Abstract
Background
The emergence of carbapenemase-producing bacteria (CPB) has become a major public health concern. Long-term care facilities (LTCF) are potential reservoirs for multidrug-resistant micro-organisms (MDRO). However, data on CPB is limited. The study aims to determine the prevalence of MDRO and risk factors for CPB colonization among residents of LTCFs.
Methods
A point-prevalence study was conducted at 14 LTCFs in Tenerife (Spain) between October 2020 and May 2021. Nasal and rectal swabs were cultured for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), carbapenemase-producing Enterobacterales, MDR Acinetobacter baumannii (MDR-Ab) and MDR Pseudomonas aeruginosa. Antimicrobial susceptibility testing and molecular detection of resistance genes were performed. Risk factors for colonization by carbapenemase-producing bacteria (CPB) were determined by univariate and multivariate analysis.
Results
A total of 760 LTCF residents were recruited. The prevalence of colonization by CPB was 9.3% (n = 71) with the following distribution: 35 (49.3%) K. pneumoniae, 26 (36.6%) MDR-Ab, 17 (23.9%) E. coli, and 1 (1.4%) C. koseri. In addition, the prevalence of colonization by MRSA was 28.1% (n = 215) and only one case of VRE was isolated. Multivariate analysis identified male sex (odds ratio [OR], 1.86; 95% confidence interval [CI], 1.86–3.11; P = 0.01), having a high health requirement (OR, 6.32; 95% CI, 1.91–20.92; P = 0.003) and previous hospitalization (OR, 3.60; 95% CI, 1.59–8.15 P = 0.002) as independent risk factors for CPB rectal carriage.
Conclusions
LTCFs are an important reservoir for MDRO, including CPB. We have identified some predictors of colonization by CPB, which enable a more targeted management of high-risk residents. Antimicrobial stewardship programmes and infection control preventive measures are needed to stop acquisition and transmission of MDRO.
Similar content being viewed by others
Background
The emergence of multidrug-resistant organisms (MDROs) is a global public health problem [1]. Initially, many of these MDROs appeared to cause hospital-acquired infections [2], but more recently they have spread into different healthcare settings, including long-term care facilities (LTCFs) [3, 4], and also into the community [5]. LTCFs are recognized as an important reservoir of methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum β-lactamase Enterobacterales (ESBL) [6]. Recently there has been growing interest in knowing the prevalence of colonization by other MDROs such as carbapenemase-producing Enterobacterales (CPE), vancomycin-resistant Enterococcus spp (VRE), MDR Acinetobacter baumannii (MDR-Ab) and MDR Pseudomonas aeruginosa (MDR-Pa) [7,8,9,10,11,12,13,14,15]. Specifically, the increasing prevalence of infections by MDR gram negative bacteria (MDR- GNB) have become a real threat in recent years. Moreover, there is a risk of LTCFs becoming a reservoir for these pathogens [16, 17].
LTCFs provide residential healthcare for people with significant disabilities, chronic illness and elderly individuals who cannot care for themselves. These institutions are also the last medical resource for patients who have survived acute illnesses in hospitals. The increase in life expectancy and, therefore, ageing of the population has meant that LTCFs have become essential in the healthcare system. However, there is evidence that a stay in a LTCF is a risk factor for the carriage of MDROs [18, 19] and this can be for several reasons: high pressure antibiotics, permanent living in a confined environment, the difficulty of diagnosing infections that present atypically and common cognitive impairment. In addition, it has been demonstrated that continuous bidirectional movement of patients between these institutions and acute care hospitals facilitates the spread and maintenance of MDRO bacteria [20, 21]. For these reasons, identifying the patients who carry MDROs and preventing the hospital from nosocomial spreading is challenging.
Antimicrobial stewardship programmes (ASP) have been widely implemented in hospitals [22], in addition to monitoring and prevention programmes with the aim of reducing the incidence of these infections. LTCFs could also benefit from these programmes. Knowledge of the epidemiology of MDROs at local level is key to implementing a successful antimicrobial stewardship intervention. However, the prevalence of MDR GNB faecal carriage in LTCFs remains unknown in most geographical areas [12].
