Abstract
Terpenoids are of great interests in a broad range of health-beneficial biological activities and various industrial applications. In plants, terpenoids are synthesized by two distinct pathways, methylerythritol phosphate (MEP) and mevalonate pathways in a separate location. MEP pathway supplies isoprene precursors isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) of terpenoid biosynthesis in plant plastids. The MEP pathway has been an engineering target to increase the metabolic flux towards higher terpenoid production in plants. 1-Hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase (HDR) is the terminal step of the MEP pathway to regulate the terpenoid biosynthesis and is encoded by three paralogous genes in Ginkgo biloba. In this study, we assessed the effect of overexpression of GbHDR1 on terpenoid metabolism in poplar plants. Overexpression of GbHDR1 in poplar plants accelerated growth and delayed winter-bud formation. Transcript levels of gibberellin, chlorophylls, and carotenoid biosynthetic genes in GbHDR1-overexpressing (GbHDR1ox) poplars were up-regulated, suggesting metabolic flux enhancement. Moreover, enhanced contents of chlorophylls and carotenoids in the leaves of the GbHDR1ox plants resulted in a higher photosynthetic rate as a consequence. Therefore, we expect the GbHDR1 overexpression will be a desirable engineering point of the MEP pathway for enhancing terpenoid metabolic flux and production in plants.
Similar content being viewed by others
Introduction
Terpenoids, also known as isoprenoids, are the largest group of natural products comprising more than 50,000 known structures. They play an essential role not only in plant physiology by participating in photosynthesis and hormonal processes but also in plant ecology by mediating various plant-herbivore, plant-pollinator, and plant-pathogen interactions [1]. Besides their natural roles, plant-derived terpenes have been found applications in pharmaceuticals, cosmetics, and as starting materials for other valued chemicals [2, 3]. In addition, they have recently shown promise as advanced biofuels because the properties of terpene-based biofuels have great potential to replace current fossil fuels [4]. Such needs for terpenoids from industry prompted researchers to seek means to use living organisms as cell factories. The diverse structures of terpenoids originate through the myriad rearrangement pathways of linear precursors originating from the condensation of surprisingly simple isoprene precursor units, isopentenyl diphosphate (IPP), and its isomer dimethylallyl diphosphate (DMAPP) [5]. To produce two isoprene precursors, plants use two separate pathways, the methylerythritol phosphate (MEP) pathway and the mevalonic acid (MVA) pathway. The former path resides in plastids [6], and the latter in peroxisomes and the cytosol [7, 8] in plants. In addition to the different localization, the role of each pathway is also distinctive. In general, the MEP pathway supplies building blocks for the mono- (C10), di- (C20), and tetraterpenes (C40), whereas MVA pathway supplies building blocks for sesqui- (C15) and triterpenes (C30).
Ginkgo biloba is one of the most ancient gymnosperms still surviving in the world. A special standardized, and purified extract (EGb 761) of G. biloba leaves that contains diterpenoids, ginkgolides and bilobalide as principal active components has been widely used as a pharmaceutical or health food with its efficacy affirmed by comprehensive pharmacological and clinical investigations [9]. MEP pathway genes from G. biloba have been cloned and characterized [10,11,12,13,14,15]. Among them, GbDXS, GbCMK, and GbHDR occur as small gene families with 2 to 3 paralogous members. The MEP pathway steps catalyzed by the family of isozymes are suggested as the important regulation points bifurcating the pathway into primary and secondary metabolisms [12, 14, 15].
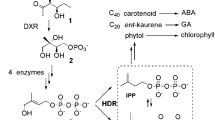
The plastidial MEP pathway synthesizes isoprene precursors more economically than the MVA pathway by utilizing the carbon source directly from the Calvin cycle. The HDR is responsible for concluding the MEP pathway by converting 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate into IPP and DMAPP (Fig. 1). Being the terminal enzyme in the MEP pathway, HDR was suggested as a key regulator of terpenoid precursor production in the MEP pathway [16, 17]. Kim et al. [15] advocated functional differentiation among HDR paralogs of G. biloba by the promoter study in Arabidopsis by demonstrating the organ-specific expression of HDR homologs [18].
Frequently, the productivity of the target terpene product in plants is very low and economic accumulation needs longer period of cultivation. However, the presence of two separate terpenoid biosynthesis pathways in plants, could provide an advantage over the microbial system to siphon isoprene precursors from one of the pathways without adversely affecting overall terpene-related pathways. Apparently, only one terpene precursor pathway in microbial systems demand highly extensive pathway manipulation to enable the microorganism withstand heavy metabolite diversion from normal isoprene precursor metabolism. Therefore, metabolic engineering of plants could provide more suitable production systems to utilize diverse terpene synthetic pathways to achieve high yield of the end-product terpenes. Terpene pathway-engineered plants, however, have seen few commercial successes to economically produce industry-relevant chemicals even though engineered microorganisms already have several lists of commercial successes [21].
