Abstract
Background
Disinfectants and antiseptics are biocides widely used in hospitals to prevent spread of pathogens. It has been reported that antiseptic resistance genes, qac’s, caused tolerance to a variety of biocidal agents, such as benzalkonium chloride (BAC) and chlorhexidine digluconate (CHDG) in Staphylococcus spp. isolates. We aimed to search the frequency of antiseptic resistance genes in clinical Staphylococcus spp. and Enterococcus spp. isolates to investigate the possible association with antiseptic tolerance and antibiotic resistance.
Methods
Antiseptic resistance genes (qacA/B, smr, qacG, qacH, and qacJ) isolated from Gram-positive cocci (69 Staphylococcus spp. and 69 Enterococcus spp.) were analyzed by PCR method. The minimum inhibitory concentrations (MICs) of BAC and CHDG were determined by agar dilution method, whereas antibiotic susceptibility was analyzed by disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) criteria.
Results
The frequency of antiseptic resistance genes was found to be high (49/69; 71.0%) in our clinical staphylococci isolates but absent (0/69; 0%) in enterococci isolates. The frequency of qacA/B and smr genes was higher (25/40; 62.5% and 7/40; 17.5%, respectively) in coagulase negative staphylococci (CNS) when compared to Staphylococcus aureus strains (3/29; 10.3%, and 4/29; 13.8%, respectively). In contrast, the frequency of qacG and qacJ genes was higher (11/29; 37.9% and 8/29; 27.5%, respectively) in S. aureus than those of CNS (5/40; 12.5%, 10/40; 25.0%) strains. qacH was not identified in none of the strains. We found an association between presence of antiseptic resistance genes and increased MIC values of BAC (>4 μg/mL) in staphylococci and it was found to be statistically statistically significant (p < 0.01). We also showed that MICs of BAC and CHDG of vancomycin-resistant enterococci (VRE) isolates were significantly higher than those of vancomycin-susceptible enterococci (VSE) isolates (p < 0.01).
Conclusions
For our knowledge, our study is the first to investigate antiseptic resistance genes in enterococci and also qacG, qacH, and qacJ genes in staphylococci isolates in Turkey. Further studies are needed to revise the biocide policy and to support infection control programs to avoid the development of new resistance mechanisms.
Similar content being viewed by others
Background
Staphylococcus aureus causes a wide range of clinical infections such as skin and soft tissue infections, surgical site infections, endocarditis, and bacteremia. Traditionally, coagulase-negative staphylococci (CNS) isolates were considered a as non-pathogenic commensals but in recent years the clinical importance of CNS has been increased significantly due to its role in some diseases like catheter related bloodstream infections. Enterococcus spp. found in the gastrointestinal tract of humans is a commensal bacteria and considered to be harmless for many years. Nowadays, it is considered to be one of the most common nosocomial pathogen especially due to strains of vancomycin resistant enterococci, VRE [1, 2]. There has been a significant increase in the incidence of nosocomial infections caused by Staphylococcus spp. and Enterococcus spp. which are multiresistant to various antibiotics in recent years [3].
Some antiseptic and disinfectant agents in the hospital setting such as quaternary ammonium compounds like benzalkonium chloride (BAC) and divalent cations like chlorhexidine digluconate (CHDG) are used to prevent infections within healthcare facilities. It has been reported in many studies that extensive use of biocidal agents reduce susceptibility to antiseptics in Staphylococcus spp. [4,5,6].
Antiseptic resistance genes (qacAB, smr, qacG, qacH, qacJ), encoding multidrug efflux pumps which are carried by plasmids, were identified in Staphylococcus genus for the first time [7,8,9,10]. Antiseptic resistance gene studies of qacA/B and smr mostly focused on S. aureus [10,11,12] but there are a limited number of studies in CNS strains [13, 14]. Moreover, the studies concerning the prevalance of qacG, qacH, and qacJ genes mostly focused on CNS obtained from animal and food sources [15, 16].
