Abstract
Background
Risk factors for nosocomial COVID-19 outbreaks continue to evolve. The aim of this study was to investigate a multi-ward nosocomial outbreak of COVID-19 between 1st September and 15th November 2020, occurring in a setting without vaccination for any healthcare workers or patients.
Methods
Outbreak report and retrospective, matched case–control study using incidence density sampling in three cardiac wards in an 1100-bed tertiary teaching hospital in Calgary, Alberta, Canada. Patients were confirmed/probable COVID-19 cases and contemporaneous control patients without COVID-19. COVID-19 outbreak definitions were based on Public Health guidelines. Clinical and environmental specimens were tested by RT-PCR and as applicable quantitative viral cultures and whole genome sequencing were conducted. Controls were inpatients on the cardiac wards during the study period confirmed to be without COVID-19, matched to outbreak cases by time of symptom onset dates, age within ± 15 years and were admitted in hospital for at least 2 days. Demographics, Braden Score, baseline medications, laboratory measures, co-morbidities, and hospitalization characteristics were collected on cases and controls. Univariate and multivariate conditional logistical regression was used to identify independent risk factors for nosocomial COVID-19.
Results
The outbreak involved 42 healthcare workers and 39 patients. The strongest independent risk factor for nosocomial COVID-19 (IRR 3.21, 95% CI 1.47–7.02) was exposure in a multi-bedded room. Of 45 strains successfully sequenced, 44 (97.8%) were B.1.128 and differed from the most common circulating community lineages. SARS-CoV-2 positive cultures were detected in 56.7% (34/60) of clinical and environmental specimens. The multidisciplinary outbreak team observed eleven contributing events to transmission during the outbreak.
Conclusions
Transmission routes of SARS-CoV-2 in hospital outbreaks are complex; however multi-bedded rooms play a significant role in the transmission of SARS-CoV-2.
Similar content being viewed by others
Background
The risks of nosocomial transmission of viral respiratory infections [1] have been known for many years and have been recognized in the SARS-CoV-2 pandemic [2]. Nosocomial transmission of SARS-CoV-2 has been reported in acute care institutions from many countries, including Canada [3,4,5,6] and highlight how rapidly SARS‐CoV‐2 can spread across hospital wards. Previous outbreaks have revealed common themes, including (1) significant disruption of health care services, (2) the need to enhance infection prevention and control (IPC) measures (3) the promotion of a culture that IPC is everyone’s responsibility and (4) that all healthcare workers (HCWs) need vigilance when assessing patients for COVID-19, appropriate donning and doffing of personal protective equipment (PPE), and to ensure appropriate environmental cleaning [7, 8]. However, evidence continues to evolve on the risk factors for nosocomial SARS-CoV-2 infections among hospitalized patients.
We investigated a multi-ward nosocomial outbreak of SARS-CoV-2 beginning in September 2020 with the following objectives: (1) to describe a nosocomial SARS-CoV-2 infection outbreak investigation on three linked cardiac wards in our acute care tertiary hospital and (2) to conduct a matched-case control study to determine ward and patient-related risk factors for nosocomial transmission of SARS-CoV-2 among cardiac patients.
Methods
Setting description of hospital and cardiac wards
Our facility is an 1100-bed tertiary teaching hospital in Calgary, Alberta. The three cardiac wards included two medical cardiac wards on the same floor separated by an elevator bank (Ward A and B) and one cardiac intensive care ward (Ward C) two floors above the medical cardiac wards with frequent patient and HCW movement between the wards. There were 294 admissions and 1,991 patient-days per month across the cardiac wards during the fiscal 2020/2021 year. Wards A and B each had six single-bed, six two-bed, and five four-bed rooms, while Ward C had four single-bed, seven two-bed, and one four-bed rooms. As per our provincial healthcare organization policy, universal admission RT-PCR laboratory testing for SARS-CoV-2 was not employed at any time during the pandemic. Universal admission symptom screening was conducted during the pandemic by our provincial healthcare organization using the COVID-19 Symptom Monitoring Tool [9]. All patients were screened at the time of initial presentation for respiratory symptoms, travel, and COVID-19 exposure to quickly identify those who required additional precautions. For all admitted patients, the COVID-19 Symptom Monitoring Tool [9] was completed by nursing staff at least once daily for the duration of the patient’s hospitalization and recorded in the patient’s medical chart. The outbreak was first declared on 19th September 2020 on Wards A and C, 48 h after five epidemiologically linked patients tested positive from SARS-CoV-2 nasopharyngeal (NP) swabs sent on Sept 17–18, 2020. The symptoms of these patients were thought initially to be due solely to their underlying cardiac disease. Then the outbreak was subsequently declared on 30th September 2020 on Ward B. There was limited community transmission during this time period (active cases, 30.7 per 10,000 population) [10].
Outbreak investigation
Case definition and contact tracing
Case definitions for confirmed or probable cases of COVID-19, outbreak and close contact definitions were based on Public Health guidelines (Additional file 1).
Data collection for outbreak investigation and response
Baseline pre-existing hospital and cardiac unit infection control measures along with details of the multidisciplinary outbreak response of investigations and control measures that were initiated at the declaration of the outbreak are outlined in Additional file 1. The multidisciplinary outbreak team met regularly until the outbreak subsided and collated investigation findings and general observations into tabular format. Index date for a case was either the date symptoms started or the date of a laboratory confirmation for SARS-CoV-2, whichever came first. Isolation information was collected from the patient’s medical record (electronic [EMR] and paper) and through discussions with the unit manager. The room on the cardiac wards where a patient with COVID-19 was deemed to have acquired the infection (room attribution) was the room where the patient stayed in within five days prior to symptom onset (based on a median incubation time of 5 days for the original Wuhan strain) [11]. Information on room movement and shared bathrooms was collected from the EMR. HCWs linked to the outbreak were interviewed by Workplace Health and Safety (WHS) using a detailed questionnaire similar to the COVID-19 Symptom Monitoring tool [9] used for patients and included additional questions for forward and backwards contact tracing. Visitors to the affected wards were notified and encouraged to be tested in the community via Public Health if symptomatic or exposed to a known case on the wards. Public Health interviewed all visitors who tested positive for contact tracing purposes and symptom ascertainment.
