Abstract
Background
Electronic continuous surveillance databases are ideal for monitoring antibiotic use (ABU) in hospitalised patients for antibiotic stewardship programmes (ASP). However, such databases are scarce in low-resource settings. Point prevalence surveys (PPS) are viable alternatives. This report describes ABU and identifies ASP implementation improvement areas in Limpopo Province, South Africa.
Methods
This cross-sectional descriptive study extracted patient-level ABU data from patients’ files using a modified global PPS tool. Data were collected between September and November 2021 at five regional hospitals in Limpopo Province, South Africa. All patients in the wards before 8 a.m. on study days with an antibiotic prescription were included. Antibiotic use was stratified by Anatomic Therapeutic Chemical and Access, Watch, Reserve classifications and presented as frequencies and proportions with 95% confidence intervals (CI). Associations between categorical variables were assessed using the chi-square test. Cramér’s V was used to assess the strength of these associations.
Results
Of 804 inpatients surveyed, 261 (32.5%) (95% CI 29.2–35.7) were prescribed 416 antibiotics, 137 were female (52.5%) and 198 adults (75.9%). One hundred and twenty-two (46.7%) patients received one antibiotic, 47.5% (124/261) received two, and 5.7% (15/261) received three or more antibiotics. The intensive care units had a higher ABU (68.6%, 35/51) compared to medical (31.3%, 120/384) and surgical (28.5%, 105/369) wards (p = 0.005, Cramér’s V = 0.2). Lower respiratory tract infection (27.4%, 104/379), skin and soft tissue infections (SST) (23.5%, 89/379), and obstetrics and gynaecology prophylaxis (14.0%, 53/379) were the common diagnoses for antibiotic prescriptions. The three most prescribed antibiotic classes were imidazoles (21.9%, 91/416), third-generation cephalosporins (20.7%, 86/416) and combination penicillin (18.5%, 79/416). Access antibiotics accounted for 70.2% (292/416) of prescriptions and Watch antibiotics for 29.6% (123/416) (p = 0.110, Cramér’s V = 0.1). Reasons for prescribing and treatment plans were documented in 64.9% (270/416) (95% CI 60.3–69.5) and 21.4% (89/416) (95% CI 17.3–25.3) of prescriptions, respectively.
Conclusions
The study serves as a baseline for ABU surveillance at the five regional hospitals in Limpopo Province. Lack of documentation indicates poor prescribing practices; ASP should address gaps by deploying evidence-based, multifaceted and stepwise interventions.
Similar content being viewed by others
Introduction
The global increase and variation in antibiotic use (ABU) [1, 2] drive the increasing global public health threat of antibiotic resistance (ABR) [3]. Even though ABR is naturally occurring, its progression is accelerated by inappropriate ABU [2, 3]. Compared to other burden of disease regions in the world, Sub-Saharan Africa (SSA) had the highest all-age death rates associated with ABR, exceeding 75 per 100,000 [1, 4]. The high ABR in SSA could be attributed to a combination of One Health domain factors, such as limited access to clean water, sanitation, and hygiene, leading to a high prevalence of priority infectious diseases such as lower respiratory tract (LRTI) and bloodstream infections [2, 5]. Factors such as poor governance, limited health financing, poverty and limited access to health services also contribute to high ABR in SSA [5, 6]. Globally, one in three hospitalised patients receives an antibiotic, however there is global variance, with low and middle-income countries having higher ABU than high-income countries [7, 8] and one in every two hospitalised patients in SSA receiving an antibiotic [1, 8, 9]. The primary cause of high ABU in SSA, in particular, can be attributed to limited hospital antibiotic stewardship programme (ASP) implementation [10,11,12,13], inappropriate prescribing (e.g., unnecessary prescribing, inappropriate selection when an antibiotic is indicated, and incorrect dosing, formulation, route of administration and treatment duration) and empirical prescribing due to a lack of or non-adherence to treatment guidelines and limited microbiological testing due to inadequate laboratory facilities, resulting in a vicious cycle of a causal-effect relationship between ABU and ABR development [1, 14, 15].
Increasing ABU necessitates measures to ensure optimal use and monitoring [2, 3, 16]. In the 2015-global action plan, the World Health Organization (WHO) recognised antibiotic stewardship as one of three pillars of a comprehensive plan for strengthening health systems and global response to antibiotic resistance, infection prevention, control, and patient medicine safety [3, 16]. Global evidence suggests that implementing hospital ASPs—a set of coordinated interventions to improve, measure, and encourage access to appropriate ABU [17]—is associated with a reduction in ABU in both hospitalised and non-hospitalised patients [10]. Antibiotic stewardship interventions are implemented by ASPs with the objectives of optimising ABU, improving patient outcomes, and lowering antibiotic resistance and healthcare costs [18]. Hospital ASPs rely on ABU surveillance data, driven from data sources such as hospital files, health insurance or pharmacy dispensing records comprising patient-level details and indications to determine current ABU levels and guide future quality improvement plans [16, 19, 20].
An electronic continuous surveillance database will be ideal for collecting ABU surveillance data to guide evidenced-based ASP interventions; however, such databases are scarce in low-resource settings [19, 21]. Without electronic continuous databases to describe ABU and prescribing quality, point prevalence surveys (PPS) are useful alternative methods [21, 22]. The PPS method is used globally to describe ABU according to the Anatomic Therapeutic Chemical (ATC) and Access, Watch, Reserve (AWaRe) classifications [23,24,25], develop and evaluate prescribing and patient care quality indicators, identify local clinical practice areas for ASP quality improvement plans, and evaluate ASP interventions in hospitalised patients [7, 8, 11, 24,25,26]. Quality indicators are objective, evidence-based healthcare measures that may be utilised with hospital administrative data to assess and monitor clinical performance and outcomes [27]. ASP interventions could control antibiotic resistance through optimal ABU when combined with ABU surveillance data and quality indicators [10, 27].
