Abstract
Background
The impact of the variant of concern (VOC) Alpha on the severity of COVID-19 has been debated. We report our analysis in France.
Methods
We conducted an exposed/unexposed cohort study with retrospective data collection, comparing patients infected by VOC Alpha to contemporaneous patients infected by historical lineages. Participants were matched on age (± 2.5 years), sex and region of hospitalization. The primary endpoint was the proportion of hospitalized participants with severe COVID-19, defined as a WHO-scale > 5 or by the need of a non-rebreather mask, occurring up to day 29 after admission. We used a logistic regression model stratified on each matched pair and accounting for factors known to be associated with the severity of the disease.
Results
We included 650 pairs of patients hospitalized between Jan 1, 2021, and Feb 28, 2021, in 47 hospitals. Median age was 70 years and 61.3% of participants were male. The proportion of participants with comorbidities was high in both groups (85.0% vs 90%, p = 0.004). Infection by VOC Alpha was associated with a higher odds of severe COVID-19 (41.7% vs 38.5%—aOR = 1.33 95% CI [1.03–1.72]).
Conclusion
Infection by the VOC Alpha was associated with a higher odds of severe COVID-19.
Similar content being viewed by others
Background
Since the end of 2020, the SARS-CoV-2 variant of concern (VOC) Alpha, also known as B.1.1.7 or VOC-202012/01 has rapidly spread across all continents [1, 2]. It has been shown that the VOC Alpha is between 43 and 90% more transmissible than variants from historical lineages (HL) 19A/B and 20A/B/C/D/E/F/G [3,4,5,6]. The effect of the VOC Alpha on COVID-19 severity is less clear, although some authors, mostly from the United Kingdom, reported an increased risk of hospitalization [7, 8] or of mortality [9,10,11,12], while others did not report changes in either symptoms, disease duration [13], or severity [14]. Variation in study designs and settings, particularly when medical infrastructures are strained, may explain these discrepancies.
In France, the VOC Alpha accounted for 3.3% of the viruses sequenced on January 8th, 2021, and reached 83% on April 15th, 2021 [15]. Therefore, while the two first epidemic waves affecting France in 2020 were related to HL, the current epidemic occurring since January 2021 is characterized by a progressive overlapping switch towards VOC Alpha dominance. The aim of this study was to assess the effect of VOC Alpha compared to HL on COVID-19 severity in a multicentre matched exposed and unexposed cohort study with retrospective data collection focusing on patients admitted to the hospital during a time when both the HL and the VOC Alpha coexisted and while there was no limitation in medical resources.
Methods
All adults (age > 18 years) hospitalized for symptomatic acute COVID-19 between Jan 1, 2021, and Feb 28, 2021, with a positive VOC Alpha screening were eligible for the study. Over the same time period, the maximum number of patients concurrently hospitalized for COVID-19 in France was 24,820, including 3492 patients in ICU, which is lower than the maximal bed capacity (108,183 beds in medical wards, including 5433 beds in ICU, and 5954 additional beds in intensive care) [16]. During the time period of the study, COVID-19 diagnosis through PCR on nasopharyngeal sampling was widely and freely available to everyone. VOC Alpha screening was performed using the ThermoFischer kit (TaqPath One-step RT-qPCR, ThermoFischer Scientific, Waltham, MA, USA) with Spike gene target RT-PCR failure or mutations-specific real-time RT-PCR (i.e. deletion 69–70 and N501Y mutation, TIB Molbiol, Berlin, Germany). The combination of spike deletion at residue 69–70 and N501Y mutation was interpreted as a suspicion of VOC Alpha. All eligible participants who objected to the use of their data were excluded from the analyses. Participants with VOC Alpha were matched in a 1:1 ratio to HL on the basis of age (± 2.5 years), sex and administrative region of hospitalization.
Data collection
Data were retrospectively collected from all participating sites of the CoCliCo (Collective of COVID-19 clinicians) network. All sites were asked to identify all adults who met the eligibility criteria and collect the site number, age and sex of these patients for centralized matching. The list of matched participants was then sent to the sites to fill out the electronic case report form. Data relevant to the study’s objectives were extracted from the patients’ medical records. Data on COVID-19 vaccination status were not collected in the study at a time where only 2.4% of the eligible population (above 75 years of age or healthcare workers) had received a complete vaccine scheme.
Outcomes
The primary outcome was the proportion of participants with a severe form of COVID-19 occurring up to day 29 after the date of hospitalization. Severity was defined by a WHO clinical progression scale > 5 (high flow oxygen therapy (HFOT), non-invasive ventilation (NIV), invasive ventilation, extra-corporeal membrane oxygenation (ECMO) or death) [17] but also by the need of a non-rebreather mask (NRB) in order to consider patients with severe COVID-19 but limitations of life-sustaining treatment. Two participants with missing primary outcome were considered as having a severe form.
The secondary endpoints were: (i) mortality; (ii) WHO clinical progression scale > 5; (iii) admission to an intensive care unit (ICU); (iv) invasive ventilation or ECMO; (v) HFOT, all up to day 29, (vi) time from symptom onset to hospitalization, and (vii) re-admission after discharge up to day 29. Maximal parenchymal lesions extension and pulmonary thrombo-embolism detected on chest CT-scan were collected.