Ventilation assessments
Ventilation, measured in air exchanges per hour (AEH) and percentage outside air were assessed on the three wards by Facilities, Maintenance, and Engineering and interpreted relative to the Canadian Safety Association standards for Heating, Ventilation, and Air Conditioning (HVAC) Systems in Health Care Facilities (CSA-Z317.2-15).
Laboratory and virological methods
Serial nasopharyngeal swabs and occasionally throat swabs were collected by experienced personnel and tested for SARS-CoV-2 using a validated real-time RT-PCR assay targeting the E gene with internal controls [12] to obtain cycle threshold (Ct) values. Clinical and environmental specimens obtained from consenting patients from the affected wards were sent to the Li Ka Shing Institute for Virology (University of Alberta) for quantitative viral culture testing as per Lin et al. [13] and PCR assays were performed according to methods previously described [12,13,14]. Environmental samples were obtained from rooms with known positive patients with a focus on high-touch areas including call bells, bedrails, telephones, cellphones, bathroom sites, commodes, and mobile medical equipment such as pulse oximeters or other oxygen monitoring probes. Symptomatic patients or HCWs were tested for SARS-CoV-2 and serial asymptomatic SARS-CoV-2 RT-PCR prevalence testing was done on all inpatients (q2- 5 days) during the outbreak only and was arranged and strongly recommended for HCWs (q5 days) who worked on the outbreak wards in the 14 days prior to and during the outbreak [9].
Whole genome sequencing
The full genome of SARS-CoV-2 strains obtained from the NP swabs of HCWs and patients from the cardiac wards was amplified by multiplex PCR according to the ARTIC V1 or V3 with clean up and no dilutions protocols [15,16,17] using the Resende oligos [18] as 2000-bp amplicons with sequencing done using Oxford Nanopore. Lineages were assigned using pangolin [19].
Case–control study
Study design and population
A retrospective matched case–control study analyzed the medical records of patients implicated in the COVID-19 outbreak and matched patient controls using incidence density sampling of a dynamic population [20, 21] from our hospital between 1st September 2020 and 15th November 2020.
Case patients were defined as admitted inpatients during the study period who were found to have a laboratory-confirmed COVID-19 infection during routine medical care and that were attributed to the cardiac wards as per outbreak protocols (Additional file 1).
Control patients were defined as inpatients present on the cardiac wards during the study period who either tested negative for SARS-CoV-2, regardless of signs or symptoms, after the outbreak was declared, or were presumed negative if they were identified prior to the outbreak declaration. Of the controls, 80% of the individual control patients were discharged after the outbreak was initially declared and therefore had serial asymptomatic testing, all of which were negative. Of the remaining controls, 20% were discharged prior to the start of the outbreak and would only have been tested for SARS-CoV-2 if they presented with symptoms. On clinical review, none of these patients had any symptoms suggestive of COVID-19 during or after their hospitalization, and in addition 71% of this group had RT-PCR tests done pre- and post-discharge which were all negative. The remaining five control patients who were not tested for SARS-CoV-2 had multiple doctors’ visits with no documentation of symptoms. Control patients were matched to cases if the timing of their stay on the outbreak wards overlapped symptom onset dates of the cases, by age within + /− 15 years of the case age and had a minimum hospital admission of at least 2 days. Controls were initially matched in a n:1 ratio with replacement, whereby each case could have a variable number of controls with some controls used as a control for multiple cases [22]. Each case was matched to controls 1:5. Controls were randomly selected for cases that had more than five matched controls.
For cases who had symptom onset during their hospital admission, exposures were examined 7-days prior to their COVID-19 symptom onset date. For cases who had symptom onset after discharge, hospital exposures were examined in the 7-days prior to discharge. For controls, the dates when outbreaks were declared were considered the index date for controls. For controls admitted to Ward A, 19 September was the index date, and for controls on Ward B, 30 September was the index date. Exposures for controls were examined in the 7-days prior to the start of the outbreak on Ward A (19th September) or Ward B (30th September), depending on when the control case was admitted. Controls admitted after the 30th of September outbreak were excluded.
Data collection
Data on case and control patients were collected using retrospective chart review of medical records using a standardized data collection instrument. Demographics data, the Braden Score, baseline medications and laboratory measures were collected from the EMR. Laboratory measures were categorized as abnormal if the results fell outside the normal ranges for each measure as defined by laboratory and clinical criteria (Additional file 1). Comorbidities were collected from admissions in the two years prior to the index date for cases and controls from the Discharge Abstract Database (DAD). Hospitalization characteristics were collected from the Admission, Discharge, Transfer database (Additional file 1).
Statistical analysis
Outbreak attack rates among admitted patients and case-fatality rates were calculated. Descriptive statistics and univariate conditional logistic regression were used to compare variables, with controls weighted inversely proportional to the number times they were matched to a case, to account for the matching with replacement. Statistical significance was set at p < 0.05. All significant variables in the univariate analysis were considered for inclusion in the multivariate conditional logistic regression analysis. Where appropriate, a sensitivity analysis for the multivariate regression was performed using different cut-offs depending on the variable. As the cases and controls were matched on time based on symptom onset date, with exposures considered in a fixed time frame prior to index date, the parameters estimated from the logistic regression are interpreted and reported as incidence rate ratios [20, 21]. The analysis was performed using R version 4.1.1 (IBM Corp, Armonk, NY, USA).
Results
Outbreak description
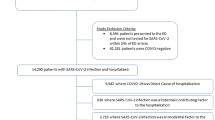
The cardiac wards had 685 admissions between 1st September 2020 and 15th November 2020, with an average length of stay of 4.6 days. During the outbreak period, there were 81 cases: 42 HCWs and 39 patients with 10 recorded patient deaths (Fig. 1).
