Abstract
Diabetes kidney damage (DKD) is a chronic inflammatory disease of the kidney induced with continuous hyperglycemia as the most prevalent consequence of diabetes. Washingtonia filifera seed oil (WFO) was used as a traditional medicine to cure various diseases in ancient Saudi. This work was carried out to investigate the potential protective impact of WFO against DKD on streptozotocin (STZ)-induced type 2 diabetic mice (C57BL/6 mice). The mice were randomly split into groups: C, C + WFO (200 mg/Kg B.W.), T2D, and T2D + WFO (200 mg/Kg B.W.). Diabetes was created in mice groups except for the control group after 6 weeks of high-fat diet (HFD) feeding. Treatments with STZ (60 mg/kg body weight) were administered three times for 6 weeks, and after that, mice were sacrificed. Kidney tissues and serum were obtained to analyze levels of insulin, metabolism of lipids [triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and free fatty acids (FFA)], antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)], creatine, and blood urea nitrogen (BUN). In addition, H&E staining had been used to investigate the histological changes of the kidneys. In T2D mice, WFO corrected aberrant serum lipids (TG, TC, HDL, LDL, and FFA), elevated antioxidative enzyme levels (CAT, SOD, and GPx), and inhibited GST to various degrees. In addition, WFO improves kidney pathological traits such as fibrosis of the kidney, hypertrophy of glomeruli, and basement membrane thickness of glomeruli. Through hypoglycemic, hypolipidemic, antioxidative, and anti-inflammatory actions, WFO might ameliorate diabetic alterations in T2D mice. WFO could significantly reduce AGE buildup in the T2D mice kidneys, therefore alleviating kidney oxidative stress and inflammatory kidney damage.
Similar content being viewed by others
Introduction
The fruit of the California fan palm (Washingtonia filifera, Family: Arecaceae) is considered underutilized. The genus Washingtonia consists of two species endemic to the western coast of Baja California, Mexico, and the United States [1,2,3]. The phytochemical profile of W. filifera fruit has shown its nutritional values. The fruit is rich in soluble sugars, carbohydrates, and minerals (i.e., phosphorus, potassium, calcium, magnesium, and zinc). In addition, W. filifera fruits are rich in bioactive constituents with antifungal, antioxidant, antibacterial, and anti-inflammatory traits. Furthermore, unlike the other palm species, the W. filifera tree is resistant to red palm weevil, encouraging its cultivation as a potential food source [4,5,6].
The nutritional value of W. filifera has been investigated, and findings indicated that W. filifera is an essential dietary staple [7]. Antioxidant properties of W. filifera extracts were studied, and novel bioactive compounds were discovered [6, 8]. The chemical profile and antioxidant traits of extracts from W. filifera leaves and flowers were investigated. Among extracts and volatile fractions, butanol and ethanol extracts from W. filifera leaves and flowers were the most powerful [3, 9,10,11].
In recent years, the quest for novel sources of innovative oils has been required. W. filifera seed oil contains high essential fatty acids and tocols [12, 13]. It also has a yellower hue than other vegetable oils and aids in protecting cells from UV radiation. Based on these and other good physicochemical properties, the seed oil used in the cosmetic and sectors of food may support it. However, to the best of our knowledge, W. filifera seed oil's potency is never investigated yet.
Inhibition of oxidative stress response has become a targeted treatment for DKD [14, 15]. Hyperglycemia and mitochondrial reactive oxygen species (ROS) overproduction have been shown as critical factors associated with the development of diabetic nephropathy. It has been reported that cold‐pressed Rosmarinus officinalis oil enhanced the redox status of glutathione in liver and brain mitochondria from streptozotocin (STZ)-induced diabetic animals and their offspring [16]. Diabetic animals exhibited augmented lipid peroxidation and depletion of reduced glutathione, while increased ROS generation was noted [17]. It has been shown that avocado oil ameliorated those defects and augmented the mitochondrial level of oleic acid [18].
The end-stage of renal disease (ESRD) is due to diabetic nephropathy in the US. Animal models of T2D provide a testing mechanism containing direct kidney tissue inspection to detect pathology [16]. Rats with abnormalities producing T2D are hard to produce, and the genetic abnormality in most of them has not yet been described [19, 20], hyperinsulinemia, hyperglycemia, hyperlipidemia, obesity, spontaneous proteinuria, and focal segmental glomerulosclerosis renal lesions (FSGS) leading to renal failure. The occurrence of substantial non-diabetic kidney abnormalities such as renal abscesses, hydronephrosis, pyelonephritis, and granulomas restricts the model's use in investigating diabetic nephropathy [19]. T2D is a metabolic anomalies, which is dysfunction of lipid and hyperglycemia are common symptoms, which have inflammation and oxidative stress. Furthermore, hyperglycemia might result in various diabetes problems [16, 21]. DKD is the most frequent consequence of T2D, and it affects ca. 35% of all people with diabetes [22]. DKD is a kidney disease with a chronic inflammation characterized by hyperglycemia, with the primary histopathological characteristics being hypertrophy of glomerulus, thickening of the basement membrane of a glomerulus, enlargement of glomerular mesangial, and fibrosis of kidney tissue [23, 24]. Hyperglycemia causes an excess of ROS, resulting in oxidative stress, which causes the formation of advanced glycation end products [25]. As a result, SIRT1 protects the kidneys by reducing oxidative stress, alleviating inflammation, and controlling lipid metabolism disorders [26, 27]. Furthermore, SIRT1 increases lipid catabolism via activation of adenosine monophosphate-activated kinase, the primary governor of metabolism [28]. In addition, AMPK/SIRT1 decreased adipogenesis by decreasing the production of sterol regulatory element-binding proteins (SREBPs), therefore reducing renal inflammation [29].
Our study aimed to highlight the chemical profile of WFO implanted in Saudi Arabia, determine its tocols profile, and investigate the anticipated protective impact of WFO towards kidney defects of diabetic mice. To the best of knowledge, this was the first investigation to study the effects of WFO on DKD in T2D mice.
Materials and methods
Collecting of W. filifera seeds
W. filifera ripen fruits were collected (December 2020) from W. filifera trees in El-Jamum city (Makkah, KSA), latitude 21°32′ N, and longitude 39°94′E. The seeds were insulated then picked up to wipe out damaging ones. Seeds were range-dehydrated for 24 h at 60 °C. The dehydrated seeds were milled, then sieved using a 1 mm fit sieve and preserved at − 15 °C.