The aim of this study is to determine the prevalence of MDROs and risk factors for colonization by carbapenemase-producing bacteria (CPB) among LTCF residents in North Tenerife (Spain). In addition, the MDROs resistance mechanism was characterized in molecular terms.
Methods
Study design
A multicentre point-prevalence study (October 2020–May 2021) was conducted at 14 LTCFs distributed throughout North Tenerife (Spain). In each LTCF, rectal and nasal swab were collected from all residents. The residents’ sociodemographic and clinical data were evaluated by means of a questionnaire. None of the LTCFs taking part had a specific action protocol to monitor and prevent MDRO transmission. A resident colonized by CPB was defined as a case, and a control was defined as those who were not colonized by CPB.
Microbiological methods
All samples were analyzed at the Microbiology Service in Hospital Universitario de Canarias, which is the reference hospital in the northern area of Tenerife. Rectal swabs were cultured directly on selective chromogenic media ChromID® CARBA SMART and ChromID® VRE (bioMèrieux, Marcy l´Etoile, France) and McConkey (bioMèrieux). Nasal swabs were cultured on ChromID® MRSA SMART (bioMèrieux) and inoculated into Brain–Heart Infusion Broth (bioMèrieux). They were reseeded in MRSA after 24 h of incubation in broth.
Identification and antimicrobial susceptibility testing of the suspicious colonies were performed with the Vitek-II® system (bioMèrieux) and reduced susceptibility/resistance to imipenem or vancomycin was confirmed by Etest (bioMèrieux). Carbapenemase production was phenotypically tested by the agar tablet/disc diffusion method (KPC/MLB and OXA-48 ConfirmKit; ROSCO Diagnostica, Taastrup, Denmark). Colistin resistance was tested by disk diffusion test and confirmed by broth microdilution (UMIC, Biocentric, France). All results were analyzed and interpreted according to the European Committee on Antimicrobial Susceptibility Testing guidelines [23]. Colonies suspected of MRSA were confirmed by the PBP2A SA culture colony Test (AlereTM Scarborough, Maine, USA).
Genes for resistance to carbapenems (NDM, VIM, KPC, OXA-48, IMP) for Enterobacterales and P. aeruginosa; and to vancomycin for enterococcus (vanA, vanB) were genotypically characterized by multiplex polymerase chain reaction (PCR) AllplexTM Entero-DR Assay (Seegene, Korea). Carbapenemase resistance in A. baumannii (OXA-51, NDM, OXA-23, OXA-40, OXA-58) and the detection of methicillin resistance genes for S. aureus (mecA, mecC) was characterized by isothermal amplification Eazyplex® with Superbug Acineto and MRSA reagents, respectively (AmplexDiagnostics, Germany).
Statistical analysis
The sample collected from 71 cases and 689 controls offers the study a power of 90% in detecting a difference between cases and controls of relative frequencies for nominal variables of at least 20%; or 3 years for the age or days for the stay in ranges of 0–5 in bilateral tests of hypothesis at a level of statistical significance P ≤ 0.05.
The characteristics of the sample as a whole are reported by summarizing its nominal variables with the frequency (relative frequency) of its component categories, and those of numerical scale with mean (P5–P95) given its distance from a normal probability distribution verified with the Kolmogorov–Smirnov test.
Nominal variable cases and controls are compared with Pearson’s χ2 test or Fisher's Exact Test. Numerical scale comparisons were made with the Mann–Whitney U test. Those variables that in these comparisons attained a significance of at least 5% in their difference will enter as potentially predictive factors of a combined colonization by CPB as an effect, first in univariate logistic regression models and then in a regression model backward stepwise multivariate binary logistics using the Wald criterion to estimate odds ratios for independent predictors of colonization.
All hypothesis contrast tests are two-sided at a level of statistical significance P ≤ 0.05 and the calculations involved in these operations are executed with the help of the statistical package for statistical data processing SPSS 25.0™ from IBM Co.® (IBM –SPSS Inc, Armonk, NY, USA).