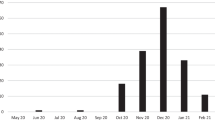
Epidemic curve by index date* for patients across the cardiac wards. Case numbers (Ward: Number): Patients (Ward A:27, Ward C:1, Ward B:11); Healthcare workers, HCWs (Ward A:33, Ward C:0, Ward B:9), and Visitors (Ward A:5, Ward C:0, Ward B:0). *Index date was either the symptom onset date or the date of laboratory confirmation for SARS-CoV-2, whichever came first
Over half of the patients with COVID-19 were males (56.4%), while HCWs with COVID-19 were mostly females (70.4%). The mean age of patients was 75 years (SD 12), and 38 years (SD 12) among HCWs. The attack rate among patients was 5.7%, with a case fatality rate of 25.6%. All patients and visitors who were found to be SARS-CoV-2 RT-PCR positive were symptomatic, while 40/42 (95.2%) HCWs were found to have symptoms [23]. Although all patients were tested serially while in hospital after the outbreak was declared, testing was not mandatory for HCWs but a total of 1497 RT-PCR tests were collected from 1,011 HCWs, of which 376 HCWs were identified as core nursing and management staff, excluding physicians, residents, allied health professionals, and lab services who were much smaller in number and many of whom were transient on the affected wards. HCW compliance for SARS-CoV-2 prevalence testing was very high during this outbreak given that this was the first major outbreak in our hospital during the pandemic and was in an unvaccinated population. Details of the symptoms in the patients, HCWs and visitors are reported elsewhere [9] and were found in 97.7% of cases, with influenza-like–illness (ILI) symptoms and signs being found in 84.9% of all RT-PCR positive cases. The outbreak network map is provided in Fig. 2.
Case Linkage by HCW and Patient Flow Map on Ward A. On 19 September 2020, a COVID-19 outbreak was declared at our facility on Ward A following identification and confirmation of a nosocomial COVID-19 case on Ward A (Patient 28) admitted on 10 September 2020, followed by two patients (Patient 1 and 4) admitted on 11 and 12 September, respectively, that enabled additional transmission events via HCWs to Ward C. Patient 4 was considered to have an exposure from a person visiting the hospital from the community. The outbreak extended to Ward B on 30 September 2020. Black Circle—patient (red outline is a patient transferred to Ward B); Brown Diamond—HCW; Blue Pentagon—visitor. Arrows—transmission pathways (dotted line indicates less likely transmission pathway); Black arrows—patient to staff; Green arrows– patient to patient; Red arrows—HCW to HCW; Orange arrows—to another unit; Blue arrows—HCW to patient; Red dashed line square around patients 1 and 4 and the HCW labelled as R—major nodes of forward transmission
Laboratory, virologic, and ventilation results
Of the 73 and 10 pre- and post-cleaning environmental swab samples collected on Ward A 11 (15.1%) and one (10%) were RT-PCR positive for SARS-CoV-2 (p = 0.6674) (Additional file 2). On Ward B, 3/55 (5.4%) randomly sampled and 9/13 (69.2%) targeted environmental swabs (stethoscope, pulse oximeter, gown, bedside tables/flooring, urine catheter bag, bedrail, inhaler) were RT-PCR positive for SARS-CoV-2, respectively. There were 64 specimens collected directly from 8 consenting patients, including clinical specimens and their immediate environment from Wards A and B, all of whom had NP Ct values < 20 (N gene; range 11.4–19.2). SARS-CoV-2 was cultured from 34/60 (56.7%) clinical and environmental specimens with titres of ranging between 5.0 × 100 and 5.2 × 105 pfu/ml (Additional file 2: Table A3).
Of the 78 NP specimens collected from both patients and HCWs who were confirmed to be related to the cardiac wards and were successfully sequenced (n = 45), 44 (97.7%) were SARS-CoV-2 lineage B.1.128. Community samples sequenced for SARS-CoV-2 during the same period differed substantially from the outbreak strain with B.1.128 representing only 8.9% of the circulating lineages at the time in our local setting and less than 1% across almost 2000 typed strains across the province at the time.
Ventilation, on Wards A, B, and C ranged from 4.3–10.7, 6.9–14.3, and 10.5–13 AEH, respectively, all with 100% outside air, meeting or exceeding the Canadian Standards Association (CSA) standards of a minimum outdoor AEH of 4 for 100% outside air.
Sources and contributing events of transmission
The multidisciplinary outbreak team, through its investigations, identified eleven potential sources and contributing events to transmission during this cardiac ward outbreak which are summarized in Table 1.
Case control study
Clinical characteristics of patients
The case control study included all 39 case patients matched to 183 controls, of which 74 were different individuals acting as control patients (Additional file 3). Four controls were excluded based on admission dates resulting in 70 different individual control patients weighted by the number of times they were matched to a case. Table 2 shows demographic data, underlying diseases, laboratory findings and mobility findings seven days prior to the index date for cases and controls. Within the seven days prior to the index date, cases were in hospital longer than controls (median 7 days vs. 4.4 days). The overall median and mean length of stay on the cardiac wards prior to the index date was similar between cases and controls (mean 12.3 vs 13.1 days, median 7.8 vs 6.0 days, respectively). Of the cases, 75.9% (31/39) had underlying chronic diseases (Additional file 3). Compared with the controls, the cases had a higher prevalence of fluid/electrolyte disorders (35.9% vs. 14.3%, p = 0.001) and neurological disorders (10.3% vs. 2.9%, p = 0.020). Prior to symptom onset, cases were also more likely to have lymphopenia (46.1% vs. 28.6%, p = 0.016), were on a diuretic longer (3.03 days vs. 2.41 days, p = 0.031) and immunosuppressive agents longer (0.52 days vs. 0.05 days, p = 0.003) than controls. Prior to symptom onset, cases were less likely than controls to walk occasionally or frequently (69.2% vs. 85.7%, p = 0.0001).
Characteristics of hospital stay
Patients who spent more than 50% of their hospital stay in a single-bed room, had a 63% (IRR 0.37, 95% CI 0.15–0.92) lower rate of acquiring COVID-19 in hospital during this outbreak (Table 3), whereas patients who spent more than 50% of their hospital stay in a multi-bedded room had nearly twice the rate of acquiring COVID-19 in hospital (IRR 1.86, 95% CI 1.17–2.94). Specifically, patients that were in multi-bedded rooms between four and seven days were significantly more likely to acquire COVID-19 (IRR 3.89, 95% CI 2.28–6.65) compared to patients who spent less than or equal to two days in a multi-bedded room.