In the present work, enhancement of terpenoid metabolic flux through the MEP pathway was successfully accomplished in poplar plants. Poplar is one of the most studied model plants and has been extensively used in plant metabolic engineering as promising bio-mass feedstock [19, 20]. To achieve this goal, we overexpressed one of the terminal enzymes, GbHDR1 from G. biloba in poplar, and the consequence of the approach was assessed in terms of downstream gene transcription. Focus was placed on genes involved in the biosynthesis of photosynthesis-related pigments and terpene-derived plant hormones. The up-regulation of genes in photosynthetic pigments and gibberellin (GA) biosynthesis positively affected the photosynthetic rate and biomass accumulation in the GbHDR1ox transgenic poplar plants.
Results
Enhanced growth performance in GbHDR1ox poplar plants
GbHDR1ox transgenic poplar seedlings, which were transferred from media plates into soil, showed rapid growth (Fig. 2A) compared with the wild type poplars (WT). At 8 weeks after potting, the height of GbHDR1ox poplars reached 24.6 cm, which is approximately 25% taller than of WT (19.6 cm) and had about 2 more leaves (16 versus 18.2 leaves for WT and GbHDR1ox) (Fig. 2B). All poplar plants were moved to the outdoor nursery after 8 weeks potting, and we regularly checked the phenotype of poplar plants. After 10 weeks of growing in the outdoor nursery, we found winter buds in the terminal shoots of all WT poplar plants (Additional file 1: Fig. S1A), but not in GbHDR1ox plants (Additional file 1: Fig. S1B). When the winter bud formed in all GbHDR1ox poplar plants, they were approximately 7% taller than WT (WT: 0.74 m versus GbHDR1ox: 0.79 m) (Additional file 1: Fig. S1C).
Increased terpenoid metabolic flux in GbHDR1ox poplar plants
We analyzed transcript levels of downstream genes relate to terpenoid metabolism in plastids such as isoprene, chlorophylls, carotenoids, and gibberellins (Fig. 3). Isoprene is one of the major terpene products in poplar leaves, and isoprene synthase (IS) is the only enzyme capable of isoprene synthesis that is believed to be emitted to cope with stress [22]. In GbHDR1ox transgenic poplars, a fivefold increase of IS transcript was observed (Fig. 3B). We also checked transcript levels of genes for photosynthetic pigments, chlorophylls and carotenoids. The transcript levels of chlorophyll synthesis-related enzymes, chlorophyll synthase (CHS) and chlorophyll a oxidase (CAO), as well as a key enzyme in carotenoid synthesis, phytoene synthase (PSY), were all up-regulated by 35%, 53% and 280% respectively. However, no statistical significances were found due to the high variations in transcript levels of CHS, CAO and PSY among the transgenic poplar plant lines (Fig. 3B). There were no transcript decreases of CHS, CAO and PSY in transgenic poplars plants. GAs are the diterpene-type plant hormones with a key intermediate of GA biosynthesis being ent-kaurene. In transgenic poplar plants, while the transcript levels of GA synthesizing and activating genes, ent-kaurene synthase (KS) and GA20-oxidase (GA20ox) increased by 170% and 85%, respectively, the level of GA2-oxidase (GA2ox), which inactivates GA, decreased by 40% (Fig. 3B).
Transcript levels of downstream terpene biosynthetic genes in poplar plants. A Overall scheme of terpene biosynthetic pathways and related enzymes, B comparison of transcript levels in WT (n = 3) and GbHDR1ox poplars (8 lines, n = 3 to 5). The white and black bar represents the WT and the transgenic plants, respectively. Error bar indicates standard deviation. The asterisk symbol indicates **P < 0.01 and *P < 0.05
Increased terpenoid photosynthetic pigments and photosynthetic rates
To verify the correlation of increased terpene-related transcript levels with the actual terpenoid contents in GbHDR1ox plants, we measured the contents of photosynthetic pigments, chlorophylls and carotenoids. Overexpression of GbHDR1 in poplar clearly resulted in enhanced chlorophylls and carotenoids contents accompanied by elevated transcript levels of related genes mentioned in the previous section. We found the enhancement of chlorophyll a and b contents by 24% and 40% (Fig. 4A), and carotenoid contents were enhanced by 20% in GbHDR1ox poplar plants compared with WT (Fig. 4B).
Plant pigment contents and photosynthetic rate of WT and GbHDR1ox transgenic poplar plants. Contents of A: chlorophylls and B: carotenoids, and C: comparison of photosynthetic rate in WT (n = 3) and GbHDR1ox poplar plants (5 lines, n = 3 to 5). Error bar represents standard deviation. The asterisk symbol indicates **P < 0.01 and *P < 0.05
As a result of increased photosynthetic pigment contents, the measured photosynthetic rates of the GbHDR1ox transgenic poplars under the given condition were higher by 30% compared to those of the WT plants (Fig. 4C).