Analysis of W. filifera seed oil
The seed oil was extracted with n-hexane using a Soxhlet extractor at 60 °C for 8 h. The mean ± standard deviation (± S.D.) expressed the values [30].
Fatty acid composition
To determine the composition of fatty acid methyl esters (FAME), 1 mL of n-hexane was added to 40 mg of WFO, then 200 mL of sodium methoxide was added then the mixture was heated in a bath for 1 min at 50 °C before being treated with 200 μL HCl (2 N). FAME peaks were obtained by injecting the upper layer (1 μL) in a GC (Agilent 6890 N, UK). FAME standard mixture was obtained from Sigma Chemical Co. (St. Louis, USA).
Tocols composition
A 0.5 g oil was diluted using 5 mL n-hexane prior to the HPLC analysis, and 5 μL samples were injected. HPLC was used to determine the tocol component of WFO following ISO 9936. The HPLC (Agilent 1100, CA, USA) is equipped with a G1313A standard autosampler, a G1354 quaternary pump, a G1321A fluorescence detector (set at excitation = 295 nm, and emission = 330 nm), and Chem station software was used for analyses. The mobile phase was n-hexane: isopropanol (99.5: 0.5, v/v) in a normal phase column (Pinnacle II silica, 3 μm, and 150 mm × 3.2 mm). The HPLC system was iso-statically operated at a 0.5 mL/min flow rate. The separations were carried out at 30 °C. α-, β-, γ-, and δ-Tocopherol standards (Sigma Chemical Co., St. Louis, USA) were used to make the mixed tocopherol standards in n-hexane (2 mg/mL). Tocotrienol peaks in WFO were detected by comparing tocols chromatograms of palm oil and coconut oil produced under similar conditions.
Animal experiments
Male mice (C57BL/6 mice, 12 weeks old, weight= 25 g) were acquired from Umm Al-Qura University's Experimental Animal Center (Makkah, KSA). Animals were kept in groups in a temperature-controlled (22°C), with a 12/12 h light/dark, and humidity of 50 ± 8%. All animals were reared under a specific clean area and had unrestricted access to water and food. All experimental methods comply with the experimental animal ethical standards suggested by the Committee of Animal Care and Use (Umm Al-Qura University, Makkah, KSA). All animals were assigned numbers at random, and each mouse’s food, drink, and body weight (B.W.) were recorded every other day through the experiment.
Induction of diabetes type 2
Following a week of confinement, the animals were haphazardly divided. For the treatment plan, three groups of animals (n = 10) were provided the exact regular doses for 6 weeks:
-
(1)
Control group (C), receiving normal saline, diet, and water.
-
(2)
Control + WFO (200 mg/kg B.W.) group.
-
(3)
T2D + HFD group, fed high fat chow comprised of basic food 48%, lard oil 26.5%, sucrose 14%, milk powder 5%, yolk powder 5.5%, 1% vitamin mixture, and sodium cholate 0.5% [31]. Following feeding 6 weeks of HFD, animals were intraperitoneally injected with a single dose of streptozotocin (STZ, 60 mg/kg prepared freshly in 0.1 M citrate buffer) every other day for a total of three times. The animals were fasted overnight prior to STZ injection. Diabetes was diagnosed 72 h after the last STZ injection in animals with fasting blood glucose (FBG) of 11.1 mmol/L.
-
(4)
T2D + WFO (200 mg/kg B.W.) group, available to HFD, with access to food and water. According to the general brain and psychological conditions of the WFO-treated mice, no side changes were reported.
Insulin sensitivity (IST) and oral glucose tolerance (OGTT)
All animals were subjected to OGTT and IST after a 10 h fasting [31, 32]. Animals received either an intraperitoneal administration of insulin (0.75 U/kg) or glucose solution by oral (2.0 g/kg body weight). For OGTT and IST, blood samples were obtained from the ends of the mouse tails at 0, 30, 60, 90, and 120 min to analyze blood sugar levels after glucose supplementation or i.p injection of insulin. The area under the curve (AUC) for IST and OGTT was calculated using the trapezoidal rule.
Kidney tissue and serum
All animals were slaughtered after the last WFO dosage. Blood samples were obtained and centrifuged at 4000 rpm (10 °C) for 10 min to recover serum, then stored at − 80 °C for further assessment. Kidneys were promptly removed, weighed, and cleaned with ice-cold saline. One of two kidneys has been homogenized in ice-cold phosphate buffer (pH 7.4) and stored at − 80 °C for further examination. The kidney was fixed in 4% formaldehyde for histopathological examination and then embedded in wax.
Biochemical analysis
During the WFO treatment, mice FBG was measured weekly with a glucometer (Roche, Germany). Urine has also been obtained weekly, one day after every treatment, and overall urinary protein content was calculated using a commercially available kit. According to the manufacturers' instructions, blood insulin and glycation (AGE) were measured using commercial kits enzyme-linked assay (ELISA). The Guerra et al. [33] equation was used to construct the homeostasis model evaluation of β-cell function (HOMA-).
High-density lipoprotein (HDL), low-density lipoprotein (LDL), blood urea nitrogen (BUN), serum triglyceride (TG), total cholesterol (TC), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-S-transferase, and levels of creatine were measured using kits obtained from Rawabi Marketing International (RMI, Riyadh, Kingdom of Saudi Arabia). In addition, total urine protein concentrations were determined. Based on the manufacturer’s specifications, all these indications were calculated.
Histology investigation
For pathological examination, tissues of the kidney were infiltrated in 4% formaldehyde for 2 days before being fixed in paraffin and sliced into 5 μm thick slices. Next, the slices were dewaxed and dehydrated for morphological examination before being stained with H&E [34]. Finally, an optical microscope was examined for stained tissues (Koninklijke Philips).