Despite the rising significance of Enterococcus spp. as nosocomial pathogen there are insufficient number of studies on qac genes in clinical isolates. Bischoff et al. [17] detected the first qacA/B gene-positive Enterococcus faecalis isolate in clinical blood samples. Thus far, there are no qac genes reported in enterococci other than qacC, qacEΔ1, qacZ and qacA/B. Increase in minimum inhibitory concentration (MIC) values to biocides does not mean “resistance” because these agents can be used at high concentrations without encountering toxicity [18]. Thus the terms “reduced susceptibility” or “increased tolerance” are more suitable for pathogens exhibiting an elevated MIC to a biocides [19].
In this study, we aimed; 1. To investigate qacAB, smr, qacG, qacH, qacJ genes in Staphylococcus spp. and Enterococcus spp. isolates, 2. To determine the susceptibility of the strains to antibiotics and two antiseptic agents, including BAC and CHDG, 3. To show relationship between MIC values of antiseptic agents/resistance to antibiotics and presence of antiseptic resistance genes. For our knowledge, this is the first study in Turkey that investigated antiseptic resistance genes in enterococci isolates and also first in terms of showing the presence of qacH, qacG, and qacJ genes in staphylococci isolates.
Methods
Bacterial isolates
In our study, 69 Staphylococcus spp. (10 methicillin-resistant S.aureus (MRSA), 19 methicillin-susceptible S.aureus (MSSA), 27 MRCNS, 13 of methicillin-susceptible CNS (MSCNS)) and 69 of Enterococcus spp. were collected from different clinical samples such as blood, cerebrospinal fluid, urine, abscess, catheter tips, nasal secretions and endotracheal aspiration fluid between January 2010–March 2011 in a university hospital with more than 1500 beds. This study was conducted in accordance with revised Helsinki Declaration and approved by the institutional clinical research ethic commitee.
All samples were cultured on suitable bacteriological media and identified by the conventional method. Staphylococcus spp. isolates were identified by API-Staph commercial identification kit (API Staph System, bioMèrieux, France).
Minimum inhibitory concentration of antiseptics
MICs of BAC and CHDG were detected by a modified agar dilution method according to the recommendations of Clinical and Laboratory Standards Institute (CLSI) [20]. The MICs of the BAC and CHDG in negative control strain (S.aureus ATCC 6538) which is negative for antiseptic resistance genes were considered as baseline and any MICs above these values were accepted as “tolerance concentration”.
Antibiotic susceptibility test
In this study, antibiotic sensitivity testing was performed via disc diffusion method by using following antibiotics; tetracycline, ciprofloxacin, tobramycin, trimethoprim-sulfamethoxazole, rifampin, and chloramphenicol. Methicillin and iMLSB (inducible macrolide-lincosamide-streptogramin B) resistance tests were implemented to Staphylocccus spp. isolates, while vancomycin resistance test to Enterococcus spp. isolates. All tests were performed according to CLSI [21] criteria.
The antibiotic susceptibility discs were purchased from OXOID (Hemakim, Istanbul, Turkey).
Detection of antiseptic resistant genes by multiplex PCR
Total genomic DNA of the strains were extracted using High Pure PCR Template Preparation Kit (Roche, Germany). All DNA extracts were stored at +4 °C prior to PCR.
A single primer pair was used for amplifying both qacA and qacB due to seven base difference between qacA and qacB genes. We made two multiplex PCR sets; one included primers of A/B and smr and second one contained primers of qacG, qacH, and qacJ genes as previously published [15, 22]. All primer sequences are shown in Table 1.
PCR Master Mix 2X (Fermentas, Canada) was used for PCR assays. According to recommendation of manufacturer, the final volume of each reaction in the PCR was 25 μL. Each reaction contained 12,5 μL master mix, for each primer; 1 μL (10 pmol) of forward primer, 1 μL (10 pmol) of reverse primer, 2 μL of DNA extract and final volume was 25 μL by adding sterile H2O. PCR of qacA/B and smr genes were performed using an initial denaturation step 96 °C for 3 min, followed by 25 cycles of 95 °C for 20 s, 53 °C for 20 s, 72 °C for 20 s, and a final extension step at 72 °C for 5 min [22]. Whereas the cycling conditions for qacG, qacH and qacJ genes were as follows: DNA denaturation at 94 °C for 10 min, 25 cycles of 95 °C for 60 s, 48 °C for 45 s, 72 °C for 60 s, and a final extension step at 72 °C for 10 min [15]. Multiplex PCR products were run in 1% gel containing ethidium bromide and photographed under ultraviolet.