Improvement of cell development in GbHDR1ox poplar plants
GA has been reported to have an effect on cell development in the secondary xylem in particular [23]. In order to confirm the hormonal effect in plant cell development, we performed phloroglucinol (PG) and berberine-aniline blue (BAB) staining in plant tissues. The section of the first stem internode of all the poplar samples had typical structure consisting of epidermis, cortex, annular vascular bundles, and pith (Fig. 5 and Additional file 1: Fig. S2). Vascular bundles contained primary phloem and lignified primary xylem with unlignified interfascicular cambium between the tissues. There was no significant difference in the development of lignified primary xylem among the samples (Fig. 5). However, lignified secondary xylem was enhanced in the middle internode section of GbHDR1ox poplars (Fig. 5). The lignified ring in the GbHDR1ox poplar is discontinuous, and this probably is the result of a local delay of cell wall lignification. Additionally, thick and dense secondary xylem was detected in the root base, which is a similar pattern observed in the middle internode. Furthermore, the BAB staining showed strong and extended signals in cross-section tissues in the GbHDR1ox transgenic poplars (Additional file 1: Fig. S2).
Phloroglucinol staining of lignified tissue in WT of GbHDR1ox transgenic poplar plants. The black arrowheads in the figures denote casparian bands. co, cortex; ep, epidermis; p, parenchyma; pi, pith; pp, primary phloem; px, primary xylem; sp, secondary xylem; sx, secondary xylem; vb, vascular bundles. Scale bar = 100 µm
Discussion
Despite the extensive application values of terpenoids, there are limitations in the stable and large-scale supply of terpenoids to meet all the demands of industry. Recent advances in metabolic engineering with the combination of synthetic biology and systems biology have enabled researchers to bring various cell factories [2, 4, 24], and several successful cases have been utilized in the biotech industry [25].
The increase of precursor supply is one of the effective strategies to achieve the flux enhancement for producing desired chemical compounds [20, 24, 26]. Overexpression of DXS, the first committing step in MEP pathway, has been frequently conducted to enhance the metabolic precursor pools for terpenoid production. This strategy, indeed, increased carbon flux and improved terpene production [27,28,29,30]. However, the following steps of the MEP pathway often hamper plant growth [31]. In the case of E. coli, overexpression of DXS causes retarded growth and poor terpenoid production, which is attributed to the depletion of DXS substrates, glyceraldehyde 3-phosphate (G3P) and pyruvate [32,33,34]. Additionally, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MECP), a MEP pathway intermediate, is known to be a limiting step due to its efflux from the E. coli cell. Activation of downstream MEP pathway enzyme reduced this efflux to effectively channel metabolite flux to the pathway end products, IPP and DMAPP [35]. Moreover, MECP was reported as a retrograde-signaling metabolite, and it induced stress-related genes in Arabidopsis [36]. In plants, removal of G3P from the Calvin cycle due to DXS-overexpression was also suggested to contribute to the unsatisfactory growth of the Arabidopsis transformant [27]. It is possible, however, that overexpression of HDR would not draw G3P directly from Calvin cycle as opposed to overexpression of DXS, and thus positively contribute to the pool of isoprene units without critically disturbing carbon flux in the Calvin cycle. In addition, enhanced photosynthetic pigment contents due to the increased pool of isoprene units, in turn, would boost the photosynthetic rate to offset the partial siphoning of G3P from the Calvin cycle to the MEP pathway. For instance, the co-expression of tomato HDR and taxadiene synthase in Arabidopsis increases carotenoid and taxadiene levels [37]. It was observed that increased HDR activity caused improved plastidial terpenoid biosynthesis in Arabidopsis [37]. Therefore, we considered that overexpression of HDR that is downstream of MECP is thus a more rational approach to increase MEP pathway carbon flux by circumventing other negative cellular responses.
Generally in gymnosperm, HDR is known to present as isozymes, and our previous study also identified that G. biloba has a small family of three HDRs [15]. In fact, each member of the HDR family has been suggested to have a distinct role in terpenoid biosynthesis. Class 1 HDRs, including GbHDR1are suggested to be involved in primary household metabolism whereas class 2 HDRs relate to secondary metabolism. Class 2 HDRs are expressed in both a developmental and spatial specific manner while no such specificities occur in class 1 HDRs (Additional file 1: Fig. S3) [15, 18, 38, 39]. Recently, we investigated the impact of class 2 GbHDR by overexpressing the GbHDR2 in tobacco plants and confirmed an increase of diterpenoid contents in GbHDR2ox tobacco plants [39]. Therefore, we expected that the GbHDR1 overexpression study would demonstrate not only the enhancement of carbon flux through the MEP pathway but also the specific functions of GbHDR1, that are related to primary metabolism.