Comet assay
The specimens were homogenized in buffer (pH 7.5), and 6 mL of the homogenate was suspended on low melting agarose (0.5%) on fully frosted slides, which were kept on ice during the polymerization. The slides were placed in a lyses solution. After the 0.5% agarose layer solidified, the slides were placed in electrophoresis (1 mM Na2EDTA, 0.3 M NaOH, pH = 13) buffer for 10 min at 0 °C to allow DNA to unwind. The slides were stained with 20 g/mL ethidium-bromide after being neutralized with tris-buffer at pH 7.5. The epifluorescence microscope (Germany) was used to examine each slide. The automatic digital analysis system analyzed 100 cells on each slide. Tail moment = tail length × % DNA in the tail. Image analysis software measures tail length and intensity [35].
Statistical analysis
Graph Prism 9.0 was used to analyze the data from 3 multiple different trials (GraphPad Software Inc., USA). The data are presented in the form of mean and standard deviation (S.D.). One-way ANOVA was utilized for the statistics, accompanied by Tukey’s post hoc test. The significance level was set at p < 0.05.
Results
Fatty acids and tocols profile of WFO
The fatty acid profile of WFO contained 18 fatty acids. The main fatty acids were oleic C18:1 (40.6%), lauric (17.8%), linoleic (16.2%), myristic (11.4%), palmitic (9.23%), and stearic (2.34%), which accounted for ca. 97.4% of total fatty acids detected in WFO. Because of the high concentration of oleic and lauric acids, WFO is classified as an oleic/lauric oil. It may have similar properties as Degletnour date seed oil [36]. WFO contains 58.1% of total unsaturated fatty acids. It can affect the membrane's physical characteristics, such as permeability and fluidity [37]. Oleic acid has a vital function in the formation of nerve cells. The body may convert it into chemicals like prostaglandins, which play an essential role in vascular function and blood clotting. Furthermore, it could help to avoid cardiovascular disease. On the other hand, linoleic acid is required to properly develop human skin [38]. The organism can convert it to a sequence of long-chain fatty acids that serve as a precursor of eicosanoids [37]. WFO has a total saturated fatty acid content of 41.6%, making it resistant to oxidative rancidity [39]. Furthermore, due to their 5α-reductase inhibitory action, lauric and myristic acids have been linked to a reduced risk of developing prostatic hyperplasia [40,41,42,43]. The current fatty acid content of WFO makes it nutritionally acceptable, and it might be utilized as an edible salad oil, cooking oil, frying oil, or margarine production.
Table 1 presents the tocopherol and tocotrienol contents of WFO. The main tocols were γ-tocotrienol (97.8 mg/100 g), 18.4 mg/100 g for δ-tocotrienol, and 12.7 mg/100 g for α-tocotrienol. γ-Tocotrienol accounts for 73.3% of total tocols, followed by δ-tocotrienol (12.6%) and α-tocotrienol (9.30%), which account for 95.2% of total tocols. WFO, like palm oil, is a good source of γ-tocotrienol [44]. WFO has 130 mg/100 g of tocols, similar to black raspberry oil (142 mg/100 g) [45].
Effect of WFO on serum insulin and metabolic parameters in T2D mice
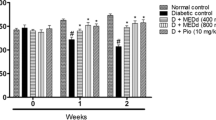
The body weight (BW) of animals was recorded weekly throughout the experiment (Fig. 1A). After 5 weeks of HFD feeding, the BW notably (p < 0.05) increased compared to the regular diet (C group). On the other hand, no significant differences in the pretreatment BW across the HFD groups were recorded. After the STZ diabetic induction, the BW of the T2D animals decreased rapidly compared with the steady increase in BW of the C group. The WFO intervention retarded the body's weight loss. Meanwhile, at the end of the experiment, the BW of WFO-treated mice was significantly (p < 0.05) more remarkable than that in the T2D group, suggesting a potential impact of WFO on retarding BW loss of T2D animals.
Compared to the C group, HFD combined with STZ resulted in a significant increase in FBG level in T2D animals, wherein FBG levels remained high till the end of the study (Fig. 1B), which is considered an indicator of diabetes [46]. However, during the first three weeks of WFO treatment, the FBG level decreased slightly. Meanwhile, after 6 weeks of WFO treatment, the FBG level decreased by 35.7% (from 22.7 ± 2.35 to 12.2 ± 2.12 mmol/L), compared to the T2D group (18.6 ± 1.59 mmol/L), indicating a strong hypoglycemic effect of WFO.
Effects of WFO on insulin sensitivity and glucose tolerance in T2D animals
To evaluate the health-promoting effects of WFO on the diabetic animals, the levels of HOMA-β and HOMA-IR were evaluated, and IST and OGTT assays were carried out. Compared with the T2D group, the HOMA-IR level in the WFO group were notably (p < 0.0001; Fig. 2A) decreased. Besides, relative to the T2D group, the mice in the WFO group possessed significantly (p < 0.0001; Fig. 2B) increased HOMA-β value. The results highlighted that WFO ameliorates insulin resistance in T2D animals to varying levels. Furthermore, the values of OGTT and IST are exhibited in Fig. 2C, D. The animals in the T2D group exhibited a greater glycemia response to glucose administration than the C group. Meanwhile, compared to T2D animals, WFO-treated animals exhibited prominently (p < 0.01; Fig. 2E) lower glycemia responses to blood glucose during the observation time (120 min). The data indicated that WFO possesses the potential to ameliorate glucose metabolic disorder in diabetic animals, reducing risk factors for kidney damage.
Effects of WFO on insulin sensitivity and glucose tolerance in T2D animals at week 13. A Calculations of HOMA-IR, B calculations of HOMA-β, C area under the curve (AUC) calculations for OGTT, D IST, E oral glucose tolerance test (OGTT), and F insulin sensitivity test (IST). Data expressed as mean ± S.D. (n = 8–10), *p < 0.05, ***p < 0.001, ****p < 0.0001 versus T2D; #p < 0.05, ##p < 0.01 versus WFO
Effect of WFO on serum lipids in T2D animals
Abnormal lipid metabolism is one of the main features of T2D. Compared with the C group, a notable increase in serum TC, TG, FFA, and LDL levels was recorded in T2D animals, while the HDL level was significantly (p < 0.01, Fig. 3A–D). The results suggested prominent dyslipidemia of T2D animals as a side effect of HFD and STZ. 6 weeks of WFO treatment, significant (p < 0.05) reversed changes in TC, TG, LDL, HDL and FFA by 25.0%, 19.5%, 28.3%, 28.1% and 33.5%, respectively were recorded.