There is evidence of ASP in the South African public and private sectors [22, 28,29,30,31,32,33,34,35,36]; however, implementation in public sector facilities is inadequate [30, 32,33,34,35]. Where ASP has been implemented, ABU surveillance has been implemented the least often compared to policy and guideline implementation and the existence of antibiotic stewardship committees [37]. Antibiotic use surveillance data has previously been collected using the PPS method in South African public sector hospitals [22, 28, 38,39,40], but ABU surveillance data remain inadequate [40, 41]. There is an over-representation of tertiary hospitals in PPS from previous global [7, 8, 11, 23] and national studies [38, 40], which could distort the reported ABU. Antibiotic use in regional hospitals in South Africa, including the Limpopo Province, is sub-optimal. Earlier national studies contained data from two regional hospitals, with the highest ABU of 76.3% when compared to other levels of care: central (29.6%), tertiary (37.3%), district (43.1%) and a countrywide ABU of 33.6% [38, 40]. To contribute knowledge on ABU surveillance data, we aimed to describe ABU to inpatients according to the ATC and AWaRe classification, describe antibiotic prescribing using quality indicators and identify ASP quality improvement areas in regional hospitals in Limpopo Province, South Africa.
Methods
Study design and setting
A cross-sectional descriptive research design was used to collect data from hospitalised patients' files from the Limpopo Province's five regional hospitals (Letaba Hospital, Mokopane Hospital, Tshilidzini Hospital, Philadelphia Hospital, and Saint Rita’s Hospital) between 07 September and 16 November 2021. Regional hospitals in the South African public sector context are secondary care facilities that operate on a 24-h basis [42]. Each of the five regional hospitals provides specialist services in paediatrics and neonatal care, obstetrics and gynaecology, anaesthesia, surgery and orthopaedics, internal medicine, psychiatry, and family medicine to 500 000–1.5 million district residents, referral services for six to seven district hospitals, and 50 to 150 primary healthcare facilities [43]. The regional hospitals receive outreach and support from tertiary hospitals and have between 200 and 800 beds [43]. The total inpatient bed capacity for the five hospitals is 1463 [43]. Therefore, the regional hospitals used in our study served as an intermediate setting to help monitor ABU in patients admitted to public sector hospitals in Limpopo Province.
Data collection and management
A paper-based PPS data collection tool was developed using the global PPS and WHO-PPS as the basis [44, 45], with additional patient care indicators based on a multidisciplinary, international consensus on generic inpatient quality indicators that may be used globally to evaluate the quality of antibiotic use among hospitalised patients [46].
The study population consisted of all patients admitted to the hospital ward before 8 a.m. on the day of the PPS with at least one systemic antibiotic prescribed. To prevent denominator inaccuracies, all patients admitted after 8 a.m. on the day of the survey were excluded. Due to the unstable and continual transfer of patients to the wards or other hospitals, patients admitted to the emergency care department were excluded.
On the antibiotic level, this study included antibacterials for systemic use (oral, parenteral, rectal, inhalation) and antiprotozoals (i.e., nitroimidazole derivatives) used as antibacterial agents. Topical antibiotics, antimycobacterials, antivirals and antifungals were excluded. All wards at the five study centres were included and categorised according to their activities: medical, surgical, and intensive care units. All outpatient departments were excluded.
The files of hospitalised patients with an antibiotic prescription were used as a data source for patient-level demographic and antibiotic prescription data. The demographic data collected for each patient on antibiotic therapy comprised age, weight and sex. For each antibiotic prescribed, the dose, route (i.e., oral, parenteral, rectal, inhalation), prescriber type (i.e., specialised, general practitioner), diagnosis, indication, and treatment start date were collected. The diagnosis of the prescribed antibiotics was collected by applying standardised categories adopted from the global PPS protocol [44, 45]. The source of infection was also categorised based on standardised definitions and included community-acquired infection, healthcare-acquired infection, surgical prophylaxis, medical prophylaxis, and other unclear indications. If the antibiotic indication was surgical prophylaxis, an extra variable indicating the period of surgical prophylaxis was collected. The period of surgical prophylaxis was categorised as follows: one dose, one day, and more than one day (prolonged surgical antibiotic prophylaxis (SAP)).
A set of prescribing quality indicators was also collected for each prescribed antibiotic: antibiotic written in the generic name (“yes/no”), prescribing reason documented in notes (“yes/no”), South African standard treatment guidelines (STGs) adherence (“yes/no/not assessable/no information”), antibiotic treatment plans documented (“yes/no”) and whether prescribed treatment was empirical or targeted. Additional patient care indicators adapted from a global consensus study [46] were also collected for each patient on antibiotic therapy: monitoring the administration of prescribed antibiotics, specimens collected for pathogen identification, availability of results in the patient file if a specimen was collected, switching of antibiotic therapy from intravenous to oral therapy based on clinical condition within 48 to 72 h, and dosage adjustment to renal function (assessed based on trough creatinine levels and creatinine clearance). All patient care indicators data were categorised as “no/yes/unknown/not applicable”. The final two patient care indicators were missed doses (the total number of antibiotic doses missed since prescribing) and reasons for missed doses (stock-out, hang-time or unknown).
To minimise denominator complexity caused by new admissions and discharges, ward data collection was completed on the same day. We visited surgical wards the day after elective surgical interventions were scheduled to collect data about prophylaxis in the previous 24 h. Data from each hospital had to be collected within one week and during weekdays.
Data analysis
Data were analysed using Statistical Package for the Social Sciences (SPSS®) version 28. Patient demographics and hospital characteristics were reported as frequencies and proportions stratified by sex (male/female), age (adults (above 18 years)/paediatric (18 years and younger), healthcare-associated infections risk factors, bed occupancy rate, and ward type. In this study, ABU was determined at the patient level (i.e., the number of inpatients with at least one antibiotic prescribed) and at the antibiotic level (total number of prescribed antibiotics) and was stratified by ward activity, source of infection, diagnosis, ATC and AWaRe classifications. The overall, mono or combination therapy and ward activity ABUs were determined by dividing the number of patients who received an antibiotic by the total number of eligible patients (admitted). ABU by ward activity was categorised as medical, surgical and intensive care units. The antibiotic per patient ratio was determined by dividing the total number of prescribed antibiotics by the number of patients with at least one prescribed antibiotic on survey days.
ABU frequencies and proportions were classified according to ATC classification level four and patient age groups and stratified by the source of infection (i.e., community-acquired infection) and diagnoses/site of infection (i.e., LRTI). To determine commonly prescribed antibiotics, individual antibiotic agents were ranked from the highest to the lowest according to their prescribing frequency and assigned their respective ATC level 4 and AWaRe classifications. ABU frequencies and proportions were also described according to the AWaRe classification and stratified by hospital, ward activity, age, prescriber type, source of infection and diagnosis. At an antibiotic level, overall antibiotic prescribing quality indicators were described using frequencies and proportions of the "yes" choice. The "yes, no, unknown, not applicable" choices were used to determine patient-level indicators of patient care quality using frequency and proportions.
