Abstract
Background
Previous studies have found that coastal eutrophication increases the influence of homogeneous selection on bacterial community assembly. However, whether seasonal changes affect the dominance of homogenous selection in bacterial community assembly in eutrophic bays remains unclear. Sansha Bay is an enclosed bay with ongoing eutrophication, located in the southeast coast of China. We investigated the bacterial community composition at two depths of the enclosed bay across seasons and the seasonal variation in community assembly processes.
Results
Diversity analyses revealed that the bacterial community composition among seasons differed significantly. By contrast, there was little difference in the community composition between the two depths. The temperature was the key environmental factor influencing the community composition. The null model indicated that the relative importance of homogeneous selection decreased in the following order: spring > winter > autumn > summer. Homogeneous selection did not always dominate the community assembly among seasons in the eutrophic bay. The effects of pure spatial variables on the community assembly were prominent in autumn and winter.
Conclusions
Our results showed the seasonal influence of eutrophication on bacterial community diversity. The seasonal variation in composition and structure of bacterial communities eclipsed the vertical variability. Eutrophication could enhance the importance of homogeneous selection in the assembly processes, but the seasonal environmental differences interfered with the steady-state maintained by ongoing eutrophication and changed the community assembly processes. Homogeneous selection was not always important in bacterial community in the eutrophic enclosed bay. The bacterial community was the most complex in summer, because the composition differed from other seasons, and the assembly process was the most intricate. These findings have contributed to understanding bacterial community composition and assembly processes in eutrophic coastal ecosystems.
Similar content being viewed by others
Background
Revealing the diversity and formation process of the microbial community is a key topic in microbial ecology (Bunse and Pinhassi 2017; Martiny et al. 2006; Nemergut et al. 2013). Compared with the microbial community diversity, which has been described as common indicators (Help et al. 1998; Hughes et al. 2001), the quantification of community assembly is more difficult and complex. Early classic ecological theories based on observations of plants and animal communities focused on the relative contribution of deterministic processes (driven by environmental conditions) and stochastic processes (e.g., birth, death, colonization, extinction, and speciation) in community assembly (Chave 2004; Leibold et al. 2004; Wang et al. 2013). With the development of the theories, a suitable conceptual framework for explaining the process of microbial community assembly takes shape gradually (Hanson et al. 2012; Nemergut et al. 2013). The community assembly is divided into four general processes, including selection, dispersal, drift, and speciation in this conceptual framework (Vellend 2010), which apply to all taxonomic scales (resolution and breadth) and contain both the deterministic and stochastic processes (Hanson et al. 2012; Nemergut et al. 2013; Zhou and Ning 2017). An operational mode called the null model is built based on this conceptual framework and it quantifies the community assembly process into five parts (Stegen et al. 2013). Variable selection means the decrease of community similarity due to the selection of different environmental conditions. Homogeneous selection implies the increase of community similarity through the selection, which is caused by similar environmental conditions. Homogenizing dispersal means that high dispersal rate between pairs of communities is the primary cause of similar community structure. Dispersal limitation means that low dispersal rate is the primary cause of dissimilar community structures, where drift (stochastic changes in species abundance) becomes more important to community composition because of low turnover rates and weak selection. “Undominated” means that neither selection nor dispersal is the dominant process driving community compositional turnover (Stegen et al. 2013, 2015; Zhou and Ning 2017). The relative importance of speciation is overlooked, because it has little influence within local communities (Leibold et al. 2004; Stegen et al. 2013).