Effect of WFO on antioxidant enzymes in T2D mice
The values of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidases (GPx), were markedly (p < 0.01, Fig. 4A–C) decreased in the T2D group compared with the C group. However, feeding WFO increased the SOD, CAT, and GPx values by 15.8% (p < 0.05), 20.5% (p > 0.05) and 14.1% (p < 0.01), respectively. Glutathione-S-transferase activity increased in the diabetic mice compared with control or WFO-treated groups. Blood urea nitrogen in serum, total urinary protein, and serum creatine was significantly (p < 0.001) increased in the T2D mice compared with the C group (Fig. 4E–G). However, WFO treatment reverses these states by various degrees.
Effects of WFO on serum lipid in T2D mice. A CAT, B SOD, C GPx, D GST activity (U/g tissue), E Blood urea nitrogen (mmol/L) in serum, F Total Urinary protein (mg/L), and G Serum creatine (mg/L). Data expressed as mean ± S.D. (n = 8–10), *p < 0.05, **p < 0.01 versus T2D; #p < 0.05, ##p < 0.01 versus WFO
Effect of WFO on histopathological renal structure in T2D mice
The kidney sections were stained with H&E to test the glomeruli cell population and histology, as glomerular endothelial cell plays an essential role in maintaining glomerular filtration function [19]. In the T2D diabetic group, the proportion of sclerosed glomeruli was considerably more significant. T2D diabetic animals were the most impacted, with much higher matrix widening. According to the findings (Fig. 5), the kidneys in the T2D group showed apparent destructive alterations, such as expanded glomerular volume, necrosis, and exfoliation in mesangial cells, compared to the C group. However, following WFO treatment, glomerular enlargement was significantly reduced, volume returned to normal, mesangial cell proliferation was reduced, and tubular epithelial cells were partially recovered. The mice in the WFO group had lower (p < 0.05, Fig. 5) kidney mass and glomerular area than in the T2D mice. By comparison to the T2D group, although no substantial (p > 0.05) difference was seen, WFO-treated animals had significantly less glomerular hypertrophy (3146.0 ± 145.2 μm2) than T2D mice against 3730.3 ± 207.09 μm2 for T2D. Furthermore, compared to the T2D group, WFO significantly reversed the decline in glomerular cell number.
Impact of WFO on kidney histology of T2D animals. A Photomicrograph of histological section staining with H&E of the kidney in control group (c-c2), control + WFO group (c + w1-c + w3), diabetic group (D-D5), and T2D + WFO group (w-w5). B Kidney weight/body weight (%) measured after administration of WFO. C Comparison of the glomerular cells number/glomerulus D Glomerular area among groups (average of 30 glomeruli/animal). Data expressed as mean ± S.D. (n = 8–10), *p < 0.05
Comet Assay of T2D mice kidney tissue
Comet Assay is a gel electrophoresis-based approach for measuring DNA injury in kidney cells. It is adaptable, responsive, and simple to perform. Although many studies utilize it to identify DNA single-strand break, variations to the approach allow detection of DNA double-strand break, base damage, crosslinks, and apoptotic nuclei. The sensitivity limit is about 50 strand breaks per diploid mammalian cell. The comet assay was initially designed to determine DNA damage and repair capacity variation within mammalian cells; this treatment may be finished within 24 h [35]. The kidney DNA showed apparent damage in kidney cells under the stress of diabetes in T2D-treated animals. On the other side, WFO-treated animals showed less damage (Fig. 6A, C, T2D, T2D + WFO).
Discussion
Washingtonia filifera is rich in antioxidants and bioactive compounds. The chemical composition and antioxidant properties of W. filifera leaf and flower extracts were investigated based on the DPPH· radical scavenging assay. Among W. filifera extracts and volatile fractions, butanol and ethanol extracts from leaves and flowers were the highest, indicating that they might be used as a source of bioactives with health-protective traits [47]. The extracts of W. filifera leaves and flowers contain flavonoids, which have a variety of health benefits, including anti-diabetic, anti-allergic, anti-inflammatory, and antiplatelet impact [48,49,50]. Phytochemical analysis of Washingtonia species discovered proteins, lipids, leucoanthocyanins, C-glycosylflavones, and flavonols [5, 51]. While flavonoid sulfates are not extensively dispersed in the plant kingdom, they are found in many Palmae species, particularly in major palm taxa like Washingtonia [52]. In addition, W. filifera sulfated flavonoids have been shown to have antibacterial action, and the antioxidant activities of W. filifera extracts were assessed, wherein two new flavonoids were found and extracted [5, 8].
Tocotrienols and tocopherols (tocols) contain vitamin E activity that makes them essential for health in terms of antioxidant qualities [53,54,55,56,57,58,59]. Vitamin E deficiency impairs nervous system development in youngsters and hemolysis [60]. Furthermore, those who do not consume enough vitamin E might be at a higher risk of developing atherosclerosis [61]. The preceding work was the first to look at the impact of WFO on renal damage in T2D animals and its protective mechanism. Findings suggested that WFO might reduce kidney damage in STZ-induced T2D mice. Several studies highlighted the impact and mechanisms of tocols and fat-soluble bioactives on STZ-induced T2D animals [16, 17, 55].
Diabetes induction with HFD paired with STZ is a well-known experimental model for T2D research [62]. A 6-week HFD combined with STZ caused polydipsia, hyperglycemia, weight loss in mice, and decreased glucose tolerance, indicating the effective development of T2D [51]. Besides, the T2D animals demonstrated insulin resistance, kidney structural damage, aberrant lipid metabolism, and renal dysfunction. All hallmarks of diabetic kidney disease, implying the development of DKD in STZ/HFD-induced T2D animals [21]. The WFO supplementation improved the physical metabolic indices of T2D animals. To varying degrees, WFO therapy enhanced the values of FBG, HOMA-IR, HOMA-β, glucose tolerance, and insulin sensitivity of T2D animals.
Our findings suggested that WFO can ameliorate HFD/STZ-induced diabetes. As a result, we hypothesize that the possible impact of WFO on diabetic nephropathy was due to its high quantities of tocols, phenolic compounds, and fat-soluble bioactives, which supports earlier research [30] and [63, 64], who discovered that phenolics could effectively alleviate and prevent diabetic kidney damage in vivo.
