPARγ, SREBP1, and C/EBPα. These results suggest the potential of Asimina triloba fruit as a valuable natural source for the development of anti-obesity functional agents.
Availability of data and materials
The datasets used in this study are available from the corresponding author on reasonable request.
Abbreviations
- PPAR:
-
Peroxisome proliferator-activated receptor
- C/EBP:
-
CCTTA/enhancer-binding protein
- SREBP1:
-
Sterol regulatory element-binding protein 1
- 3T3-L1:
-
Murine preadipocytes
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- PBS:
-
Phosphate-buffered saline
- IBMX:
-
3-Isobutyl-1-methylxanthine
- FAS:
-
Fatty acid synthase
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- PVDF:
-
Polyvinylidene fluoride
- DC:
-
Detergent compatible
- ECL:
-
Enhanced chemiluminescence
- AT:
-
Asimina triloba Fruit 70% ethanolic extract
- p-AMPK:
-
Phospho-AMP-activated protein kinase
References
King D (2011) The future challenge of obesity. Lancet 378:743–744. https://doi.org/10.1016/S0140-6736(11)61261-0
Arcari DP, Santos JC, Gambero A, Ribeiro ML (2013) The in vitro and in vivo effects of yerba mate (Ilex paraguariensis) extract on adipogenesis. Food Chem 141:809–815. https://doi.org/10.1016/j.foodchem.2013.04.062
Tsai AG, Bessesen DH (2019) Obesity (Japanese version). Ann Intern Med 170:JITC33–JITC48. https://doi.org/10.7326/IsTranslatedFrom_AITC201903050_Japanese
Dietz WH (2011) Reversing the tide of obesity. Lancet 378:744–746. https://doi.org/10.1016/S0140-6736(11)61218-X
Liu H, Liu M, Jin Z, Yaqoob S, Zheng M, Cai D et al (2019) Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct 10:3603–3614. https://doi.org/10.1039/C9FO00027E
Rosen ED, Spiegelman BM (2000) Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16:145–171. https://doi.org/10.1146/annurev.cellbio.16.1.145
Lee J-E, Schmidt H, Lai B, Ge K (2019) Transcriptional and epigenomic regulation of adipogenesis. Mol Cell Biol 39:e00601-e618. https://doi.org/10.1128/MCB.00601-18
Farmer S (2005) Regulation of PPARγ activity during adipogenesis. Int J Obes 29:S13–S16. https://doi.org/10.1038/sj.ijo.0802907
Lefterova MI, Lazar MA (2009) New developments in adipogenesis. Trends Endocrinol Metab 20:107–114. https://doi.org/10.1016/j.tem.2008.11.005
Chen C-Y, Su C-W, Li X, Liu Y, Pan Q, Cao T et al (2021) Lipid extract from a vegetable (Sonchus Oleraceus) attenuates adipogenesis and high fat diet-induced obesity associated with AMPK activation. Front Nutr 8:624283. https://doi.org/10.3389/fnut.2021.624283
Lora J, Larranaga N, Hormaza JI (2018) Genetics and breeding of fruit crops in the Annonaceae family: Annona spp. and Asimina spp. Adv Plant Breed Strategies Fruits 3:651–672. https://doi.org/10.1007/978-3-319-91944-7_16
Brannan RG, Coyle MN (2021) Worldwide introduction of North American pawpaw (Asimina triloba): evidence based on scientific reports. Sustain Agric Res 10:19–24. https://doi.org/10.5539/sar.v10n3p19
Donno D, Beccaro GL, Mellano MG, Cerutti AK, Bounous G (2014) Chemical fingerprinting as nutraceutical quality differentiation tool in Asimina triloba L. fruit pulp at different ripening stages: an old species for new health needs. J Food Nutr Res 53:1
Nam JS, Park SY, Oh HJ, Jang HL, Rhee YH (2019) Phenolic profiles, antioxidant and antimicrobial activities of pawpaw pulp (Asimina triloba [L.] Dunal) at different ripening stages. J Food Sci 84:174–182. https://doi.org/10.1111/1750-3841.14414