For statistical inferences, ABU, prescribing and patient care indicators were represented as frequencies and proportions with 95% confidence intervals. We used the Chi-square test with a p-value of 0.05 for statistical significance to assess the relationship between categorical variables (e.g., between ABU and ward activity and between Access and Watch antibiotic groups and ward activity, age group and prescriber type). For practical significance interpretation, Cramér's V ≥ 0.6 was deemed a strong association, 0.3–0.5 moderate, 0.1–0.2 weak and < 0.1 no association.
Results
Patient demographics and hospital characteristics
In the five hospitals, 804 of the 1463 available beds were occupied, resulting in a bed occupancy rate of 55.0%. In 2020, the five hospitals had 340 660 inpatient days. Two-hundred and sixty-one (261) of the 804 hospitalised patients satisfied the study's inclusion criteria (inpatients with at least one antibiotic prescription) (Table 1).
Baseline demographic data are shown in Table 2. Most patients were female (52.5%, 137/261) and adults (above 18 years) (75.9%, 198/261) (Table 2). The study population had the following healthcare-associated infection risk factors: urinary catheters (35.3%, 92/261), surgery since admission (22.6%, 59/261), or were transferred from another hospital (21.8%, 57/261) (Table 1).
Overall antibiotic use
Table 3 presents a summary of the overall ABU; of the 804 admitted patients, 261 were prescribed at least one antibiotic, giving an ABU of 32.5% (95% CI 29.2–35.7), with a total of 416 antibiotic prescriptions, yielding an antibiotic per patient ratio of 1.6: 1. One hundred and twenty-two (46.7%) patients received one antibiotic, compared to 124 (47.5%) on two and 15 (5.7%) on three or more antibiotics. Of the five study sites, Hospital C had the highest ABU at 39.5% (47/119) (95% CI 30.7–48.3) and Hospital E had the lowest ABU at 28.9% (46/159) (95% CI 21.9–35.9). The intensive care units had a higher ABU (68.6%, 35/51) compared to the medical (31.3%, 120/384) and surgical (28.5%, 105/369) wards (Table 4). Overall, there was a statistically significant relationship between ABU and ward type (p = 0.000), with a weak association between the two variables (Cramér’s V = 0.2) (Table 4). In terms of medical activity wards, Hospital B had the lowest ABU (15/103, 14.6%), and Hospital C had the highest ABU (28/58, 48.3%). Hospital E had the lowest ABU (13/78, 16.7%), and Hospital B had the highest ABU (40/89, 44.9%) in surgical activity wards.
Antibiotic use according to the Anatomical Therapeutic Chemical classification level 4, indications and diagnosis
The most common indication for antibiotic prescriptions was community-acquired infection (55.5%, 231/416), followed by healthcare-acquired infection (17.8%, 74/416) and surgical prophylaxis (14.2%, 59/416) (Table 5). The majority of macrolides (88.9%, 16/18), three-quarters (77.2%, 61/79) of combination penicillin and two-thirds (66.3%, 57/86) of third-generation cephalosporins (3GCs) prescriptions were for community-acquired infection. All prescriptions for carbapenems (n = 13) were indicated for healthcare-acquired infection. The most prevalent antibiotic classes in SAP were extended-spectrum penicillins (31.0%, 22/71) and imidazole derivatives (29.7%, 27/91), whereas the majority (94.7%, 18/19) of sulphonamides-trimethoprim combinations were prescribed for medical prophylaxis (Table 5), for adults with human immunodeficiency virus infection (Additional file 1: Table S1). Community-acquired infection was the most common indication in both adults (191/321, 59.6%) and paediatrics (42.1%, 40/95) (Additional file 1: Table S1). The second common indication was healthcare-acquired infection in adults (17.1%, 55/321) and medical prophylaxis in paediatrics (32.6%, 31/95) (Additional file 1: Table S1).
Table 6 presents the ten common diagnoses for antibiotic prescriptions and their antibiotic classes (ATC level 4). LRTIs (27.4%, 104/379), SST (23.5%, 89/379), and obstetrics and gynaecology prophylaxis (14.0%, 53/379) were the three most common diagnoses for antibiotic prescriptions. Overall, imidazoles (22.7%, 86/379) were the most frequently prescribed antibiotic class in the top 10 common diagnoses list, followed by 3GCs (19.5%, 74/379) and combination beta-lactam penicillins (18.7%, 71/379). Combination penicillin (32.7%, 34/104), 3GCs (28.9%, 30/104), and macrolides (13.5%, 14/104) were the most frequently prescribed antibiotic classes for LRTI. Combination penicillins (30.3%, 27/89), imidazoles (27.0%, 24/89) and 3GCs (24.7%, 22/89) were the most frequently prescribed antibiotic classes for skin and soft tissue (SST) diagnosis. For prophylaxis in obstetrics and gynaecology, nearly half (49.1%, 26/53) of prescribed antibiotics were imidazoles, and extended-spectrum penicillin contributed two-fifths (41.5%, 22/53) of the antibiotic prescriptions. Adults had the same top three diagnoses as the total sample, while paediatrics had medical prophylaxis in neonates (42.3%, 30/71), LRTI (26.8%, 19/71), and sepsis (15.5%, 11/71) (Additional file 1: Table S1).
Commonly prescribed antibiotic classes and agents according to the Anatomical, Therapeutic Chemical classification
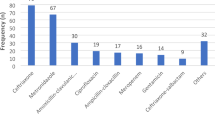
The top three antibiotic classes prescribed were imidazoles (21.9%, 91/416), 3GC (20.7%, 86/416) and combination penicillins (18.5%, 79/416) (Table 5). The overall five commonly prescribed antibiotic agents (ATC level 5) were metronidazole (21.9%, 91/416), ceftriaxone (20.7%, 86/416), amoxicillin with an enzyme inhibitor (18.5%, 77/416), ampicillin (12.7%, 53/416) and gentamycin (7.2%, 30/416) (Table 7). The top five antibiotics prescribed to children and adults were similar, except for gentamycin (28.0%, 23/82) and meropenem (8.5%, 7/82) in paediatrics and sulphamethoxazole-trimethoprim (6.6%, 18/272) in adults (Additional file 1: Table S1).
