Abstract
Background
We aimed to identify interventions used to implement antimicrobial stewardship practices among hospitalized patients in least-developed countries.
Methods
The research team searched PubMed, EMBASE, and Cochrane Central Register of Controlled Trials for studies of AMS interventions in the least developed and low-income countries, published between 2000 and 2023. Included studies had a population of hospitalized patients of all age groups in least-developed countries, implemented an AMS intervention, and reported its impact on prescription practices, clinical outcomes, or microbiological results. The risk of bias was assessed using the integrated quality criteria for review of multiple study designs. A total of 443 articles were identified, 386 articles were screened, 16 full-text papers were reviewed, and 10 studies were included in the analysis.
Results
The ten studies included three controlled before and after, two qualitative, one controlled interrupted time series, two non-controlled interrupted time series, one quasi-experimental study, and one randomized controlled trial. Three studies implemented either enabling, persuasive, or structural interventions respectively. The rest used bundled strategies, including a combination of persuasive, enabling, structural, and restrictive interventions. Bundled interventions using enabling and persuasive strategies were the most common. These involved creating a prescription guideline, training prescribers on updated methods, and subsequent review and feedback of patient files by members of an AMS team. Improved microbiological surveillance was important to most studies but, sustained improvement in appropriate prescriptions was dependent on enabling or persuasive efforts. Studies noted significant improvements in appropriate prescriptions and savings on the costs of antibiotics. None evaluated the impact of AMS on AMR.
Conclusion
AMS practices generally involve multiple strategies to improve prescription practices. In the setting of least-developed countries, enabling and persuasive interventions are popular AMS measures. However, measured outcomes are heterogeneous, and we suggest that further studies assessing the impact of AMS should report changes in AMR patterns (microbiological outcomes), patient length of stay and mortality (patient outcomes), and changes in prescription practices (prescription outcomes). Reporting on these as outcomes of AMS interventions could make it easier for policymakers to compare which interventions have desirable outcomes that can be generalized to similar settings.
Similar content being viewed by others
Introduction
Globally, over 5 million people die from diseases or complications of conditions associated with a micro-organism that is resistant to the medication given to treat it [1]. In Sub-Saharan Africa, AMR has been associated with about 16,000 disability-adjusted life years (DALYs) because many infections are resistant to first-line or empirical antibiotics [2,3,4]. Despite differences in resistance patterns across the world, AMR affects all people because drug-resistant infections spread beyond geographical barriers and are becoming increasingly harder to control [5].
Unfortunately, many low-income countries, lack the resources to identify and monitor antimicrobial resistance patterns [6,7,8,9]. In fact, over 40% of African countries have no data on antimicrobial resistance patterns, and, with 78% of antibiotics in low and middle income countries being self-medicated and unregulated, it is increasingly harder monitor antibiotic use [8, 10,11,12].
Despite these challenges, many studies have shown that it is possible to implement antimicrobial stewardship programs to regulate antibiotic use in hospitals to curb antimicrobial resistance.
Studies that have introduced antimicrobial stewardship interventions have grouped them into enabling, persuasive, structural, and restrictive interventions [13]: enabling AMS programs involve teaching clinical staff about better prescription practices; persuasive AMS programs allow for auditing of prescriptions and the duration of treatment and constructively discussing these with the prescriber; structural programs involve the judicious use of diagnostics to ensure antibiotics are prescribed for the right organism; and restrictive interventions involve hospital or regional level policies that restrict availability of antibiotics to specific groups, prescribers or organisms [13].
Systematic reviews on antimicrobial stewardship have highlighted methods to curb antimicrobial resistance in different settings globally. Among low and middle income countries, clinical decision making tools were found to be efficient methods of improving prescription practices [10].
Other systematic reviews on interventions to improve antimicrobial prescribing in hospital inpatients found that; antimicrobial stewardship programs decreased the duration of treatment and reduced length of stay, AMS improved adherence to prescription recommendations, restrictive interventions were associated with increased compliance, and that enabling interventions of audit and feedback and were highly effective [14,15,16,17]. In these studies, reduction in duration of treatment was not associated with an increase in mortality and this represents an economic incentive to control antibiotic use in resource constrained settings.
However, the gap in these studies is that systematic reviews on low- and middle-income countries focus on middle income countries of China, Indonesia and south Africa, which represent middle income countries [9, 10, 14, 17].