Statistical analyses
For an 80% power, a 5% type I error and an expected severity of 20% in patients infected with HL, 1100 exposed and 1100 unexposed individuals to VOC Alpha were needed to detect a 25% higher risk of severity in participants infected with VOC Alpha compared to HL with 1:1 matching, while the number of matched pairs corresponding to 30%, 40% and 50% higher risk of severity were 769, 444, and 291 respectively.
The analysis population consisted of all individuals exposed to VOC Alpha with a matched unexposed control. The characteristics of exposed and unexposed individuals to VOC Alpha were compared with a Mc Nemar test for categorical variables and Wilcoxon paired test for continuous variables.
Unadjusted and adjusted odds ratios (OR) were calculated using a logistic regression model stratified on each matched pair to assess the association between VOC Alpha infection and the occurrence of a severe form of COVID-19. The following factors associated with COVID-19 severity were accounted for in multivariable models: age, BMI, smoking and comorbidities (cardiovascular disease, chronic lung disease, asthma, chronic kidney disease, chronic liver disease, chronic neurological disease, active cancer, solid organ or hematopoietic cell transplantation, autoimmune disease, HIV infection, and diabetes). Treatments received during the course of hospitalization could be the result of a worse course of the disease and therefore be on the causal pathway of a more severe disease; therefore, receiving corticosteroids was not included in the main model.
Time-to-event methods, including Kaplan–Meier estimates and Cox proportional-hazards models, were used to analyze all secondary outcomes. Unadjusted and adjusted hazard ratios (HR) were calculated using Cox proportional hazard model stratified on each pair to assess whether VOC Alpha was associated with the outcome. Death was not accounted as a competing risk in the main analysis, and was accounted for in sensitivity analyses for high flow oxygen therapy, ICU admission, and mechanical ventilation or ECMO. Analyses were conducted with SAS software version 9.4 (SAS Statistical Institute, Cary, North Carolina). All statistical tests were 2-tailed, with α = 0.05.
Results
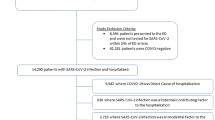
In this multicentre matched exposed-unexposed cohort study, 882 participants with VOC Alpha infection were eligible and 650 were enrolled and matched on the basis of sex, age and administrative region, to 650 contemporaneous participants infected by HL (Fig. 1).
Characteristics of patients are presented in Table 1 according to SARS-CoV-2 lineage. The median age was 70 years (range 25 to 101) and 61.3% were males. Participants with VOC Alpha infection less often had at least one of the specific comorbidities listed above, and were less often smokers, than their matched participants. The proportion of patients first admitted in the ICU did not differ between groups (16.7% in the VOC Alpha group vs 13.8% in the HL group, P = 0.12). Median oxygen saturation level before initiation of oxygen therapy was not different (90% (range 32–99) in the VOC Alpha group vs 91% (range 30–99) in the HL group, P = 0.12), as well as the acme of C-reactive protein level during the first three days of hospitalization (median 93 mg/L (range 1–584) in the VOC Alpha group vs 89 mg/L (range 1–423) in the HL group, P = 0.34). Proportions of patients who received corticosteroids and other immunomodulatory therapies were higher in the VOC Alpha group (respectively 84.3% and 8.4%) than in the HL group (respectively 76.6%, P < 0.001 and 5.2%, P < 0.02). The proportion of participants who received anticoagulant and antibiotic therapy did not differ (93.3% versus 91.3% and 60.3 versus 60.6% respectively). Only 5 participants in each group received monoclonal antibodies or antiviral drugs.
The proportion of severe COVID-19 (defined as a WHO score > 5 or the need of a NRB) within 29 days of hospitalization was 41.7% in the VOC Alpha group and 38.5% in the HL group (aOR 1.33 95% confidence interval (95% CI): [1.02–1.72] in the multivariable analysis) and similar results were observed for the 2 components of the primary endpoints (Table 2). Regarding secondary outcomes within 29 days after hospitalization, the mortality rate was 24% in the VOC Alpha group and 19% in the HL group (aHR 1.21 [0.93–1.58] in the multivariable analysis), and the proportion of patients reaching a WHO score > 5 was 26.2% in the VOC Alpha group and 20.5% in the HL group (aHR 1.24 [1.00–1.55] in the multivariable analysis). All other secondary endpoints were not significantly associated with infection by the VOC Alpha. The entire multivariable model and Kaplan–Meier curves are provided as Additional file 1.
Acme of parenchymal extent on chest CT-scan was higher in the VOC Alpha group (50%, range 0–95) than in the HL group (40%, range 0–99, univariable analysis P = 0.04). The proportion of patients diagnosed with pulmonary embolism did not differ (5.3% in the VOC Alpha group, and 6.0% in the HL group, univariable analysis P = 0.29).