Antibiotic use by 2021 World Health Organization AWaRe classification
The antibiotic prescribing pattern according to the WHO AWaRe classification is summarised in Table 8. Access antibiotics accounted for 70.2% (292/416) of prescribed antibiotics, while Watch antibiotics accounted for 29.6% (123/416). Access antibiotics were prescribed at a relatively high proportion in surgical wards (77.8%, 133/171), while Watch antibiotics were prescribed at a relatively high proportion in medical wards (37.4%, 70/187). A statistically significant relationship was found between ward activity and the prevalence of Access and Watch antibiotics (p = 0.005), with a weak association between the variables (Cramér’s V = 0.2). Paediatric patients were prescribed a higher proportion of Access antibiotics (79.0%, 75/95), while adult patients were prescribed a higher proportion of Watch antibiotics (32.1%, 103/321). The relationships between age groups and the prescribing prevalence of Access and Watch antibiotics were statistically significant (p = 0.000), with a strong association between the variables (Cramér’s V = 1.0).
Quality antibiotic prescribing and patient care indicators
Indicators for antibiotic prescribing and the quality of patient care are presented in Table 9. One-third (32.9%, 137/416) of antibiotic prescriptions were written using the generic name. Most (87.7%, 365/416) (95% CI 84.6–90.9) antibiotic prescriptions were parenteral formulations. A third (33.9%, 20/59) (95% CI 21.8–46.0) of surgical prophylaxis antibiotic prescriptions were prolonged beyond 24 h. The national STGs were adhered to in 93.3% (388/416) (95% CI 90.9–95.7) of prescriptions. Reasons for prescribing were documented in 64.9% (270/416) (95% CI 60.3–69.5) of antibiotic prescriptions, while the documentation of antibiotic treatment plans (stop/review date) was 21.4% (89/416) (95% CI 17.5–25.3). Only 3.4% (14/416) (95% CI 1.6–5.1) of antibiotic prescriptions targeted specific pathogens.
Diagnostic specimens were taken in only 11.9% (31/261) (95% CI 8.0–15.8) of the patients, whereas results were available in 45.2% (14/31) (95% CI 27.6–62.7) of the cases. However, 95.7% (250/261) (95% CI 93.5–98.2) of patients were current on antibiotic therapy, totalling 41 missing doses. Thirty-six of 261 patients (13.8%) (95% CI 9.6–18.0) were identified for probable intravenous to oral switch therapy, whereas 17 patients (6.5%) (95% CI 3.5–9.5) were identified for renal dose adjustment, and six patients (2.5%) (95% CI 0.5–4.1) were identified for therapeutic drug monitoring.
Discussion
This study used an adapted PPS tool to describe ABU in five public sector regional hospitals in Limpopo Province, South Africa, as a baseline for future comparison. Antibiotics were prescribed to approximately one-third (32.5%) of patients, which is similar to earlier South African public sector studies (31.0% and 33.6%) [39, 40]. The controlled public sector medicine procurement that implements national STGs may explain the similarities between ABU in our study and earlier South African studies [39, 47]. The ABU in this study was also compared to global trends in hospitalised adults (34.4%) and paediatrics (36.7%) [7, 8].
One hundred and twenty-two (46.7%) patients received one antibiotic, 124 (47.5%) received two, and 15 (5.7%) received three or more antibiotics. Carefully chosen combination therapy is useful, and reporting it may indicate ASP intervention areas [22, 38,39,40].
In our study, the intensive care unit ABU was the highest (68.6%), followed by medical (31.5%) and surgical (28.5%) wards. A similar trend was observed in a Western Cape Province tertiary hospital [39]. The intensive care unit antibiotic ABU (68.6%) in our study is consistent with the global prevalence (70.0%) [48]. We did not investigate the existence, functionality, or effect of ASP interventions. Therefore, it is unclear what contributed to the variation in ABU between the five study sites and ward activity [10]. Globally, 54.0% of intensive care unit patients are suspected or confirmed to be infected with Gram-negative organisms, associated with a 30.0% mortality [48], which may explain the high ABU in intensive care units in this study. Consequently, ASPs should promote the timely selection and administration of appropriate antibiotic therapy and de-escalation of empiric broad-spectrum antibiotics to reduce adverse patient outcomes [49].
Similar to global trends [8], the most common indication for antibiotic prescriptions in our study was community-acquired infections, followed by healthcare-acquired infections and surgical antibiotic prophylaxis, with variability between adults and paediatrics. Most prescriptions of broad-spectrum antibiotics were prescribed for community-acquired infections in accordance with the national STG [47].
Overall LRTIs, SST, obstetrics and gynaecology prophylaxis were the most common diagnoses for antibiotic prescriptions. This was comparable to previous South African studies [22, 39]. Globally, LRTIs, SST, and sepsis were the most common diagnostic reasons for antibiotic prescriptions in hospitalised patients [8, 11]. The high prevalence of LRTI diagnoses in this study corroborates global trends [50, 51]. In this study, LRTI antibiotic therapy mainly comprised a combination penicillin, 3GCs, and macrolides and is in line with national treatment guidelines [47, 52, 53].
Globally, including in South Africa and Limpopo Province, traffic accidents, self-harm, and interpersonal violence are the leading causes of injury to SST [50, 54, 55]. These injuries may result in contaminated open wounds and cellulitis that require antibiotic treatment, which is consistent with the findings of this study, demonstrating that SST was the second most common diagnosis for ABU [54, 55]. The South African STGs recommend using beta-lactams as empirical treatment for SST due to their good activity against Staphylococcus aureus and Streptococcus species [47]. In this study, combination penicillins were the most prescribed antibiotic class in SST. The second antibiotic class for SST was imidazole derivatives, which is concerning as anaerobic cultures were not typically isolated in SST in South Africa, and not aligned to the South African STGs [47, 56].
Imidazoles were the frequently prescribed antibiotic class, followed by 3GCs and combination penicillins, correlating with global trends [2, 8]. The imidazole derivatives were also common antibiotic classes in SSA countries indicated parenterally for SST and obstetrics and gynaecology prophylaxis [9, 47]. In our study, imidazoles were prescribed mainly for prophylaxis in obstetrics and gynaecology, followed by SST and gastrointestinal infections in accordance with the South African STG [47].