Positive control strains (qacA/B, smr, qacG, qacH, qacJ) were provided kindly by Jostein Bjorland from Norwegian School of Veterinary Science. S.aureus ATCC 6538 was used as negative control strain.
Statistical analysis
The data analysis was conducted with the statistical package, IBM SPSS Statistics 22 (IBM.
SPSS, Turkey). The normal distribution of variables were checked by the Shapiro–Wilk test. Comparison of quantitive data between two groups were performed by Mann Whitney U test, while Continuity Correction and Fisher’s Exact tests were used for comparison of qualitative data between two groups. P value less than 0.05 was considered statistically significant.
Results
We found that 15/29 (51.7%) Staphylococcus aureus and 34/40 (85.0%) CNS isolates, for a total of 49/69 (70.0%) Staphylococcus spp. isolates harbored at least one antiseptic resistance genes. Among the 49 strains positive for an antiseptic resistance gene; qacA/B genes were the most dominant (28/49; 57.1%) followed by qacJ (18/49; 36.7%), qacG (16/49; 32.6%), and smr (11/49; 22.4%) genes. We also found that 30/49 (61.2%) gene positive Staphylococcus spp. isolates had only one gene type, whereas the rest of the isolates (19/49; 38.7%) carried more than one gene type. None of the Staphylococcus spp. isolates carried the qacH gene. In addition, all Enterococcus spp. isolates were negative for any resistance genes (Table 2).
The MICs of BAC ranged from 1 to 16 μg/mL, and the MICs of CHDG ranged from 0.75 to 12 μg/mL in Staphylococcus spp. Baseline MIC concentrations of BAC and CHDG in the negative control strain (S.aureus ATCC 6538) were 4 and 1.5 μg/mL, respectively. Based on these measurements, we found a significant difference in MIC >4 μg/mL BAC between Staphylococcus spp. isolates positive and negative for an antiseptic resistance gene (p: 0.002; p < 0.01) but this relationship was not statistically significant for MICs of CHDG (>1.5 μg/mL; p: 0.925; p > 0.01) (Table 3).
All Enterococcus spp. isolates in this study were negative for antiseptic resistance genes. MIC levels of BAC and CHDG were 8–16 μg/ml and 6–12 μg/mL, respectively. We found that MICs of BAC and CHDG of VRE isolates were significantly higher than those of vancomycin-susceptible enterococci (VSE) isolates (p < 0.01) (Table 4).
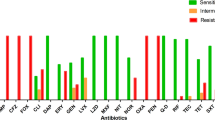
A comparison of antibiotic resistance and the presence of antiseptic resistance genes in Staphylococcus spp. are shown in Table 5.
Discussion
MRSA, MRCNS, and VRE strains are opportunistic pathogens transmitted by the hands of health care workers. BAC and CHDG are handwashing and skin antiseptics used extensively to the control and prevent hospital infections. Many studies have shown both the presence of plasmid-mediated antiseptic resistance genes such as qacA/B, smr, qacG, qacH, and qacJ and reduced susceptibility to antiseptic agents. Other researchers have reported that plasmids carry antiseptic resistance genes together with antibiotic resistance genes and contribute to the development of resistance in pathogens [23,24,25]. In this study, we tried to find a possible association between the presence of antiseptic resistance genes and reduced susceptibility to antiseptics or resistance to various antibiotics in clinical isolates of Staphylococcus spp. and Enterococcus spp.. We observed that 15 (51.7%) of 29 S.aureus and 34 (85.0%) of 40 CNS isolates, for a total of 49 (71%) out of 69 staphylococci isolates harbored at least one antiseptic resistance gene. We also determined that among the 49 Staphylococcus spp. isolates positive for a gene, qacA/B genes were the most dominant (57.1%) followed by qacJ (36.7%), qacG (32.6%), and smr (22.4%) genes. The qacH gene was not found in any tested isolates. Our Enterococcus spp. isolates were free from any antiseptic resistance genes.