Patient risk factors for nosocomial COVID-19 outbreak
The multivariate analysis (Table 4) revealed that fluid/electrolyte or neurological disorders, days on immunosuppressive agents, and percent of exposure in a multi-bedded room were independent risk factors for nosocomial COVID-19. A sensitivity analysis for the multivariate regression was performed using different cut-offs (25%, 75%) for percentage of exposure time in multi-bedded rooms. The rate ratio of a nosocomial COVID-19 case increased from 1.89 (95% CI 1.04–3.43) at the > 25% cut-off to 3.32 (95% CI 1.47–7.02) at the > 75% cut-off for percentage of time spent in a multi-bedded room (Additional file 3).
Control measures and interventions
Control measures included but were not limited to: active versus passive fit-for-work screening among HCWs with staff symptom screening and temperature checks twice per shift; continuous masking and eye protection by HCWs; enhanced education on PPE including a PPE Safety Coach Program [24]; ward logs for tracking staff and physicians entering the wards, continuation of asymptomatic testing every five days for as long as the HCW worked on any impacted ward, exclusion of non-essential HCWs (e.g. students, volunteers) on the affected wards; and HCW cohorting on all outbreak wards, aided by a single-site order restricting HCWs to only work on a specific ward without moving between wards. Other control measures included, visitation restrictions, enhanced cleaning of high touch or shared equipment, symptom screening twice daily for patients on all outbreak wards, discontinuation of precautions by the most responsible healthcare practitioners, with IPC approval, use of dedicated bedside commodes in two-bed and multi-bedded rooms with shared toilets and blocking beds to reduce the number of multi-bedded rooms being used.
Discussion
Our study is one of several observational studies exploring risk factors for patient COVID-19 acquisition in hospitals [25,26,27,28,29,30,31,32]. There were several factors which were identified during this outbreak in a setting without vaccination for any HCWs or patients which may have facilitated the transmission events to occur. Failure to isolate symptomatic patients on symptom onset likely led to transmission via close contact [33] through either respiratory droplets/particles across a continuum of sizes, and/or contact (direct and indirect) routes of transmission within shared rooms/bathrooms. A retrospective cohort study found in a crude analysis of 122 patients across three outbreak wards that being exposed to a symptomatic COVID-19 patient within the same 4-bed bay regardless of proximity in the room was associated with doubling the risk of becoming a case (crude RR, 2.3, 95% CI 1.42–3.65) [27]. In a matched case–control by Aghdassi et al. [28], the multivariate analysis revealed that presence on a ward that experienced a COVID-19 outbreak (aOR 15.9, 95% CI 2.5–100.8) and documented contact with a COVID-19 case (aOR 23.4, 95% CI 4.6–117.7) to be the primary factors for nosocomial COVID-19 infections in patients [28]. This latter study and the results of our outbreak investigation supports the need to preemptively isolate patients known to be exposed to cases.
Based on our findings, patients who spent > 50% of their admission in a multi-bedded room had 3.2 times the rate of acquiring COVID-19. Other observational studies have demonstrated that multi-bedded rooms versus one-to-two bedded rooms and the use of shared toilets were more common among nosocomial COVID-19 cases compared to controls [29, 31, 34, 35]. The duration of time in a multi-bedded room was a major risk factor and the finding of a dose–response relationship adds epidemiologic strength of association to this finding. Another study from Singapore in a large cohort of nosocomial SARS-CoV-2 infections in patients housed in 5–6-bed cubicles, during time periods encompassing both SARS-CoV-2 Delta and Omicron variants found that sharing a common toilet with ≥ 1 cohorted cubicle was an independent risk factor for a transmission event (aOR, 1.92; 95% CI, 1.02–3.62) along with performance of aerosol-generating procedures and a cycle-threshold value of < 20 on RT-PCR testing [36]. This latter finding corroborates our finding of a 3.2-fold increased rate of acquiring SARS-CoV-2 with exposure to a multi-bedded room with a shared toilet and adds additional support given that it is irrespective of variant strain of SARS-CoV-2.
Another outbreak factor was the delayed recognition of initial cases with illness symptoms compatible with COVID-19 due to crossover with symptoms common to cardiac patients with heart failure (shortness of breath, cough, chest pain, dyspnea). It was difficult to know if clinical judgement, situational factors (e.g. staffing shortages, workarounds), or compromised HCW psychological and physical safety (e.g. stress, fatigue, burnout) resulted in suboptimal point-of-care risk assessment resulting in missed, delayed, or incorrect diagnosis of COVID-19 among these patients leading to preventable exposures and increased transmission [37]. Precise case identification is essential to isolate vulnerable individuals and hence contain transmission [38, 39].
A surprising finding was that individuals with underlying fluid and electrolyte disorders had nearly four times the rate of acquiring COVID-19. It has been hypothesized that the renin–angiotensin–aldosterone system and its core factor ACE2 which regulates electrolyte homeostasis may play a role in the acquisition of COVID-19 [40, 41]. Dehydration, chronic hypertonicity, and/or hypovolemia before COVID-19 infection can alter levels and/or activities of hormones that depend on cell volume (e.g. insulin) and/or balances total body water (e.g. aldosterone), which increases ACE2 receptors potentially making individuals more susceptible to infection [41]. It is also possible that by virtue of their underlying cardiac conditions, these patients may have had a higher degree of pre-existing fluid and electrolyte disorders.
Many studies have reported on the identification of SARS-CoV-2 RNA on inanimate surfaces; however, some authors have suggested that the risk of transmission of SARS-CoV-2 through fomites is low [42]. A recently published systematic review identified that infectious SARS-CoV-2 is indeed present on fomites in multiple settings, especially high frequency touched surfaces. Infectious SARS-CoV-2 on fomites was significantly more likely when the RT-PCR Ct values for clinical specimens and fomite samples was < 30 and most frequently detected within the first week of symptom onset in immunocompetent individuals [43, 44]. Other studies have corroborated the finding of infectious virus being very strongly correlated with low Ct values, irrespective of the variant [45, 46].