Discussion
In this multicentre matched exposed-unexposed cohort study, we found a 33% (95%CI: 3–72%) higher odds of severe COVID-19 in participants infected by a VOC Alpha, while the increase in the risk of death within 29 days after hospitalization was not significant (21% (95% CI: − 7% to + 58%).
These results are in line with the literature showing an increased severity of VOC Alpha compared to HL. The European Surveillance System analyzed 19,207 cases of VOC Alpha and 3,348 HL cases reported between Sept 14, 2020, and March 14, 2021, from seven European countries [7]. In this study, patients infected with VOC Alpha were found to have a 1.7 times higher risk of being hospitalized for COVID-19. Hospitalized patients were also shown to be younger (by 10 years in median) and to be less comorbid than patients hospitalized for COVID-19 related to HL in this study and others [7, 11, 14]. The latter was also true in our study. Comparison of data concerning the age of infected patients is more complex because of a different epidemiological context and vaccine strategies, hence we do not provide additional data on that matter, given that participants were matched on age in our study. We can however underline that the impact of VOC Alpha on the higher occurrence of severe COVID-19 is reinforced by the fact that our patients have less comorbidities than the matched non-exposed patients.
The effect of VOC Alpha on the risk of mortality is still the subject of debate. Both the study from the OpenSAFELY electronic health records [11] and three community-based studies [9, 10, 12] performed in the United Kingdom from Oct 1, 2020, to Feb 14, 2021, which compared VOC Alpha to HL, showed an increased hazard of death of 1.55 to 1.67. These studies could have been biased by the epidemiological context and overwhelmed hospital capacities, that may have increased the impact of COVID-19 in the most severely ill patients. Of note, although our study may have been underpowered to detect a significant increase in the risk of death, the confidence interval of the mortality hazard in our study is compatible with the reported confidence intervals reported in the United Kingdom with a higher bound of 1.58. Conversely, rates of ICU admission or death did not differ significantly in any age group in the study from The European Surveillance System [7]. In addition, there was no evidence of an association between severe disease, death and lineage in a hospital-based cohort study of patients acutely admitted to hospitals in London from Nov 9, 2020, to Dec 20, 2020, before the peak of hospital admissions [14], but that study was small and the baseline date was different for participants with symptoms (date of symptoms) and those without (date of hospitalization). These discrepancies between studies underline the need to consider the geographical area, and the potential impact of the epidemiological pressure on healthcare facilities that could have increased morbimortality of COVID-19.
Why VOC Alpha is associated with an increased severity in human beings is unknown. Our study highlights clinically relevant details depicting the course and pathogenesis of COVID-19 related to VOC Alpha. Patients with VOC Alpha infection were not hospitalized sooner after the onset of first symptoms, and were not more frequently admitted to ICU first than patients infected with HL. However, we found a higher maximal parenchymal extent of ground-glass opacities on chest CT-scan. In the meantime, these patients were more likely to reach a WHO score > 5. This suggests that increased pathogenicity of VOC Alpha is not linked with a shortened delay between the first symptoms and the hospital admission. Patients were hospitalized at the beginning of their second week of symptoms, at a time which is considered to be the “inflammatory phase” of the disease. At the same time, high nasopharyngeal viral loads are central to pathogenesis of viral infections and have been shown in SARS to be associated with the onset of symptoms, oxygen desaturation, mechanical ventilation, and death [18]. Recent studies showed that COVID-19 patients infected by VOC Alpha had a viral load 3 to 10 times higher than the HL in nasopharyngeal samples [14, 19, 20]. This higher viral load in SARS-CoV-2 VOC Alpha infection can result from a higher virus binding affinity to the angiotensin-converting enzyme 2 receptor [21], which likely enhances entry to epithelial host cells in the respiratory tract and the lungs and could trigger a stronger immune response causing a more severe disease compared to HL. This might be illustrated by the higher parenchymal extent of ground-glass opacities on chest CT-scan, although the acme of C-reactive protein serum level during the first three days of hospitalization did not differ between groups. In the present study, SARS-CoV-2 nasopharyngeal viral loads, estimated by real-time PCR Ct values, were not recorded due to the heterogeneity of RT-PCR assays used in this multicentre study, which renders difficult their interpretations due to the inter-assays variations.
In this study we controlled for several potential confounding factors by using a short study period and matching on the basis of administrative region to account for the potential impact of the local burden of the epidemic on the care system which can influence the clinical outcomes. Given the strong effect of age on the severity of the disease we matched participants exposed to the VOC Alpha to participant exposed to HL on age within 2.5 years and we also adjusted the analysis according to age. We also accounted for the presence of comorbidities and smoking, factors known to be associated with a more severe course of the disease. Only 650 of the 882 patients infected with VOC Alpha initially listed as eligible by the clinical sites could finally be enrolled and matched. Although it is unlikely for this drop to be strongly linked with the outcomes, we cannot exclude some selection bias. Socio-economic status and origin were not collected and could not be accounted for, although they are associated with severity of disease. Given the retrospective nature of data collection, we had to restrict data collection to variables available in medical records of most participants. For instance, although obesity was accounted for, we could not collect the exact BMI which would have been more precise. As in any observational studies, the remaining role of additional confounders cannot be ruled out. Finally, we considered the results of SARS-CoV-2 screening test strategies and not of viral genome sequencing, however a very high level of agreement has been described in the literature between the presence of deletion 69–70 and the VOC Alpha [9].
