Summary
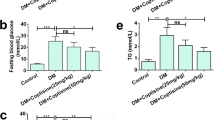
The oxidative stress response plays an important role in the occurrence and development of diabetic kidney disease (DKD). It has become a new treatment target for DKD. In the current study, the effects of carbamylated erythropoietin (CEPO) on renal oxidative stress and damage in diabetic rats were examined. Thirty Sprague Dawley rats were intraperitoneally administered with 60 mg/kg streptozotocin to establish the diabetes model. The diabetic rats were randomly allocated into 4 groups (n=6 each): diabetes model group (DM group), DM + CEPO treatment group (DC group), DM + CEPO + EPO receptor (EPOR) blocking peptide treatment group (DCEB group), and DM + CEPO + CD131 blocking peptide treatment group (DCCB group). Meanwhile, a normal control group (NC group, n=6) was set up. Kidney tissues and blood samples were obtained for evaluation of oxidative stress and renal function. The results showed that diabetic rats exhibited increased oxidative stress in the kidney and early pathological changes associated with DKD. Treatment with CEPO reduced oxidative stress and attenuated renal dysfunction. However, diabetic rats treated with the combination of CEPO and EPOR blocking peptide or CD131 blocking peptide showed increased oxidative stress and reduced renal function when compared with CEPO treatment alone group. These results suggested that CEPO can protect against kidney damage in DKD by inhibiting oxidative stress injury via EPOR-CD131 heterodimers.
Similar content being viewed by others
References
Sanajou D, Ghorbani Haghjo A, Argani H, et al. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur J Pharmacol, 2018,833:158–164
Kajal A, Singh R. Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLoS One, 2019, 14(3):e0213147
Guerin-Dubourg A, Cournot M, Planesse C, et al. Association between Fluorescent Advanced Glycation End-Products and Vascular Complications in Type 2 Diabetic Patients. Biomed Res Int, 2017,2017:7989180
Abdel-Moneim A, Mahmoud B, Nabil A, et al. Correlation between oxidative stress and hematological profile abnormalities in diabetic nephropathy. Diabetes Metab Syndr, 2019,13(4):2365–2373
Villegas-Rivera G, Roman-Pintos LM, Cardona-Munoz EG, et al. Effects of Ezetimibe/Simvastatin and Rosuvastatin on Oxidative Stress in Diabetic Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Oxid Med Cell Longev, 2015, 2015:756294
Lazavi F, Mirmiran P, Sohrab G, et al. The barberry juice effects on metabolic factors and oxidative stress in patients with type 2 diabetes: A randomized clinical trial. Complement Ther Clin Pract, 2018,31:170–174
Hwang YC, Kim SW, Hur KY, et al. Predictive Factors for Efficacy of AST-120 Treatment in Diabetic Nephropathy: a Prospective Single-Arm, Open-Label, Multi-Center Study. J Korean Med Sci, 2019,34(15):e117
Lee EY, Lee MY, Hong SW, et al. Blockade of oxidative stress by vitamin C ameliorates albuminuria and renal sclerosis in experimental diabetic rats. Yonsei Med J, 2007,48(5):847–855
Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J, 2013,15(1):195–218
Takayanagi R, Inoguchi T, Ohnaka K. Clinical and experimental evidence for oxidative stress as an exacerbating factor of diabetes mellitus. J Clin Biochem Nutr, 2011,48(1):72–77
Bhoopalan SV, Huang LJ, Weiss MJ. Erythropoietin regulation of red blood cell production: from bench to bedside and back. F1000Res, 2020,18(9):1153
Kittur FS, Lin Y, Arthur E, et al. Recombinant asialoerythropoetin protects HL-1 cardiomyocytes from injury via suppression of Mst1 activation. Biochem Biophys Rep, 2019,17:157–168
Hache G, Garrigue P, Bennis Y, et al. ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability. Shock, 2016,46(4):390–397
Huang CT, Chen SH, Lin SC, et al. Erythropoietin reduces nerve demyelination, neuropathic pain behavior and microglial MAPKs activation through erythropoietin receptors on Schwann cells in a rat model of peripheral neuropathy. Glia, 2018,66(11):2299–2315
Lee BJ, Jun HO, Kim JH, et al. Astrocytic cystine/glutamate antiporter is a key regulator of erythropoietin expression in the ischemic retina. Faseb J, 2019,33(5): 6045–6054