The unavailability of Ginkgo transformation system to date justified the use of a heterologous plant system, which would lead to an evaluation of the application potential of GbHDR1 in plant metabolic engineering. The poplar tree is an ideal plant model system with various advantages such as fast growth and high biomass. Wood composition of the plants is especially desirable in engineering plants for fuel uses. [40]. In addition, the triploid poplar trees used in this study are free from biosafety issues because they prevent gene escape due to infertility. Furthermore, extensive poplar studies provide many accessible genetic tools and information to use the system straightforwardly for metabolic engineering research. Thus, we attempted to overexpress the GbHDR1 in poplar to acquire stable transgenic plants of GbHDR1ox. In the initial growth stage, the GbHDR1ox poplar plants exhibited rapid growth accompanied by increased height and leaf number compared with the WT plants (Fig. 2). GbHDR1ox poplar plants formed winter buds late compared to the WT poplars, which suggests high tolerance against low temperatures in GbHDR1ox poplars. Poplars temporally cease shoot growth to avoid permanent growth termination by forming winter buds to protect from low and freezing temperatures.
The enhanced growth of GbHDR1ox plants suggested the increased carbon flux towards several terpenoid metabolites such as isoprene, GA, chlorophylls and carotenoids. For example, an increase of IS transcript levels suggested increased C5 isoprene precursor pools in GbHDR1ox poplars (Fig. 3B). Additionally, up-regulation of IS transcription indirectly reflected enhanced isoprene emission from leaves. The emission of isoprene is known to help plants overcome abiotic stress such as drought and temperature by protecting photosynthesis [41]. This fact suggests that the leaves of GbHDR1ox poplar can be an excellent platform to produce volatile terpenoids even under various stress conditions.
Enhanced GA (diterpene) biosynthesis in GbHDR1ox poplars could promote plant elongation, and increased levels of photosynthetic pigments such as chlorophylls (diterpene side chain) and carotenoids (tetraterpene) would stimulate photosynthesis. We thus analyzed the transcript levels of key genes in the biosynthetic pathway of these terpenoid metabolites as illustrated in Fig. 3A. The data in the present study clearly indicate that overexpression of GbHDR1 in poplar brought about up-regulation of downstream genes in a feed-forward activation manner (Fig. 3A).
Wille et al. [42] suggested that the MEP pathway and the production of plastidial pigments have a significant degree of coordination at the gene expression level in a feed-forward manner. Thus, overexpression of DXS and HDR leads to increased levels of chlorophylls and carotenoids [27, 37]. The phytol side chain, a diterpene of MEP pathway origin, is an essential component of light-harvesting pigments, chlorophylls a and b, that anchors the pigments to the thylakoid membrane [43]. In the present study, overexpression of GbHDR1 in poplar evoked upregulation of CHS and CAO and also resulted in enhanced accumulation of chlorophylls a and b. Plant carotenoids derived from the MEP pathway play a crucial role in photosynthesis as accessory pigments [44]. In GbHDR1ox transgenic poplar plants, we observed increased transcription levels of GbPSY, a key enzyme in carotenoid biosynthesis, with an concomitant increase of carotenoid contents.
It is evident in the present experiment that, besides simple law of mass action, up-regulation of downstream genes was operating in the GbHDR1ox plants to effectively drain the accumulated MEP pathway of end-products. During deetiolation of tomatoes, strong upregulation of HDR is accompanied by increased transcription of PSY [37]. Therefore, enhanced carotenoid accumulation due to HDR overexpression in the present work resembles the PSY-activating mechanism in chloroplasts. The upregulation of chlorophyll and carotenoid biosynthetic genes in turn increased chlorophyll and carotenoid contents in transgenic plants. Such increase of photosynthetic pigment contents would enhance the photosynthesis rate. Indeed, overexpression of GbHDR1 led to elevated photosynthetic rates in the leaves of poplar (Fig. 4C).
Upregulation of KS and GA20ox transcription suggests elevated GA biosynthesis in the GbHDR1ox poplar plants (Fig. 3B). Because the biosynthesis of gibberellin is self-regulating, the effect of GbHDR1 overexpression on gibberellin-catabolizing GA2ox must be considered, as well [45]. In this regard, it is very indicative that GbHDR1 overexpression in poplar resulted in the upregulation of KS and GA20ox with concomitant GA2ox downregulation (Fig. 3B). The results from both plants thus strongly imply increased active GA content in the GbHDR1ox plants. An increase in bioactive GA level is known to promote plant growth [23] and the inhibition of growth cessation under short-day photoperiod in poplar trees [46]. Our data in GbHDR1ox poplars also displayed rapid growth and delayed terminal bud formation (Additional file 1: Fig. S1). Moreover, a previous GA20ox overexpression study in poplar proved that the increased bioactive GA level can improve the secondary growth in xylem fibers [23] and is consistent with our cross-section data of GbHDR1ox poplar that showed enhanced secondary xylem (Fig. 5 and Additional file 1: Fig. S2).