Data presented from our viral cultures showing very high quantitative burdens of infectious virus both from patients and their immediate surroundings in conjunction with very low Ct values lends support that direct and indirect (fomite) transmission played a role as a route of transmission. For this outbreak, the culturable virologic and RT-PCR patient and environmental data would lend support to contact transmission occurring within multi-bedded rooms and/or shared bathrooms, especially in the setting of continuous surgical mask wearing by all HCWs, and ventilation parameters exceeding standards and with 100% outside air. We cannot exclude mixed modes of transmission, but the relative protection provided for nosocomial acquisition by patients within single rooms argues against long range airborne transmission. We cannot exclude poor hand hygiene and suboptimal PPE practices by HCWs which have been implicated previously as associated with nosocomial transmission of SARS-CoV-2 by HCWs, even with the use of full PPE [44, 47].
Our outbreak investigation identified multiple exposures among HCWs from symptomatic patients before they were diagnosed with COVID-19, despite the use of continuous masking by all HCWs but without other components of PPE, which may have contributed to acquisition of COVID-19 among HCWs. Doffing of PPE in the appropriate manner and sequence is critical to prevent self-contamination [48,49,50].
Our study is not without limitations. The retrospective nature of our case–control study precludes conclusions of causation for acquisition of COVID-19. Nosocomial cases were investigated more thoroughly during the outbreak, whereas data on controls were collected retrospectively. Selection bias is a common limitation of case–control studies; however, we believe this bias was mitigated by selecting controls matching by age with similar clinical health statuses and ensuring controls overlapped their time in hospital with the case patients. Both cases and controls would have been exposed to similar outbreak control measures. Confounding bias may exist. Information on HCWs was insufficient to include in the study. Although we did not employ universal admission RT-PCR testing for SARS-CoV-2 as per local policy, recent recommendations argue against its routine use for asymptomatic persons in healthcare facilities [51].
There have been many reports on hospital-based outbreaks of COVID-19 [4, 25, 27,28,29, 31, 39, 52,53,54,55,56], however a strength of our report is that it incorporated a case–control study to explore contributing ward and patient-related factors to the acquisition of COVID-19, occurring in a setting without vaccination for any HCWs or patients. Our outbreak has similarities with other COVID-19 nosocomial outbreaks including unidentified cases on a ward [39, 56], positive HCWs who may have sub-optimal adherence to IPC measures [52, 54], and the role of multi-bedded rooms in SARS-CoV-2 transmission [27, 29, 31, 34, 35] Further, our report included patient symptoms, environmental sampling, whole genome sequencing, viral culture and has identified the novel finding of fluid and electrolyte disorders increasing the likelihood of COVID-19 acquisition.
Conclusion
In conclusion, conducting outbreak investigations and evaluating hypotheses epidemiologically is critical in identifying sources of and measures to mitigate transmission of SARS-CoV-2. Key learnings for future outbreaks include but are not limited to (1) recognizing structural and organizational elements in hospitals i.e. multi-bedded rooms, frequent patient movements, routes of patient movement that may contribute to potential spread and include them in pandemic responses; (2) recognizing the contribution of contaminated surfaces and especially mobile medical equipment; the need for careful cleaning and disinfection; and the need for hand hygiene with compliance monitoring, (3) prompt identification of COVID-19 patients; (4) using consistent approaches to ending contact/droplet precautions; (5) minimizing patient transfers; and (6) maintaining adequate training of HCWs in the principles of infection prevention and control including vigilance in donning and doffing of PPE.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available so as to not compromise patient identity.
Abbreviations
- ACE2:
-
Angiotensin converting enzyme 2
- AEH:
-
Air exchanges per hour
- ARECCI:
-
A Project Ethics Community Consensus Initiative
- CHREB:
-
Conjoint Health Research Ethics Board
- COVID-19:
-
Coronavirus disease 2019
- CSA:
-
Canadian Standards Association
- Ct:
-
Cycle threshold
- DAD:
-
Discharge Abstract Database
- EMR:
-
Electronic medical record
- HCWs:
-
Healthcare workers
- HVAC:
-
Heating, ventilation, and air conditioning
- IPC:
-
Infection prevention and control
- IRR:
-
Incidence rate ratio
- NP:
-
Nasopharyngeal
- OR:
-
Odds ratio
- ORION:
-
Outbreak Reports and Intervention studies of Nosocomial infection
- PPE:
-
Personal protective equipment
- RNA:
-
Ribonucleic acid
- RT-PCR:
-
Reverse-transcription polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome Coronavirus-2
- SD:
-
Standard deviation
- WHS:
-
Workplace Health and Safety
References
Paphitis K, Achonu C, Callery S, Gubbay J, Katz K, Muller M, et al. Beyond flu: Trends in respiratory infection outbreaks in Ontario healthcare settings from 2007 to 2017, and implications for non-influenza outbreak management. Can Commun Dis Rep. 2021;47(56):269–75. https://doi.org/10.14745/ccdr.v47i56a04.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. https://doi.org/10.1001/jama.2020.1585.
Abbas M, Robalo Nunes T, Martischang R, Zingg W, Iten A, Pittet D, et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10(1):7. https://doi.org/10.1186/s13756-020-00875-7.
Kanji JN, Chan YLE, Boychuk LR, Boyington C, Turay S, Kobelsky M, et al. SARS-CoV-2 outbreak in a Canadian suburban tertiary hospital necessitating full facility closure: a descriptive observational study. CMAJ Open. 2022;10(1):E137–45. https://doi.org/10.9778/cmajo.20210064.
Susky EK, Hota S, Armstrong IE, Mazzulli T, Kestenberg S, Casaubon LK, et al. Hospital outbreak of the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) delta variant in partially and fully vaccinated patients and healthcare workers in Toronto, Canada. Infect Control Hosp Epidemiol. 2021:1–4. doi:https://doi.org/10.1017/ice.2021.471.
Lessells R, Moosa Y, de Oliveira T. Report into a nosocomial outbreak of coronavirus disease 2019 (COVID-19) at Netcare St. Augustine's Hospital. KwaZulu-Natal, South Africa: University of KwaZulu-Natal and the KwaZulu-Natal Research Innovation and Sequencing Platform; 2020 15 May 2020.