Long-term excessive fat consumption can induce nephrotoxicity, directly expressed as lipid ectopic deposition, dyslipidemia, and insulin resistance, accompanied by an inflammatory response, oxidative stress, and eventually pathological kidney injury and renal failure [65].
Consistent with earlier research, our findings indicated that STZ/HFD caused hypertriglyceridemia in T2D mice, as well as aberrant serum TG, TC, FFA, LDL, and HDL levels, all of which are critical contributors to dyslipidemia. WFO treatment, on the other hand, effectively restored the aberrant levels of blood lipids (i.e., FFA, TG, TC, LDL, and HDL). In addition, dyslipidemia may stimulate the generation of ROS, resulting in inflammation and oxidative stress [66].
The levels of antioxidant enzymes (including CAT, SOD, and GPx) in T2D mice were significantly lower, indicating a reduction in antioxidant capacity and a redox imbalance caused by oxidative stress in T2D animals. However, WFO therapy boosted the activities of CAT, SOD, and GPx. Furthermore, glutathione-S-transferase activity increased in the diabetic group compared with control or WFO-treated groups. In addition, blood urea nitrogen in serum, total urinary protein, and serum creatine were increased in the T2D animals compared with the C group. However, WFO treatment reverses these states by various degrees. As previously noted, the breakdown of the glomerular basement membrane (GBM) is a critical pathological character of diabetic kidney disease [23].
Podocytes are glomerular cells found on the lateral side of GBM. The podocytes' split diaphragm connects the cells to establish a barrier toward protein loss [67]. Podocin is a slit diaphragm protein that plays a vital function in the slit diaphragm's integrity [67]. Our findings show that WFO reduces renal dysfunction in T2D mice, related to the protective impact on glomerular podocytes. The sustained hyperglycemia may promote the buildup of AGE in the kidney, a necessary etiological and pathogenic process in DKD [68]. AGE was found to be significantly accumulated in the kidneys of T2D mice, accompanied by histopathological changes (thickening of glomerular basement membranes and glomerular hypertrophy), increases in blood urine nitrogen, serum creatinine, and urinary protein indicating renal dysfunction and glomerular structure damage. Furthermore, the findings revealed that AGE was linked to the pathogenic processes of DKD [69].
By attaching to its receptor (RAGE), AGEs induce various cellular responses, including inflammation, matrix formation, and fibrogenesis [70]. AGE is a significant generator of oxidative radicals [71]. AGE may cause an increase in the generation of ROS, lowering the expression of antioxidative enzymes such as SOD2, HO-1, and GPx, which are implicated in diabetic kidney injury [72]. When WFO-treated animals were compared to T2D mice, there was an increase in the expression of CAT, SOD2, and GPx. According to these findings, WFO effectively controlled oxidative stress in diabetic animals. Nrf2 modulates antioxidant defense system enzymes such as SOD2, GPx, and CAT, all of which are major downstream targets of Nrf2 and have considerable anti-inflammatory and antioxidant characteristics [73, 74]. Our results supported that Nrf2 modulated renal oxidative stress in STZ/HFD-induced T2D animals [75]. The treatment with WFO significantly raised the decreased Nrf2 levels in the kidneys of T2D animals that were thought to be the cause of the elevated antioxidant enzyme activities in the WFO-treated animals.
Under diabetic circumstances, lipid dysfunction and hyperglycemia are invariably followed by an inflammatory response in the kidney, and hence inflammation plays an essential part in the etiology of DKD [24]. Nuclear factor-kappa B (NF-kB) is a transcription factor that plays an essential role in developing inflammation [48]. NF-kB p65 enters the nucleus, triggering transcription of target genes that include various inflammatory factors, including TNF-, COX-2, IL-1, and iNOS [48]. The release of these pro-inflammatory mediators stimulates ROS creation, which is linked to the advancement of diabetic nephropathy [76]. TNF-, for example, was discovered to actively contribute to DKD as a mediator by increasing the generation of ROS, triggering apoptosis in renal cells, and modifying the permeability of glomerular albumin.
Diabetic kidney injury is usually linked to abnormal energy and lipid metabolism [63, 64, 77]. Energy and lipid metabolism are regulated by adenosine monophosphate (AMP)-activated kinase (AMPK) and sirtuin 1 (SIRT1) [62]. SIRT1 belongs to histone deacetylases, and its robust deacetylation capacity allows it to regulate a wide range of physiological activities, including inflammation, lipid metabolism, insulin resistance, and cell death [78]. Furthermore, SIRT1 may boost lipid catabolism by activating AMPK, and it can also control glycolipid metabolism to keep energy balance [50]. Furthermore, the SIRT1/AMPK signaling pathway has been shown to limit adipogenesis by lowering the production of sterol regulatory element-binding proteins (SREBPs), consequently reducing renal inflammation [29]. The current work discovered that WFO might notably upregulate the activities of SIRT1 in the kidneys of T2D animals, thus increasing the PGC-1 level.
Conclusions
It could be concluded that WFO modulated dyslipidemia, hyperglycemia, oxidative stress, and inflammation in T2D animals, alleviating pathological kidney injury. Furthermore, WFO selectively decreased renal AGE-induced oxidative stress via activating the Nrf2 signaling pathway. In addition, WFO may markedly control the NF-κB pathway, inhibiting NF-kB-mediated renal inflammatory injury. Besides, it was shown that WFO had a preventive potential against diabetic kidney injury in T2D animals.
Availability of data and materials
Not applicable.
Abbreviations
- AGE:
-
Advanced glycation end products
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- AUC:
-
Area under the curve
- COX-2:
-
Cyclooxygenase-2
- DKD:
-
Diabetic kidney damage
- GPx:
-
Glutathione peroxidase
- HDL:
-
High-density lipoprotein
- HFD:
-
High-fat diet
- IL-1β:
-
Interleukin-1β
- LDL:
-
Low-density lipoprotein
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- OGTT:
-
Oral glucose tolerance test
- p-AMPK:
-
Phosphorylated AMPK
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- T2D:
-
Streptozotocin (STZ)-induced diabetes type 2
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TNF-α:
-
Tumor necrosis factor-α
- WFO:
-
Washingtonia filifera Oil
References
Jones DL (1995) Palms Throughout the World. Smithsonian Books. Washington DC, ISBN 10:1560986166.