Increased photosynthesis and elevated GA levels are indicative of stem lengthening of stem in the GbHDRox1 plants. Recently, it was shown that the presence of the N-terminal region of the GbHDR1 enzyme is responsible for the shift of the optimal pH of the enzyme toward the basic side so the enzyme operates under alkaline condition of chloroplast stroma [47]. Therefore, GbHDR1 best functions while the photosynthetic apparatus is operating. The close relationship of the enzyme with primary metabolism, embodied by expedited growth as shown in the present study, is suggestive.
Overexpression of GbHDR1 would be an applicable strategy to boost accumulation of the target terpenes especially in various plant cell platforms. Plant system as a cell factory platform have many advantages for engineering the complicated plant terpene metabolic pathways [24, 26, 40]. Additionally, they use light for photosynthesis and no externally added sugars are required to synthesize secondary metabolites, which enable eco-friendly and sustainable way of plant origin chemical production. In particular, terpenoid production as fuels, which require vast amount of biomass, would be economically viable when using a plant system like the poplar cell factory.
Materials and methods
Plant materials and growth conditions
The non-flowering triploid poplar, Populus glandulosa BH1 (Populus alba × Populus tremula var. glandulosa BH1) was used in this study. Poplar seedlings were maintained in a culture room at 25 ± 2 °C under cool white fluorescent light for 16 h per day. The WT and transgenic poplars were moved from indoors to the outdoor nursery after 8 weeks of growing in pots, and they were grown in general outdoor conditions without any environmental protection.
Establishment of transgenic plants
A binary vector containing the kanamycin-resistance gene (pBI121, Clontech, USA) was used for the gene construction. The GbHDR1-coding region was amplified by PCR using primers designed to add XbaI and BamHI sites to the 5′ and 3′ ends, respectively (Additional file 1: Table S1). The amplicon was inserted into the pBI121 binary vector under the control of the cauliflower mosaic virus (CaMV) 35S promoter (Additional file 1: Fig. S4). The transformation vector was mobilized by freeze–thaw in Agrobacterium tumefaciens strains LBA4404 for poplar [48]. Poplar transformation was performed by co-cultivating the stem segments of the BH1 clone with LBA4404 strain carrying GbHDR1ox construct. The transformed calli were selected by kanamycin selection, and shoot and roots were regenerated in appropriate media with antibiotics before transferring to a 5-inch pot. The transgenic poplar plants were eventually transferred to the pots with sterilized potting mix and watered at 7-day intervals. The entire procedure for the establishment of transgenic poplars is briefly shown given with the information in Additional file 1: Fig. S5. To confirm the introduction of GbHDR1 in the transformants, PCR reaction analysis was performed with cDNA of GbHDR1 transgenic poplars. A band of about 1450 bp, the expected size of GbHDR1 ORF, was amplified from each GbHDR1ox transgenic poplar (Additional file 1: Fig. S4).
RNA extraction and quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from the leaves of the plants using the TRIzol (Life Technologies, USA) method [49]. The reverse transcriptase polymerase chain reaction (RT-PCR) was performed according to the protocol of Kim et al. [38]. To assess the transcript levels in poplar plants, PaIS (Populus alba x Populus tremula isoprene synthase), PtKS (Populus trichocarpa kaurene synthase), PtGA20ox (P. trichocarpa GA20 oxidase), PtGA2ox (P. trichocarpa GA2 oxidase), PtCHS (P. trichocarpa chlorophyll synthase), PtCAO (P. trichocarpa chlorophyll a oxygenase), and PgPSY (P. trichocarpa phytoene synthase) were used to design qRT-PCR primers (Additional file 1: Table S1). The qRT-PCR was carried out on a Rotor-Gene 2000 Real-Time Amplification System (Corbett Research, Australia) using the SYBR Green PCR system (Takara, Japan) according to the manufacturer’s protocol. The thermal cycling profile consisted of: stage 1, 95 °C for 15 min; stage 2, 95 °C for 10 s and 52 °C for 30 s. Stage 2 was repeated for 40 cycles. The relative expression level was quantified using P. trichocarpa ubiquitin (PtUBQ) as a reference.
Analyses of chlorophylls, carotenoids, and photosynthetic rate
The leaves from 5-week old poplar plantlets were collected and ground with liquid nitrogen. The macerated paste was extracted with 40 mL of 80% acetone at 4 °C by shaking in the dark for 15 min in a 50-mL Falcon tube. Then the tube was centrifuged at 5000 rpm for 15 min at 4 °C before the optical density value of the supernatant was measured at 470, 647, and 663 nm. The contents of the pigments were calculated according to the equation given by Lichtenthaler and Buschmann [50].