In this systematic review, we aim to identify the protocols, policies and practices used for antimicrobial stewardship in hospitalised patients of all ages in the least developed and low-income countries and to provide comprehensive information on how AMS can be carried out in these settings.
Methods
We conducted a systematic review of AMS practices in least developed and low-income countries. The protocol for this review was registered and published with the international prospective register of systematic reviews (PROSPERO): CRD42020210634 [18].
Search strategy
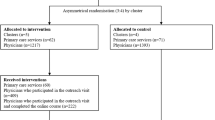
Between November 2020 and 11th September 2023, two reviewers (GM and MM) independently identified studied studies by searching PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). Records were screened for the inclusion and exclusion criteria at abstract, and full-text review as demonstrated in the Prisma Flow Chart in Fig. 1. Search terms and databases used are described in Additional file 1.
Antimicrobial Stewardship in Least-Developed and Low-Income Countries – Prisma Flow Diagram. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Inclusion and exclusion criteria
We included original research from studies that implemented AMS interventions in hospitalized patients published in English between 2000 and 2023, conducted in a least developed or low-income country based on the Development Assistance Committee (DAC) classification [19]. We extracted data from randomized controlled trials, controlled before and after trials, interrupted time series studies, cohort, and qualitative studies. Our study population was hospitalized children and adults of all ages with bacterial infections, including patients with surgical and obstetric conditions.
We included structural, enabling, persuasive, and restrictive interventions and searched for behavioral, clinical, and microbiological outcomes. Structural interventions are those where the intervention was technology to guide antibiotic treatment [13]. These include new laboratory equipment, mobile phone applications or algorithms used to discern bacterial infections and their levels of antibiotic susceptibility. Persuasive interventions mostly involve reviewing prescriptions and providing feedback to the prescriber on how they could improve the appropriateness of the drug chosen and the duration of treatment [13]. Enabling interventions implement ways to educate prescribers but do not review their prescriptions. This can be done through treatment guidelines, classes or seminars on antimicrobial resistance and prescription guidelines [13]. Restrictive interventions are those that require approval for certain antibiotics. This can be from the pharmacy level, infectious disease specialist level or AMS team level. We also included studies which used a combination of any of these types of interventions.
The exclusion criteria were interventions on patients in communities, pharmacies, and dispensaries. We also excluded studies that only reported on prevalence of AMR, hospital related infections or prescription practices without implementing an intervention. We documented whether AMR was an endpoint and how that was measured.
The studies were assessed for microbiological, clinical, behavioral, and prescriptive outcomes. Microbiological outcomes include changes in resistance patterns. Clinical outcomes included changes in length of stay, days of treatment, hospital related infections (including surgical site infections). Prescriptive outcomes are changes in prescription practices. Other outcomes that were identified from the studies include the costs of treatment.
We adapted the Cochrane collaboration data collection form to extract data and used the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to present our findings [20,21,22]. Reviewers independently used the Integrated Quality Criteria for Review of Multiple Study Designs (ICROMS) to assess for risk of bias and consolidated findings with a third reviewer [23]. The Prisma 2020 checklist for this study is presented in Additional file 2. No automation materials were used during the entire process of this study.
Results
We identified 443 articles, and after 57 duplicates were removed, we screened 386 records (Fig. 1). The included studies were published between 2015 and 2022, and were from Malawi, Tanzania, Nepal, Cambodia, Ethiopia, Uganda, Liberia, Bangladesh and Mali and involved a total of 3295 patients (Table 1). Two studies focused on obstetric patients, two on pediatric patients only, three on adults over 15 years, and three on patients of all ages. Among the study designs included were two were controlled before and after, two were qualitative, one was a controlled interrupted time series, one non-controlled interrupted time series, and one non-controlled before and after study. Enabling interventions were the most common type of intervention used and were present in eight of the ten studies. Three studies used one type of intervention; enabling, structural or persuasive respectively; other studies used bundled interventions involving at least two types of interventions. All outcomes from the included studies are reported in Table 1. Due to variation in how outcomes were reported, a meta-analysis was not done.