Gilman EF, Watson DG (2006) Washingtonia filifera : Desert Palm 1. Agric Eng, pp 1–3.
Selim NM, El-Hawary SS, El Zalabani SM, Shamma RN, Mahdy NES, Sherif NH, Fahmy HA, Mekkawy MH, Yasri A, Sobeh M (2020) Impact of Washingtonia robusta Leaves on Gamma Irradiation-Induced Hepatotoxicity in Rats and Correlation with STING Pathway and Phenolic Composition. Pharmaceuticals (Basel) 13(10):320. https://doi.org/10.3390/ph13100320
Flynn T, Lorence DH (2002) Additions to the flora of the Hawaiian Islands. Bishop Mus Occas Pap 69:14–16
Dewir YH, El-Mahrouk ME, Seliem MK, Murthy HN (2020) Bioactive compounds of California Fan Palm Washingtonia filifera (Linden ex André) H. Wendl. ex de Bary. In: Murthy HN, Bapat VA (eds) Bioactive compounds in underutilized fruits and nuts. Springer International Publishing, Cham, pp 63–74. https://doi.org/10.1007/978-3-030-30182-8_7
Era B, Floris S, Sogos V, Porcedda C, Piras A, Medda R, Fais A, Pintus F (2021) Anti-aging potential of extracts from Washingtonia filifera seeds. Plants 10:151. https://doi.org/10.3390/plants10010151
Cornett JW (1987) Nutritional value of desert fan palm fruits. Principes. 31(4):159–161
Hemmati AA, Kalantari H, Siahpoosh A, Ghorbanzadeh B, Jamali H (2015) Anti-inflammatory effect of hydroalcoholic extract of the Washingtonia filifera seeds in carrageenan-induced paw edema in rats. Jundishapur J Nat Pharm Prod 10(1):1–5
Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MSRB, Folador P, Damazio RG et al (2008) Flavonoids: prospective drug candidates. Mini Rev Med Chem 8(13):1429–1440
Cazarolli LH, Folador P, Moresco HH, Brighente IMC, Pizzolatti MG, Silva FRMB (2009) Stimulatory effect of apigenin-6-C-beta-L-fucopyranoside on insulin secretion and glycogen synthesis. Eur J Med Chem 44(11):4668–4673
Cazarolli LH, Folador P, Moresco HH, Brighente IMC, Pizzolatti MG, Silva FRMB (2009) Mechanism of action of the stimulatory effect of apigenin-6-C-(2″-O-alpha-l-rhamnopyranosyl)-beta-L-fucopyranoside on 14C-glucose uptake. Chem Biol Interact 179(2–3):407–412
Daulatabad CD, Ankalgi RF (1985) Component acids of palmae seed oils. Fette Seifen Anstrichm. 87:126–128. https://doi.org/10.1002/lipi.19850870310
Nehdi IA (2011) Characteristics and composition of Washingtonia filifera (Linden ex André) H. Wendl. seed and seed oil. Food Chem 126(1):197–202
Dang J, Tu Y, Wang J, Yang Y (2021) Carbamylated erythropoietin alleviates kidney damage in diabetic rats by suppressing oxidative stress. Curr Med Sci. 41(3):513–521. https://doi.org/10.1007/s11596-021-2370-x
Bénardeau A, Kahnert A, Schomber T, Meyer J, Pavkovic M, Kretschmer A, Lawrenz B, Hartmann E, Mathar I, Hueser J, Kraehling JR, Eitner F, Hahn MG, Stasch J-P, Sandner P (2021) Runcaciguat, a novel soluble guanylate cyclase activator, shows renoprotection in hypertensive, diabetic, and metabolic preclinical models of chronic kidney disease. Naunyn Schmiedebergs Arch Pharmacol 394(12):2363–2379. https://doi.org/10.1007/s00210-021-02149-4
El-Beeh ME, Aljabri M, Orabi HF, Qari SH, Ramadan MF (2019) Ameliorative impact of cold-pressed Rosmarinus officinalis oil against liver toxicity and genotoxic effects in streptozotocin-induced diabetic rats and their offspring. J Food Biochem 43:e12905. https://doi.org/10.1111/jfbc.12905
El-Hadary AE, Elsanhoty RM, Ramadan MF (2019) In vivo protective effect of Rosmarinus officinalis oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats. PharmaNutrition 9(2019):100151. https://doi.org/10.1016/j.phanu.2019.100151
Ortiz-Avila O, Figueroa-García MD, García-Berumen CI, Calderón-Cortés E, Mejía-Barajas JA, Rodriguez-Orozco AR, Mejía-Zepeda R, Saavedra-Molina A, Cortés-Rojo C (2017) Avocado oil induces long-term alleviation of oxidative damage in kidney mitochondria from type 2 diabetic rats by improving glutathione status. J Bioenerg Biomembr 49(2):205–214. https://doi.org/10.1007/s10863-017-9697-9
Betz BB, Jenks SJ, Cronshaw AD, Lamont DJ, Cairns C, Manning JR, Goddard J, Webb DJ, Mullins JJ, Hughes J, McLachlan S, Strachan MWJ, Price JF, Conway BR (2016) Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int 89(5):1125–1135
Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. Initial characterization of a rat model of diabetic nephropathy. Diabetes. 2004. 53(3):735–42. https://pubmed.ncbi.nlm.nih.gov/14988259. Accessed 22 Oct 2021.