To date, two studies [26, 27] have been published on the antiseptic resistance genes of staphylococci in Turkey in addition to our previous study in 2012 [25]. Duran et al. [26] reported that the frequency of qacA/B and smr genes among amikacin-resistant S.aureus was 47.4% and 28.9% in Turkey, respectively, and this finding was statistically significant (p < 0.05). In the same study, the frequency of qacA/B and smr in CNS was 37.9% and 20.7%, respectively (p < 0.05). In another study conducted in Turkey, Aykan et al. [27] reported that 11.6% of MRSA isolates harbored qacA/B resistance genes. In this study, we found that 10% of our MRSA strains harbored the qacA/B gene, and our results were compatible with the results of Aykan et al. [27]. In our previous study, (25) we found that smr genes were more prevalent (36.0%) in MRSA whereas qacA/B genes more prevalent (4.0%) in MSSA strains. In addition, the presence of the iMLSB resistance phenotype in 8/18 (44.5%) smr-positive strains compared to 2/32 (6.25%) smr-negative strains was statistically significant (p < 0.001) [25]. However, unlike our previous work, smr and qacA/B genes existed in equal frequency (10.0%) in MRSA strains in this study. In addition, smr genes were more prevalent (15.7%) than qacA/B (10.5%) in MSSA strains. Moreover, we could not find any significant relationship between the presence of antiseptic resistance genes and antibiotic resistance.
Mayer et al. [7] reported results of the SENTRY European study group on the distribution of qacA/B and smr in 297 MRSA and 200 MSSA strains isolated between 1997 and 1999 in 24 different European university hospitals in 14 countries. They found that 42.0% of S.aureus (63.0% of MRSA and 12.0% of MSSA) harbored qacA/B genes, and qacA/B genes were more prevalent than the smr gene, which was detected in 5.8% of S. aureus (6.4% of MRSA and 5.0% of MSSA). They emphasized that the prevalence of antiseptic resistance genes is a widespread problem in European hospitals. Our study demonstrated that the presence of qacA/B genes in clinical S.aureus strains was lower (10.3%) than smr genes, which were more prevalent (13.8%) than European strains. Vali et al. [10] reported that in the UK, smr (44.2%) genes were most prevalent, followed by qacA/B (8.3%) and qacH (3.3%), and that qacA/B and smr were detected concomitantly in 4.2% of isolates; however they did not find qacG in 120 clinical MRSA strains. In contrast to Vali et al. we found a high frequency of qacG (7/10; 70.0%) in MRSA strains; but the qacH gene was not seen. We also detected qacA/B genes concomitantly with smr in 5.2% (1/19) of MSSA and 7.4% (2/27) of MRCNS, with qacG in 50.0% (5/10) of MRSA, and qacJ in 11.2% (3/27) of MRCNS and 38.4% (5/13) of MSCNS.
Longtin et al. [28] reported that smr genes were more frequent (7.0%) than qacA/B genes (2%) in 334 MRSA isolates collected from two Canadian intensive care units between 2005 and 2009, and no strain contained both genes. Noguchi et al. [22] detected qacA/B genes in 14.0% and smr genes in 28.0% of 71 clinical MRSA isolates in Japan. Alam et al. [29] reported that in 522 clinical S. aureus isolates from a hospital in Japan, qacA/B was more prevalent in MRSA (32.6%) and more prevalent in MSSA (7.5%) than smr genes, which had a frequency of 3.3% in MRSA and 5.9% in MSSA.
Zhang et al. [30] reported that 50.0% of MRSA and 16% of MSSA strains were positive for qacA/B gene, and this difference was statistically significant (p: 0.003). In our study, smr and qacA/B genes existed in equal frequency (10.0%) in MRSA strains, and smr genes were more prevalent (15.7%) than qacA/B (10.5%) in MSSA strains.
Zhang et al. [30] reported that CNS carried more qacA/B genes (56.7%) and smr genes (18.1%) than S. aureus (41.2% and 11.8%, respectively) in strains isolated from nares of nurses in a hospital in Hong Kong. In the same study, they detected a significant difference in the frequency of qacA/B between MRCNS (66.9%) and MSCNS (35.1%) strains (p < 0.001). They observed similar findings for smr (p: 0.001). In our study, the frequency of qacA/B and smr genes in MRCNS (63% and 14.8%, respectively) was very close to that of MSCNS (61.5% and 23.0%, respectively).
