Abstract
Background
Rapid emergence of multidrug resistant Staphylococcus aureus has resulted to difficulty in treatment of infections caused by such strains. The aim of this meta-analysis study was to determine the pooled prevalence of resistance of S. aureus to different antibiotics in Nigeria.
Methods
Literature search for studies was done using Google scholar, PubMed, Science direct, and African Journal Online. The prevalence of S. aureus resistance to different antibiotics was evaluated using the meta-analysis proportion command in MedCalc software version 20.0 adopting a rand effect model. I2 statistic and Egger test in MedCalc was used to evaluate the heterogeneity and the presence of publication bias among studies respectively.
Results
A total of 40, 682 studies were retrieved through the database search of which 98 studies met the study inclusion criteria. Prevalence of resistance of S. aureus to different antibiotics ranges from 13 to 82%. Results showed a very high degree of resistance to penicillin G (82% [95% confidence interval (CI) 61%, 0.96%]), cloxacillin (77% [95% CI 64%, 88%]), amoxacillin (74% [95% CI 66%, 81%]), cefuroxime (69% [95% CI 51%, 85%]), ampicillin (68% [95% CI 53%, 81%]). Moderately resistance to erythromycin (47% [95% CI 40%, 53%]), chloramphenicol (47% [95% CI 37%, 56%]), methicillin (46% [95% CI 37%, 56%]), ofloxacin (24% [95% CI 18%, 31%]) and rifampicin 24% [95% CI 6%, 48%]). Low resistance was observed in vancomycin 13% (95% CI 7%, 21%). For each individual meta-analysis, high heterogeneity was observed with I2 range (79.36–98.60%) at p-values ≤ 0.01). Egger’s tests for regression intercept in funnel plots indicated no evidence of publication bias.
Conclusion
This meta-analysis study established that S. aureus in Nigeria has developed resistance to commonly used antibiotics such as the beta-lactam class antibiotics, sulphonamides, tetracyclines, chloramphenicol, and vancomycin. Hence it is imperative to develop programs to promote rational use of antimicrobial agents, infection prevention and control to reduce the incidence of antimicrobial resistance.
Similar content being viewed by others
Background
Staphylococcus aureus (S. aureus) is well adapted to various environments due to their metabolic versatility and pharmic resistance ability. S. aureus colonize the skin and nasopharyngeal membranes as normal microbiota in healthy individuals [1]. However, they cause myriad of detrimental infections when they invade the internal tissues or enter the bloodstream. S. aureus is an important pathogen involved in both hospital-acquired and community-acquired infections and causes many infectious diseases ranging from mild skin and soft tissue infections, bones and joint infections, infective endocarditis, cardiovascular disorders, osteomyelitis, bacteremia, and fatal pneumonia in both healthy and individuals with underlying diseases [2]. The high incidence of both community and nosocomial staphylococcal infections coincide with the emergence of multidrug resistant S. aureus which renders antibiotic treatments ineffective [3].
S. aureus has become resistant to various antibiotics over the past years especially to the beta-lactam class of antibiotics [4]. Emergence of methicillin resistant S. aureus (MRSA) and vancomycin resistant S. aureus (VRSA) constitutes a serious global public health problem. Currently, VRSA and MRSA strains are classified as very potent and dangerous agents that can potentially cause devastating damage worldwide in the absence of effective treatment options [5].
Various mechanisms of resistance utilized by S. aureus include: production of beta-lactamase enzymes to deactivate beta-lactam antibiotics, efflux pump for extruding antibiotics such as tetracyclines [6], reduced accumulation of macrolides antibiotics [7], production of aminoglycosides modifying enzymes to inactivate aminoglycoside antibiotics, alteration of DNA gyrase and topoisomerase IV expression of floroquinolones antibiotics, and expression of Mec genes which alters penicillin binding proteins [8].
In Nigeria, the prevalence of multi-drug resistant pathogens continue to be on the increase due to several factors such as drug misuse, self medication, lack of trained medical personnel, and poverty. As the world battles the persistent rise in antimicrobial resistance (AMR), it is pertinent that adequate data and information about AMR is known which can serve as the basic foundation for setting out effective interventions to contain the crisis of AMR. From the literature, no prior meta-analysis has been done on S. aureus resistance to different antibiotics routinely use in Nigeria. Due to the various infections caused by S. aureus, it is pertinent to determine the pooled prevalence of resistance of S. aureus to various routinely used antibiotics in Nigeria. This will help in improving treatment options and enlighten the populace on the menace and the possible cause of treatment failures due to the increasing rise of multidrug resistant strains. The aim of this meta-analysis was to determine the pooled prevalence of S. aureus resistance to various routinely used antibiotics in Nigeria.
Methods
Study design
Meta-analysis was adopted to evaluate the prevalence of S. aureus of resistance to various antibiotics in Nigeria using the appropriate studies that rely solely on S. aureus from the title. The prevalence of resistance of S. aureus to various routinely used antibiotics in Nigeria is a country wide study as it covers studies from the six geo-political regions of Nigeria. Meta-analysis was adopted because it is a quantitative study of pooled prevalence of resistance of S. aureus to routinely use antibiotics in Nigeria.
Search strategy
Electronic search engines including Google scholar, PubMed, ScienceDirect, and African Journal Online (AJOL) were used to search for available studies from 23rd March to May 2022. Relevant key words such as Staphylococcus, antibiotic resistance, antibacterial resistance, antimicrobial resistance, drug resistance, drug susceptibility, Nigeria were used during the search. These key words were used in different combinations (Staphylococcus OR S. aureus AND antibiotic resistance OR antibacterial resistance OR antimicrobial resistance OR drug resistance AND Nigeria) in various electronic databases using the Boolean operators. The reference lists of included articles were also check to identify studies relevant to the current study.
Inclusion and exclusion criteria
The titles of search results of all retrieved articles were screened independently by two authors with the aim of including studies that address the research question. The articles were inserted into Zotero version 5.0.95.1 referencing application which helped in detecting duplicate articles. The title of the study which solely focused on prevalence of antimicrobial resistance of S. aureus was grouped as eligible for inclusion. S. aureus resistance in any state in Nigeria and studies only done in Nigeria represented in the title is the first criteria for inclusion. However, studies that focused on many microbial strains antimicrobial resistance were excluded.
In general, retrieved studies selected from predefined criteria were screened further using the inclusion criteria: studies that were research articles and used cross sectional design, studies that used human samples, studies that conducted antimicrobial susceptibility tests using the Clinical Laboratory Standard Institute (CLSI) guidelines, studies written in English language and studies with full text.
Exclusion criteria in this meta-analysis include: studies conducted on non-human samples, studies with isolates below 20, duplicate studies, studies that did not conduct antimicrobial susceptibility tests using the Clinical Laboratory Standard Institute (CLSI) guidelines studies not written in English, and review articles.