Wilson J. Infection prevention and control in the COVID-19 pandemic: what have we learnt? J Infect Prev. 2021;22(1):5–6. https://doi.org/10.1177/1757177420984914.
Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11(3):e045343. doi:https://doi.org/10.1136/bmjopen-2020-045343.
O'Grady HM, Dixit D, Khawaja Z, Snedeker K, Ellison J, Erebor J, et al. Asymptomatic Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection in adults is uncommon using rigorous symptom characterization and follow-up in an acute care adult hospital outbreak. Infect Control Hosp Epidemiol. 2022(Online ahead of print):1–25. doi:https://doi.org/10.1017/ice.2022.168.
Williamson T, Eastwood C, Quan H, Southern D. The COVID-19 response: Alberta's pandemic response Calgary, Canada: Centre for Health Informatics; 2020. Available from: https://covid-tracker.chi-csm.ca/.
Elias C, Sekri A, Leblanc P, Cucherat M, Vanhems P. The incubation period of COVID-19: a meta-analysis. Int J Infect Dis. 2021;104:708–10. https://doi.org/10.1016/j.ijid.2021.01.069.
Pabbaraju K, Wong AA, Douesnard M, Ma R, Gill K, Dieu P, et al. Development and validation of RT-PCR assays for testing for SARS-CoV-2. J Assoc Med Microbiol Infec Dis Can. 2021;6(1):16–22. https://doi.org/10.3138/jammi-2020-0026.
Lin Y-C, Malott RJ, Ward L, Kiplagat L, Pabbaraju K, Gill K, et al. Detection and quantification of infectious severe acute respiratory coronavirus-2 in diverse clinical and environmental samples. Sci Rep. 2022;12(1):5418. https://doi.org/10.1038/s41598-022-09218-5.
Rajakumar I, Isaac DL, Fine NM, Clarke B, Ward LP, Malott RJ, et al. Extensive environmental contamination and prolonged severe acute respiratory coronavirus-2 (SARS CoV-2) viability in immunosuppressed recent heart transplant recipients with clinical and virologic benefit with remdesivir. Infect Control Hosp Epidemiol. 2021:1–3. doi:https://doi.org/10.1017/ice.2021.89.
nCoV-2019 sequencing protocol V.1. protocols.io. 2020. Available from: https://www.protocols.io/view/ncov-2019-sequencing-protocol-bp2l6n26rgqe/v1?version_warning=no.
Tyson JR, James P, Stoddart D, Sparks N, Wickenhagen A, Hall G, et al. Improvements to the ARTIC multiplex PCR method for SARS-CoV-2 genome sequencing using nanopore. bioRxiv. 2020. doi:https://doi.org/10.1101/2020.09.04.283077.
nCoV-2019 sequencing protocol v3 (LoCost) V.3. 2020. Available from: https://www.protocols.io/view/ncov-2019-sequencing-protocol-v3-locost-bp2l6n26rgqe/v3.
Long reads nanopore sequencing to recover SARS-CoV-2 whole genome v.3. University College London. 2020. Available from: https://www.protocols.io/view/long-reads-nanopore-sequencing-to-recover-sars-cov-5jyl8mkw9g2w/v3.
Rambaut A, Holmes EC, O’Toole A, Hill V, McCrone JT, Ruis C, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5(11):1403–7. https://doi.org/10.1038/s41564-020-0770-5.
Labrecque JA, Hunink MMG, Ikram MA, Ikram MK. Do case-control studies always estimate odds ratios? Am J Epidemiol. 2021;190(2):318–21. https://doi.org/10.1093/aje/kwaa167.
Knol MJ, Vandenbroucke JP, Scott P, Egger M. What do case-control studies estimate? Survey of methods and assumptions in published case-control research. Am J Epidemiol. 2008;168(9):1073–81. https://doi.org/10.1093/aje/kwn217.
Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84(1):151–61. https://doi.org/10.1162/003465302317331982.
Dixit D, Conly J, Snedeker K, Etches N, Weiss A, Suddes M, et al. Nosocomial SARS-CoV-2 infection in health care workers: descriptive analysis of a large acute care hospital COVID-19 outbreak on cardiac units. . J Assoc Med Microbiol Infect Dis Can. 2021;6(Suppl):Abstract P17. doi:https://doi.org/10.3138/jammi.6.s1.abst.
Alberta Health Services. Provincial PPE Safety Coach Program Edmonton, Alberta: Alberta Health Services; 2020 [Available from: https://www.albertahealthservices.ca/ipc/Page17279.aspx.
Biernat MM, Zinczuk A, Biernat P, Bogucka-Fedorczuk A, Kwiatkowski J, Kalicinska E, et al. Nosocomial outbreak of SARS-CoV-2 infection in a haematological unit - High mortality rate in infected patients with haematologic malignancies. J Clin Virol. 2020;130:104574. doi:https://doi.org/10.1016/j.jcv.2020.104574.
Iio R, Kaneko T, Mizuno H, Isaka Y. Clinical characteristics of COVID-19 infection in a dialysis center during a nosocomial outbreak. Clin Exp Nephrol. 2021;25(6):652–9. https://doi.org/10.1007/s10157-021-02025-8.
Leeman DS, Ma TS, Pathiraja MM, Taylor JA, Adnan TZ, Baltas I, et al. Severe acute respiratory coronavirus virus 2 (SARS-CoV-2) nosocomial transmission dynamics, a retrospective cohort study of two healthcare-associated coronavirus disease 2019 (COVID-19) clusters in a district hospital in England during March and April 2020. Infect Control Hosp Epidemiol. 2021:1-7. doi:https://doi.org/10.1017/ice.2021.483.
Aghdassi SJS, Schwab F, Pena Diaz LA, Brodzinski A, Fucini GB, Hansen S, et al. Risk factors for nosocomial SARS-CoV-2 infections in patients: results from a retrospective matched case-control study in a tertiary care university center. Antimicrob Resist Infect Control. 2022;11(1):9. https://doi.org/10.1186/s13756-022-01056-4.
Thoma R, Kohler P, Haller S, Schlegal M, Flury D. Ward-level risk factors associated with nosocomial coronavirus disease 2019 (COVID-19) outbreaks: a matched case-control study. Antimicrobial Stewardship Healthcare Epidemiol. 2022;2(e49):1–3. https://doi.org/10.1017/ash.2022.11.