Conclusion
VOC Alpha is associated with an increased severity, and potentially leads to an increased mortality. These considerations have huge implications for vaccine allocation policies. Vaccination should now urgently be made accessible to patients who were not previously prioritized in order to reach herd immunity. These results also point to the importance of limiting the circulation of the virus until a very large proportion of the population is vaccinated.
Availability of data and materials
Data requests should be sent to Prof Dominique Costagliola. Data access must be approved by the French data protection authority, la Commission Nationale de l’Informatique et des Libertés. For further information, please see: https://www.cnil.fr/.
Abbreviations
- VOC:
-
Variant of concern
- COVID-19:
-
Coronavirus disease-19
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- WHO:
-
World Health Organization
- aOR:
-
Adjusted odds ratios
- HR:
-
Hazard ratios
- HL:
-
Historical lineages
- CoCliCo:
-
Collective of COVID-19 clinicians
- HFOT:
-
High flow oxygen therapy
- NIV:
-
Non-invasive ventilation
- ECMO:
-
Extra-corporeal membrane oxygenation
- NRB:
-
Non-rebreather mask
References
Available from: https://cov-lineages.org/global_report_B.1.1.7.html.
Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N Engl J Med. 2021. https://doi.org/10.1056/NEJMc2100362.
Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:6538.
Gaymard A, Bosetti P, Feri A, Destras G, Enouf V, Andronico A, et al. Early assessment of diffusion and possible expansion of SARS-CoV-2 Lineage 20I/501Y.V1 (B.1.1.7, variant of concern 202012/01) in France, January to March 2021. Euro Surveill. 2021. https://doi.org/10.2807/1560-7917.ES.2021.26.9.2100133.
Leung K, Shum MH, Leung GM, Lam TT, Wu JT. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2021. https://doi.org/10.2807/1560-7917.ES.2020.26.1.2002106.
Zhao S, Lou J, Cao L, Zheng H, Chong MKC, Chen Z, et al. Quantifying the transmission advantage associated with N501Y substitution of SARS-CoV-2 in the UK: an early data-driven analysis. J Travel Med. 2021;28(2). https://doi.org/10.1093/jtm/taab011.
Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, et al. Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Euro Surveill. 2021;26(16). https://doi.org/10.2807/1560-7917.ES.2021.26.16.2100348.
Nyberg T, Twohig KA, Harris RJ, Seaman SR, Flannagan J, Allen H, et al. Risk of hospital admission for patients with SARS-CoV-2 variant B.1.1.7: cohort analysis. BMJ. 2021;373:n1412.
Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372: n579.
Davies NG, Jarvis CI, Group CC-W, Edmunds WJ, Jewell NP, Diaz-Ordaz K, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–4. https://doi.org/10.1038/s41586-021-03426-1.
Grint DJ, Wing K, Williamson E, McDonald HI, Bhaskaran K, Evans D, et al. Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Euro Surveill. 2021. https://doi.org/10.2807/1560-7917.ES.2021.26.11.2100256.
Patone M, Thomas K, Hatch R, Tan PS, Coupland C, Liao W, et al. Mortality and critical care unit admission associated with the SARS-CoV-2 lineage B.1.1.7 in England: an observational cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/S1473-3099(21)00318-2.
Graham MS, Sudre CH, May A, Antonelli M, Murray B, Varsavsky T, et al. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health. 2021;6(5):e335–45. https://doi.org/10.1016/S2468-2667(21)00055-4.
Frampton D, Rampling T, Cross A, Bailey H, Heaney J, Byott M, et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/S1473-3099(21)00170-5.
Available from: https://www.santepubliquefrance.fr/etudes-et-enquetes/enquetes-flash-evaluation-de-la-circulation-des-variants-du-sars-cov-2-en-france.
Available from: https://geodes.santepubliquefrance.fr/#c=indicator&f=0&i=covid_hospit.rea&s=2021-06-08&t=a01&view=map2.
Characterisation WHOWGotC, Management of C-i. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192-e7.
Hung IF, Cheng VC, Wu AK, Tang BS, Chan KH, Chu CM, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis. 2004;10(9):1550–7.
Calistri P, Amato L, Puglia I, Cito F, Di Giuseppe A, Danzetta ML, et al. Infection sustained by lineage B.1.1.7 of SARS-CoV-2 is characterised by longer persistence and higher viral RNA loads in nasopharyngeal swabs. Int J Infect Dis. 2021;105:753–5.
Teyssou E, Soulie C, Visseaux B, Lambert-Niclot S, Ferre V, Marot S, et al. The 501Y.V2 SARS-CoV-2 variant has an intermediate viral load between the 501Y.V1 and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect. 2021;83(1):119–45. https://doi.org/10.1016/j.jinf.2021.04.023.