Data extraction
Relevant data such as name of author (s) and publication year, study design, study place, clinical sample size, isolate source, total number of Staphylococcus aureus isolates tested in each research article, and total No. of isolates resistant each antibiotics. In situations where the proportion of susceptible isolates was reported, then the No. of resistant Staphylococcus aureus isolates was calculated by subtracting the percentage susceptibility from 100 and then dividing the result by 100 and multiplying to the total number of isolates. However, in situation where the proportion of the resistant isolates was given, then the No. of resistant Staphylococcus aureus isolates was calculated by dividing the proportion of the resistant isolates by 100 and multiply with the total number of isolates. The formula is given as thus:
To ascertain the reporting of all relevant information in this meta-analysis, we followed the Preferred reporting Items for Systematic Review and Meta-analysis (PRISMA) [9] (Additional file 1: S1) guidelines.
Statistical analysis procedures
In this meta-analysis, statistical analyses were performed using MedCalc statistical software version 20.0.1. The pooled prevalence of antibiotic resistance of S. aureus was evaluated using the meta-analysis proportion command in MedCalc. A total of 23 separate meta-analyses were carried out to evaluate the pooled prevalence of S. aureus resistance to 23 different antibiotics. Between 6 and 77 studies were included in the 23 different meta-analyses. I2 statistic command in MedCalc was used to evaluate the heterogeneity among the included studies. Random effect and fixed effect are two models used to estimate pooled prevalence in meta-analysis. In this study, due to the characteristically high heterogeneity of the included studies, the random effect model was used for meta-analysis at 95% CIs. Egger test was employed for assessing the presence of publication bias [10].
The Freeman-Tukey double arcsine transformation was used to ensure studies which report proportions near or at 0 and 1 were not being excluded. In addition, studies that report unusually high prevalence of resistance when compared to others, a sensitivity analysis was perform by removing the studies. If the point estimate of pooled prevalence after removing a study that reported unusually high prevalence of resistance lies within the 95% CI of the overall pooled estimate for all studies combined, the study is considered as having no significant influence on the overall estimate and vice versa.
Results
Characteristics of included studies
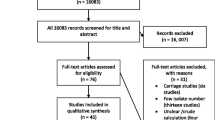
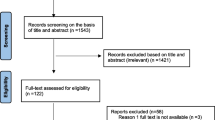
Studies search record from electronic databases yielded 40, 682 of which 35, 400, 2, 180, 1,706, and 1396 were from Google scholar, AJOL, PubMed, and Science Direct, respectively. Articles from Google Scholar gave 35,400 results comprising of many studies irrelevant or that does not fit to the study aim; hence, they were screened randomly from titles alone. Screening of the titles reduced the number of eligible articles to 134 for full text assessment. After going through the full texts, 36 articles were excluded (reported small number of isolates and isolates not from human samples). Thus, 98 studies met the inclusion criteria of the study (Fig. 1).
About 46, 640 S. aureus isolates were tested against different antibiotics and 23,048 isolates were resistant to various antibiotics. Isolates sources include: nasal, blood, vaginal, ear, wound, urine, throat, pimples, hand, and mixed samples were collected from both symptomatic patients [61] and asymptomatic people [37]. Eighty six studies used primary data while twelve used records from hospitals. The characteristics of each study included is summarized Table 1.
Heterogeneity survey and publication bias
The included studies were conducted in the six geo-political zones of Nigeria; a total of 98 studies comprising of 26 from South South, 23 South West, 20 South East, 18 North West, 8 North Central and 3 North East. Quality assessment (risk of bias) was done in line with the following criteria: studies which used CLSI guideline for antibiotic resistant assessment, studies that used more than 20 S. aureus isolates and studies that used adequate sample representative of the region where testing was done. Agar diffusion based method was used to determine the resistance level of S. aureus isolates in all included studies. High heterogeneity was observed for each of the meta-analyses performed with I2 ranging from 79.36 to 98.60%; at p-values ≤ 0.01). This is due to vast difference in sample sizes; some studies used 20 isolates while some used 400 isolates which impacted on the resistance profile of each antibiotic. Also, number of clinical samples and recovered S. aureus isolates differ in all studies and these disparities resulted in high heterogeneity. More studies were conducted in the Southern (South South, South West, and South East) part of Nigeria giving rise to high heterogeneity. Studies were done in different hospitals within these regions with different prevalence estimates. Random sampling was used in most of the studies and different clinical samples were collected. More than one clinical sample per patient was collected in 51 studies while one clinical sample was collected per patient in 47 studies. Egger’s test for a regression intercept gave a p-value range of 0.06 to 0.99, indicating no evidence of publication bias (Additional file 2: S2) following Eggers’ test rule which state that ‘P-value less than 0.05 indicates the presence of publication bias’.
Prevalence of S. aureus resistance to different antimicrobial agents
In this meta-analysis, the pooled prevalence of S. aureus resistance to twenty-three different antibiotics and the number of studies included in each meta-analysis is summarized in Table 2. Prevalence of resistance of S. aureus to each antibiotic based on pharmacological classification is given below for antibiotics routinely used in Nigeria.
Prevalence of resistance S. aureus to rifamycins (rifampicins)
Seven studies involving the prevalence of resistance to rifampicin was analyzed. The pooled prevalence of resistance of S. aureus to rifampicin in Nigeria is 24% (95% confidence interval [CI] 6%, 48%). The forest plot (rifampicin) is presented in Fig. 2.
Prevalence of resistance of S. aureus to glycopeptides (vancomycin)
The pooled prevalence of S. aureus resistance to vancomycin is 13% (95% CI 7%, 21%) and the forest plot is presented in Fig. 3. Sensitivity results after exclusion of four studies [20, 22, 27, 36] that reported high prevalence of S. aureus resistant to vancomycin is 7% (95% CI 3.3%, 12%). Hence, there was significant decrease in poled prevalence.
Prevalence of resistance of S. aureus to beta-lactams antibiotics
Estimation of the pooled prevalence of S. aureus resistance to penicillin antibiotics (penicillin G, methicillin, amoxicillin, cloxacillin, ampicillin, and amoxacilin/caluvanic acid are here presented. Resistance to penicillin G, amoxicillin, cloxacillin, ampicillin, and augmentin were estimated based on 15, 40, 22, 28 and 20 studies respectively. Pooled prevalence resistance rates were highest in penicillin G at 82% (95% CI 61%, 96%). Resistance to cloxacillin [77% (95% CI 64%, 88%)], to amoxicillin [74% (95% CI 66%, 81%)], to ampicillin [68% (95% CI 53%, 81%)] and to amoxacilin/caluvanic [62% (95% CI 50%, 73%)]. However, resistance rate was moderate for methicillin [46% (95% CI 37%, 56%)]. Forest plots for antibiotics (methicillin and penicillin G) resistance are shown in Fig. 4 and 5, respectively while the forest plots for amoxicillin, ampicillin, amoxicillin/clavulanic acid and cloxacillin resistance are presented in Additional file 3: S3, Additional file 4: S4, Additional file 5: S5 and Additional file 6: S6 respectively.
Higher prevalence of resistance among cephalosporin antibiotic was observed in cefuroxime 69% (95% CI 51%, 85%) followed by ceftazidime 61% (95% CI 46%, 75%). Resistance to ceftriaxone is 44% (95% CI 34%, 54%) and to cefoxitine is 43% (95% CI 31%, 546%). The forest plot for ceftriaxone resistance is presented in Fig. 6 while the forest plots for cefuroxime and cefoxitine resistance are presented respectively in Additional file 7: S7 and Additional file 8: S8.