The five commonly prescribed antibiotic agents were metronidazole, ceftriaxone, amoxicillin with an enzyme inhibitor, ampicillin and gentamycin, which is consistent with earlier South African studies [38,39,40]. The extensive prescribing of 3GCs (i.e., ceftriaxone) in this study is concerning due to the high 3GCs resistance (70.0%) in bloodstream infections and Klebsiella pneumonia predominance in South Africa [57]. Meropenem was in the top five paediatric antibiotics (possibly for sepsis [21]), which is concerning given the high prevalence (80.0%) of carbapenem-resistant Acinetobacter baumannii in South Africa [57].
According to the WHO's 2021 AWaRe classification [23], 70.2% of prescribed antibiotics in our study were from the Access group. This is consistent with local antibiotic sales data [58]. The Watch ABU in our study was 29.2%, similar to national findings in adults (30.0%) and paediatrics (27.8%) [38, 40]. Ward activity and age group were statistically associated with ABU of Access and Watch antibiotics.
Antibiotic prescribing quality indicators are important for identifying the appropriateness of prescribing and establishing and monitoring the effectiveness of ASP interventions [7]. Parenteral route antibiotic prescriptions (87.7%) in this study was higher than global trends (~ 72–79%) [7, 8] and early South African studies ranging from 46 to 76% [22, 38, 40]. The high parenteral antibiotic prescribing in our study requires ASPs to develop and implement early intravenous to oral switch therapy guidelines (particularly for amoxicillin with an enzyme inhibitor and metronidazole) to minimise risks associated with catheter-related healthcare-acquired infections, high healthcare costs, and extended length of hospital stay [59,60,61,62].
In our study, there were 59 prescriptions for SAP, of which 34.5% were prolonged (> 24 h), which is lower than findings from early South African adult (73.2%) and paediatric (66.7%) studies [38, 40]. Prolonged SAP is a global concern in terms of inappropriate use for the prevention of surgical site infections [63]. The preventative efficacy of SAP is widely demonstrated in international guidelines [64]; however, prolonged SAP does not provide an advantage to surgical site infection care and may increase the risk of adverse events such as acute renal injury and Clostridium difficile infection; hence, an area for persuasive prospective audit and feedback ASP interventions to reduce SAP[10, 65, 66].
The adherence to antibiotic prescribing to clinical guidelines is associated with improved patient outcomes, including decreased mortality, length of stay, improved quality and lower costs in the direction of targeted prescribing [67, 68]. The adherence to national STGs was 93.3% in our study, which was similar to findings from previous public sector South African studies (90.2% and 98.0%) [22, 39] and higher than global benchmark (77.4%) [8]. The controlled public sector medicine procurement using national STGs and essential medicines lists may explain the high adherence to national STGs [47].
The documentation of antibiotic prescribing reasons was 64.9%, which is lower than the global benchmark (76.9%) [8] and findings at a Western Cape Province tertiary hospital (86.0%) [39], but comparable to hospitalised adults (64.3%) nationwide [40]. In this study, 32.9% of antibiotic prescriptions were written using non-proprietary names. The documentation of antibiotic reasons and prescribing by their non-proprietary name facilitates communication between healthcare professionals and enables the monitoring of treatment plans [8, 16]. The documentation of antibiotic treatment plans was 21.4%, double the 11.0% from a Western Cape Province tertiary hospital [39], but below the global benchmark (38.3%) [8]. The treatment plan prescribing indicator promotes therapy review within 48 to 72 h to avoid inappropriate (extended period, appropriate agent and route of administration) ABU[8]. Adherence to treatment plan documentation was low in our study and could be an area for a persuasive educational and administrative ASP intervention to encourage practice change [16, 69].
In our study, the proportion of antibiotic prescriptions targeting specific pathogen(s) was 3.4%, lower than global levels (9.8% to 22.3%) [7, 8, 26], as well as previous South African studies (8.3% to 28.8%) [38, 40]. The low-targeted prescribing observed together with low specimen collection (11.9%) and fewer results (45.2%) in our study may be attributable to a lack of point-of-care diagnostics and inadequate laboratory capacity, which may result in inappropriate diagnosis and antibiotic therapy [1, 14, 15].
Our study revealed additional areas for clinical ASP interventions, including intravenous to oral switch therapy, renal-dose adjustment, and therapeutic drug monitoring, which are considered advanced and costly ASP interventions [16]. Finally, our study commenced on 7 September 2021, at the tail-end of the Delta wave of the Coronavirus disease of 2019 (COVID-19) pandemic in South Africa and ended on 16 November 2021, before the Omicron variant wave began (23 November 2023) [70]; however, ABU was similar to pre-pandemic studies in South Africa [40]. Modelling of global antibiotic sales data shows an increase of 0.3% per 1000 individuals for a 10% increase in COVID-19 cases [71]. Given that our study was conducted between two COVID-19 infection waves [70], we believe that the pandemic may not have had a significant influence on the ABU during our study period.
The following limitations were noted in this study. A PPS is limited by its cross-sectional study design since data were collected over a short period; this approach did not account for seasonal variation in diseases and antibiotic prescriptions. A more accurate estimate of inpatient ABU may have been obtained by conducting this survey over several periods using serial or a seasonal repeated PPS. This study was conducted in regional (secondary) hospitals, and its findings cannot be generalised to other levels of care (primary or tertiary hospitals) as global and national evidence suggests ABU heterogeneity by hospital type [8, 38, 40]. Our findings cannot be generalised to the private sector settings, considering that antibiotic prescribing and STGs adherence vary between the two sectors in South Africa [72].
Abbreviations
- ATC:
-
Anatomic Therapeutic Chemical
- 3GC:
-
Third-generation cephalosporin
- ABU:
-
Antibiotic use
- ASP:
-
Antibiotic Stewardship programme
- AWaRe:
-
Access, Watch, Reserve
- COVID-19:
-
Coronavirus disease of 2019
- LRTI:
-
Lower respiratory tract infection
- PPS:
-
Point prevalence survey
- SAP:
-
Surgical antibiotic prophylaxis
- SSA:
-
Sub-Saharan Africa
- SST:
-
Skin and soft tissue
- STG:
-
Standard treatment guideline
- WHO:
-
World Health Organization
References
Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EPA, Hamadani BHK, Zaraa S, et al. Global antibiotic consumption and usage in humans, 2000–2018: a spatial modelling study. Lancet Planet Health. 2021;5(12):e893-904. https://doi.org/10.1016/S2542-5196(21)00280-1.
Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463–70. https://doi.org/10.1073/pnas.1717295115.