Abbas M, Robalo Nunes T, Cori A, Cordey S, Laubscher F, Baggio S, et al. Explosive nosocomial outbreak of SARS-CoV-2 in a rehabilitation clinic: the limits of genomics for outbreak reconstruction. J Hosp Infect. 2021;117:124–34. https://doi.org/10.1016/j.jhin.2021.07.013.
Dinh C, Gallouche M, Terrisse H, Gam K, Giner C, Nemoz B, et al. Risk factors of nosocomial Covid-19 in a French university hospital: a case-control study. Preprint: Research Square; 2022.
Lakhani K, Minguell J, Guerra-Farfan E, Lara Y, Jambrina U, Pijoan J, et al. Nosocomial infection with SARS-CoV-2 and main outcomes after surgery within an orthopaedic surgery department in a tertiary trauma centre in Spain. Int Orthop. 2020;44(12):2505–13. https://doi.org/10.1007/s00264-020-04798-1.
Onakpoya IJ, Heneghan CJ, Spencer EA, Brassey J, Pluddemann A, Evans DH, et al. SARS-CoV-2 and the role of close contact in transmission: a systematic review. F1000Res. 2021;10:280. doi:https://doi.org/10.12688/f1000research.52439.3.
Martin A, Kampouri E, Senn L, Sartori C. Epidemiology and risk factors of nosocomial COVID infections in the Service of internal medicine at CHUV. Rev Med Suisse. 2021;17(760):2049–54.
Karan A, Klompas M, Tucker R, Baker M, Vaidya V, Rhee C. The risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission from patients with undiagnosed Coronavirus Disease 2019 (COVID-19) to roommates in a large academic medical center. Clin Infect Dis. 2022;74(6):1097–100. https://doi.org/10.1093/cid/ciab564.
Wee LE, Ko KKK, Conceicao EP, Aung MK, Aung MO, Yang Y, et al. Enhanced infection prevention measures including universal N95 usage and daily testing: the impact on SARS-CoV-2 transmission in cohorted hospital cubicles through successive Delta and Omicron waves. Clin Infect Dis. 2022;75(5):917–9. https://doi.org/10.1093/cid/ciac320.
Gandhi TK, Singh H. Reducing the risk of diagnostic error in the COVID-19 era. J Hosp Med. 2020;15(6):363–6. https://doi.org/10.12788/jhm.3461.
Chen T, Hanna J, Walsh EE, Falsey AR, Laguio-Vila M, Lesho E. Syncope, Near syncope, or nonmechanical falls as a presenting feature of COVID-19. Ann Emerg Med. 2020;76(1):115–7. https://doi.org/10.1016/j.annemergmed.2020.04.037.
Roberts SC, Foppiano Palacios C, Grubaugh ND, Alpert T, Ott IM, Breban MI, et al. An outbreak of SARS-CoV-2 on a transplant unit in the early vaccination era. Transpl Infect Dis. 2022;24(2):e13782. doi:https://doi.org/10.1111/tid.13782.
Nahkuri S, Becker T, Schueller V, Massberg S, Bauer-Mehren A. Prior fluid and electrolyte imbalance is associated with COVID-19 mortality. Commun Med. 2021;1(1):51. https://doi.org/10.1038/s43856-021-00051-x.
Stookey JD, Allu PKR, Chabas D, Pearce D, Lang F. Hypotheses about sub-optimal hydration in the weeks before coronavirus disease (COVID-19) as a risk factor for dying from COVID-19. Med Hypotheses. 2020;144:110237. doi:https://doi.org/10.1016/j.mehy.2020.110237.
Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis. 2020;20(8):892–3. https://doi.org/10.1016/S1473-3099(20)30561-2.
Onakpoya IJ, Heneghan CJ, Spencer EA, Brassey J, Pluddemann A, Evans DH, et al. SARS-CoV-2 and the role of fomite transmission: a systematic review. F1000Res. 2021;10:233. doi:https://doi.org/10.12688/f1000research.51590.3.
O’Grady HM, Harrison R, Snedeker K, Trufen L, Yue P, Ward L, et al. A two-ward acute care hospital outbreak of SARS-CoV-2 delta variant including a point-source outbreak associated with the use of a mobile vital signs cart and sub-optimal doffing of personal protective equipment. J Hosp Infect. 2022;131:1–11. https://doi.org/10.1016/j.jhin.2022.09.019.
Bouton TC, Atarere J, Turcinovic J, Seitz S, Sher-Jan C, Gilbert M, et al. Viral dynamics of Omicron and Delta SARS-CoV-2 variants with implications for timing of release from isolation: a longitudinal cohort study. Clin Infect Dis. 2022. https://doi.org/10.1093/cid/ciac510.
Young BE, Ong SWX, Ng LFP, Anderson DE, Chia WN, Chia PY, et al. Viral dynamics and immune correlates of Coronavirus Disease 2019 (COVID-19) severity. Clin Infect Dis. 2021;73(9):e2932–42. https://doi.org/10.1093/cid/ciaa1280.
Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk factors of healthcare workers with Coronavirus Disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020;71(16):2218–21. https://doi.org/10.1093/cid/ciaa287.
Chughtai AA, Chen X, Macintyre CR. Risk of self-contamination during doffing of personal protective equipment. Am J Infect Control. 2018;46(12):1329–34. https://doi.org/10.1016/j.ajic.2018.06.003.
Saran S, Gurjar M, Garg A. Identifying and implementing strategies to reduce the risk of self-contamination of health care workers caused by doffing of personal protective equipment during the COVID-19 pandemic. Disaster Med Public Health Prep. 2020:1–4. doi:https://doi.org/10.1017/dmp.2020.396.
Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database of Systematic Reviews. 2020(4). doi:https://doi.org/10.1002/14651858.CD011621.pub4.
Talbot TR, Hayden MK, Yokoe DS, Malani AN, Amer HA, Kalu IC, et al. Asymptomatic screening for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) as an infection prevention measure in healthcare facilities: Challenges and considerations. Infect Control Hosp Epidemiol. 2022:1–6. doi:https://doi.org/10.1017/ice.2022.295.