Prevalence of resistance of S. aureus to floroquinolones
Three antibiotics (ciprofloxacin, ofloxacin, and norfloxacilin) from floroquinolones were included in the study. For ciprofloxacin, 44 studies were used to estimate the pooled resistance, 25 were used for ofloxacin and 9 studies were used for norfloxacilin. The pooled prevalence of resistance of S. aureus to ciprofloxacin [31% (95% CI 24%, 38%)], ofloxacin [24% (95% CI 18%, 31%)], and to norfloxacillin [33% (95% CI 17%, 52%)]. The forest plot for ofloxacin resistance is presented in Fig. 7 while the forest plot for ciprofloxacin and norfloxacilin included in Additional file 9: S9 and Additional file 10: S10.
Prevalence of resistance of S. aureus to protein synthesis inhibitors
Tetracycline a reversible protein synthesis inhibitor showed the highest resistance rate [65% 995% CI 56%, 76%)] followed by erythromycin (macrolides) [47% (95% CI 40%, 53%)] and chloramphenicol [47% (95% CI 37%, 56%)], respectively. Aminoglycosides (gentamycin and streptomycin) and lincosamides (clindamycin) showed relatively lower level of resistance. The pooled prevalence of resistance to streptomycin [45% (95% CI 34%, 57%)], to clindamycin [35% (95% CI 23%, 49%)] and to gentamycin [31% (95% CI 25%, 37%)]. The forest plot for chloramphenicol resistance is presented in Fig. 8 while the forest plots for tetracycline, erythromycin, gentamycin, streptomycin, and clindamycin resistance are presented in Additional file 11: S11, Additional file 12: S12, Additional file 13: S13, Additional file 14: S14, and Additional file 15: S15 respectively.
Prevalence of resistance of S. aureus to antimetabolites
High resistance was observed among the antimetabolites antibiotics. Pooled prevalence of S. aureus resistance to cotrimoxazole was found to be 66% (95% CI 55%, 76%) and to trimethoprim is 55% (95% CI 35%, 74%). The forest plot for cotrimoxazole resistance is presented in Fig. 9 while the forest plot for trimethoprim is presented in Additional file 16: S16.
Comparison of the prevalence of S. aureus resistance to different antibiotics
The trend of prevalence of S. aureus resistance to different antibiotics addressed in this meta-analysis is shown in Fig. 10. From observation, the prevalence of resistance of S. aureus to the different antibiotics in this study ranges from 13 (vancomycin) to 82% (penicillin G).
The order of resistance in increasing order based on the pooled prevalence of S.aureus resistance to different antibiotics was observed to be vancomycin, ofloxacin, rifampicin, ciprofloxacilin, gentamycin, norfloxacillin, clindamycin, cefoxitine, ceftriaxone, streptomycin, methicillin, chloramphenicol, erythromycin, trimethoprim, ceftazidim, amoxicillin-clavulanic acid, tetracycline, cotrimoxazole, ampicillin, cefuroxime, amoxacilin, cloxacillin, and pencilin G.
Discussion
Antimicrobial resistance continues to be on the rise which constitutes a serious public health problem globally. Many microbes have developed resistance to many different antimicrobial agents over time. This meta-analysis estimated the pooled prevalence of resistance of Staphylococcus aureus to 23 different antibiotics routinely used in Nigeria. Ninety eight studies [98] were included in this meta-analysis study with variation in the number of studies included in each meta-analysis which ranged from 6 to 77. In general, the 98 studies evaluated the rate of S. aureus resistance to different antibiotics based on 46,640 isolates of which 23, 048 were resistant to various antibiotics. Prevalence of resistance of S. aureus to different antibiotics ranges from 13 to 82%. Results from the meta-analysis showed that resistance of S. aureus to routinely used antibiotics in Nigeria was alarmingly high. From the studies, it was found that 82% S. aureus were resistant to penicillin G. However, it was observed from the studies that 24% of S. aureus were resistant to ofloxacin and rifampicin. In general, clinical samples (nasal, urine, wound, pimple, ear, blood, and vaginal swab) were collected from both symptomatic patients [61] and asymptomatic people [37].
High heterogeneity was observed for each of the meta-analyses performed with I2 ranging from 79.36 to 98.90% at p-values ≤ 0.01). This is because many studies used varying number of isolates/sample sizes. Some studies used 20 isolates while some used 400 isolates which impacted on the resistance profile of each antibiotic. This can better be illustrated in the prevalence of resistance of S. aureus to vancomycin. Sensitivity test was carried out to by removing studies that reported very high prevalence of S. aureus to vancomycin and the overall pooled prevalence reduced from 13 to 7%. This showed the degree of heterogeneity among studies. Possible cause of heterogeneity is due to different number of clinical samples and number of isolates recovered which were subjected to antibiotic sensitivity tests. Also random sampling of clinical samples can also be the possible cause. Publication bias was evaluated for all meta-analysis of the 23 antibiotics and publication bias was not found. Egger test is use to estimate asymmetry of data using funnel plots. p-value less than 0.05 using Egger criteria indicate no presence of publication bias even though erythromycin had p-value of 0.017 which is below 0.05. This is because a p-value of 0.017 for the Egger test means that the results found have a 1.7% chance to occur when there is no 'small sample bias.
The pooled prevalence of S. aureus resistance to Beta-lactams class of antibiotics was extremely high especially for penicillins. S. aureus showed highest resistance to penicillin G (82%) and 69% resistance to cefuroxime (cephalosporin). The pooled estimate of S. aureus resistance to penicillin G is comparable with the reported estimation of worldwide resistance of 90–95% [108]. This is not surprising due to the fact that penicillin G is the first antibiotic to be discovered. Bacteria are able to develop resistance to antibiotics due to selective pressure from antibiotics. Selective pressure from penicillin led to the production of beta-lactamase to conuter the effect of beta-lactam antibiotics. Consequently, semi-synthetic beta-lactam antibiotics such as ampicillin, Amoxicillin/clavulanic acid and amoxicillin with different side chains were developed to counter such bacteria strains. However, S. aureus resistance to amoxicillin and ampicillin is relatively high from our results. Lower rate of resistance was observed among beta-lactamase-resistant antibiotics (methicillin, ceftriaxone, cefoxitine). Also, lower rate of resistance to clindamycin might be attributed to infrequent use of the antibiotic. Amoxicillin-clavulanic acid was developed as a combination of an antibiotic (amoxicillin) and non-antibiotic (clavulanic acid). Clavulanic acid inhibit beta-lactamase enzyme which prolong the antibacterial activity of amoxicillin component; however, results from the meta-analysis showed high resistance of S. aureus to amoxicillin/clavulanic acid.