World Health Organization. Global action plan on antimicrobial resistance. https://apps.who.int/iris/handle/10665/193736. Accessed 20 Dec 2022.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55. https://doi.org/10.1016/S0140-6736(21)02724-0.
Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018;2(9):e398–405. https://doi.org/10.1016/S2542-5196(18)30186-4.
Allel K, Day L, Hamilton A, Lin L, Furuya-Kanamori L, Moore CE, et al. Global antimicrobial-resistance drivers: an ecological country-level study at the human-animal interface. Lancet Planet Health. 2023;7(4):e291–303. https://doi.org/10.1016/S2542-5196(23)00026-8.
Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, ARPEC project group. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71(4):1106–17. https://doi.org/10.1093/jac/dkv418.
Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, Jarlier V, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–29. https://doi.org/10.1016/S2214-109X(18)30186-4.
Saleem Z, Godman B, Cook A, Khan MA, Campbell SM, Seaton RA, et al. Ongoing efforts to improve antimicrobial utilization in hospitals among African countries and Implications for the future. Antibiotics. 2022;11(12):1824. https://doi.org/10.3390/antibiotics11121824.
Zay Ya K, Win PTN, Bielicki J, Lambiris M, Fink G. Association between antimicrobial stewardship programs and antibiotic use globally: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(2):e2253806. doi:https://doi.org/10.1001/jamanetworkopen.2022.53806.
Pauwels I, Versporten A, Vermeulen H, Vlieghe E, Goossens H. Assessing the impact of the Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS) on hospital antimicrobial stewardship programmes: results of a worldwide survey. Antimicrob Resist Infect Control. 2021;10(1):138. https://doi.org/10.1186/s13756-021-01010-w.
Howard P, Pulcini C, Levy Hara G, West RM, Gould IM, Harbarth S, et al. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2015;70(4):1245–55. https://doi.org/10.1093/jac/dku497.
Akpan MR, Isemin NU, Udoh AE, Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist. 2020;22:317–24. https://doi.org/10.1016/j.jgar.2020.03.009.
Siachalinga L, Godman B, Mwita JC, Sefah IA, Ogunleye OO, Massele A, et al. Current Antibiotic Use Among Hospitals in the sub-Saharan Africa Region. Findings and Implications Infect Drug Resist. 2023;16:2179–90. https://doi.org/10.2147/IDR.S398223.
Abubakar U, Salman M. Antibiotic use among hospitalized patients in Africa: a systematic review of point prevalence studies. J Racial Ethn Health Disparities. 2023;8:1–22. https://doi.org/10.1007/s40615-023-01610-9.
World Health Organization. Antimicrobial stewardship programmes in healthcare facilities in low- and middle-income countries: a WHO practical toolkit. https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf?sequence=1&isAllowed=y. Accessed 20 Dec 2022.
Dyar OJ, Huttner B, Schouten J, Pulcini C; ESGAP (ESCMID Study Group for Antimicrobial stewardship). What is antimicrobial stewardship? Clin Microbiol Infect. 2017;23(11):793–8. https://doi.org/10.1016/j.cmi.2017.08.026.
Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8:35. https://doi.org/10.1186/s13756-019-0471-0.
Godman B, Egwuenu A, Wesangula E, Schellack N, Kalungia AC, Tiroyakgosi C, et al. Tackling antimicrobial resistance across sub-Saharan Africa: current challenges and implications for the future. Expert Opin Drug Saf. 2022;21(8):1089–111. https://doi.org/10.1080/14740338.2022.2106368.
Moulin E, Boillat-Blanco N, Zanetti G, Plüss-Suard C, de Vallière S, Senn L. Point prevalence study of antibiotic appropriateness and possibility of early discharge from hospital among patients treated with antibiotics in a Swiss University Hospital. Antimicrob Resist Infect Control. 2022;11(1):66. https://doi.org/10.1186/s13756-022-01104-z.
Kruger D, Dlamini NN, Meyer JC, Godman B, Kurdi A, Lennon M, et al. Development of a web-based application to improve data collection of antimicrobial utilization in the public health care system in South Africa. Hosp Pract (1995). 2021;49(3):184–93. https://doi.org/10.1080/21548331.2021.1889213.
Dlamini NN, Meyer JC, Kruger D, Kurdi A, Godman B, Schellack N. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa: a pilot study and implications. Hosp Pract (1995). 2019;47(2):88–95. https://doi.org/10.1080/21548331.2019.1592880.
World Health Organization. 2021 AWaRe classification. https://www.who.int/publications/i/item/2021-aware-classification. Accessed 21 Dec 2022.
World Health Organization collaborating centre for drug statistics methodology. ATC/DDD Index 2023. https://www.whocc.no/atc_ddd_index/. Accessed 14 Feb 2023.
Pauwels I, Versporten A, Drapier N, Vlieghe E, Goossens H; Global-PPS network. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother. 2021;76(6):1614–24. https://doi.org/10.1093/jac/dkab050.
Prusakov P, Goff DA, Wozniak PS, Cassim A, Scipion CEA, Urzúa S, et al. A global point prevalence survey of antimicrobial use in neonatal intensive care units: The no-more-antibiotics and resistance (NO-MAS-R) study. EClinicalMedicine. 2021;32:100727. https://doi.org/10.1016/j.eclinm.2021.100727.
Campbell SM, Wettermark B, Andersen, M. Defining and developing quality indicators for drug utilization. In: Elseviers M, Wettermark B, Almarsdóttir AB, Andersen M, Benko R, et al., editors. Drug utilization research: methods and applications. Oxford. Wiley; 2016. p. 126–138.
Skosana PP, Schellack N, Godman B, Kurdi A, Bennie M, Kruger D, et al. A national, multicentre web-based point prevalence survey of antimicrobial use in community healthcare centres across South Africa and the implications. Hosp Pract (1995). 2022;50(4):306–17. https://doi.org/10.1080/21548331.2022.2114251.
Boyles TH, Naicker V, Rawoot N, Raubenheimer PJ, Eick B, Mendelson M. Sustained reduction in antibiotic consumption in a South African public sector hospital; Four-year outcomes from the Groote Schuur Hospital antibiotic stewardship program. S Afr Med J. 2017;107(2):115–8. https://doi.org/10.7196/SAMJ.2017.v107i2.12067.
Brink AJ, Messina AP, Feldman C, Richards GA, van den Bergh D; Netcare Antimicrobial Stewardship Study Alliance. From guidelines to practice: a pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J Antimicrob Chemother. 2017;72(4):1227–34. https://doi.org/10.1093/jac/dkw523.