Results
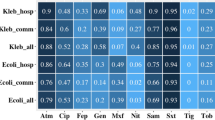
Among 14 LTCFs (10 publics and 4 private), 764 residents were selected to participate in the study. However, we failed to obtain rectal samples from four residents, resulting in a total 760 residents included in the study (Table 1). A total of 71 (9.3%) and 689 (90.7%) residents were classified as cases and controls, respectively. Cases were colonized by CPB in the following proportions: 35 (49.3%) K. pneumoniae, 26 (36.6%) MDR-Ab, 17 (23.9%) E.coli and 1 (1.4%) C. koseri. In addition, 26 (36.6%) were also colonized by MRSA and two (2.8%) by MDR-Pa. Of the controls, 188 (27.3%), 12 (1.7%) and 1 (0.1%) were colonized by MRSA, MDR-Pa and VRE, respectively. The results obtained in terms of characterization of resistance mechanism are shown in Table 2.
The clinical and epidemiological characteristics of the two groups are shown in Table 3. Cases were significantly more likely than controls to be male (P = 0.025), have active infection (P = 0.025), urinary incontinence (P = 0.044), faecal incontinence (P = 0.014), previous antibiotic use (P = 0.040), high/medium health requirement (P = 0.005/0.05), prior hospital admission within the last 3 months (P = 0.002) and previous MDRO (P = 0.013).
On multivariate analysis (Table 4), the only variables retained as independent risk factors for colonization by CPB were male sex (OR, 1.86; 95% CI, 1.86–3.11; P = 0.01), high/medium health requirement (OR, 6.32; 95% CI, 1.91–20.92; P = 0.003/OR, 3.78; 95% CI,1.09–13.04; P = 0.036) and previous hospitalization (OR, 3.60; 95% CI, 1.59–8.15; P = 0.002).
Discussion
This study was conducted due to the increasing prevalence of infections by CPBs in acute care hospitals in our geographical area. We sought to know whether these LTCFs also constitute a reservoir of CPB, in addition to identifying risk factors for colonization by CPB.
In this multicentre point-prevalence study, a remarkably high rate of colonization by CPB was observed among LTCF residents in North Tenerife. In the literature, there are few recent studies about CPB in Europe showing a high geographical variation [8, 12]. Most studies reported low CPB prevalence rates (0.06–1.7%) among residents of LTCFs [11, 13, 24,25,26]. However, some studies in Israel (12%), Spain (4.1%) and Italy (28.4%) determined a high prevalence [4, 17, 27]. Our findings are in line with other studies in Spain, which confirm that LTCFs are turning into reservoirs of CPB [16].
Our multivariate analysis identified male sex, a high or medium health requirement and previous hospitalization as important risk factors for CPB rectal colonization. Several studies have identified male sex as a risk factor for MDRO colonization [21, 28]. However, the reason why male sex is a risk factor remains unknown. Rodríguez-Villadores et al. [8] explain that this may be due to a higher frequency of risk factors among male residents, who have more comorbidities compared to female residents.
Establishing a level of dependence for the probability of MDRO colonization is difficult, mainly due to the variability of scores used across the studies. Some commonly used methods are Katz, Barthel, Karnofsky or the French index. In our study, all LTCFs have a bed distribution according to health requirements (high, medium or low) and, therefore, residents were classified according to the criteria of the centres themselves. Despite the lack of a dependence threshold related to MDRO colonization, there appears to be compelling evidence to indicate that an increased level of dependence is associated with an increased risk of being colonized by MDROs [8].
Several previous studies have identified prior hospital admission as a risk factor for MDRO colonization [12, 29]. Depending on the study, they establish the limit at three, six or twelve previous months. As in our study, hospital admission in the previous 3 months was also reported to be a risk factor for MDRO colonization [30]. It remains unknown how long after hospital admission this risk increases. Therefore, previous hospitalization should be considered a risk factor for MDRO colonization among LTCF residents. However, to what extent this risk could be increased by days of hospitalization remains unknown [8].