Another semi-synthetic penicillin resistant antibiotic called methicillin was developed which is resistant to hydrolysis of beta-lactamase was developed. The term Methicillin Resistant Staphylococcus aurues (MRSA) is synonymous with multi-drug resistance (MDR) because MRSA are invariably resistant to different antibiotics. Acquisition of mec A gene that encodes penicillin binding protein confers resistance to S. aureu [109]. The pooled prevalence of S. aureus to methicillin (46% [95% CI 37%, 56%]) in Nigeria is similar to 2014 global surveillance reports of the world health organization (WHO) [110] 2014. Which depicted MRSA prevalence ranged 33–95% in Africa. Similarly, the pooled estimate of 46% in our study is also in agreement with the pooled prevalence estimate of MRSA in continents such as North America, Asia, and Europe which ranges from 23.1 to 47.4% [109]. The high pooled prevalence in our study might be due to certain factors and variables such as the inclusion of nosocomial and community acquired infections in the original studies analyzed. Generally, nosocomial infection causing pathogens are believed to possess higher resistance rate due to prolonged and higher exposure to different antimicrobial agents and exchange of genetic materials. Thus, there is greater transmission of resistant genes through various means within the hospital settings [111]. The implication of infections cause by MRSA is difficulty in treatment which often requires alternative antimicrobial agents which are most times very expensive.
The pooled prevalence of S. aureus resistance to vancomycin (13% at 95% CI [0.7%, 21%]) in this meta-analysis is high and a cause for concern when compared to global prevalence estimate [4]. The prevalence of vancomycin resistant S. aureus (VRSA) in Africa was reported to be 2.5% [4]. This is quite low when compared to the result from this study which is very high (13%). With this increased resistance, the use of vancomycin to treat MRSA is becoming problematic and poses serious health challenge. The rise in VRSA might be due to the indiscriminate use of vancomycin in Nigeria. By the way, Four studies [20, 22, 28, 36] reported a very high prevalence of VRSA; however, sensitivity analysis showed that they had high significant influence on the overall pooled prevalence estimate. Removing the three studies reduces the pooled prevalence of S. aureus resistance to vancomycin from 13 to 7%. Analyzing studies that depicted high prevalence of resistance of S. aureus to vancomycin showed that the same author conducted and published the three studies in peer reviewed journals. Urine samples were mainly used for S. aureus isolation by the author in the three studies of which [20, 22] were from symptomatic urinary tract infection patients who visited the hospitals and [27] from healthy volunteers. Urinary tract infection is a common infection and a reason for antibioticl use; consequently, resistant microbial strains have emerged. This reason might be attributed to the high prevalence of S. aureus resistant to vancomycin in the three studies. Exposure to resistant strains especially in hospital settings might have resulted in the increased resistance to vancomycin in the three studies [112, 113]. This is because in Nigeria, expired or waste antibiotics are not properly discharged. This could result in selective pressure on inhabitant microorganisms which results in development of various resistant mechanisms.
Generally, the global pattern of antimicrobial resistance varies among different geographical locations and socioeconomic level [114, 115]. Variations in studies can be attributed to design, time, and population involved. Heterogeneity tests at p ≤ 0.01 showed significant variation among included studies in this meta-analysis. Therefore, it is reasonable to assert that the study population might be infected with the same strains of S. aureus within the same location at a specified period. This is because most of the studies were conducted within a specified period of time and area.
Mechanisms of resistance of S. aureus include: production of beta-lactamase enzymes to deactivate beta-lactam sensitive antibiotics, efflux pump for extruding antibiotics such as tetracyclines [6], reduced accumulation of macrolides antibiotics [7], production of aminoglycoside modifying enzymes to inactivate aminoglycosides antibiotics, alteration of DNA gyrase and topoisomerase IV expression for floroquinolones antibiotics, and expression of Mec genes which alters penicillin binding proteins. From the results and mechanism of resistance of S. aureus, it can be said that S. aureus found in Nigeria are highly resistant to the beta-lactam class of antibiotics.
The pooled prevalence of S. aureus resistance to the floroquinolones class of antibiotics such as ciprofloxacin, ofloxacin, and norfloxacin was lower especially for ciprofloxacin which is commonly used within Nigeria. However, high pooled prevalence of S. aureus resistance to antimetabolites class of antibiotics (cotrimoxazole and trimethoprim) was observed.
From the meta-analysis, S. aureus mediated infection in Nigeria can be treated using vancomycin, floroquinolones, and aminoglycosides. MRSA has been a concern in Nigeria especially with the incidence of VRSA. Newer alternative antibiotics such as linezolid, telavancin, ceftaroline, tigecycline and daptomycin are rarely used in Nigeria. Various factors such as lack of infection prevention which lead to reoccurrence of infection, inappropriate use of antibiotics, poor hospital facilities, lack of routine susceptibility test before antibiotic administration, and self medication contributes to the rapid emergence and re-emergence of AMR. Tackling this factors, will go a long way in the fight against the continue rise of MDR pathogens in general.
Study limitations
Most of the included studies share similar characteristics. The search was limited to only titles that deal with antibiotic resistance. Selection was done randomly especially in Google Scholar with had 35, 400 studies results from the search. The meta-analysis was done once for each antibiotics and sub-grouping to reduce high heterogeneity and publication bias was not done due to too many meta-analysis already done. The included studies used in-vitro antimicrobial assays which has limitations such as difficulties in interpreting data, variability of testing media (differences in cation content, acidic or alkaline), and difficulty in knowing the pharmacokinetics of an antibiotic or post effect of an antibiotic (a situation where bacteria growth is inhibited even when the antibiotic concentration falls below the MIC). Most of the studies were done in teaching hospitals and tertiary institutions in big cities; hence both symptomatic and asymptomatic individuals are involved. For symptomatic individuals, most of the studies were done in teaching hospitals were patients with chronic and recurrent infections are treated; resistance level could be overestimated.
Conclusion
The results of this meta-analysis showed that S. aureus is resistant to many routinely used antibiotics in Nigeria. It is highly resistant to beta-lactams, tetracyclines, and antimetabolites antibiotics. Resistance of S. aureus to vancomycin remains a serious health problem due to limited treatment options. There is a lot of variation in resistance estimates between studies. High heterogeneity was observed in each meta-analysis for each antibiotic which was attributed to various factors such as different clinical sample and recovered isolates sizes, random sampling and method used for resistance investigation. Hence it is imperative to develop programs to promote rational use of antimicrobial agents, infection prevention and control to reduce the incidence of AMR. In addition, furthers researches focusing on identifying the dynamics promoting microbial resistance, infectious microbial strains and molecular/genetic basis of resistance should be encouraged.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its supporting information.
Abbreviations
- AMR:
-
Antimicrobial resistance
- CLSI:
-
Clinical Laboratory Standard Institute
- CI:
-
Confidence interval
- MRSA:
-
Methicillin resistant Staphylococcus aureus
- S. aureus :
-
Staphylococcus aureus
- VRSA:
-
Vancomycin resistant Staphylococcus aureus
References
Rasigade JP, Vandenesch F. Vandenesch, Staphylococcus aureus: a pathogen with still unresolved issues. Infect Genet Evol. 2014;21:510–4.
Bamigboye BT, Olowe OA, Taiwo SS. Phenotypic and molecular identification of vancomycin resistance in clinical Staphylococcus aureus isolates in Osogbo, Nigeria. Eur J Microbiol Immunol. 2018;8:25–30.
McGuinness WA, Malachowa N, DeLeo FR. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med. 2017;90(2):269–681.
Shariati A, Dadashi M, Moghadam MT. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. 2020;10:12689.
Khan A, Wilson B, Gould IM. Current and future treatment options for community-associated MRSA infection. Expert Opin Pharmacother. 2018;19(5):457–70.