Oshima N, Onimaru H, Yamagata A, et al. Erythropoietin, a putative neurotransmitter during hypoxia, is produced in RVLM neurons and activates them in neonatal Wistar rats. Am J Physiol Regul Integr Comp Physiol, 2018,314(5):R700–R708
Elliot-Portal E, Laouafa S, Arias-Reyes C, et al. Brain-derived erythropoietin protects from intermittent hypoxia-induced cardiorespiratory dysfunction and oxidative stress in mice. Sleep, 2018,41(7):1–13
Elshiekh M, Kadkhodaee M, Seifi B, et al. Additional effects of erythropoietin pretreatment, ischemic preconditioning, and N-acetylcysteine posttreatment in rat kidney reperfusion injury. Turk J Med Sci, 2019,49(4): 1249–1255
Siems W, Carluccio F, Radenkovic S, et al. Oxidative stress in renal anemia of hemodialysis patients is mitigated by epoetin treatment. Kidney Blood Press Res, 2005,28(5–6):295–301
Bartnicki P, Fijalkowski P, Majczyk M, et al. Effect of methoxy polyethylene glycol-epoetin beta on oxidative stress in predialysis patients with chronic kidney disease. Med Sci Monit, 2013,19:954–959
Hemani S, Lane O, Agarwal S, et al. Systematic Review of Erythropoietin (EPO) for Neuroprotection in Human Studies. Neurochem Res, 2021,46(4):732–739
Mun KC, Golper TA. Impaired biological activity of erythropoietin by cyanate carbamylation. Blood Purif, 2000,18(1):13–17
Jo HR, Kim YS, Son H. Erythropoietin and carbamylated erythropoietin promote histone deacetylase 5 phosphorylation and nuclear export in rat hippocampal neurons. Biochem Biophys Res Commun, 2016,470(1):220–225
Boesch S, Nachbauer W, Mariotti C, et al. Safety and tolerability of carbamylated erythropoietin in Friedreich’s ataxia. Mov Disord, 2014,29(7):935–939
Dorotea D, Kwon G, Lee JH, et al. A pan-NADPH Oxidase Inhibitor Ameliorates Kidney Injury in Type 1 Diabetic Rats. Pharmacology, 2018,102(3–4):180–189
Ilatovskaya DV, Blass G, Palygin O, et al. A NOX4/TRPC6 Pathway in Podocyte Calcium Regulation and Renal Damage in Diabetic Kidney Disease. J Am Soc Nephrol, 2018,29(7):1917–1927
Wang Y, Li Y, Yang Z, et al. Pyridoxamine Treatment of HK-2 Human Proximal Tubular Epithelial Cells Reduces Oxidative Stress and the Inhibition of Autophagy Induced by High Glucose Levels. Med Sci Monit, 2019,25:1480–1488
Diab El-Harakeh M, Njeim R, Youssef A, et al. Novel triazine-based pyrimidines suppress glomerular mesangial cells proliferation and matrix protein accumulation through a ROS-dependent mechanism in the diabetic milieu. Bioorg Med Chem Lett, 2019,29(13): 1580–1585
Cheng YS, Chao J, Chen C, et al. The PKCbeta-p66shc-NADPH oxidase pathway plays a crucial role in diabetic nephropathy. J Pharm Pharmacol, 2019;71(3):338–347
Østergaard JA, Cooper ME, Jandeleit-Dahm KAM. Targeting oxidative stress and anti-oxidant defence in diabetic kidney disease. J Nephrol, 2020,33(5):917–929
Miller JL, Church TJ, Leonoudakis D, et al. Discovery and Characterization of Nonpeptidyl Agonists of the Tissue-Protective Erythropoietin Receptor. Mol Pharmacol, 2015,88(2):357–367
Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA, 2004,101(41):14907–14912
Bennis Y, Sarlon-Bartoli G, Guillet B, et al. Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost, 2012,10(9):1914–1928
Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal, 2007,19(3):634–645
Hahn N, Knorr DY, Liebig J, et al. The Insect Ortholog of the Human Orphan Cytokine Receptor CRLF3 Is a Neuroprotective Erythropoietin Receptor. Front Mol Neurosci, 2017,10:223
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
This work was supported by the National Natural Sciences Foundation of China (No. 81500639) and the Science Foundation of Hubei Society of Microcirculation (No. 2019HX0020).
Rights and permissions
About this article
Cite this article
Dang, Jz., Tu, Yf., Wang, J. et al. Carbamylated Erythropoietin Alleviates Kidney Damage in Diabetic Rats by Suppressing Oxidative Stress. CURR MED SCI 41, 513–521 (2021). https://doi.org/10.1007/s11596-021-2370-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2370-x