Ramanathan M, Ferguson ID, Miao W, Khavari PA. SARS-CoV-2 B.1.1.7 and B.1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect Dis. 2021;21(8):1070. https://doi.org/10.1016/S1473-3099(21)00262-0.
Acknowledgements
We wish to thank all the patients and staff from all the units that participated in the study, and the ANRS-MIE (Agence Nationale de Recherches sur le SIDA et les hépatites virales- Maladies Infectieuses Emergentes) for its support. CoCliCo study group: Guillaume Martin-Blondel1,2, Pierre Delobel1, Gaspard Grouteau1, Jean Roch Le Henaff1, Vincent Mear1, Sandra Lagarrigues1, Alais Frelat1,Thomas De Nadai14, Zara Steinmeyer14, Arnaud Del Bello15, Stéphanie Ruiz16, Benjamine Sarton16, Elise Noel-Savina17, Jacques Izopet18, Nathan Peiffer-Smadja3, Michael Thy3, Mathilde Gare3, Diane Le Pluart3, François-Xavier Lescure3, Christophe Rioux3, Laurène Deconinck3, Yazdan Yazdanpanah3, BenoitVisseaux3, Diane Descamps5, Charlotte Charpentier5, Jean-Marc Chapplain6, Pierre Tattevin6, Thomas Perpoint7, Maude Bouscambert-Duchamp19, Hodane Yonis20, Paul Chabert20, Hugues Cordel8, Youssouf Mohamed-Kassim8, Nolan Hassold8, Segolène Brichler21, Julien Caliez22, Thomas Rambaud23, Marilucy Lopez-Sublet24, Frédéric Adnet25, Gilles Pialoux9, Christia Palacios9, Marwa bachir9, Marine Nadal9, Mathieu turpin26, Antoine Parrot27, Djeneba Fofana28, Jérome Pacanowski10, Karine Lacombe10, Emmanuelle Gras10, Laura Levi10, Laure Surgers10, Ines Devred10, Nadia Valin10, Thibault Chiarabini10, Jean Luc Meynard10, Adeline Bauvois11, Clara Duran11, Elyanne Gault11, Jean-Emmanuel Kahn11, Elisabeth Rouveix11, Guillaume Geri29, Didier laureillard12, Albert Sotto12, Paul Loubet12, Claire Roger30, Julien Poissy13, Marc Lambert13, Ady Assaf31, Laurence Bocket32, Firouzé Bani-Sadr33, Yohan N'Guyen33, Juliette Romaru33, Maxime Hentzien33, Thomas Gabas34, Amélie Chabrol34, Cecilia Billiou35, Philippe Menager36, Christophe Billy37, Jean-Jacques Laurichesse37, Fabrice Ketty N Simba37, Pauline Caraux Paz38, Liliane Tinang39, Agathe Bounhiol39, Catherine Burnat40, Sandrine Soriot-Thomas41, Damien Basille41, Jean Philippe Lanoix41, Yoan Zerbib41, Yoann Zerbib41, Anne Pouvaret42, Fanny Lanternier42, Helene Mascitti43, Aurélien Dinh43, Benjamin Davido43, Hôpital Foch, Suresnes, France, Service de Biologie Clinique : Philippe Lesprit44, Eric Farfour44, Mathilde Neuville45, Linda Nait Allaoua46, Michèle Lejaille46, Nathalie De Castro47, Jean-Michel Molina47, Diane Ponscarme47, Mariagrazia Tateo47, Geoffroy Liegeon47, Ines Boussen47, Pauline Huriez47, André Cabié48, Valentine Campana48, Isabelle Calmont48, Jean-Marie Turmel48, Guitteaud Karine48, Pierre-François Sandrine48, Athéna Marquise48, Ornella Cabras48, Mélanie Lehoux48, Cyrille Chabartier49, Vincent Dubee50, Diama Ndiaye50, Caroline Lefeuvre51, Achille Kouatchet52, Duc Nguyen53, Camille Tumiotto54, Pierre Sioniac55, Alexandre Boyer55, Jean-François Faucher56, Edouard Desvaux57, Sylvie Rogez58, Paul Le Turnier59, François Raffi59, Emmanuel Canet60, Antoine Roquilly60, Louise Castain61, Solène Secher61, Véronique Mondain62, Lionel Piroth63, Christelle Auvray64, Pascal Chavanet64, Marielle Buisson64, Sophie Mahy64, François-Xavier Catherine64, Clementine Esteve64, Michel Duong64, Carole Charles64, Sandrine Gohier64, Céline Schaffer65, Olivier Robineau66, Perrine Bortolotti66, Maxime Pradier66, Francois Goehringer67, Alice Corbel67, Jeanne Kotzyba67, Kévin Alexandre68, Gaetan Beduneau69, Elodie Alessandri-Gradt70, Martin Martinot71, Simon Gravier71, Ciprian Ion71, Victoire de Lastours72, Roza Rahli72, Valérie Garrait73, Laurent Richier73, Mounira Smati-lafarge74, Guillemette Frémont75, Pierre Louis Nivose75, Marie Hélène André75, Magdalena Gerin75, Aicha Hamdi76, Naomi Sayre77, Stephanie Cossec77, Sophie Alviset77, Pierre Alain Billy77, Marie Gousseff78, Emmanuel Forestier79, Anne-Laure Destrem79, Olivier Rogeaux79, Alexie Bosch79, Sabrina Bryant79, Gaëlle Bourgeois79, Ophélie Dos Santos Schaller79, Marie-Christine Carret79, Nicolas Ettahar80, Haciba Moudjahed81, Nathalie Leone82, Mehdi Djennaoui83, Nicolas Lefebvre84, Axel Ursenbach84, François Danion84, Yvon Ruch84, Morgane Solis85, Hamid Merdji86, Loïc Kassègne87, Fanny Pommeret88, Emeline Colomba Blameble88, Merad Manssouria89, Annabelle Stoclin89, Franck Griscelli90, Sophie Deriaz91, Eric Oziol91, Laurent Favier91, Julien Obiols91, Pascal Gicquel92, Christophe Rapp93, Laurence Louvet93, Paul Ihout93, Jean-Benoit Zabbé94, Laurent Bellec95, Tomasz Chroboczek96, Sandrine Mégessier96, Marie Lacoste96, Benjamin Viala96, Thibaut Challan-Belval96, Chloé Wackenheim96, Paule Letertre-Gibert96,Olivier Grossi97