Sompolinsky D, Zaidenzaig Y, Ziegler-Schlomowitz R, Abramova N. Mechanism of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1970;62(3):351–62.
Yamagishi S, Nakajima Y, Inoue M, Oka Y. Decrease in accumulation of macrolide antibiotics as a mechanism of resistance in Staphylococcus aureus. Jpn J Microbiol. 1971;15(1):39–52.
Li XF, Fan XJ, Guo XJ, Feng P, Lu XJ, Gao YY, et al. A study on the mechanism of drug resistance in Staphylococcus aureus. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37(3):365–8.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. 2009;151:264–9.
Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Akortha EE, Ikenebomeh MJ. Nasal colonization of symptomatic pneumonia patients in University of Benin Teaching Hospital, Benin City, Nigeria by multiple antibiotic resistant Staphylococcus aureus. Afr J Microbiol Res. 2010;4(11):1071–5.
Idris MA, Kumurya AS, Mohammed Y. Detection of methicillin-resistant staphylococcus aureus among bacterial isolated from children aged 0–5 years with septicaemia in Kano, north western, Nigeria. FUDMA J Microbiol. 2018;1(1):51–7.
Stanley CN, Ugboma HAA, Ibezim EC, Attama AA. Prevalence and antibiotic susceptibility of Staphylococcus aureus and other staphylococcal infections in pregnant women attending antenatal clinic in a tertiary hospital in port Harcourt, Nigeria. Infect Dis Ther. 2013;2(1):1–6.
Odu NN, Okonko IO. Nasal carriage and antibiotics susceptibility of Staphylococcus aureus in healthy students of University of Port Harcourt, Rivers State, Nigeria. N Y Sci J. 2012;5(7):56–63.
Isibor JO, Otabor E. Asymptomatic carriage of plasmid-mediated multidrugresistant Staphylococcus aureus in nasal tracts of persons in a semi-urban area of Nigeria. Am J life Sci. 2014;2(4):200–4.
Nworie A. Nasal carriage of methicillin resistant Staphylococcus aureus amongst meat sellers in Abakaliki Metropolis, Ebonyi State, Nigeria. Microbiol Res Int. 2013;1(3):48–53.
Egbuobi RC, Wachuku CK, Dike-Ndudim JN, Ogamaka AI, Okorie HM, Nwagbaraocha MA, et al. Evaluation of antibacterial profile of methicillin resistant Staphylococcus aureus (MRSA) isolated from hospitals in Imo state, Nigeria. Int J Med Med Sci. 2014;6(2):69–74.
Olowo-Okere A, Atana RF, Abass A, Shuaibu AS, Yahya UH, Tanko N. Incidence and antibiotic susceptibility profile of Staphylococcus aureus isolates from wounds of patients at specialist hospital, Sokoto, Nigeria. J Med Bacteriol. 2017;6(4):44–50.
Olorode OA, Ogba OM, Nanighe SO. Molecular detection of methicillin resistance genes (Mec A; Pvl) in methicillin resistant Staphylococcus aureus isolates from Federal Medical Centre, Yenagoa, Bayelsa State, Nigeria. J Curr Med Res Opin. 2021;4(10):1035–41.
Onanuga A, Awhowho GO. Antimicrobial resistance of Staphylococcus aureus strains from patients with urinary tract infections in Yenagoa, Nigeria. J Pharm Bioallied Sci. 2012;4(3):226–30.
Ayodeji AA, Omoniyi AK. Multi-drug resistant Staphylococcus aureus in clinical cases in Ile-Ife, Southwest Nigeria. Int J Med Med Sci. 2009;1(3):68–72.
Onanuga A, Onaolapo AJ. Antimicrobial Susceptibility of Community-associated Staphylococcus aureus Isolates from Healthy Women in Zaria, Nigeria. Trop J Pharm Res. 2008;7(1):929–34.
Chigbu C, Ezeronye OU. Antibiotic resistant Staphylococcus aureus in Abia State of Nigeria. Afr J Biotechnol. 2013;2(10):374–8.
Yah SC, Enabule OI, Eggafona NO. The screening of multidrug resistance (MDR) susceptibilities of Staphyloccocus aureus and Staphyloccocus epidermidis to methicillin and vancomycin in teaching hospitals in Nigeria. Int J Biol Chem Sci. 2007;2(4):257–61.
Yah SC, Yusuf EO, Haruna T. Patterns of antibiotics susceptibility of isolates and plasmid analysis of Staphylococcus from surgical site infections in Nigeria. Sudan JMS. 2009;3(4):810–8.
Onwubiko NE, Sadiq NM. Antibiotic sensitivity pattern of Staphylococcus aureus from clinical isolates in a tertiary health institution in Kano, Northwestern Nigeria. Pan Afr Med J. 2011;8(4):1–7.
Onanuga A, Temedie TC. Nasal carriage of multi-drug resistant Staphylococcus aureus in healthy inhabitants of Amassoma in Niger delta region of Nigeria. Afr Health Sci. 2011;11(2):176–81.
Onanuga A, Avosuahi RO, Onaolapo JA. Prevalence and susceptibility pattern of methicillinresistant Staphylococcus aureus isolates among healthy women in Zaria. Nigeria Afr J Biotechnol. 2005;4(11):1321–4.
Akanbi RO, Mbe JU. Occurrence of methicillin and vancomycin resistant Staphylococcus aureus in University of Abuja teaching hospital, Abuja, Nigeria. Afr J Clin Exp Microbiol. 2013;14(1):10–3.
Alli OAT, Ogbolu DO, Akorele E, Onemu OM, Okanlawon BM. Distribution of mecA gene amongst Staphylococcus aureus isolates from Southwestern Nigeria. Afr J Biomed Res. 2011;14:9–14.
Iroha IR, Okoye E, Osigwe CA, Moses IB, Ejikeugwu CP, Nwakaeze AE. Isolation, Phenotypic characterization and prevalence of ESBL-producing Escherichia coli and Klebsiella species from orthopedic wounds in National Orthopedic Hospital Enugu (NOHE), South East Nigeria. J Pharm Care Health Syst. 2017;4(4):1000184.
Eke S, Abdulkadiri S, Okoro CJ, Ekoh SN, Mbachi NG. The prevalence and resistivity pattern of Staphylococcus aureus Isolates from apparently healthy university students in Ekpoma, Edo, Nigeria. Int J Basic Appl Innov Res. 2012;1(4):183–7.
Ekundayo EO, Ndubuisi RN. In-vitro antimicrobial susceptibility pattern of Staphylococcus aureus isolates in Umuahia, Abia state, Nigeria. Afr J Clin Exp Microbiol. 2015;16(3):62–6.
Obasuyi O, Akerele JO. High incidence of multidrug-resistant strains of methicillinresistant Staphylococcus aureus isolated from clinical samples in Benin-city, Nigeria. Afr J Clin Exp Microbiol. 2015;16(3):124–7.
Akerele JO, Obasuyi O, Omede D. Prevalence of methicillin-resistant Staphylococcus aureus among healthy residents of Ekosodin community in Benin-City, Nigeria. Trop J Pharm Res. 2015;14(8):1495–9.
Badger-Emeka LI, Emeka PM, Dibua UME. Plasmid profile of multi antibiotic resistant Staphylococcus aureus isolated from diabetic wounds from patients at Nsukka, South astern, Nigeria. Afr J Biotectnol. 2014;13(43):4148–54.