Our univariate analysis identified active infection, urinary and faecal incontinence, previous antibiotic use and previous MDRO colonization as factors associated with colonization by CPB. However, multivariate analysis did not reveal these to be independent risk factors. These are traditional factors associated with MDRO colonization in the literature and previous use of antibiotics is the main associated factor [6, 31,32,33].
Since the aim of the study was to assess colonization factors exclusively by CPB and control residents by MDR-Pa and VRE were scarce, we decided to include them in the statistical analysis. Nevertheless, we performed this analysis without including theses control residents and obtained the same significant risk factors.
This study has several limitations. First, as the study was performed during the COVID-19 pandemic, we had enormous difficulty accessing the centres for sample collection and filling out the questionnaires, delaying the study deadlines. Second, the cross-sectional survey design did not enable us to investigate the dynamic of MDRO colonization (acquisition, persistence and clearance of carriage). Third, disk diffusion by colistin did not allow the detection of resistance to this antibiotic since there are not breakpoints for EUCAST and CLSI, requiring confirmation by another technique.
Conclusions
Our study documents a high prevalence of colonization by MDROs, including CPB, among LTCF residents in North Tenerife. This emphasizes the role of these centres as reservoirs for MDROs. Male sex, a high health requirement and prior hospital admission were all identified as independent risk factors for CPB rectal colonization. These results strengthen the importance of establishing a standardized protocol to manage colonized patients between acute hospital centres and LTCFs. In addition, antimicrobial stewardship programmes and infection control preventive measures accounting for related risk factors in LTCFs are required to stop the acquisition and transmission of MDROs in healthcare facilities.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the requirement to protect patient confidentiality.
Abbreviations
- ASP:
-
Antimicrobial stewardship programmes
- CPB:
-
Carbapenemase-producing-bacteria
- CPE:
-
Carbapenemase-producing Enterobacterales
- ESBL:
-
Extended-spectrum β-lactamase Enterobacterales
- LTCF:
-
Long-term care facilities
- MDR:
-
Multidrug-resistant
- MDR-Ab:
-
Multidrug-resistant Acinetobacter baumannii
- MDR-GNB:
-
Multidrug-resistant gram negative bacteria
- MDRO:
-
Multidrug-resistant micro-organisms
- MDR-Pa:
-
Multidrug-resistant Pseudomonas aeruginosa
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- OR:
-
Odds ratio
- VRE:
-
Vancomycin-resistant enterococci
References
Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(SUPPL. 5):397–428.
Montesinos I, Salido E, Delgado T, Lecuona M, Sierra A. Epidemiology of methicillin-resistant Staphylococcus aureus at a university hospital in the Canary Islands. Infect Control Hosp Epidemiol. 2003;24(9):667–72.
Gómez Alonso B, Rodríguez Álvarez C, Castro Hernández B, Arias Rodríguez A, Aguirre Jaime A, Lecuona FM. Hospital emergency health service care as a risk factor for methicillin-resistant Staphylococcus aureus in residents of long-term care facilities. Emergentes. 2016;28(6):381–6.
Ben-David D, Masarwa S, Navon-Venezia S, Mishali H, Fridental I, Rubinovitch B, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol. 2011;32(9):845–53.
Gijón D, Curiao T, Baquero F, Coque TM, Cantón R. Fecal carriage of carbapenemase-producing Enterobacteriaceae: a hidden reservoir in hospitalized and non-hospitalized patients. J Clin Microbiol. 2012;50(5):1558–63.
Aschbacher R, Pagani E, Confalonieri M, Farina C, Fazii P, Luzzano F, et al. Review on colonization of residents and staff in Italian long-term care facilities by multidrug-resistant bacteria compared with other European countries. Antimicrob Resist Infect Control. 2016;5(1):1–9.
Jeong H, Kang S, Cho HJ. Prevalence of multidrug-resistant organisms and risk factors for carriage among patients transferred from long-term care facilities. Infect Chemother. 2020;52(2):183–93.
Rodríguez-Villodres Á, Martín-Gandul C, Peñalva G, Guisado-Gil AB, Crespo-Rivas JC, Pachón-Ibáñez ME, et al. Prevalence and risk factors for multidrug-resistant organisms colonization in long-term care facilities around the world: a review. Antibiotics. 2021;10(6):680.