1Service des Maladies Infectieuses et Tropicales, Centre Hospitalier Universitaire de Toulouse, Toulouse, France; 2Institut Toulousain des Maladies Infectieuses et Inflammatoires (Infinity), INSERM UMR1291—CNRS UMR5051—Université Toulouse III, Toulouse, France; 3Service des Maladies Infectieuses et Tropicales, Hôpital Bichat-Claude-Bernard, APHP, Paris, France; 4Sorbonne Université, INSERM, Institut Pierre Louis d’Épidémiologie et de Santé Publique (IPLESP), Paris, France; 5Service de Virologie, Université de Paris, INSERM, IAME, UMR 1137, AP-HP, Hôpital Bichat-Claude Bernard, F-75018 Paris, France; 6Service des Maladies Infectieuses et Tropicales, Centre Hospitalier Universitaire de Rennes, Rennes, France; 7Service des Maladies Infectieuses et Tropicales, Hospices Civils de Lyon, Lyon, France; 8Service des Maladies Infectieuses et Tropicales, Hôpital Avicenne, AP-HP, Bobigny, France; 9Service des Maladies Infectieuses et Tropicales, Hôpital Tenon, APHP, Paris, France; 10Service des Maladies infectieuses et tropicales, Hôpital Saint-Antoine, APHP, Paris, France; 11Service de Médecine Interne, Hôpital Ambroise Paré, APHP, Boulogne-Billancourt, France; 12Service des Maladies Infectieuses et Tropicales, Centre Hospitalier Universitaire de Nîmes, Nîmes, France; 13Univ. Lille, Inserm U1285, CHU Lille, Pôle de médecine intensive réanimation, CNRS, UMR 8576—UGSF—Unité de Glycobiologie Structurale et Fonctionnelle, F-59000 Lille, France; 14Départmement de Gériatrie, Centre Hospitalier Universitaire de Toulouse, Toulouse, France; 15Département de Néphrologie, Centre Hospitalier Universitaire de Toulouse, Toulouse, France; 16Service de Réanimation, Centre Hospitalier Universitaire de Toulouse, Toulouse, France; 17Servivce de Pneumologie, Centre Hospitalier Universitaire de Toulouse, Toulouse, France; 18Laboratoire de Virologie, Centre Hospitalier Universitaire de Toulouse, Toulouse, France; 19Laboratoire de Virologie, Hospices Civils de Lyon, Lyon, France; 20Service de Réanimation, Hospices Civils de Lyon, Lyon, France; 21Laboratoire de Virologie, Hôpital Avicenne, AP-HP, Bobigny, France; 22Service de Pneumologie, Hôpital Avicenne, AP-HP, Bobigny, France; 23Service de Réanimation, Hôpital Avicenne, AP-HP, Bobigny, France; 24Service de Médecine Interne, Hôpital Avicenne, AP-HP, Bobigny, France; 25Service des Urgences et SAMU 93, Hôpital Avicenne, AP-HP, Bobigny, France; 26Service de Réanimation, Hôpital Tenon, APHP, Paris, France; 27Service de Pneumologie, Hôpital Tenon, APHP, Paris, France; 28Laboratoire de Virologie, Hôpital Tenon, APHP, Paris, France; 29Service de Réanimation, Hôpital Ambroise Paré, APHP, Boulogne-Billancourt, France; 30Service de Réanimation, Centre Hospitalier Universitaire de Nîmes, Nîmes, France; 31Service des Maladies Infectieuses, Centre Hospitalier Universitaire de Lille, Lille, France; 32Laboratoire de Virologie, Centre Hospitalier Universitaire de Lille, Lille, France; 33Service des Maladies Infectieuses, CHU de Reims, Reims, France; 34Service de Maladies Infectieuses et Tropicales, Centre Hospitalier Sud Francilien, Corbeil, France; 35Service de Réanimation, Centre Hospitalier Sud Francilien, Corbeil, France; 36Service de Pneumologie, Centre Hospitalier Sud Francilien, Corbeil, France; 37Service de Médecine Interne, CH François Quesnay, Mantes, France; 38Service de Maladies Infectieuses et Tropicales, Hôpital intercommunal, Villeneuve St George, France; 39Centre de Recherche Clinique, Hôpital intercommunal, Villeneuve St George, France; 40Unité d'hygiène, Hôpital intercommunal, Villeneuve St George, France; 41Centre de Recherches Cliniques CHU Amiens Picardie, Amiens, France; 42Service des Maladies Infectieuses, APHP Hôpital Necker, Paris, France; 43Service de Dermatologie et vénérologie, APHP Hôpital Raymond Poincaré, Garches, France; 44Service de Biologie Clinique, Hôpital Foch, Suresnes, France; 45Service de Réanimation, Hôpital Foch, Suresnes, France; 46Délégation à la Recherche Clinique et à l'Innovation, Hôpital Foch, Suresnes, France; 