Ayeni FA, Gbarabon T, Andersen C, Nørskov-Lauritsen N. Comparison of identification and antimicrobial resistance pattern of Staphylococcus aureus isolated from Amassoma, Bayelsa state, Nigeria. Afr Health Sci. 2015;15(4):1282–8.
Torimiro N, Torimiro SEA. Antibiotic resistance profile in community-associated Staphylococcus aureus strains isolated from a Nigerian peri-urban community. Afr J Biotechnol. 2012;11(94):16071–6.
Bale M, Babatunde SK, Adedayo MR, Ajiboye AE, Ajao AT. Characterization of methicillin-resistant Staphylococcus aureus isolates from apparently healthy individuals in Malete, Kwara state, Nigeria. Afr J Clin Exp Microbiol. 2019;20(1):17–24.
Adesoji AT, Onuh JT, Bagu J, Itohan SA. Prevalence and antibiogram study of Staphylococcus aureus isolated from clinical and selected drinking water of Dutsin-Ma, Katsina state, Nigeria. Afr Health Sci. 2019;19(1):1385–92.
Ariom TO, Iroha TR, Moses IB, Iroha CS, Ude UT, Kalu AC. Detection and phenotypic characterization of methicillin-resistant Staphylococcus aureus from clinical and community samples in Abakaliki, Ebonyi State, Nigeria. Afr Health Sci. 2019;19(2):2026–36.
Ajani TA, Elikwu CJ, Nwadike V, Babatunde T, Anaedobe CG, Shonekan O, et al. Nasal carriage of methicillin resistant Staphylococcus aureus among medical students of a private institution in Ilishan-Remo, Ogun State, Nigeria. Afr J Clin Exp Microbiol. 2020;21(4):311–7.
Olorunfemi PO, Onaolapo JA, Ibrahim YKE. Prevalence and antibiotic susceptibility of community acquired methicillin resistant Staphylococcus aureus from healthy students of University of Jos. J Pharm Bioresour. 2020;17(2):131–41.
Onanuga A, Adamu OJ, Odetoyin B, Hamza JA. Nasal carriage of multi-drug resistant panton valentine leukocidin positive Staphylococcus aureus in healthy individuals of Tudun-Wada, Gombe State, Nigeria. Afr J Infect Dis. 2021;15(1):24–33.
Ramalan AS, Joseph FN, Owoseni MC, Ya’aba Y, Chuku A. Prevalence and antibiotic resistance profile of Staphylococcus aureus and Escherichia coli among patients attending urology Clinic of Dalhatu Araf specialist hospital (dash) Lafia, Nasarawa state, Nigeria. Bayero J Pure Appl Sci. 2020;13(1):80–9.
Udobi CE, Obajuluwa AF, Onaolapo AJ. Prevalence and Antibiotic resistance pattern of methicillin-resistant Staphylococcus aureus from an Orthopaedic Hospital in Nigeria. BioMed Res Int. 2013;4.
Obasola EF, Ezeamagu CO, Oyelade AA, Adeniyi AO. Comparative study of antibiotic resistance of Staphylococcus species isolated from clinical and environmental samples. AU J Tech. 2010;13(3):165–9.
Moses AE, Ohagim IP, Inyang UC. Nasal methicillin-resistant Staphylococcus aureus carriage in pre and post-operative patients in a tertiary health facility in Uyo, South-south, Nigeria. World J Pharm Res. 2017;6(5):160–71.
Nsofor CA, Nwokenkwo VN, Nwaokpa C. Nasal carriage of Staphylococcus aureus among apparently Healthy School Children in Owerri Metropolis, Nigeria. Basic Res J Microbiol. 2015;2(3):34–8.
Adetayo TO, Deji-Agboola AM, Popoola MY, Atoyebi TJ, Egberongbe KJ. Prevalence of methicillin resistant Staphylococcus aureus from clinical specimens in Ibadan, Nigeria. Int J Eng Sci. 2014;3(9):1–11.
Ejikeugwu C, Nwezeagu FJ, Edeh C, Eze P. Detection of constitutive and inducible-clindamycin-resistance in clinical isolates of Staphylococcus aureus from a Federal Teaching Hospital in Abakaliki, Nigeria. J Bacteriol Infect Dis. 2018;2(1):31–4.
Anucha JC, Morikwe UC, Ikegbunam MN, Ezejiegu CK, Ugwu MC. Age and gender- based antibiotic susceptibility patterns of Staphylococcus aureus among HIV patients in some selected hospitals in Anambra Central, Nigeria. Gen Mol Med. 2021;3(2):1–5.
Agwu E, Ihongbe J, Inyang N. Prevalence of quinolone-susceptible Pseudomonas aeruginosa and Staphylococcus aureus in delayed-healing diabetic foot ulcers in Ekpoma, Nigeria. Cell Tis Prod. 2010;22(4):100–5.
Adesida AS, Okeyide AO, Abioye A, Omolopo I, Egwuatu TO, Amisu KO, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus among elderly people in Lagos, Nigeria. Avicenna J Clin Microb Infect. 2016;3(4):e39272.
Mofolorunsho CK, Ocheni M, Omatola CO, Agieni AG. Staphylococcus aureus prevalence and antibiotic susceptibility profile in Anyigba, North-Central Nigeria. Am J Infect Dis. 2015;11(4):93–7.
Osiyemi JA, Osinupebi OA, Akinduti PA, Ejilude O, Makanjuola SO, Sunmola NO, et al. Antibiotic resistance profile of methicillin-resistant Staphylococcus aureus in Abeokuta, Nigeria. J Adv Microbiol. 2018;9(2):1–9.
Ibe C, Onyeagba RA, Ugochukwu SC. Antibiotic resistance patterns and plasmid profiles of methicillin resistant Staphylococcus aureus isolates from human samples. Br Microbiol Res J. 2014;4(2):185–94.
Onaolapo JA, Olayinka BO, Adeshina GO, Igwe JC, Obajuluwa AF. Antimicrobial susceptibility pattern of Staphylococcus aureus isolates from orthopaedic patients in Abuth Zaria. J Food Indust Microbiol. 2016;2(1):1–6.
Ugwu MC, Anie CO, Ibezim EC, Esimone CO. Antimicrobial evaluation of methicillin-resistant Staphylococcus aureus nasal carriage amongst healthy students in Agbor, Delta State, Nigeria. Arch Clin Microbiol. 2016;7(2):13.
Tula MY, Okoro AJ, Okojie RO, Iyoha O. Antimicrobial susceptibility pattern and plasmid-mediated antibacterial resistance in Staphylococcus aureus and coagulase-negative staphylococci. Highl Med Res J. 2016;16(2):80–6.
Anyanwu NC, Abdullahi IO, Ameh JB, Ella EE. Molecular detection of PVL, msrA genes and antibiotic susceptibility pattern of staphylococcus aureus from skin and soft tissue infections in Zaria, Nigeria. Sci J Microbiol. 2013;2(2):43–52.
Onyeagwara N, Obasuy O, Ottih I, Akerele JO. Nasal carriage of methicillin-resistant Staphylococcus aureus among hospitalized otorhinolaryngological patients in Benin City of Nigeria. Arch Int Surg. 2014;4(2):99–103.