Single interventions
Enabling intervention
Clinicians and researchers at a referral hospital in Mbeya, Tanzania created an antimicrobial prescription guidebook to ensure good prescribing practices among clinicians between 2017 and 2019 [24]. Before creating the book, they conducted comprehensive baseline studies to understand the state of AMR and prescribing practices in their area. They conducted clinician surveys to understand the knowledge of AMR and AMS at the hospital, chart reviews to assess prescribing patterns, culture and sensitivity reviews to understand resistance patterns at the hospital, and pharmacy surveys to assess the availability of over-the-counter antibiotics in the region. The study found that 7 of 38 junior clinicians did not know about AMS, 50% of empirical treatments were not aligned to national guidelines, about 66% of in-hospital antimicrobial courses were not completed, and, there was high resistance of Escherichia coli to cotrimoxazole [24]. Using this information, the antimicrobial prescription book was made available physically and electronically to enable access during clinical work. The impact of these interventions on prescribing practices and AMR was not measured.
Persuasive intervention
Nauriyal et al. [25] conducted a post-prescription review and feedback program in three hospitals to improve antibiotic prescribing for in patient adults (over 15 years) with wounds or burns in Nepal. Infectious disease specialists trained physician champions and updated the antibiotic guidebook to cover treatment for wounds and burns. Baseline chart reviews were done for 6 months (January 2018–June 2018), followed by a one-month (July 2018) implementation, and a six-month post-intervention chart review phase (August 2–18 to January 2019). The result of this intervention was that days of treatment with intravenous antibiotics were reduced (10.1 days at baseline and 8.8 days post-intervention, t = 3.56; p < 0.001) [25]. There was significant improvement in prescription practices through appropriate prescriptions, improved documentation, de-escalation, and adherence to prescribing guidelines.
Structural intervention
A smart-phone based diarrheal etiology prediction tool (DEP) was developed to help prescribers differentiate between viral and bacterial causes of diarrhea in children [26]. A randomized cross over study was then conducted to determine if this reduced antibiotic prescriptions in children under five in Bangladesh and Mali. There was no statistically significant difference in antibiotic prescription between children with the DEP arm and the control arm (RD − 4.2%, 95% CI − 10.7 to 1.0%) [26].
Bundled interventions
Persuasive and enabling interventions
At a hospital in Nepal, a joint persuasive and enabling intervention involved creating an antimicrobial prescribing guideline and post-prescription review and feedback [27]. Pre-intervention screening was done for 221 patient charts which revealed that 31.6% of antibiotic prescriptions were unjustified [27]. The guideline included empirical and definitive antibiotic therapy and was based on a hospital antibiogram created using hospital antibiotic sensitivities. After the intervention, 230 charts were reviewed and 78% of prescribers followed recommendations made by prescription champions to improve prescriptions, de-escalation and documentation of antibiotic use [27].
In Ethiopia, an audit feedback intervention that recruited 1264 patients over 10 months was used for prescriptions for sepsis, febrile neutropenia, and hospital- and community-acquired pneumonia, in general medicine and pediatric wards [28]. The enabling intervention was antimicrobial prescribing guidelines made into an accessible app, and the persuasive intervention was reviews of the treatment of 25% of admitted patients. Following the discussion of these cases, the antibiotic therapy was either stopped, changed, adjusted for the duration of treatment or a consult with an infectious disease specialist was recommended [28]. After the intervention, there was an increase in days of antibiotic treatment from 8.7 ± 6.9 days to 12.8 ± 11.7 days, and the mean days of therapy per 1000 patient days (DOT/1000) doubled from 754 ± 99.8/1000 patient days to 1549 ± 175.2/1000 patient days [28]. There was a 20% increase in length of stay from 19.8 ± 12.0 days during the intervention period, to 24.1 ± 13.9 days after the intervention (p < 0.001) and an increase in all-cause mortality from 6.9 to 14.7% post-intervention (p < 0.01) [28]. The authors attributed these findings to an increased knowledge of second-line antibiotics, and their increased use leading to longer hospital stays. The increase in all-cause mortality was attributed to the absence of infectious disease specialist involvement during the intervention. These specialists likely provided critical treatment advice to reduce mortality during the intervention [28].
In Cambodia, bundled AMS interventions were implemented in a paediatric hospital [29]. The enabling intervention was transforming antimicrobial prescription guidelines to a mobile phone app and the persuasive intervention was using point prevalence surveys to inform antibiotic prescriptions [29]. The most common hospital-acquired infections were hospital-acquired pneumonia, ventilator-associated pneumonia, lower and upper respiratory tract infection, and necrotizing enterocolitis in neonates [29]. This study noted a 75% increase in appropriate antibiotic prescriptions and a downward trend in mortality after antimicrobial surveillance started.