Gas exchange measurements were made on fully opened leaves with the portable photosynthesis system Li-Cor 6400 (Li-cor, USA) 10 weeks after potting for poplar, grown in a growth chamber with light intensity at 77.7 µmol m−2 s−1 (Li-cor 250A Light Meter). Light for the measurement was provided by a Red/Blue LED light source (Li-Cor 6400-02B). Leaves were equilibrated at a photon irradiance of 100 µmol quanta m−2 s−1 for 30 min. During the experimental run, leaf chamber CO2 concentration and humidity were maintained at 380 µmol mol−1 and 23 mmol mol−1, respectively, and leaf temperature was maintained at 23 °C. The samples were taken from five plants belonging to the same line, and a t-test was performed for statistical validation.
Histochemical analysis of poplar plants
Poplar cuttings with one bud were aseptically grown on wood plant media (WPM) in test tubes for 65 days until the stem length reached about 10 cm. The stem was sectioned under a dissecting microscope with a razor blade. For PG staining, sections were treated with one drop of concentrated HCl for 2–3 min followed by one drop of 5% phloroglucinol for 3–5 min. Lignified xylem walls appeared cherry red under a bright-field microscope (Leica DME with Nikon E5400 digital camera) [51]. BAB staining was carried out by adding one drop of 0.1% berberine hemisulfate to the section, which was allowed to stand for about 1 h followed by washing with water [52]. The staining was completed by immersion in one drop of 0.5% aniline blue for 30 min followed by washing with water. Lignified cell walls appeared as stagnant yellow, Casparian bands vivid yellow, and suberized cell walls brown on a fluorescent microscope (Olympus IX71 with excitation filter G 365 nm, absorption filter U-WB, dichromatic mirror DM500, compensation excitation filter BP450–480, and compensation absorption filter BA515). The photograph was taken with a digital camera RZ200C-21 (China).
Statistical analysis
The data obtained in this experiment were subject to statistical analysis. The student’s t-test was performed by using SAS (version 9.1). Statistical significances were denoted as * (P ≤ 0.05) and ** (P ≤ 0.01).
Conclusion
In conclusion, we established GbHDR1ox transgenic poplar plants. The GbHDR1 overexpression enabled improvement in terpenoid metabolic flux through the MEP pathway, which was supported by the up-regulation of transcript levels of downstream genes in terpene biosynthesis as well as increased terpenoid photosynthetic pigments. This study is noteworthy in terms of the functional study of gymnosperm HDR isozymes, and it also suggests an efficient way to boost terpenoid biosynthesis in plastids. Therefore, HDR is an ideal target for MEP pathway manipulation towards improved production of terpenoids and biomass in heterologous cell factories.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Tholl D (2015) Biosynthesis and Biological Functions of Terpenoids in Plants. In: Schrader J, Bohlmann J (eds) Biotechnology of Isoprenoids. Springer International Publishing, Cham, pp 63–106
Tippmann S et al (2013) From flavors and pharmaceuticals to advanced biofuels: production of isoprenoids in Saccharomyces cerevisiae. Biotechnol J 8(12):1435–1444
Leavell MD, McPhee DJ, Paddon CJ (2016) Developing fermentative terpenoid production for commercial usage. Curr Opin Biotechnol 37:114–119
Mewalal R et al (2016) Plant-derived terpenes: a feedstock for specialty biofuels. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2016.08.003
McGarvey DJ, Croteau R (1995) Terpenoid metabolism. Plant Cell 7(7):1015
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16(5):565–574
Sapir-Mir M et al (2008) Peroxisomal localization of Arabidopsis isopentenyl diphosphate isomerases suggests that part of the plant isoprenoid mevalonic acid pathway is compartmentalized to peroxisomes. Plant Physiol 148(3):1219–1228
Simkin AJ et al (2011) Peroxisomal localisation of the final steps of the mevalonic acid pathway in planta. Planta 234(5):903–914
Hori T et al (2012) Ginkgo Biloba A Global Treasure: From Biology to Medicine Science & Business Media. Springer, Berlin
Kim S-M et al (2005) Functional identification of Ginkgo biloba 1-deoxy-D-xylulose 5-phosphate synthase (DXS) gene by using Escherichia coli disruptants defective in DXS gene. J Appl Biol Chem 48(2):101–104
Kim S-M et al (2006) Cloning and characterization of 2-C-methyl-D-erythritol 2, 4-cyclodiphosphate synthase (MECS) gene from Ginkgo biloba. Plant Cell Rep 25(8):829–835