Ngwai YB, Bakare OR. Asymptomatic bacteriuria and antimicrobial susceptibility of Staphylococcus aureus from university students in Keffi, Nigeria. Int J Microbiol Bioinform. 2012;2(1):6–8.
Umar M, Abdulkarim IM, Yaya A, Bello HI, Yusuf G, Mathias ML. Biochemical characterization and antibiotic susceptibility profile of Staphylococcus aureus isolated from patients attending Barau Dikko Specialist Hospital, Kaduna State, Nigeria. Int J Novel Res Life Sci. 2015;2(6):1–9.
Obajuluwa AF, Olayinka BO, Adeshina JA, Afolabi-Balogun NB. Plasmid profile of Staphylococcus aureus from orthopaedic patients in Ahmadu Bello University Teaching Hospital Zaria, Nigeria. Afr J Microbiol Res. 2015;9(33):1941–6.
Iduh UM, Chollom CS, Nuhu A, Spencer THI, Nura MB, Ashcroft OF, et al. Nosocomial infections in post-operative wounds due to Staphylococcus aureus and Pseudomonas aeruginosa in Benue State Nigeria. Afr J Microbiol Res. 2015;9(36):1989–96.
Ibanga IA, Akan OD, Iyanga FZ, Mantu EC, Asuquo M. Multi-drug resistance pattern of Staphylococcus aureus from pediatric ward, General Hospital, Ikot-Ekpaw, Mkpat Enin LGA, Akwa-Ibom State, Nigeria. Arch Clin Microbiol. 2020;11(105):1–4.
Emeakaroha MC, Nkwocha IG, Adieze NC, Adieze IE. Antimicrobial susceptibility pattern of Staphylococcus aureus, and their nasal and throat carriage among food handlers at the Federal University of Technology, Owerri Nigeria. Int J Biomol Biomed. 2017;6(3):1–7.
Bisi-Johnson MA, Kolawole DO, Shittu OA. Epidemiological analysis of clinical isolates of Staphylococcus aureus in IIe-Ife, Nigeria. Pak J Bio Sci. 2005;8(7):1016–20.
Ayepola OO, Nurudeen AO, Egwari LO, Becke K, Schaumburg F. Molecular characterization and antimicrobial susceptibility of Staphylococcus aureus isolates from clinical infection and asymptomatic carriers in Southwest Nigeria. PLoS ONE. 2015;10(9): e0137531.
Odogwu DA, Kadarko PS, Muazu JS, Bassey BE, Abimiku RH, Nkene IH, et al. Antimicrobial resistance profile and molecular detection of MecA gene in methicillin resistant Staphylococcus aureus from patients in selected general hospitals in Abuja municipal, Nigeria. GSC Biol Pharm Sci. 2019;7(3):93–106.
Adeiza SS, Onaolapo JA, Olayinka BO. Prevalence, risk-factors, and antimicrobial susceptibility profile of methicillin-resistant Staphylococcus aureus (MRSA) obtained from nares of patients and staff of Sokoto state-owned hospitals in Nigeria. GMS Hyg Infect Control. 2020;15:1–17.
Ismail HY, Bello HS, Mustafa A, Adamu A. Multidrug resistance pattern of Staphylococcus aureus isolates in Maiduguri metropolis. Sci Rev. 2015;1(2):16–20.
Ibrahim S, Adam SA, Alero AA, Umar S. Prevalence and antibiotic sensitivity pattern of Staphylococcus aureus Isolated from wound and otitis media among patients attending Aminu Kano Teaching Hospital, Kano, Nigeria. Microbiol Res J Int. 2018;25(2):1–9.
Olowe OA, Kukoyi OO, Taiwo SS, Ojurongbe O, Opaleye OO, Bolaji OS, et al. Phenotypic and molecular characteristics of methicillin-resistant Staphylococcus aureus isolates from Ekiti State, Nigeria. Infect Drug Res. 2013;6:87–92.
Oche AD, Oyegoke PI, Akpudo MO, Olayinka BO. Antibiotics resistance pattern of Staphylococcus aureus isolated from in-patients of an Orthopaedic Hospital in North-Western Nigeria. Int J Epidemiol Res. 2021;8(4):160–6.
Onelum B, Odetoyin B, Onipede A, Oyelese A. The role of methicillin-resistant Staphylococcus aureus in clinical infections in Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, South Western Nigeria. J Microbiol Exp. 2015;2(2):00041.
Akindut AP, Osiyemi JA, Banjo TT, Ejilude O, El-Ashke M, Adeyemi AG, et al. Clonal diversity and spatial dissemination of multi-antibiotics resistant Staphylococcus aureus pathotypes in Southwest Nigeria. PLoS ONE. 2021;16(2):e0247013.
Oladipo AO, Oladipo OG, Bezuidenhout CC. Multi-drug resistance traits of methicillin-resistant Staphylococcus aureus and other Staphylococcal species from clinical and environmental sources. J Water Health. 2019;17(6):932–43.
Ogefere HO, Umaru G, Ibadin EE, Omoregie R. Prevalence of methicillin-resistant staphylococci among apparently healthy students attending a tertiary institution in Benin City, Nigeria. Niger J Basic Appl Sci. 2019;27(1):114–21.
Motayo BO, Akinduti PA, Okerentugba PO, Innocent-Adiele HC, Onoh CC, Nwanze JC, et al. Methicillin resistance and beta-lactamase production in Staphylococcus aureus isolated from different clinical samples in Abeokuta, Nigeria. Acad Arena. 2012;4(6):25–8.
Onyeka FI, Nwobodo DC, Umenne IC, Atada EE, Ojukwu CA, Aniekwe MA, et al. Antibiotic resistance pattern of Staphylococcus aureus isolated from nostrils of healthy undergraduates of Madonna University Elele Campus, Rivers State, Nigeria. Microbes Infect Dis. 2021;2(2):280–5.
Ugwu CM, Odimegwu DC, Ibezim EC, Esimone CO. Antibiotic Resistance patterns of Staphylococcus aureus isolated from nostrils of healthy human subjects in a Southeastern Nigeria Locality. Maced J Med Sci. 2009;2(4):294–300.
Nsofor CA, Nwokenkwo VN, Ohale CU. Prevalence and antibiotic susceptibility pattern of Staphylococcus aureus isolated from various clinical specimens in South East Nigeria. MOJ Cell Sci Rep. 2016;3(2):00054.
Mbim EN, Mboto CI, Edet UO, Umego CF, George UE, Temidayo L. Comparative evaluation of methicillin-resistant Staphylococcus aureus (MRSA) isolates from hospital and community settings in Nigeria. J Adv Microbiol. 2017;4(4):1–9.
Ogbolu DO, Alli TOA, Bello LA, Ibrahim AO. Emergence of vancomycin intermediate Staphylococcus aureus (VISA) in clinical isolates of methicillin resistant S. aureus from South Western Region of Nigeria. Int J Trop Dis Health. 2015;10(4):1–5.
Osinupebi OA, Osiyemi JA, Deji-Agboola AM, Akinduti PA, Ejilude O, Makanjuola SO, et al. Prevalence of methicillin-resistant Staphylococcus aureus in Abeokuta, Nigeria. South Asian J Res Microbiol. 2018;1(1):1–8.