47Service des Maladies Infectieuses, Hôpital St Louis, Paris, France; 48Inserm CIC 1424, CHU de Martinique, Fort de France, France; 49Service de Réanimation, CHU de Martinique, Fort de France, France; 50Service des Maladies Infectieuses, Hôpital Universtiaire, Angers, France; 51Laboratoire de Virologie, Hôpital Universtiaire, Angers, France ; 52Service de réanimation, Hôpital Universtiaire, Angers, France; 53Service de Maladies Infectieuses et Tropicales , CHU Bordeaux, Bordeaux, France; 54Laboratoire de Virologie, CHU Bordeaux, Bordeaux, France; 55Service de Réanimation, CHU Bordeaux, Bordeaux, France; 56Service de Maladies Infectieuses et Tropicales, CHU Limoges, Limoges, France ; 57Service de Gériatrie, CHU Limoges, Limoges, France ; 58Laboratoire de Virologie, CHU Limoges, Limoges, France; 59Service de Maladies Infectieuses et Tropicales, Hôpital Hôtel-Dieu, Nantes, France; 60Service de Réanimation, Hôpital Hôtel-Dieu, Nantes, France; 61Laboratoire de virologie, Hôpital Hôtel-Dieu, Nantes, France; 62Service d'Infectiologie, Hôpital de l'Archet, CHU de Nice, Hôpital de l'Archet, Nice, France; 63Département d'infectiologie, CHU Dijon, Bourgogne, Dijon, France; 64Laboratoire de Virologie : CHU Dijon, Bourgogne, Dijon, France; 65Pole de Biologie, CHU Dijon, Bourgogne, Dijon, France; 66Service Universitaire des Maladies Infectieuses, Centre hospitalier de Tourcoing, Tourcoing, France; 67Service de Maladies Infectieuses et Tropicales, Centre Hospitalier Régional Universitaire de Nancy, Vandoeuvre lès Nancy, France; 68Service des Maladies Infectieuses, CHU Rouen, Rouen, France; 69Service de Réanimation, CHU Rouen, Rouen, France; 70Laboratoire de Virologie, CHU Rouen, Rouen, France; 71Service de Maladies Infectieuses et Tropicales Hôpitaux Civils de Colmar, Colmar, France; 72Service de Médecine Interne, APHP, Hôpital Beaujon, Clichy, France; 73Service de Médecine Interne, Centre Hospitalier Intercommunal de Créteil, Créteil, France; 74Laboratoire de Microbiologie Centre Hospitalier Intercommunal de Créteil, Créteil, France; 75Service de Médecine Interne et Maladies Infectieuses, Centre Hospitalier Intercomunal André Grégoire, Montreuil, France; 76Service de Réanimation, Centre Hospitalier Intercomunal André Grégoire, Montreuil, France; 77Service de Maladies Infectieuses et Tropicales , CH Saint Denis, St Denis, France; 78Service de Médecine Interne, Centre Hospitalier Bretagne Atlantique, Vannes, France; 79Service des Maladies Infectieuses, Centre Hospitalier Métropole Savoie, Chambéry, France; 80Service de Maladies Infectieuses et Tropicales, Centre Hospitalier de Valenciennes, Valenciennes, France ; 81Laboratoire de biologie médicale, Centre Hospitalier de Valenciennes, Valenciennes, France; 82Unité de recherche clinique, Centre Hospitalier de Valenciennes, Valenciennes, France; 83Département d'information médicale, Centre Hospitalier de Valenciennes, Valenciennes, France; 84Service des Maladies infectieuses, Hôpitaux Universitaires de Starsbourg, Strastbourg, France ; 85Laboratoire de Virologie, Hôpitaux Universitaires de Starsbourg, Strastbourg, France ; 86Service de Réanimation, Hôpitaux Universitaires de Starsbourg, Strastbourg, France ; 87Service de Pneumologie, Hôpitaux Universitaires de Starsbourg, Strastbourg, France; 88Département d'Oncologie Médicale, Gustave Roussy Cancer Campus, Villejuif, France; 89Service de Réanimation, Gustave Roussy Cancer Campus, Villejuif, France ; 90Laboratoire de Virologie, Gustave Roussy Cancer Campus, Villejuif, France ; 91Service de Médecine Interne, Infectiologie Centre Hospitalier de Béziers, Béziers, France; 92Service de Médecine Polyvalente, Centre Hospitalier de Châteaubriant, France; 93Service de Médecine Interne, Hopital Américain de Paris, Neuilly sur Seine, France ; 94Service de Maladies Infectieuses et Tropicales, Centre hospitalier de Périgueux, Périgueux, France; 95Service d'Infectiologie, Centre Hospitalier du Centre Bretagne, Pontivy, France; 96Service de Maladies Infectieuses et Tropicales, Centre Hospitalier Alpes Leman, Contamine sur Arve, France; 97Service de Médecine Interne et Infectiologie, Hôpital Privé du Confluent, Nantes, France
Funding
The study was funded by the ANRS Maladies Infectieuses Emergentes who had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