Kim S-M et al (2006) Identification of class 2 1-deoxy-D-xylulose 5-phosphate synthase and 1-deoxy-D-xylulose 5-phosphate reductoisomerase genes from Ginkgo biloba and their transcription in embryo culture with respect to ginkgolide biosynthesis. Planta Med 72(03):234–240
Kim S-M et al (2006) Cloning and functional characterization of 2-C-methyl-D-erythritol 4-phosphate cytidyltransferase (GbMECT) gene from Ginkgo biloba. Phytochem 67(14):1435–1441
Kim S-M et al (2008) Two copies of 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase (CMK) gene in Ginkgo biloba: molecular cloning and functional characterization. Planta 228(6):941–950
Kim S-M et al (2008) 1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase (IDS) is encoded by multicopy genes in gymnosperms Ginkgo biloba and Pinus taeda. Planta 227(2):287–298
Olofsson L et al (2011) Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol 11(1):1
Rodríguez-Concepción M et al (2012) Biosynthesis of isoprenoid precursors in Arabidopsis. Isoprenoid Synthesis in Plants and Microorganisms. Springer, pp 439–456
Kang M-K et al (2013) Distinct expression patterns of two Ginkgo biloba 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase/isopentenyl diphospahte synthase (HDR/IDS) promoters in Arabidopsis model. Plant Physiol Biochem 62:47–53
Sannigrahi P, Ragauskas AJ, Tuskan GA (2010) Poplar as a feedstock for biofuels: a review of compositional characteristics. Biofuels, Bioprod Biorefin 4(2):209–226
van Beilen JB (2008) Transgenic plant factories for the production of biopolymers and platform chemicals. Biofuels, Bioprod Biorefin 2(3):215–228
Chen Y et al (2015) Enabling technologies to advance microbial isoprenoid production. Adv Biochem Eng Biotechnol 148:143–160
Ghirardo A et al (2014) Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol 165(1):37–51
Eriksson ME et al (2000) Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat Biotech 18(7):784–788
Vickers CE et al (2014) Metabolic engineering of volatile isoprenoids in plants and microbes. Plant Cell Environ 37(8):1753–1775
Nielsen J, Keasling JD (2016) Engineering Cellular Metabolism. Cell 164(6):1185–1197
Yoon JM, Zhao L, Shanks JV (2013) Metabolic engineering with plants for a sustainable biobased economy. Annu Rev Chem Biomol Eng 4:211–237
Estévez JM et al (2001) 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276(25):22901–22909
Muñoz-Bertomeu J et al (2006) Up-regulation of 1-deoxy-D-xylulose-5-phosphate synthase enhances production of essential oils in transgenic spike lavender. Plant physiol 142(3):890–900
Simpson K et al (2016) Differential contribution of the first two enzymes of the MEP pathway to the supply of metabolic precursors for carotenoid and chlorophyll biosynthesis in carrot (Daucus carota). Front Plant Sci. https://doi.org/10.3389/fpls.2016.01344
Shi M et al (2016) Enhanced diterpene tanshinone accumulation and bioactivity of transgenic salvia miltiorrhiza hairy roots by pathway engineering. J Agri Food Chem 64(12):2523–2530
Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci 98(15):8915–8920
Farmer WR, Liao JC (2001) Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol Prog 17(1):57–61
Brown AC et al (2010) The nonmevalonate pathway of isoprenoid biosynthesis in Mycobacterium tuberculosis is essential and transcriptionally regulated by Dxs. J Bacteriol 192(9):2424–2433
Kim SW, Keasling J (2001) Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol Bioeng 72(4):408–415
Zhou K et al (2012) Metabolite profiling identified methylerythritol cyclodiphosphate efflux as a limiting step in microbial isoprenoid production. PLoS ONE 7(11):e47513
Xiao Y et al (2012) Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149(7):1525–1535
Botella-Pavía P et al (2004) Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 40(2):188–199
Kim Y-B et al (2009) Regulation of resin acid synthesis in Pinus densiflora by differential transcription of genes encoding multiple 1-deoxy-D-xylulose 5-phosphate synthase and 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase genes. Tree Physiol. https://doi.org/10.1093/treephys/tpp002
Kim YB et al (2021) Overexpression of Ginkgo biloba Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate reductase 2 gene (GbHDR2) in Nicotiana tabacum cv Xanthi. Biotech 11(7):337
Kempinski C et al (2015) Metabolic engineering of higher plants and algae for isoprenoid production. Biotechnology of Isoprenoids. Springer, pp 161–199
Ryan AC et al (2014) Isoprene emission protects photosynthesis but reduces plant productivity during drought in transgenic tobacco (Nicotiana tabacum) plants. New Phytol 201(1):205–216
Wille A et al (2004) Sparse graphical Gaussian modeling of the isoprenoid gene network in Arabidopsis thaliana. Genome Biol 5(11):1