In Malawi, a quasi-experimental study was done to reduce cephalosporin use in adults at a tertiary hospital [30]. A multidisciplinary team supervised the intervention which involved a baseline prescription survey, creating an antibiotic treatment guideline available as a mobile application, a post-implementation survey, and prescription feedback given by infectious disease specialists. Blood cultures were done to monitor sensitivity patterns and Salmonella Typhi, Salmonella Typhimurium and Klebsiella pneumoniae were the most isolated pathogens. The outcome was a 27% reduction in the use of third-generation cephalosporins and a 9% decline in prescriptions that were made without an indicated focus of infection among 203 patient charts that were screened [30]. Although there was no difference in mortality and length of stay, the intervention was estimated to have saved about $15,000 in the costs of antibiotics [30].
Structural and restrictive interventions
A study in Uganda that used structural and restrictive interventions targeting surgical site infections from cesarean sections highlighted the value of wound care as part of IPC to prevent AMR [31]. The structural intervention was swabbing post-cesarean section wounds for culture and sensitivity to guide antibiotic prescription. Following a year of the intervention, 90% of patients suspected to have sepsis had culture and sensitivity performed on wounds from their wounds [31]. This was attributed to a new policy that restricted the prescription of high-end antibiotics to those necessitated by culture and sensitivity. Apart from clinical outcomes, the study reported improved care of surgical wounds and collaboration between clinicians, nurses, laboratory staff, and pharmacists as important outcomes which contributed to AMS in the post-natal ward [31]. Another outcome was that the procurement of antibiotics changed to reflect reported antibiotic sensitivities [31].
Structural and enabling interventions
In Tanzania a tertiary hospital combined IPC and AMS programs for post-caesarean section and surgical site infections for 1377 patients [32]. The intervention involved appropriate pre and post-operative antibiotic administration, infection prevention measures during surgery, and training in AMS and IPC [32]. The result was a decrease in overall surgical site infections during the post-intervention survey, with a decrease in gram-positive infections (OR 0.263; 95% CI 0.126–0.548; p < 0.001) and a decrease in the prevalence of methicillin-resistant S. aureus (MRSA) from 79 to 21.4% (OR 0.072; 95% CI 0.016–0.314; p < 0.001) [32].
Researchers in Liberia used structural and enabling interventions to introduce AMS to three hospitals [33]. The structural intervention was establishing a microbiology laboratory for culture and sensitivity, available to the three hospitals. The enabling intervention was the creation of a multidisciplinary AMS team to create prescription guidelines, and train prescribers during AMS ward rounds that occurred three times a week. Despite high use of empirical antibiotics and challenges in adopting prescribing practices, the structural changes meant that a blood culture was conducted for 79.7% of patients suspected to have an infectious disease [33].
Resources for antimicrobial stewardship practices
Antimicrobial surveillance was key to formulating locally relevant AMS protocols. In Tanzania, surveillance revealed high E. coli resistance, particularly against trimethoprim-sulfamethoxazole and penicillin’s [32, 34]. In Nepal, the majority of isolates were E. coli (42%), and Klebsiella spp. (16%) and were highly resistant to penicillin’s and third-generation cephalosporins [27]. Third-generation cephalosporins were antibiotics with high resistance to 75% of clinical isolates [29].
Three studies described creating a multidisciplinary team as a key feature of the AMS intervention. Hall et al. described a team of physicians and nurses selected to monitor the implementation of AMS practices [24]. In Joshi et al. physician champions, who were doctors in medical, surgical, and obstetric specialties, were trained to monitor prescription patterns within their respective wards [27]. In one study in Ethiopia, pharmacists led the surveillance and production of AMS protocols together with an infectious disease specialist [28]. Our findings complement a systematic review which showed that pharmacist led intervention improved adherence to prescription guidelines [18].
Risk of bias assessment
Table 2 shows the risk of bias scoring according to the Integrated Quality Criteria for review of Multiple Studies (ICROMS). Two studies did not meet the ICROMS criteria. One qualitative study did not demonstrate the outcomes of the study, and one non-controlled before and after did not sufficiently describe its baseline assessment group [24, 33].