GMB, FXL, LA CC, JMC, TP, JP, DD and DC designed the study. GMB, JMC, TP, GG, HC, GP, JP, MT, AB, DL and members of the CoCliCo study group collected the data. LA, FH, MG and DC analyzed the data. All authors interpreted the data. GMB wrote the first version of the manuscript, with contributions from LA, CC, JP, DD and DC. GMB, LA, FH, MG, JP and DC verified the data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the National Institutional Review Board of the SPILF (IRB00011642, reference: COVID 2021–05) and was registered on ClinicalTrials.gov (NCT04863547). All methods were performed in accordance with the guidelines and regulations applicable in France for reuse of medical data. All of the data obtained were de-identified, and need to obtain informed consent was waived by the National Institutional Review Board of the French Society of Infectious Diseases (SPILF) due to the retrospective nature of the data collection. However, all participants were informed about the study and had the ability to object to the use of their data.
Consent for publication
Not applicable.
Competing interests
DC reports HIV grants from Janssen (2017–2018, 2019–2020), personal fees from Janssen (2018) and Gilead (2018, 2020) for lectures on HIV outside the submitted work. CC reports personal fees from Janssen (2018), MSD (2019), Gilead (2018–2020), Theratechnologies (2020) and ViiV Healthcare (2018–2020). HC reports personal fees from MSD (2020) and ViiV Healthcare (2020) for lectures on HIV. GMB reports support for attending meetings and personal fees from BMS, MSD, Janssen, Sanofi, Pfizer and Gilead for lectures outside the submitted work. JP reports support for attending meetings and personal fees from Gilead, Pfizer and Eumedica Gilead for lectures. DD reports personal fees from Gilead, ViiV Healthcare and Janssen for participation on an advisory Board. Other authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Unadjusted and adjusted analysis of factors associated with COVID-19 severity by Day 29 using a stratified logistic regression model on each matched pair. Table S2. Multivariable analysis of factors associated with mortality by Day 29 using a stratified Cox regression model on each matched pair. Table S3. Multivariable analysis of factors associated with WHO scale >5 by Day 29 using a stratified Cox regression model on each matched pair. Table S4. Multivariable analysis of factors associated with non-rebreather mask by Day 29 using a stratified Cox regression model on each matched pair. Table S5. Multivariable analysis of factors associated with high flow oxygen therapy by day 29 using a stratified Cox regression model on each matched pair. Table S6. Multivariable analysis of factors associated with ICU admission by day 29 using a stratified Cox regression model on each matched pair. Table S7. Multivariable analysis of factors associated with Mechanical ventilation or ECMO by day 29 using a stratified Cox regression model on each matched pair. Table S8. Multivariable analysis of factors associated with time from symptoms onset to hospitalization using a stratified Cox regression model on each matched pair. Table S9. Multivariable analysis of factors associated with duration on hospitalization using a stratified Cox regression model on each matched pair. Table S10. Multivariable analysis of factors associated with readmission using a stratified Cox regression model on each matched pair. Figure S1. Kaplan–Meir plot for all cause of mortality. Figure S2. Kaplan–Meir plot for WHO scale >5. Figure S3. Kaplan–Meir plot for non-rebreather mask. Figure S4. Kaplan–Meir plot for high flow oxygen therapy. Figure S5. Kaplan–Meir plot for intensive care admission. Figure S6. Kaplan–Meir plot for Mechanical ventilation or ECMO. Figure S7. Kaplan–Meir plot for hospitalization (time from symptoms onset to hospitalization). Figure S8. Kaplan–Meir plot for hospital discharge (duration on hospitalization). Figure S9. Kaplan–Meir plot for readmission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Martin-Blondel, G., Lescure, FX., Assoumou, L. et al. Increased risk of severe COVID-19 in hospitalized patients with SARS-CoV-2 Alpha variant infection: a multicentre matched cohort study. BMC Infect Dis 22, 540 (2022). https://doi.org/10.1186/s12879-022-07508-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07508-x