Lee CM, Lai CC, Chiang HT, Lu MC, Wang LF, Tsai TL, et al. Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan. J Microbiol Immunol Infect. 2017;50(2):133–44.
Lim CJ, Cheng AC, Kennon J, Spelman D, Hale D, Melican G, et al. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case-control study. J Antimicrob Chemother. 2014;69(7):1972–80.
Latour K, Huang TD, Jans B, Berhin C, Bogaerts P, Noel A, et al. Prevalence of multidrug-resistant organisms in nursing homes in Belgium in 2015. PLoS ONE. 2019;14(3):1–18.
Chen HY, Jean SS, Lee YL, Lu MC, Ko WC, Liu PY, et al. Carbapenem-resistant enterobacterales in long-term care facilities: a global and narrative review. Front Cell Infect Microbiol. 2021;11(April):601968.
Kohler P, Fulchini R, Albrich WC, Egli A, Balmelli C, Harbarth S, et al. Antibiotic resistance in Swiss nursing homes: analysis of national surveillance data over an 11-year period between 2007 and 2017. Antimicrob Resist Infect Control. 2018;7(1):1–9.
Reuben J, Donegan N, Wortmann G, DeBiasi R, Song X, Kumar P, et al. Healthcare Antibiotic Resistance Prevalence—DC (HARP-DC): a regional prevalence assessment of carbapenem-resistant Enterobacteriaceae (CRE) in healthcare facilities in Washington, District of Columbia. Infect Control Hosp Epidemiol. 2017;1738(8):921–9.
Cheng VCC, Chen JHK, Ng WC, Wong JYH, Chow DMK, Law TC, et al. Emergence of carbapenem-resistant Acinetobacter baumannii in nursing homes with high background rates of MRSA colonization. Infect Control Hosp Epidemiol. 2016;37(8):983–6.
Palacios-Baena ZR, Oteo J, Conejo C, Larrosa MN, Bou G, Fernández-Martínez M, et al. Comprehensive clinical and epidemiological assessment of colonisation and infection due to carbapenemase-producing Enterobacteriaceae in Spain. J Infect. 2016;72(2):152–60.
Ruiz-Garbajosa P, Hernández-García M, Beatobe L, Tato M, Méndez MI, Grandal M, et al. A single-day point-prevalence study of faecal carriers in long-term care hospitals in Madrid (Spain) depicts a complex clonal and polyclonal dissemination of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2016;71(2):348–52.
March A, Aschbacher R, Sleghel F, Soelva G, Kaczor M, Migliavacca R, et al. Colonization of residents and staff of an Italian long-term care facility and an adjacent acute care hospital geriatric unit by multridrug-resistant bacteria. New Microbiol. 2017;40(4):258–63.
Giufrè M, Ricchizzi E, Accogli M, Barbanti F, Monaco M, Pimentel de Araujo F, et al. Colonization by multidrug-resistant organisms in long-term care facilities in Italy: a point-prevalence study. Clin Microbiol Infect. 2017;23(12):961–7.
March A, Aschbacher R, Dhanji H, Livermore DM, Böttcher A, Sleghel F, et al. Colonization of residents and staff of a long-term-care facility and adjacent acute-care hospital geriatric unit by multiresistant bacteria. Clin Microbiol Infect. 2010;16(7):934–44.
Nucleo E, Caltagirone M, Marchetti VM, D’Angelo R, Fogato E, Confalonieri M, et al. Colonization of long-term care facility residents in three Italian Provinces by multidrug-resistant bacteria. Antimicrob Resist Infect Control. 2018;7(1):1–11.
Rodríguez-Baño J, Paño-Pardo JR, Alvarez-Rocha L, Asensio Á, Calbo E, Cercenado E, et al. Programas de optimización de uso de antimicrobianos (PROA) en hospitales españoles: documento de consenso GEIH-SEIMC, SEFH y SEMPSPH. Enferm Infecc Microbiol Clin. 2012;30(1):22-e1.
EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version1.0; 2013. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf. Accessed 9 May 2022.
. Collaboration agreement between the Insular Institute of social and socio-health care of the Hon., Tenerife Council and Granadilla de Abona City Council for the provision of services in residential centers and day care for people in a situation of dependency, and in general for people elderly or disabled. November 24, 2016. https://sede.granadilladeabona.es/portal/sede/RecursosWeb/DOCUMENTOS/1/0_5477_1.pdf.
Van Dulm E, Tholen ATR, Pettersson A, Van Rooijen MS, Willemsen I, Molenaar P, et al. High prevalence of multidrug resistant Enterobacteriaceae among residents of long term care facilities in Amsterdam, the Netherlands. PLoS ONE. 2019;14(9):1–14.
Dandachi I, Salem Sokhn E, Najem E, Azar E, Daoud Z. Carriage of beta-lactamase-producing Enterobacteriaceae among nursing home residents in north Lebanon. Int J Infect Dis. 2016;45:24–31.
Ambretti S, Bassetti M, Clerici P, Petrosillo N, Tumietto F, Viale P, et al. Screening for carriage of carbapenemresistant Enterobacteriaceae in settings of high endemicity. Antimicrob Resist Infect Control. 2019;8(136):1–11.
Jans B, Schoevaerdts D, Huang TD, Berhin C, Latour K, Bogaerts P, et al. Epidemiology of multidrug-resistant microorganisms among nursing home residents in Belgium. PLoS ONE. 2013;8(5):1–8.
Denis O, Jans B, Deplano A, Nonhoff C, De Ryck R, Suetens C, et al. Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) among residents of nursing homes in Belgium. J Antimicrob Chemother. 2009;64(6):1299–306.
Del Rosario-Quintana C, Tosco-Núñez T, Lorenzo L, Martín-Sánchez AM, Molina-Cabrillana J. Prevalencia y factores asociados a la colonización de microorganismos multirresistentes en centros de larga estancia de Gran Canaria. Rev Esp Geriatr Gerontol. 2015;50(5):232–6.
Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JDD, Quentin C, Caibo ES, et al. A multinational survey of risk factors for infection with extended-spectrum β-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49(5):682–90.
Flokas ME, Alevizakos M, Shehadeh F, Andreatos N, Mylonakis E. Extended-spectrum β-lactamase-producing Enterobacteriaceae colonisation in long-term care facilities: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017;50(5):649–56.
Zhao SY, Zhang J, Zhang YL, Wang YC, Xiao SZ, Gu FF, et al. Epidemiology and risk factors for faecal extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) carriage derived from residents of seven nursing homes in western Shanghai, China. Epidemiol Infect. 2016;144(4):695–702.
Legeay C, Hue R, Berton C, Cormier H, Chenouard R, Corvec S, et al. Control strategy for carbapenemase-producing Enterobacteriaceae in nursing homes: perspectives inspired from three outbreaks. J Hosp Infect. 2019;101(2):183–7.
Acknowledgements
In addition to providing reagents and funding for this study, bioMérieux reviewed the manuscript prior to submission.
Funding
This work has been possible thanks to a medical research grant from Fundación MAPFRE Guanarteme, 2019 and funding from bioMerieux SA.
Author information
Authors and Affiliations
Contributions
MCF and RAR carried out the collection of the samples and all the laboratory analyses. MCF and AMA wrote the main manuscript text. AAJ performed the statistical analysis. MBCH organized the analyses in molecular biology. MJRL and YPF participated in the collection and processing of the samples. MLF participated in the design and coordination of the study. All authors reviewed the paper critically, and comments and suggestions were incorporated. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed with the approval of the Institutional Review Board of Hospital Universitario de Canarias (Tenerife, Spain), code CHUC_2019_91.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Callejón Fernández, M., Madueño Alonso, A., Abreu Rodríguez, R. et al. Risk factors for colonization by carbapenemase-producing bacteria in Spanish long-term care facilities: a multicentre point-prevalence study. Antimicrob Resist Infect Control 11, 163 (2022). https://doi.org/10.1186/s13756-022-01200-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-022-01200-0