Ye et al. [16] examined the frequency of qacG, qacH and qacJ in 237 S. aureus (including 12 MRSA) and 604 CNS isolates (139 of which were methicillin resistant). They found that S. aureus isolates from a nurse (1.9%) harbored qacJ. Of the qacG-positive isolates, one of them harbored qacA/B and another harbored smr. In the same study, they detected that MICs of gene-positive strains were 2-fold higher than those of negative controls, and the presence of a second qac gene further elevated the MICs. The frequency of resistance to gentamicin, fusidic acid, clindamycin, and tetracycline increased in gene-positive isolates, but the MRSA isolates did not harbor these genes.
Liu et al. [11] found that 94.6% (53/56) of QAC-tolerant S.aureus isolates was positive for the qacA/B gene. The frequencies of smr and qacH were 3.6% and 7.1%, respectively. QacG was not detected in any isolates. The researches concluded that S. aureus isolates of China could survive at proper in-use concentrations of some biocides.
Zhang et al. [30] found an association between the presence of antiseptic resistance genes, and resistance to some anibiotics (cefoxitin, penicillin, ciprofloxacin, SXT, tetracycline, clindamycin) and reduced susceptibility to antiseptics (BAC: MIC ≥4 μg/mL, and CHDG: MIC ≥2–4 μg/mL). We also found a significant difference (p < 0.01) between the presence of antiseptic resistance genes and the BAC value (MIC > 4 μg/mL) but not antibiotic resistance (p > 0.05) in staphylococci isolates.
A limited number of antiseptic resistance gene studies have involved clinical Enterococcus spp. isolates. Bischoff et al. [17] found that 1 out of 42 (2.38%) E. faecalis isolates from blood samples carried qacA/B; 1 out of 109 (0.92%) E. faecalis isolates from stool samples harbored smr; and no Enterococcus spp. isolates were positive for qacG, qacH, or qacJ genes. In contrast to Bischoff et al. [17] we could not find any qacA/B and smr genes in Enterococcus spp. Thus far, no qac genes have been reported in enterococci other than qacC, qacEΔ1, qacZ and qacA/B. We also did not find qacG, qacH, or qacJ genes in enterococci isolates.
Bhardaj et al. [31] reported that MIC values of E. faecium and E. faecalis to CHDG ranged from 0.5 to 16 μg/mL. They also found that VanA-type resistance genes (VRE) are induced by sub-bactericidal levels of CHDG. It is a major concern whether exposure to sub-MIC CHDG results in cross-resistance to antibiotics in clinical use. MICs of BAC and CHDG in our Enterococci spp. isolates ranged from 8 to 16 μg/mL and 6 to 12 μg/mL, respectively. However there was a statistically significant difference (p < 0.01) of MICs in BAC and CHDG between VRE and VSE strains.
Our study has some limitations. This study focused on antiseptic resistance genes such as qacA/B, smr, qacG, qacH, and qac J, which are mostly isolated in Gram-positive bacteria, but other genes, such as qacEΔ, qac F, and qacZ, should be investigated in the future. In addition, the small sample size might have affected the possible relationship between antiseptic resistance genes and the resistance pattern of antibiotics and antiseptic agents.
Conclusion
The frequency of antiseptic resistance genes was high (71.0%) in our Staphylococcus spp. and absent (0%) in Enterococcus spp. isolates. The frequency of qacA/B and smr genes was higher (62.5% and 17.5%, respectively) in CNS isolates compared to S. aureus strains (10.3% and 13.8%, respectively). In contrast, the frequency of qacG and qacJ genes was higher (37.9% and 27.5%, respectively) in S. aureus strains compared to CNS strains (12.5% and 25.0%, respectively). We found statistically significant (p < 0.01) association between the presence of antiseptic resistance genes and the MIC of BAC (>4 μg/mL) in staphylococci. They may be a marked difference in the frequency and type of antiseptic resistance genes between countries or even between different hospitals in the same country, and therefore each hospital should review its antimicrobial policy and support continuing education to avoid developing new antimicrobial resistance mechanisms.