Discussion
This systematic review of AMS practices in least developed and low-income countries showed that only a handful of countries have evaluated AMS in their settings; and that of these practices, bundled interventions including an enabling approach are the most studied. Enabling interventions, where teaching tools are used to guide the choice of antibiotic prescribed, were the most common. Microbiological surveillance revealed the presence of hospital acquired infections and resistance patterns that needed to be addressed and was an important tool to provide feedback to clinicians and policymakers at hospital level. None of the studies evaluated the impact of AMS on AMR.
These results showed that multidisciplinary involvement reinforced judicious prescription practices among clinicians, when pharmacists and nurses were involved in developing AMS protocols. Feedback on prescription practices can occur at ward, laboratory, and pharmacy levels and can be provided by nurses, clinical officers, pharmacists, or doctors [35, 36]. Pharmacy-led interventions, when pharmacists participated in patient care during ward rounds and in formulating AMS protocols, have demonstrated a reduction in inappropriate prescribing and better adherence to AMS protocols [36]. This demonstrates the importance of multi-disciplinary AMS teams to improve patient care and can be of particular benefit in settings with staff shortages [37, 38]. Dedicated AMS team members, like “AMS champions”, at critical levels of health care in low-resource settings can be used to advocate for, and implement measures that can improve the practice of AMS protocols in these settings [27, 39].
Several studies incorporated IPC measures into their AMS programs to reduce hospital-acquired infections [29, 31]. Incorporating IPC in surgical care is a recurring theme in curbing AMR that resulted in reduced surgical site infections and MRSA infections [32, 40]. In surgical settings, enabling AMS interventions were combined with structural changes to patient management, leading to improved wound care and reduced recurrent hospital infections. Similarly, studies in Kenya, Uganda, Zambia and South Africa used antiseptic pre- and post-operative antiseptics for patients to decrease the likelihood of post-operative surgical site infections [41, 42]. The COVID-19 pandemic demonstrated how important IPC measures are for both medical and surgical patients, and should form an important part of any AMS program [37, 43, 44].
The included studies created their own AMS protocols based on a biogram from their own surveillance data to understand local resistance patterns [24, 27, 31]. Although microbiology is important so that hospital-specific organisms can be targeted, many low-income settings lack the laboratory infrastructure to support this [45] and more than 40% of African countries have no data on AMR [8]. The WHO Access, Watch, and Reserve (AWaRe) protocol guides antibiotic prescribing according to the risk of resistance, and this can be used to develop hospital-level AMS protocols [46], and be incorporated into mobile apps like those used in Ethiopia and Cambodia [28, 29]. In the absence of a biogram, this could be an accessible, evidence-based way to guide prescriptions.
Although all studies reported positive outcomes of varying degrees, after applying AMS strategies, it is difficult to extrapolate the impact on large populations because of the varying nature of the interventions. A 2022 systematic review involving high and middle-income countries found that AMS interventions reduced the length of stay, and days of antibiotic treatment [17]. In resource-limited settings, the lack of data on improvements in mortality and length of hospital stay reflect the complex nature of the diseases that occur [24, 30]. To make studies comparable and generalizable to large populations, outcomes from AMS interventions should not be limited to judicious antimicrobial use alone, but could also include clinical, microbiological, and cost-effectiveness data [13].
Incorporating feedback on prescriptions was a key mechanism in AMS practices. In all settings, providing constant feedback to prescribers about the appropriateness of their prescriptions was important to achieve sustained appropriate prescribing and improvement of clinical outcomes. In Nepal, 78% of prescription recommendations were adopted and feedback had a positive impact on patient outcomes [27]. While there were reported decreases in hospital-acquired infections and surgical site infections in Cambodia and Tanzania [29, 32], studies in Nepal and Ethiopia reported an increase in days of antibiotic use and hospital stay after prescription training [27, 28]. This indicates the need to create mechanisms for constant feedback on prescription practices at all levels of health care. This also shows that even without microbiological surveillance, reporting on new infections and complications might inform health institutions on the effect of AMS practices.
At national and regional levels, feedback mechanisms should include reporting AMR patterns as the lack of these data has impeded the development of interventions to address AMR [8]. The WHO Global Antimicrobial resistance and use Surveillance System (GLASS) can be used by researchers and policymakers to monitor AMR trends and guide AMS protocols at national policy level [1].