Chetty S, Reddy M, Ramsamy Y, Dlamini VC, Reddy-Naidoo R, Essack SY. Antimicrobial Stewardship in Public-Sector Hospitals in KwaZulu-Natal, South Africa. Antibiotics. 2022;11(7):881. https://doi.org/10.3390/antibiotics11070881.
Chetty S, Reddy M, Ramsamy Y, Naidoo A, Essack S. Antimicrobial stewardship in South Africa: a scoping review of the published literature. JAC Antimicrob Resist. 2019;1(3):dlz060. https://doi.org/10.1093/jacamr/dlz060.
Junaid E, Jenkins L, Swanepoel H, North Z, Gould T. Antimicrobial stewardship in a rural regional hospital—growing a positive culture. S Afr Med J. 2018;108(7):546–50. https://doi.org/10.7196/SAMJ.2018.v108i7.13149.
van den Bergh D, Messina AP, Goff DA, van Jaarsveld A, Coetzee R, de Wet Y, et al. A pharmacist-led prospective antibiotic stewardship intervention improves compliance to community acquired pneumonia guidelines in 39 public and private hospitals across South Africa. Int J Antimicrob Agents. 2020;56(6):106189. https://doi.org/10.1016/j.ijantimicag.2020.106189.
Engler D, Meyer JC, Schellack N, Kurdi A, Godman B. Compliance with South Africa’s Antimicrobial Resistance National Strategy Framework: are we there yet? J Chemother. 2021;33(1):21–31. https://doi.org/10.1080/1120009X.2020.1789389.
Peters SM, Sheik S, Werner JL, Davies MA, Willems B. Antimicrobial stewardship in the Western Cape: a situational analysis of existing facility-level initiatives. S Afr Med J. 2021;111(5):421–5. https://doi.org/10.7196/SAMJ.2021.v111i5.14645.
Charani E, Castro-Sanchéz E, Bradley S, Nathwani D, Holmes AH, Davey P. Implementation of antibiotic stewardship in different settings - results of an international survey. Antimicrob Resist Infect Control. 2019;8:34. https://doi.org/10.1186/s13756-019-0493-7.
Skosana PP, Schellack N, Godman B, Kurdi A, Bennie M, Kruger D, Meyer JC. A national, multicentre, web-based point prevalence survey of antimicrobial use and quality indices among hospitalised paediatric patients across South Africa. J Glob Antimicrob Resist. 29:542–50. https://doi.org/10.1016/j.jgar.2021.12.003.
Finlayson, H., Versporten, A., Whitelaw, A., Goossens, H, Taljaard J. The global point prevalence survey of antimicrobial consumption and resistance (Global-PPS): results of antimicrobial prescribing in a South African tertiary hospital. 2016. https://www.global-pps.com/wp-content/uploads/ECCMID-2016_South-Africa.pdf. Accessed 14 Jan 2023.
Skosana PP, Schellack N, Godman B, Kurdi A, Bennie M, Kruger D, et al. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev Anti Infect Ther. 2021;19(10):1353–66. https://doi.org/10.1080/14787210.2021.189894610.
Engler D, Meyer JC, Schellack N, Kurdi A, Godman B. Antimicrobial Stewardship activities in public healthcare facilities in South Africa: a baseline for future direction. Antibiotics (Basel). 2021;10(8):996. https://doi.org/10.3390/antibiotics10080996.
Limpopo Department of Health (South Africa). Annual performance plan 2018/19 2018. Available from: Limpopo Department of Health (South Africa). Annual performance plan 2018. https://provincialgovernment.co.za/department_annual/809/2019-limpopo-health-annual-report.pdf. Accessed 10 Jan 2023.
National Health Act 2003, Republic of South Africa. National Health Act 2003: Regulations: Categories of hospitals. https://www.gov.za/documents/national-health-act-regulations-categories-hospitals. Accessed 20 Dec 2022.
Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (2019 GLOBAL-PPS). https://www.global-pps.com/wp-content/uploads/2019/02/Global-PPS-2019-protocol.pdf. Accessed 20 Aug 2020.
World Health Organization. WHO methodology for point prevalence survey on antibiotic use in hospitals version 1.1. https://apo.who.int/publications/i/item/WHO-EMP-IAU-2018.01. Accessed 20 Aug 2020.
Monnier AA, Schouten J, Le Maréchal M, Tebano G, Pulcini C, Stanic Benic M, et al. Quality indicators for responsible antibiotic use in the inpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother. 2018;73(suppl_6):vi30–9. https://doi.org/10.1093/jac/dky116.
National Department of Health, Republic of South Africa. Hospital-level (adults) standard treatment guidelines and essential medicines list 2nd edition – 2019. https://www.knowledgehub.org.za/elibrary/hospital-level-adults-standard-treatment-guidelines-and-essential-medicines-list-2nd. Accessed 10 Jan 2023.
Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–87. https://doi.org/10.1001/jama.2020.2717.
Kollef MH, Shorr AF, Bassetti M, Timsit JF, Micek ST, Michelson AP, et al. Timing of antibiotic therapy in the ICU. Crit Care. 2021;25(1):360. https://doi.org/10.1186/s13054-021-03787-z.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. https://doi.org/10.1016/S0140-6736(20)30925-9.
GBD 2019 LRI Collaborators. Age-sex differences in the global burden of lower respiratory infections and risk factors, 1990–2019: results from the Global Burden of Disease Study 2019. Lancet Infect Dis. 2022 Nov;22(11):1626–47. https://doi.org/10.1016/S1473-3099(22)00510-2.
Boyles TH, Brink A, Calligaro GL, Cohen C, Dheda K, Maartens G, et al. South African guideline for the management of community-acquired pneumonia in adults. J Thorac Dis. 2017;9(6):1469–502. https://doi.org/10.21037/jtd.2017.05.31.
Zar HJ, Moore DP, Andronikou S, Argent AC, Avenant T, Cohen C, et al. Diagnosis and management of community-acquired pneumonia in children: South African Thoracic Society guidelines. Afr J Thorac Crit Care Med. 2020;26(3). https://doi.org/10.7196/AJTCCM.2020.v26i3.104.
Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Glass T, et al. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. Lancet Glob Health. 2016;4(9):e642–53. https://doi.org/10.1016/S2214-109X(16)30113-9.