Danda RS, Habiba NM, Rincon-Choles H, Bhandari BK, Barnes JL, Abboud HE, Pergola PE (2005) Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int 68(6):2562–2571
Kowalski A, Krikorian A, Lerma EV (2015) Diabetes and chronic kidney disease. Dis Mon 61(9):378–386
Fioretto P, Mauer M. Diabetic nephropathy-challenges in pathologic classification. Nat Rev Nephrol. 2010. 6(9):508–10. https://www.nature.com/articles/nrneph.2010.96
Navarro-González JF, Mora-Fernández C (2011) Inflammatory pathways. Contrib Nephrol 170:113–123. https://doi.org/10.1159/000325646
Weng L, Chen T-H, Zheng Q, Weng W-H, Huang L, Lai D, Fu Y-S, Weng CF (2021) Syringaldehyde promoting intestinal motility with suppressing α-amylase hinders starch digestion in diabetic mice. Biomed Pharmacother 141:111865. https://doi.org/10.1016/j.biopha.2021.111865
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (2013) Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25(10):1939–1948
Wakino S, Hasegawa K, Itoh H (2015) Sirtuin and metabolic kidney disease. Kidney Int 88(4):691–698
Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9(5):407–416
Sharma K (2014) Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl 4(1):113–117
Oomah BD, Ladet S, Godfrey DV, Liang J, Girard B (2000) Characteristics of raspberry (Rubus idaeus L) seed oil. Food Chem 69(2):187–193
Xia X, Xu J, Wang X, Wang H, Lin Z, Shao K, Fang L, Zhang C, Zhao Y. Jiaogulan tea (Gpostemma pentaphyllum) potentiates the antidiabetic effect of white tea via the AMPK and PI3K pathways in C57BL/6 mice. Food Funct. 2020. 26;11(5):4339–55. https://pubs.rsc.org/en/content/articlehtml/2020/fo/d0fo00395f.
Poucher SM, Cheetham S, Francis J, Zinker B, Kirby M, Vickers SP (2012) Effects of saxagliptin and sitagliptin on glycaemic control and pancreatic β-cell mass in a streptozotocin-induced mouse model of type 2 diabetes. Diabetes Obes Metab 14(10):918–926. https://doi.org/10.1111/j.1463-1326.2012.01619.x
da Costa Guerra JF, Maciel PS, de Abreu IC, Pereira RR, Silva M, de Morais CL, Pinheiro-Sant’Ana HM, de Lima WG, Silva ME, Pedrosa ML (2015) Dietary açai attenuates hepatic steatosis via adiponectin-mediated effects on lipid metabolism in high-fat diet mice. J Funct Foods 1(14):192–202
Lillie RD, Pizzolato P, Donaldson PT (1976) Nuclear stains with soluble metachrome metal mordant dye lakes. Histochemistry 49:23–35
Olive PL, Banáth JP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1(1):23–29
Besbes S, Blecker C, Deroanne C, Drira NE, Attia H (2004) Date seeds: chemical composition and characteristic profiles of the lipid fraction. Food Chem 84(4):577–584
Nasri N, Khaldi A, Fady B, Triki S (2005) Fatty acids from seeds of Pinus pinea L.: composition and population profiling. Phytochemistry 66(14):1729–1735
Bruckert E (2001) Fonctionnalite des lipides dans le contexte d’une relation alimentation-sante les phytostérols, place dans la prise en charge du patient hyperlipidémique. Oléagineux, Corps Gras, Lipides 8(4):312–316. https://doi.org/10.1051/ocl.2001.0312
Jayadas NH, Nair KP (2006) Coconut oil as base oil for industrial lubricants-evaluation and modification of thermal, oxidative and low temperature properties. Tribol Int 39(9):873–878
Liang T, Liao S (1992) Inhibition of steroid 5α-reductase by specific aliphatic unsaturated fatty acids. Biochem J 285(2):557–562
Niederprüm HJ, Schweikert HU, Zänker KS (1994) Testosterone 5α-reductase inhibition by free fatty acids from Sabal serrulata fruits. Phytomedicine 1(2):127–133
Raynaud JP, Cousse H, Martin PM (2002) Inhibition of type 1 and type 2 5alpha-reductase activity by free fatty acids, active ingredients of Permixon. J Steroid Biochem Mol Biol 82(2–3):233–239
VeereshBabu SV, Veeresh B, Patil AA, Warke YB (2010) Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur J Pharmacol 626(2–3):262–265
Al-Saqer JM, Sidhu JS, Al-Hooti SN, Al-Amiri HA, Al-Othman A, Al-Haji L, Ahmed N, Mansour IB, Minal J (2004) Developing functional foods using red palm olein. IV. Tocopherols and tocotrienols. Food Chem 85(4):579–583. https://doi.org/10.1016/j.foodchem.2003.08.003
Adhikari P, Hwang KT, Shin MK, Lee BK, Kim SK, Kim SY, Lee KT, Kim SZ (2008) Tocols in caneberry seed oils. Food Chem 111(3):687–690. https://doi.org/10.1016/J.FOODCHEM.2008.04.038
Wang Q, Zhou J, Xiang Z, Tong Q, Pan J, Wan L, Chen J (2019) Anti-diabetic and renoprotective effects of Cassiae Semen extract in the streptozotocin-induced diabetic rats. J Ethnopharmacol 239:111904. https://doi.org/10.1016/J.JEP.2019.111904
Hammami S, Salem S Ben, Jarraya H, Devi PUM, Nefzi A, Mighri Z. Chemical composition and antioxidant properties of washingtonia filifera leaves and flowers. 2012.