This systematic review was limited by the paucity of data on AMS in least developed and low-income countries. The included studies did not use standardized metrics or report on patient outcomes consistently and few provided quantitative data on outcomes, including AMR, as has been discussed extensively in other reviews [10, 42]. Therefore, we were unable to quantify the impact of AMS protocols on the populations studied. To address this in future studies, we suggest standardized reporting of outcomes of AMS interventions. This means reporting on a minimum set of outcomes such as baseline and follow-up patterns of AMR (microbiological outcomes), reporting clinical outcomes reflected by patients’ baseline and follow-up length of stay and mortality rates (patient outcomes), and reporting changes in prescription practices (prescription outcomes). This would make it easier to compare results from different studies and help decision-makers in health institutions to decide on which interventions would benefit their population.
Conclusion
In conclusion, AMS has been demonstrated to be effective in high-income countries in addressing AMR, but we identified only a handful of studies evaluating AMS interventions in least developed and low-income countries. None evaluated the impact of AMS on AMR. Clinicians and policymakers looking to implement AMS interventions in resource-constrained settings could consider firstly, creating multidisciplinary AMS teams incorporating infection prevention strategies in clinical wards and surgical theatres. Secondly, where possible, it is important to have antimicrobial surveillance. This could be done continuously where resources are available or at pre-specified time points to guide formulation of AMR guidelines which should be available physically and electronically. Regular and constructive feedback from the health care team, nurses and pharmacists included, could improve the performance of the clinical team. Information on clinical complications and the cost of changing antibiotics could be included in reports of AMR patterns. Lastly, we suggest that studies on AMS interventions should have standardized reporting on microbiological, clinical, and prescription practice outcomes. This would make results comparable and help policymakers to decide on which interventions would suit their setting.
Availability of data and materials
All information used for data extraction and quality analysis is available upon request to the corresponding author.
References
World Health Organization. Priorities on antimicrobial resistance. 2022.
Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55.
Makoka MH, Miller WC, Hoffman IF, Cholera R, Gilligan PH, Kamwendo D. Bacterial infections in Lilongwe, Malawi: aetiology and antibiotic resistance. BMC Infect Dis. 2012;12(1):67.
Gandra S, Tseng KK, Arora A, Bhowmik B, Robinson ML, Panigrahi B, et al. The mortality burden of multidrug-resistant pathogens in India: a retrospective. Observational Study. 2019;69(4):563–70.
Berndtson AE. Increasing globalization and the movement of antimicrobial resistance between countries. Surg Infect. 2020;21:579–85.
Leopold SJ, Van Leth F, Tarekegn H, Schultsz C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. J Antimicrob Chemother. 2014;69:2337–53.
Fenta T, Engidawork E, Amogne W, Beyene BA. Evaluation of current practice of antimicrobial use and clinical outcome of patients with pneumonia at a tertiary care hospital in Ethiopia: a prospective observational study. PLOS ONE. 2020;15(1):1–18.
Tadesse BT, Ashley EA, Ongarello S, Havumaki J, Wijegoonewardena M, González IJ, et al. Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis. 2017;17(1):1–17.
Medugu N, Iregbu K, Tam PI, Obaro S. Aetiology of neonatal sepsis in Nigeria, and relevance of Group b streptococcus: a systematic review. PLOS ONE. 2018;13(7):1–16. https://doi.org/10.1371/journal.pone.0200350%0AEditor.
Abo YN, Freyne B, Kululanga D, Bryant PA. The impact of antimicrobial stewardship in children in low- and middle-income countries: a systematic review. Pediatr Infect Dis J. 2022;41(3):S10–7.
Torres NF, Chibi B, Kuupiel D, Solomon VP, Mashamba-Thompson TP, Middleton LE. The use of non-prescribed antibiotics; prevalence estimates in low-and-middle-income countries: a systematic review and meta-analysis. Arch Public Heal. 2021;79(1):1–15.
Belachew SA, Hall L, Erku DA, Selvey LA. No prescription? No problem: drivers of non-prescribed sale of antibiotics among community drug retail outlets in low and middle income countries: a systematic review of qualitative studies. BMC Public Health. 2021;21(1):1–13.
Van DC, Arnoldine J. Antibiotic stewardship interventions in hospitals in low-and middle- income countries: a systematic review. World Heal Organ. 2017;2018:266–80.
Foxlee ND, Townell N, Heney C, McIver L, Lau CL. Strategies used for implementing and promoting adherence to antibiotic guidelines in low-and lower-middle-income countries: A systematic review. Trop Med Infect Dis. 2021;6(3):166.