Achoki T, Sartorius B, Watkins D, Glenn SD, Kengne AP, Oni T, et al. Health trends, inequalities and opportunities in South Africa’s provinces, 1990–2019: findings from the Global Burden of Disease 2019 Study. J Epidemiol Community Health. 2022;76(5):471–81. https://doi.org/10.1136/jech-2021-217480.
Makwela AB, Grootboom WM, Abraham V, Witika B, Godman B, Skosana PP. Antimicrobial management of skin and soft tissue infections among surgical wards in South Africa: Findings and implications. Antibiotics (Basel). 2023;12(2):275. https://doi.org/10.3390/antibiotics12020275.
National Department of Health, Republic of South Africa. Surveillance for antimicrobial resistance and consumption of antimicrobials in South Africa. https://www.knowledgehub.org.za/system/files/elibdownloads/2022-06/AMR%20and%20AMC%20report%20for%202021%20in%20South%20African_June2022.pdf. Accessed 21 Dec 2022.
Mthombeni TC, Burger JR, Lubbe MS, Julyan M. Antibiotic consumption by Access, Watch and Reserve index in public sector of Limpopo province, South Africa: 2014–2018. S Afr J Infect Dis. 2022;37(1):463. https://doi.org/10.4102/sajid.v37i1.463.
Babonji A, Darwesh B, Al-Alwai M. Implementation of pharmacist-managed early switch from intravenous to oral therapy using electronic identification at a tertiary academic hospital. Saudi Pharm J. 2021;29(4):324–36. https://doi.org/10.1016/j.jsps.2021.03.006.
Gasparetto J, Tuon FF, Dos Santos OD, Zequinao T, Pipolo GR, Ribeiro GV, et al. Intravenous-to-oral antibiotic switch therapy: a cross-sectional study in critical care units. BMC Infect Dis. 2019;19(1):650. https://doi.org/10.1186/s12879-019-4280-0.
McCarthy K, Avent M. Oral or intravenous antibiotics? Aust Prescr. 2020;43(2):45–8. https://doi.org/10.18773/austprescr.2020.008.
Béïque L, Zvonar R. Addressing Concerns about Changing the Route of Antimicrobial Administration from Intravenous to Oral in Adult Inpatients. Can J Hosp Pharm. 2015;68(4):318–26. https://doi.org/10.4212/cjhp.v68i4.1472.
Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e288–303. https://doi.org/10.1016/S1473-3099(16)30402-9.
Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e276–87. https://doi.org/10.1016/S1473-3099(16)30398-X.
Branch-Elliman W, O’Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019;154(7):590–8. https://doi.org/10.1001/jamasurg.2019.0569.
Sefah IA, Denoo EY, Bangalee V, Kurdi A, Sneddon J, Godman B. Appropriateness of surgical antimicrobial prophylaxis in a teaching hospital in Ghana: findings and implications. JAC Antimicrob Resist. 2022;4(5):dlac102. doi:https://doi.org/10.1093/jacamr/dlac102.
Campbell SM, Meyer JC, Godman B. Why compliance to national prescribing guidelines is important especially across Sub-Saharan Africa and suggestions for the future. J Biomed Pharm Sci. 2021;4(6):316.
Wathne JS, Harthug S, Kleppe LKS, Blix HS, Nilsen RM, Charani E, et al. The association between adherence to national antibiotic guidelines and mortality, readmission and length of stay in hospital inpatients: results from a Norwegian multicentre, observational cohort study. Antimicrob Resist Infect Control. 2019;8:63. https://doi.org/10.1186/s13756-019-0515-5.
Nnadozie UU, Umeokonkwo CD, Maduba CC, Igwe-Okomiso D, Onah CK, Madubueze UC, et al. Antibiotic use among surgical inpatients at a tertiary health facility: a case for a standardized protocol for presumptive antimicrobial therapy in the developing world. Infect Prev Pract. 2020;2(4):100078. https://doi.org/10.1016/j.infpip.2020.100078.
Al Hasan SM, Saulam J, Mikami F, Kanda K, Yokoi H, Hirao T. COVID-19 outbreak trends in South Africa: A comparison of Omicron (B.1.1.529), Delta (B.1.617.2), and Beta (B.1.351) variants outbreak periods. J Infect Public Health. 2022;15(7):726–33. https://doi.org/10.1016/j.jiph.2022.05.011.
Nandi A, Pecetta S, Bloom DE. Global antibiotic use during the COVID-19 pandemic: analysis of pharmaceutical sales data from 71 countries, 2020–2022. EClinicalMedicine. 2023;57:101848. https://doi.org/10.1016/j.eclinm.2023.101848.
Mohlala G, Peltzer K, Phaswana-Mafuya N, Ramlagan S. Drug prescription habits in public and private health facilities in 2 provinces in South Africa. East Mediterr Health J. 2010;16(3):324–8.
Elshenawy RA, Umaru N, Alharbi AB, Aslanpour Z. Antimicrobial stewardship implementation before and during the COVID-19 pandemic in the acute care settings: a systematic review. BMC Public Health. 2023;23(1):309. https://doi.org/10.1186/s12889-023-15072-5.
Acknowledgements
The authors acknowledge the cooperation and support they received from all Limpopo Department of Health healthcare workers at the five study centres during data collection. The authors also acknowledge Prof Marike Cockeran of the North-West University Statistical Consultations Services for undertaking data analysis.
Funding
Open access funding provided by North-West University. The study did not receive any funding.
Author information
Authors and Affiliations
Contributions
TM designed the study. JB, ML and MJ were involved in planning and supervising the study. TM collected and prepared data for statistical analysis. JB and ML assisted in interpreting the results. TM drafted the first version of the manuscript. All authors provided essential feedback on the quality research process and data analysis for the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval of the study was obtained from the Health Research Ethics Committee of the North-West University (NWU-00312-20-A1), with participants’ consent waived. Data collection access permission was granted by the Head of the Limpopo Department of Health (LP-202003-012).
Consent for publication
Not applicable.
Competing interests
TM. works as an administrator at the central office of the Limpopo Department of Health and is not a practising health professional at any of the study centres. The other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1: Antibiotic use among inpatients at five regional hospitals in Limpopo Province, stratified by patient age.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mthombeni, T.C., Burger, J.R., Lubbe, M.S. et al. Antibiotic prescribing to inpatients in Limpopo, South Africa: a multicentre point-prevalence survey. Antimicrob Resist Infect Control 12, 103 (2023). https://doi.org/10.1186/s13756-023-01306-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01306-z