Abbreviations
- BAC:
-
Benzalkonium chloride
- CHDG:
-
Chlorhexidine digluconate
- CLSI:
-
Clinical and Laboratory Standards Institute
- iMLSB:
-
Inducible macrolide-lincosamide-streptogramin B
- MIC:
-
The minimum inhibitory concentration
- MRCNS:
-
Methicillin-resistant coagulase-negative staphylococci
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MSCNS:
-
Methicillin-susceptible coagulase-negative staphylococci
- MSSA:
-
Methicillin-susceptible Staphylococcus aureus
- SXT:
-
Trimethoprim-sulfamethoxazole
- VRE:
-
Vancomycin resistant enterococci
- VSE:
-
vancomycin susceptible enterococci
References
Casey AL, Lambert PA, Elliott TS. Staphylococci. Int J Antimicrob Agents. 2007;29:23–32.
Fisher K, Phillips C. The ecology, epidemiology and virulence of enterococcus. Microbiology. 2009;155:1749–57.
Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36:697–705.
McBain AJ, Rickard AH, Gilbert P. Possible implications of biocide accumulation in the environment on the prevalence of bacterial antibiotic resistance. J Ind Microbiol Biotechnol. 2002;29:326–30.
Gilbert P, Mcbain AJ. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin Microbiol Rev. 2003;16:189–208.
Maillard JY. Antimicrobial biocides in the healthcare environment: efficacy, usage, policies and perceived problems. Ther Clin Risk Manag. 2005;1:307–20.
Mayer S, Boos M, Beyer A, Fluit AC, Schmitz FJ. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and susceptible European isolates of Staphylococcus Aureus. J Antimicrob Chemother. 2001;47:896–7.
Noguchi N, Suwa J, Narui K, Sasatsu M, Ito T, Hiramatsu K, Song JH. Susceptibilities to antiseptic agents and distribution of antiseptic-resistance genes qacA/B and smr of methicillin-resistant Staphylococcus Aureus isolated in Asia during 1998 and 1999. J Med Microbiol. 2005;54:557–65.
Bjorland J, Steinum T, Sunde M, Waage S, Heir E. Novel plasmid-borne gene qacJ mediates resistance to quaternary ammonium compounds in equine Staphylococcus Aureus, Staphylococcus Simulans and Staphylococcus Intermedius. Antimicrob Agents Chemother. 2003;47:3046–52.
Vali L, Davies SE, Lai LLG, Dave J, Amyes SGB. Frequency of biocide resistance genes, antibiotic resistance and the effect of clorhexidine exposure on clinical methicillin resistant Staphylococcus Aureus isolates. J Antimicrob Chemother. 2008;61:524–32.
Liu Q, Liu M, Wu Q, Li C, Zhou T, Ni Y. Sensitivities to biocides and distribution of biocide resistance genes in quaternary ammonium compound tolerant Staphylococcus Aureus isolated in a teaching hospital. Scand J Infect Dis. 2009;41:403–9.
Miyazaki NH, Abreu AO, Marin VA, Rezende CA, Moraes MT, Villas Bôas MH. The presence of qacA/B gene in Brazilian methicillin-resistant Staphylococcus Aureus. Mem Inst Oswaldo Cruz. 2007;102:539–40.
Teixeira CF, Pereira TB, Miyazaki NH, Villas Boas MH. Widespread distribution of qacA/B gene among coagulase-negative staphylococcus spp. in Rio de Janeiro, Brazil. J Hosp Infect. 2010;75:333–44.
Leelaporn A, Paulsen IT, Tennent JM, Littlejohn TG, Skurray RA. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J Med Microbiol. 1994;40:214–20.
Bjorland J, Steinum T, Kvitle B, Waage S, Sunde M, Heir E. Widespread distribution of disinfectant resistance genes among staphylococci of bovine and caprine origin in Norway. J Clin Microbiol. 2005;43:4363–8.
Ye HF, Zhang M, O'Donoghue M, Boost M. Are qacG, qacH and qacJ genes transferring from food isolates to carriage isolates of staphylococci? J Hosp Infect. 2012;80:95–6.