Wade T, Roberts N, Ban JW, Waweru-Siika W, Winston H, Williams V, et al. Utility of healthcare-worker-targeted antimicrobial stewardship interventions in hospitals of low- and lower-middle-income countries: a scoping review of systematic reviews. J Hosp Infect. 2023;131:43–53. https://doi.org/10.1016/j.jhin.2022.09.008.
Davey P, Ca M, Cl S, Charani E, Mcneil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients (Review) summary of findings for the main comparison. Cochrane Database Syst Rev. 2017; Art. No. (2).
Sadeq AA, Hasan SS, AbouKhater N, Conway BR, Abdelsalam AE, Shamseddine JM, et al. Exploring antimicrobial stewardship influential interventions on improving antibiotic utilization in outpatient and inpatient settings: a systematic review and meta-analysis. Antibiotics. 2022;11(10):1306.
Mzumara GW, Mambiya M, Iroh Tam PY. Antimicrobial stewardship interventions in least developed and low-income countries: A systematic review protocol. BMJ Open. 2021;11(8):1–6.
Salvador E, Helena S. DAC list of ODA recipients effective for reporting on 2018, 2019 and 2020 flows. 2020.
Moher D, Liberati A, Tetzlaff J, Altman DGTPG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(6):e1000097.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLOS Med. 2021;18(3):1–15.
Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, et al. PRISMA for abstracts: Reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10(4):e1001419.
Zingg W, Secci FV, Edwards R, Drumright LN, Sevdalis N, Holmes AH. Innovative tools for quality assessment: integrated quality criteria for review of multiple study designs. Public Health. 2015;133:19–37. https://doi.org/10.1016/j.puhe.2015.10.012.
Hall JW, Bouchard J, Bookstaver PB, Haldeman MS, Kishimbo P, Mbwanji G, et al. The Mbeya antimicrobial stewardship team: implementing antimicrobial stewardship at a zonal-level hospital in Southern Tanzania. Pharmacy. 2020;8:107.
Nauriyal V, Rai SM, Joshi RD, Thapa BB, Kaljee L, Prentiss T, et al. Evaluation of an antimicrobial stewardship program for wound and burn care in three hospitals in Nepal. Antibiotics. 2020;9(12):1–11.
Nelson EJ, Khan AI, Keita AM, Brintz BJ, Keita Y, Sanogo D, et al. Improving antibiotic stewardship for diarrheal disease with probability-based electronic clinical decision support: a randomized crossover trial. JAMA Pediatr. 2022;176(10):973–9.
Joshi RD, Zervos M, Kaljee LM, Shrestha B, Maki G, Prentiss T, et al. Evaluation of a hospital-based post-prescription review and feedback pilot in Kathmandu. Nepal Am J Trop Med Hyg. 2019;101(4):923–8.
Gebretekle GB, Haile Mariam D, Abebe Taye W, Mulu Fentie A, Amogne Degu W, Alemayehu T, et al. Half of prescribed antibiotics are not needed: a pharmacist-led antimicrobial stewardship intervention and clinical outcomes in a referral hospital in Ethiopia. Front Public Heal. 2020;8(April):1–11.
Hearn P, Miliya T, Seng S, Ngoun C, Day NPJ, Lubell Y, et al. Prospective surveillance of healthcare associated infections in a Cambodian pediatric hospital. Antimicrob Resist Infect Control. 2017;6:1–9.
Lester R, Haigh K, Wood A, Macpherson EE, Maheswaran H, Bogue P, et al. Sustained reduction in third-generation cephalosporin usage in adult inpatients following introduction of an antimicrobial stewardship program in a large, urban hospital in Malawi. Clin Infect Dis. 2020;71(9):E478–86.
Ackers L, Ackers-johnson G, Seekles M, Odur J, Opio S. Opportunities and challenges for improving anti-microbial stewardship in low- and middle-income countries; lessons learnt from the maternal sepsis intervention in Western Uganda. Antibiotics. 2020;9(6):315.
Gentilotti E, De Nardo P, Nguhuni B, Piscini A, Damian C, Vairo F, et al. Implementing a combined infection prevention and control with antimicrobial stewardship joint program to prevent caesarean section surgical site infections and antimicrobial resistance: A Tanzanian tertiary hospital experience. Antimicrob Resist Infect Control. 2020;9(1):1–11.