Tanaka R, Tanaka A (2011) Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1807(8):968–976
Lu S, Li L (2008) Carotenoid metabolism: biosynthesis, regulation, and beyond. J Integr Plant Biol 50(7):778–785
Rieu I et al (2008) Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell 20(9):2420–2436
Eriksson ME et al (2015) Transgenic hybrid aspen trees with increased gibberellin (GA) concentrations suggest that GA acts in parallel with FLOWERING LOCUS T2 to control shoot elongation. New Phytol 205(3):1288–1295
Shin B-K, Ahn J-H, Han J (2015) N-Terminal region of GbIspH1, Ginkgo biloba IspH type 1, may be involved in the pH-dependent regulation of enzyme activity. Bioinorg Chem Appl. https://doi.org/10.1155/2015/241479
Weigel D, Glazebrook J (2006) Transformation of agrobacterium using the freeze-thaw method. CSH Protoc. https://doi.org/10.1101/pdb.prot4666
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:F4.3.1–F4.3.8
Liljegren S (2010) Phloroglucinol stain for lignin. CSH Protocols
Brundrett MC, Enstone DE, Peterson CA (1988) A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146(2):133–142
Acknowledgements
All authors would like to express special appreciation and thanks to Prof. Soo-Un Kim. Although he is no longer with us, his research achievements and dedication to science development will be remembered and respected.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant (2021R1A5A8029490); the Korea Foundation for Women In Science, Engineering and Technology (WISET) Returners into R&D Program grant; the intramural grant (2Z06670) from the Korea Institute of Science and Technology (KIST), Republic of Korea.
Author information
Authors and Affiliations
Contributions
M–KK, S-UK, and S-MK. designed the experiments. M–KK, J-YK, LH, CY, and ZJ. performed experiments. YIC. provided the poplar plants and supervised the management of transgenic poplar plants. YJP. helped with data analysis. M–KK, S-UK, and S-MK wrote the manuscript and all authors approved and commented on the manuscript. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There is no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1: Fig. S1
Winter bud formation and final height of poplar plants at growth cessation. After 2-month growing in outdoor nursery, winter bud was visible at the top apex in A WT poplar plants (n=3), but not in the B GbHDR1ox poplar plants (5 lines, n=3–5) and C: Final height after completion of winter buds both in WT and GbHDR1ox poplar plants. WT: Wild type, GbHDR1ox: GbHDR1ox transgenic poplars. The asterisk symbol indicates **P < 0.01 and *P < 0.05. Fig. S2 Berberine-aniline blue staining (BAB) of WT and GbHDR1ox transgenic poplar plants. co, cortex; ep, epidermis; p, parenchyma; pi, pith; pp, primary phloem; px, primary xylem; sp, secondary xylem; sx, secondary xylem; vb, vascular bundles. Scale bar = 100 µm. GbHDR1 Fig. S3 Phylogenetic tree of plant HDRs. MEGA 6.0 software was used for sequence alignment and phylogenetic analysis with neighbor joining algorithm and Bootstrap with 1000 replications. (Le: Lycopersicon esculentum, Ls: Lactuca sativa, St: Solanum tuberosum, At: Arabidopsis thaliana, Vv: Vitis vinifera, Pot: Populus trichocarpa, Ap: Adonis palaestina, Hb: Hevea brasiliensis, Sr: Stevia rebaudiana, Hv: Hordeum vulgare, Os: Oryza sativa, So: Saccharum officinarum, Pt: Pinus taeda, Pd: Pinus densiflora, Gb: Ginkgo biloba, Ps: Picea sitchensis). Fig. S4 Vector construction for GbHDR1ox transgenic plants (X: XbaI, B: BamHI). Direct PCR amplification of GbHDR1 ORF sequence in the transgenic poplar plants, respectively. All lines carried GbHDR1 gene. (M: maker, WT: wild type, GbHDR1ox: GbHDR1ox transgenic poplars). Fig. S5 Scheme for the GbHDR1ox poplar regeneration procedures. CIM1 (callus induction medium 1): MS medium with 10 mM 2,4-D, 1.0 mM NAA, 0.1 mM BAP, sucrose (20 g/L), agar (15 g/L), and antibiotics (50 mg/L kanamycin and 100 mg/L cefotaxim) CIM2: CIM1 without antibiotics, SIM (stem induction medium): 10 mM Zeatin, 1.0 mM NAA, 0.1 mM BAP, sucrose and antibiotics, RIM (root induction medium):1/2 MS medium with agar and antibiotics. Table S1. Primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, MK., Kim, JY., Choi, YI. et al. Enhanced metabolic flux of methylerythritol phosphate (MEP) pathway by overexpression of Ginkgo biloba 1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate Reductase 1 (GbHDR1) gene in poplar. Appl Biol Chem 65, 50 (2022). https://doi.org/10.1186/s13765-022-00718-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00718-6