Khan H, Jawad M, Kamal MA, Baldi A, Xiao J, Nabavi SM, Daglia M (2018) Evidence and prospective of plant derived flavonoids as antiplatelet agents: Strong candidates to be drugs of future. Food Chem Toxicol 1(119):355–367
Mi Y, Zhao X, Liu F, Sun C, Sun Z, Liu L (2021) Changes in soil quality, bacterial community and anti-pepper phytophthora disease ability after combined application of straw and multifunctional composite bacterial strains. Eur J Soil Biol 1:105
Xiao J (2018) Stability of dietary polyphenols: it’s never too late to mend? Food Chem Toxicol 1(119):3–5
Gao D, Zhao M, Qi X, Liu Y, Li N, Liu Z, Bian Y (2016) Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch Pharm Res 39:221–230
Harborne JB (1975) Flavonoid sulphates: a new class of sulphur compounds in higher plants. Phytochemistry 14(5–6):1147–1155
Lercker G, Rodriguez-Estrada MT (2000) Chromatographic analysis of unsaponifiable compounds of olive oils and fat-containing foods. J Chromatogr A 881(1–2):105–129. https://doi.org/10.1016/s0021-9673(00)00455-6
Ramadan MF, Amer MM, Awad AE (2008) Coriander (Coriandrum sativum L.) seed oil improves plasma lipid profile in rats fed a diet containing cholesterol. Eur Food Res Technol 227:1173–1182. https://doi.org/10.1007/s00217-008-0833-y
El-Hadary AE, Ramadan Hassanien MF (2016) Hepatoprotective effect of cold-pressed Syzygium aromaticum oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats. Pharm Biol 54(8):1364–1372. https://doi.org/10.3109/13880209.2015.1078381
Issaoui M, Delgado AM (2019) Grading, labeling and standardization of edible oils. In: Ramadan MF (ed) Fruit oils chemistry and functionality. Springer, Cham. https://doi.org/10.1007/978-3-030-12473-1_2
Ramadan MF (2019) Chemistry and functionality of fruit oils: an introduction. In: Ramadan M (ed) Fruit oils: chemistry and functionality. Springer, Cham. https://doi.org/10.1007/978-3-030-12473-1_1
Delgado A, Al-Hamimi S, Ramadan MF, De Wit M, Durazzo A, Nyam KL, Issaoui M (2020) Contribution of tocols to food sensorial properties, stability, and overall quality. J Food Qual. https://doi.org/10.1155/2020/8885865
Durazzo A, Nazhand A, Lucarini M, Delgado AM, De Wit M, Nyam KL, Santini A, Ramadan MF (2021) Occurrence of tocols in foods: an updated shot of current databases. J Food Qual. https://doi.org/10.1155/2021/8857571
Holt RR, Uriu-Adams JY, Keen CL (2012) Zinc. In: Macdonald IA, Zeisel SH (eds) Present knowledge in nutrition, 10th edn. Wiley, Blackwell, pp 521–539. https://doi.org/10.1002/9781119946045.ch34
Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC (1993) Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 328(20):1450–1456. https://doi.org/10.1056/NEJM199305203282004
Zhang B, Sun W, Yu N, Sun J, Yu X, Li X, Xing Y, Yan D, Ding Q, Xiu Z (2018) Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J Funct Foods. 46:256–267
Li M, Zhou F, Xu T, Song H, Lu B (2018) Acteoside protects against 6-OHDA-induced dopaminergic neuron damage via Nrf2-ARE signaling pathway. Food Chem Toxicol 119:6–13
Li X, Jiang X, Sun J, Zhu C, Bai W (2018) Recent advances of medical foods in China: the opportunities and challenges under standardization. Food Chem Toxicol 119:342–354
Izquierdo-Lahuerta A, Martínez-García C, Medina-Gómez G (2016) Lipotoxicity as a trigger factor of renal disease. J Nephrol 29(5):603–610
Wicks SE, Nguyen TT, Breaux C, Kruger C, Stadler K (2016) Diet-induced obesity and kidney disease- In search of a susceptible mouse model. Biochimie 1(124):65–73
Migliorini A, Angelotti ML, Mulay SR, Kulkarni OO, Demleitner J, Dietrich A, Sagrinati C, Ballerini L, Peired A, Shankland SJ, Liapis H, Romagnani P, Anders HJ (2013) The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am J Pathol 183(2):431–440
Kanwar YS, Sun L, Xie P, Liu FY, Chen S (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol Mech Dis 6:395–423. https://doi.org/10.1146/annurev.pathol4110807092150
Yadav A, Jahan A, Yadav TP, Sachdev N, Chitkara A, Asare R (2013) Effect of glucocorticoids on serum lipid profile and endothelial function and arterial wall mechanics. Indian J Pediatr 80(12):1007–1014. https://doi.org/10.1007/s12098-013-1035-6
Oraby MA, El-Yamany MF, Safar MM, Assaf N, Ghoneim HA (2019) Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed Pharmacother 1(109):910–920
Bhattacharjee N, Borah A (2016) Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson’s disease. Neurochem Int 1(101):48–55
Huang K, Chen C, Hao J, Huang J, Wang S, Liu P, Huang H (2015) Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol Cell Endocrinol 5(399):178–189
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116. https://doi.org/10.1146/ANNUREV.PHARMTOX.46.120604.141046
Todorovic M, Wood SA, Mellick GD (2016) Nrf2: a modulator of Parkinson’s disease? J Neural Transm 123(6):611–619. https://doi.org/10.1007/s00702-016-1563-0
Norris KM, Okie W, Kim WK, Adhikari R, Yoo S, King S, Pazdro R (2016) A high-fat diet differentially regulates glutathione phenotypes in the obesity-prone mouse strains DBA/2J, C57BL/6J, and AKR/J. Nutr Res 36(12):1316–1324
Assiri AMA, El-Beeh ME, Amin AH, Ramadan MF (2017) Ameliorative impact of Morus alba leaves’ aqueous extract against embryonic ophthalmic tissue malformation in streptozotocin-induced diabetic rats. Biomed Pharmacother 1(95):1072–1081
Igarashi Y, Iida S, Dai J, Huo J, Cui X, Sawashita J, Mori M, Miyahara H, Higuchi K (2021) Glavonoid-rich oil supplementation reduces stearoyl-coenzyme A desaturase 1 expression and improves systemic metabolism in diabetic, obese KK-Ay mice. Biomed Pharmacother 140:111714. https://doi.org/10.1016/j.biopha.2021.111714
Ghizzoni M, Haisma HJ, Maarsingh H, Dekker FJ (2011) Histone acetyltransferases are crucial regulators in NF-κB mediated inflammation. Drug Discov Today 16(11–12):504–511
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: 19-SCI-1-01- 0037.
Funding
This research was funded by the Deanship of Scientific Research at Umm Al-Qura, grant number 19-SCI-1-01-0037.
Author information
Authors and Affiliations
Contributions
Conceptualization, MEE., AAE, SHQ.; MEE, methodology, MEE, AAE; validation, MEE, AAE; investigation, MEE, MFR; writing—original draft preparation, MFR; writing—review and editing, SHQ; MEE; visualization, MEE; funding acquisition; MEE. All authors have read and agreed to the published version of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Committee of Animal Care and Use (Umm Al-Qura University, Makkah, KSA).
Consent for publication
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Beeh, M.E., El-Badawi, A.A., Qari, S.H. et al. Protective and health-promoting impact of Washingtonia filifera oil on the kidney of STZ-induced diabetic mice. Appl Biol Chem 65, 41 (2022). https://doi.org/10.1186/s13765-022-00713-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00713-x