Ajoke OI, Okeke OI, Odeyemi OA, Okwori AEJ. Prevalence of methicillin-resistant Staphylococcus aureus from healthy community individuals volunteers in Jos south, Nigeria. J Microbiol Biotechnol Food Sci. 2012;1(6):1389–405.
Onyebueke EA, Onyemelukwe NF, Oladeji DS. Antibiotic susceptibility pattern of Staphylococcus species implicated in urinary tract infection in Enugu state Nigeria. Pharm OnLine. 2019;1:166–76.
Adetutu AA, Oritsewehinmi B, Khiwili OM, Moradeke AO, Odochi AS, Adeola OE. Studies on Staphylococcus aureus isolated from pimples. Pak J Biol Sci. 2017;20:350–4.
Bale MI, Babatunde SK, Awe S. prevalence of methicillin resistant Staphylococcus aureus bacteriuria among pregnant women attending secondary health hospitals in LLorin, Nigeria. J Adv Microbiol. 2021;21(9):2456–7116.
Nmema EE. Risk factors for infection of Staphylococcus aureus: nasal carriage, skin carriage and multi-antibiotic resistance in healthy individuals. Br J Med Med Res. 2017;21(9):1–8.
Ike B, Ugwu MC, Ikegbunam MN, Nwobodo D, Ejikeugwu C, Gugu T, et al. Prevalence, antibiogram and molecular characterization of comunity-acquired methicillin-resistant Staphylococcus aureus in AWKA, Anambra Nigeria. Open Microbiol J. 2016;10:211–21.
Ugwu MC, Mokwe NM, Ejikeugwu P, Enemor EC, Eze CO, Ugwu BC, et al. Antibiogram of Staphylococcus aureus from healthy school pupils in Agulu, Southeastern Nigeria. Int J Res Pharm Biosci. 2015;2(4):5–9.
Emeka-Nwabunnia I, Chiegboka NA, Udensi UJ, Nwaokorie FO. Vancomycin-resistant Staphylococcus aureus isolates from HIV positive patients in Imo State, Nigeria. Sci J Public Health. 2015;3(5):1–7.
Alli OAT, Ogbolu DO, Mustapha JO, Akinbami R, Ajayi AO. The non-association of Panton-valentine leukocidin and mecA genes in the genome of Staphylococcus aureus from hospitals in South Western Nigeria. Indian J Med Microbiol. 2012;30(2):159–64.
Sadauki AH, Olorukooba AA, Balogun MS, Dalhat MM, Waziri H, Abdulaziz MM, et al. Nasal carriage of meticillin-resistant Staphylococcus aureus among children living with HIV attending infectious diseases clinics in Kano. Nigeria Infect Prev Pract. 2022;4:1–8.
O’Malley SM, Emele FE, Nwaokorie FO, Idika N, Umeizudike AK, Emeka-Nwabunnia I, et al. Molecular typing of antibiotic-resistant Staphylococcus aureus in Nigeria. J Infect Public Health. 2015;8:187–93.
Emeka-Nwabunnia I, Ejigeme K, Oguoma OI. Antibiotic resistance pattern of methicillin-resistant Staphylococcus aureus isolated from children under the age of five years in Anambra State. Niger J Biotechnol. 2019;36(1):213–21.
Ako-Nai AK, Adeyemi FM, Aboderin OA, Kassim OO. Antibiotic resistance profile of staphylococci from clinical sources recovered from infants. Afr J Biotechnol. 2015;4(6):816–22.
Frank-Peterside N, Mukoro M. The current susceptibility pattern of methicillin resistant Staphylococcus aureus to antibiotics in Port Harcourt. Global J Pure Appl Sci. 2010;16(4):375–9.
Yahaya H, Ahmad AS, Ibrahim A, Abdullahi AS. Phenotypic Detection of Macrolide, Lincosamide and Streptogramin B resistance among Staphylococcus aureus clinical isolates in A Northern Nigeria Tertiary Hospital. Alq J Med App Sci. 2022;5(1):193–8.
Onanuga A, Eboh DD, Okou GT. Antibiogram and virulence characteristics of multi-drug resistant Staphylococcus aureus from nasal cavity of healthy students of Niger Delta University, Amassoma, Bayelsa State, Nigeria. J Clin Diagn Res. 2019;13(7):24–9.
Oginni IO, Olayinka AA. Distribution and antibiotics resistance pattern of community-acquired methicillin-resistance Staphylococcus aureus in Southwestern Nigeria. Adv Exp Med Biol. 2022;1369:81–91.
Nwankwo EOK, Sale A, Magagi A, Ihesiulor G. Methicillin resistant S. aureus (MRSA) and their antibiotic sensitivity pattern in Kano, Nigera. Afr J Clin Exp Microbiol. 2010;11(1):129–36.
Olufunmiso C, Tolulope I, Roger C. Multidrug and vancomycin resistance among clinical isolates of Staphylococcus aureus from different teaching hospitals in Nigeria. Afr Health Sci. 2017;17(3):798–806.
Olajide RA, Ameh JA, Simon MK. Antibiotic resistance and susceptibility pattern/profile of Staphylococcus aureus and Klebsiella species to popularly—used and readily available antibiotics in Zaria, Nigeria. J Phytother Pharm. 2012;1:16–21.
Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15(8):639–52.
Li S, Li J, Qiao Y, Ning X, Zeng T, Shen X. Prevalence and invasiveness of community-acquired methicillin-resistant Staphylococcus aureus: a metaanalysis. Indian J Pathol Microbiol. 2014;57(3):418–22.
WHO. Antimicrobial resistance: global report ton surveillance. 2014.
Struelens MJ. The epidemiology of antimicrobial resistance in hospital acquired infections: problems and possible solutions. BMJ. 1998;317(7159):652–4.
Guta M, Aragaw K, Merid Y. Bacteria from infected surgical wounds and their antimicrobial resistance in Hawassa University referral teaching hospital, southern Ethiopia. Afr J Microbiol Res. 2014;8(11):1118–24.
Dessalegn L, Shimelis T, Tadesse E, Gebre-selassie S. Aerobic bacterial isolates from post-surgical wound and their antimicrobial susceptibility pattern: a hospital based cross-sectional study. J Med Res. 2014;3(2):18–23.
DACA. Antimicrobials use, resistance and containment baseline survey: syntheses of findings. In: 2009th ed. Addis Ababa; 2009.
Falagas ME, Karageorgopoulos DE, Leptidis J, Korbila IP. MRSA in Africa: filling the global map of antimicrobial resistance. PLoS ONE. 2013;8(7):e68024.
Acknowledgements
Not applicable
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
CKE, CNE and UMED conceptualized the research idea. CKE and SCE conducted literature search, selection and data extraction. CKE performed the statistical analyses. CKE prepared the draft manuscript. All authors revised, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ezeh, C.K., Eze, C.N., Dibua, M.E.U. et al. A meta-analysis on the prevalence of resistance of Staphylococcus aureus to different antibiotics in Nigeria. Antimicrob Resist Infect Control 12, 40 (2023). https://doi.org/10.1186/s13756-023-01243-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01243-x