Bischoff M, Bauer J, Preikschat P, Schwaiger K, Mölle G, Hölzel C. First detection of the antiseptic resistance gene qacA/B in Enterococcus Faecalis. Microb Drug Resist. 2012;18:7–12.
Meyer B, Cookson B. Does microbial resistance or adaptation to biocides create a hazard in infection prevention and control? J Hosp Infect. 2010;76:200–5.
Weber DJ, Rutala WA. Use of germicides in the home and the healthcare settings: is there a relationship between germicide use and antibiotic resistance? Infect Control Hosp Epidemiol. 2006;27:1107–19.
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M7-A7. 7th ed. Wayne, PA: CLSI; 2006.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Approved Standard M100-S17. Wayne, PA: CLSI; 2007.
Noguchi N, Hase M, Kitta M, Sasatsu M, Deguchi K, Kono M. Antiseptic susceptibility and distribution of antiseptic resistance genes in methicillin-resistant Staphylococcus Aureus. FEMS Microbiol Lett. 1999;172:247–53.
Wassenaar TM, Ussery D, Nielsen LN, Ingmer H. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur J Microbiol Immunol (Bp). 2015;5:44–61.
Prag G, Falk-Brynhildsen K, Jacobsson S, Hellmark B, Unemo M, Söderquist B. Decreased susceptibility to chlorhexidine and prevalence of disinfectant resistance genes among clinical isolates of Staphylococcus Epidermidis. APMIS. 2014;122:961–7.
Nakipoğlu Y, Iğnak S, Gürler N, Gürler B. The prevalence of antiseptic resistance genes (qacA/B and smr) and antibiotic resistance in clinical Staphylococcus Aureus strains. Mikrobiyol Bul. 2012;46:180–9.
Duran N, Temiz M, Duran GG, Eryılmaz N, Jenedi K. Relationship between the resistance genes to quaternary ammonium compounds and antibiotic resistance in staphylococci isolated from surgical site infections. Med Sci Monit. 2014;20:544–50.
Aykan SB, Cağlar K, Engin ED, Sipahi AB, Sultan N, Yalınay ÇM. Investigation of the presence of disinfectant resistance genes qacA/B in nosocomial methicillin-resistant Staphylococcus Aureus isolates and evaluation of their in vitro disinfectant susceptibilities. Mikrobiyol Bul. 2013;47:1–10.
Longtin J, Seah C, Siebert K, McGeer A, Simor A, Longtin Y, Low DE, Melano RG. Distribution of antiseptic resistance genes qacA, qacB, and smr in methicillin-resistant Staphylococcus Aureus isolated in Toronto, Canada, from 2005 to 2009. Antimicrob Agents Chemother. 2011;55:2999–01.
Alam MM, Ishino M, Kobayashi N. Analysis of genomic diversity and evolution of the low-level antiseptic resistance gene smr in Staphylococcus Aureus. Microb Drug Resist. 2003;9:1–7.
Zhang M, O’Donoghue MM, Ito T, Hiramatsu K, Boost MV. Prevalence of antiseptic-resistance genes in Staphylococcus Aureus and coagulase-negative staphylococci colonising nurses and the general population in Hong Kong. J Hosp Infect. 2011;78:113–7.
Bhardwaj P, Ziegler E, Palmer KL. Chlorhexidine induces VanA-type vancomycin resistance genes in enterococci. Antimicrob Agents Chemother. 2016;60:2209–21.
Acknowledgements
We thank Dr. Jostein Bjorland from Norwegian School of Veterinary Science for providing qac genes positive control strains.
Funding
The present work was supported by the Research Fund of Istanbul University with Project No. 7214.
Availability of data and materials
All the data supporting conclusions are available in Tables 1, 2, 3, 4 and 5.
Author’s contributions
All authors participated in the design of the work. SI performed the test and data collection. SI and YN wrote the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study was approved by the Clinical Research Ethic Commitee of the Istanbul University in 18.11.2009 with the number 2009/2850–86.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ignak, S., Nakipoglu, Y. & Gurler, B. Frequency of antiseptic resistance genes in clinical staphycocci and enterococci isolates in Turkey. Antimicrob Resist Infect Control 6, 88 (2017). https://doi.org/10.1186/s13756-017-0244-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-017-0244-6