Alabi AS, Picka SW, Sirleaf R, Ntirenganya PR, Ayebare A, Correa N, et al. Implementation of an antimicrobial stewardship programme in three regional hospitals in the south-east of Liberia: lessons learned. JAC-Antimicrobial Resist. 2022;4(3):dlac069.
Hall JW, Bouchard J, Bookstaver PB, Haldeman MS, Kishimbo P, Mbwanji G, et al. The Mbeya antimicrobial stewardship team: implementing antimicrobial stewardship at a zonal-level hospital in southern Tanzania. Pharmacy. 2020;8(2):107.
Gebretekle GB, Mariam DH, Abebe W, Amogne W, Tenna A, Fenta TG, et al. Opportunities and barriers to implementing antibiotic stewardship in low and middle-income countries: lessons from a mixed-methods study in a tertiary care hospital in Ethiopia. PLOS ONE. 2018;13(12):1–15.
Otieno PA, Campbell S, Maley S, Obinju Arunga T, Otieno Okumu M. A systematic review of pharmacist-led antimicrobial stewardship programs in sub-Saharan Africa. Int J Clin Pract. 2022;2022.
Kanj SS, Ramirez P, Rodrigues C. Beyond the pandemic: the value of antimicrobial stewardship. Front Public Heal. 2022;10(June):1–5.
Charani E, Smith I, Skodvin B, Perozziello A, Lucet JC, Lescure FX, et al. Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries—A qualitative study. PLOS ONE. 2019;14(1):1–20.
Nguyen KV, Thi Do NT, Chandna A, Nguyen TV, Pham CV, Doan PM, et al. Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health. 2013;13:1158.
Dramowski A, Aucamp M, Beales E, Bekker A, Cotton MF, Fitzgerald FC, et al. Healthcare-associated infection prevention interventions for neonates in resource-limited settings. Front Pediatr. 2022;10(July):1–12.
Allegranzi B, Aiken AM, Zeynep Kubilay N, Nthumba P, Barasa J, Okumu G, et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before–after, cohort study. Lancet Infect Dis. 2018;18(5):507–15.
Brink AJ, Messina AP, Feldman C, Richards GA, Becker PJ, Goff DA, et al. Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis. 2016;16(9):1017–25. https://doi.org/10.1016/S1473-3099(16)30012-3.
Stoessera N, Emarya K, Soklina S, Peng-Ana K, Sophala S, Chhomratha S, Dayb NPJ, Limmathurotsakulb D, Ngeta P, Pangnaritha Y, Sonaa S, Kumara V, Moorea CE, Chanpheaktra N, Parrya CM. The value of intermittent point-prevalence surveys of healthcare-associated infections for evaluating infection control interventions at Angkor Hospital for Children, Siem Reap, Cambodia. Trans R Soc Trop Med Hyg. 2013;9(5):248–53.
Peters A, Schmid MN, Parneix P, Lebowitz D, De Kraker M, Sauser J. Impact of environmental hygiene interventions on healthcare—associated infections and patient colonization: a systematic review. Antimicrob Resist Infect Control. 2022;11:38. https://doi.org/10.1186/s13756-022-01075-1.
Kimbowa IM, Ocan M, Eriksen J, Nakafeero M, Obua C, Lundborg CS, et al. Characteristics of antimicrobial stewardship programmes in hospitals of Uganda. PLoS One. 2022;17(5 May):1–14.
The World Health Organisation. The WHO AWaRe Antibiotic Book. วารสารวิชาการมหาวิทยาลัยอีสเทิร์นเอเชีย. 2022; 4:88–100. Available from: https://www.who.int/publications/i/item/9789240062382.
Acknowledgements
Not applicable.
Funding
This work was supported by a Wellcome Trust Programme Grant (grant number 091909/Z/10/Z) and the Malawi-Liverpool-Wellcome Programme Core Award (grant number 206454) from the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
GM and PI conceptualized the scope of the systematic review. GM and MM were independent reviewers and PI was the third reviewer. GM wrote the manuscript and PI reviewed and provided feedback for the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search Strategy.
Additional file 2.
PRISMA 2020 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mzumara, G.W., Mambiya, M. & Iroh Tam, PY. Protocols, policies and practices for antimicrobial stewardship in hospitalized patients in least-developed and low-income countries: a systematic review. Antimicrob Resist Infect Control 12, 131 (2023). https://doi.org/10.1186/s13756-023-01335-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01335-8