Rana K, Sharma B, Lakshmi PVM, Kaur M, Singh MP, Singh R, et al. Nosocomial outbreak of SARS-CoV-2 in a non-COVID zone of a tertiary care hospital of North India: Need to upgrade infection control practices. J Prim Care Community Health. 2021;12:21501327211050750. https://doi.org/10.1177/21501327211050753.
Harada S, Uno S, Ando T, Iida M, Takano Y, Ishibashi Y, et al. Control of a nosocomial outbreak of COVID-19 in a university hospital. Open Forum Infect Dis. 2020;7(12):ofaa512. doi:https://doi.org/10.1093/ofid/ofaa512.
Lee U, Kim SE, Lee SY, Wi HN, Choi O, Park JW, et al. Source analysis and effective control of a COVID-19 outbreak in a university teaching hospital during a period of increasing community prevalence of COVID-19. J Korean Med Sci. 2021;36(24):e179. doi:https://doi.org/10.3346/jkms.2021.36.e179.
Moore G, Rickard H, Stevenson D, Aranega-Bou P, Pitman J, Crook A, et al. Detection of SARS-CoV-2 within the healthcare environment: a multi-centre study conducted during the first wave of the COVID-19 outbreak in England. J Hosp Infect. 2021;108:189–96. https://doi.org/10.1016/j.jhin.2020.11.024.
Lesho EP, Walsh EE, Gutowski J, Reno L, Newhart D, Yu S, et al. A cluster-control approach to a coronavirus disease 2019 (COVID-19) outbreak on a stroke ward with infection control considerations for dementia and vascular units. Infect Control Hosp Epidemiol. 2021;42(11):1333–9. https://doi.org/10.1017/ice.2020.1437.
Acknowledgements
We thank Paul Dieu, Christina Ferrato, Kara Gill, Raymond Ma, and Johanna Thayer from the Genomics and Bioinformatics or Research Department, Alberta Public Health Laboratory – South, Calgary, AB, Canada and the Canadian COVID-19 Genomics Network (CanCOGeN) for their support. We also wish to thank the Calgary Zone Workplace Health and Safety Occupational Health Nursing team, with special thanks to Durwin Luc, the Public Health Communicable Disease team, the Foothills Medical Centre Site Command Post and the cardiology medical team for their assistance with queries related to healthcare worker and visitor data acquisition. We would also like to acknowledge the work done by Dr. Uma Chandran and Winnie Winter in creating the initial draft and updates of the COVID-19 symptom identification and monitoring tool (COVID-19SIMT). The HCW component of this study was presented in abstract form as a poster at AMMI Canada-CACMID Annual Meeting in 2021. (https://jammi.utpjournals.press/doi/pdf/10.3138/jammi.6.issue-s1). We acknowledge the Infection Prevention and Control and Public Health team members who facilitated rapid contact tracing and epidemiological investigations related to the outbreak. The site leadership teams were also instrumental in facilitating the coordinated and effective outbreak response.
Funding
This study was funded in part by the University of Calgary Infectious Diseases Research and Innovation Fund for COVID-19 and the Canadian COVID-19 Genomics Network (CanCOGeN).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.L., J.M.C., H.M.O., J.El.; Methodology: J.L., J.M.C., H.M.O., J.E.; Data Curation: J.L., J.M.C., H.M.O., D.D., Z.K., K.S., J.El., J.Er., D.S., K.R., K.W., M.C., B.B., K.P., Y.L., D.E., Formal Analysis: J.L., J.M.C., H.M.O., L.A., D.D., Supervision: J.L., J.M.C., Investigation: H.M.O., Z.K., K.S., J.Er., M.C., B.B., K.P., Y.L., D.E. Validation: D.D., Z.K., K.S., M.C., B.B. Writing original draft: J.L., J.M.C., H.M.O. Writing-review and editing: J.L., J.M.C., H.M.O., L.A., D.D., Z.K., K.S., J.El., J.Er., P.J., A.W., D.S., K.R., K.W., M.C., B.B., K.P., Y.L., D.E.; Resources: H.M.O., P.J., A.W., D.S., K.R., K.W., D.E.; Funding acquisition: J.M.C. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The outbreak investigations were conducted in the setting of a formal Epidemiologic Investigation under Public Health in the Province of Alberta. The ARECCI (A Project Ethics Community Consensus Initiative) assessment scored this project as fitting with a quality improvement project for which Institutional Review Board approval was not required. The case control component and collection and culturing of patients was approved by the University of Calgary’s Conjoint Health Research Ethics Board (CHREB ID: REB21-0666 and REB20-0444, respectively). The ORION framework for reporting an outbreak or intervention study of a nosocomial organism was followed in this report.
Consent for publication
Not applicable.
Competing interests
All authors will have filled out an ICMJE Disclosure form. JL reports Program funds from the Centre of Health Informatics at the University of Calgary used to provide in-Kind analytical support for her COVID-19 research, not related to this manuscript and funds paid to the University of Calgary to support professional development, specifically IPAC Canada 2022 Virtual conference fees were reimbursed by the Public Health Agency of Canada’s Canadian Nosocomial Infection Surveillance Program. All other authors do not have relevant conflicts of interest to declare related to any aspect of the submitted manuscript. All authors have reviewed the version of the manuscript which has been submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Definitions: Lists full definitions for outbreak, case definition, and data sources. It also provides additional details about data collection.
Additional file 2
. Environmental sampling results: Includes three tables of the environmental sampling results before and after cleaning, and cultivatable virus detected from clinical and environmental specimens.
Additional file 3
. Additional case-control details and results: Includes case-control matching details, breakdown of comorbidities between cases and matched controls, and independent risk factors for nosocomial COVID-19 using different cut-off points for the percent exposure on multi-bedded rooms.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Leal, J., O’Grady, H.M., Armstrong, L. et al. Patient and ward related risk factors in a multi-ward nosocomial outbreak of COVID-19: Outbreak investigation and matched case–control study. Antimicrob Resist Infect Control 12, 21 (2023). https://doi.org/10.1186/s13756-023-01215-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01215-1