McLaughlin JL (2008) Paw paw and cancer: annonaceous acetogenins from discovery to commercial products. J Nat Prod 71:1311–1321. https://doi.org/10.1021/np800191t
Nam JS, Park SY, Lee HJ, Lee SO, Jang HL, Rhee YH (2018) Correlation between acetogenin content and antiproliferative activity of pawpaw (Asimina triloba [L.] Dunal) fruit pulp grown in Korea. J Food Sci 83:1430–1435. https://doi.org/10.1111/1750-3841.14144
Nam JS, Jang HL, Rhee YH (2017) Antioxidant activities and phenolic compounds of several tissues of pawpaw (Asimina triloba [L.] Dunal) grown in Korea. J Food Sci 82:1827–1833. https://doi.org/10.1111/1750-3841.13806
Chae SY, Seo SG, Yang H, Yu JG, Suk SJ, Jung ES et al (2015) Anti-adipogenic effect of erucin in early stage of adipogenesis by regulating Ras activity in 3T3-L1 preadipocytes. J Funct Foods 19:700–709. https://doi.org/10.1016/j.jff.2015.09.060
de Ferranti S, Mozaffarian D (2008) The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 54:945–955. https://doi.org/10.1373/clinchem.2007.100156
Yang J-Y, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ et al (2008) Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci 82:1032–1039. https://doi.org/10.1016/j.lfs.2008.03.003
Morrison RF, Farmer SR (2000) Hormonal signaling and transcriptional control of adipocyte differentiation. J Nutr 130:3116S-S3121. https://doi.org/10.1093/jn/130.12.3116S
Ambati S, Yang JY, Rayalam S, Park HJ, Della-Fera MA, Baile CA (2009) Ajoene exerts potent effects in 3T3-L1 adipocytes by inhibiting adipogenesis and inducing apoptosis. Phytother Res Int J Devoted Pharmacol Toxicol Eval Nat Prod Derivat 23:513–518. https://doi.org/10.1002/ptr.2663
Hardie DG (2004) The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci 117:5479–5487. https://doi.org/10.1242/jcs.01540
Du J, Zhong B, Subbiah V, Barrow CJ, Dunshea FR, Suleria HA (2021) Lc-esi-qtof-ms/ms profiling and antioxidant activity of phenolics from custard apple fruit and by-products. Separations 8:62. https://doi.org/10.3390/separations8050062
Nani A, Murtaza B, Sayed Khan A, Khan NA, Hichami A (2021) Antioxidant and anti-inflammatory potential of polyphenols contained in Mediterranean diet in obesity: molecular mechanisms. Molecules 26:985. https://doi.org/10.3390/molecules26040985
Iobe H, Koike A, Takeda S, Watanabe K, Saito-Matsuzawa Y, Sone H et al (2023) Effects of pawpaw (Asimina triloba) seed extract on the differentiation and fat accumulation of 3T3-L1 cells under different glucose conditions. J Nutr Sci Vitaminol 69:53–61. https://doi.org/10.3177/jnsv.69.53
Acknowledgements
This study was supported by a research Grant from Korea Food Research Institute (No. E0210300) and the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MIST) (Nos. GA224600, 2022M3H9A1084670).
Funding
This study was supported by a research Grant from Korea Food Research Institute (No. E0210300) and the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MIST) (Nos. GA224600, 2022M3H9A1084670).
Author information
Authors and Affiliations
Contributions
Laboratory experiments: CJL, JH; Data analysis: CJL, YSK, SYC, GY; Wrote the manuscript: CJL, SYC; Designed and supervised the study: SYC, YSK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, C.J., Kim, YS., Hur, J. et al. Asimina triloba (pawpaw) fruit extract suppresses adipocyte differentiation and lipogenesis-related protein expression in 3T3-L1 cells. Appl Biol Chem 66, 75 (2023). https://doi.org/10.1186/s13765-023-00837-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-023-00837-8