Sansha Bay is a subtropical enclosed bay located in the southeast coast of Fujian Province, China. The bay has the sole narrow outlet connected to the East China Sea and provides suitable environmental conditions for aquaculture. Large-scale aquaculture has been emerging in the twenty-first century. Various aquaculture industries have been distributed in Sansha Bay, including the large yellow croaker (Pseudosciaena crocea), macroalgae, abalone, etc. The aquaculture area was expanding year by year and it increased 1.6 times from 2010 to 2018 (Chen et al. 2019; Ji et al. 2021). The continuous intensive aquaculture causes long-term eutrophication in this bay, including excessive nitrogen and phosphorus concentrations (Sun et al. 2015; Wang et al. 2020a; Xie et al. 2020). Pollution from coastal industries and urbanization also aggravate eutrophication. Therefore, the local bacterial community is not only affected by season and spatial variation, but also by anthropogenic activities and their results (e.g., eutrophication).
Previous investigations have identified that deterministic processes become important in marine bacterial communities as eutrophication intensifies (Dai et al. 2017; Li et al. 2020). Homogenous selection has contributed a lot to the assembly of aquatic microbial communities in eutrophic environments (Li et al. 2019; Nyirabuhoro et al. 2020; Wu et al. 2021). It is found that the marine bacterial community composition and assembly processes show distinct seasonal changes under oligotrophic and mesotrophic conditions, without significant spatial variations (Gilbert et al. 2012; Pinhassi et al. 2006; Wang et al. 2020b; Ward et al. 2017). In this work we studied the bacterial communities from the surface and bottom layers of Sansha Bay across seasons. We hypothesized that (i) Seasonal variation played a dominant role in bacterial community composition instead of vertical depth variation; (ii) Homogeneous selection always dominated community assembly processes during ongoing eutrophication, and the assembly didn’t reflect the seasonal variation clearly.
Materials and methods
Study area and sampling
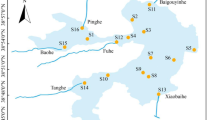
Samples were collected at 15 stations in Sansha Bay, Fujian Province (119.5°–120.0° E, 26.5°–26.8° N; Fig. 1) from four cruises (winter: January 2019; spring: April 2019; summer: July 2019; autumn: October 2019). We collected water samples from the surface (0.5 m) and the bottom (2–55.5 m) at each station. A total of 120 water samples were collected. Water samples were prefiltered through a 200-μm-pore mesh and then filtered onto a 0.22-μm-pore polycarbonate membrane (47 mm diameter; Millipore; USA). These membranes were stored at – 80 ℃ immediately after being transported into the laboratory until DNA extraction (Mo et al. 2018). Salinity, water temperature, and depth were measured in situ with the Conductivity–Temperature–Depth (CTD) oceanic profilers (AML Base X, Canada). Total phosphorus (TP), phosphate phosphorus (PO43−–P), active silicon (DSi), total nitrogen (TN), dissolved inorganic nitrogen (DIN), nitrate nitrogen (NO3−–N), ammonium nitrogen (NH4+–N), chlorophyll-a (Chl-a), and pH were measured according to the standard methods (Office of the State Oceanic Administration 2006). The eutrophication level was classified by the N/P ratio method (Guo et al. 1998; Niu et al. 2021).
DNA extraction, PCR and sequencing
We extracted total genomic DNA using the FastDNA spin kit (MP Biomedicals, USA). The hypervariable V3–V4 regions of the 16S rRNA gene were amplified by PCR primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNVGGGTWTCTAAT-3′). The PCR reaction was set as followed: initial denaturation at 94 ℃ for 5 min, followed by 30 cycles of denaturation at 94 ℃ for 30 s, annealing at 57 ℃ for 30 s and extension at 72 ℃ for 15 s, and a final extension at 72 ℃ for 10 min. The triplicate PCR products for each sample were mixed in equal quantities and electrophoresed. The target bands were recovered and purified using the GeneJET gel purification kit (Thermo Scientific, USA). The concentration of purified DNA samples was measured using a Qubit 2.0 fluorescence quantifier (Thermo Fisher Scientific, Waltham, USA), and the same amount of DNA was mixed for each sample to build the database. The library was paired-end for sequencing on the Illumina HiSeq platform (Illumina Inc., San Diego, USA).
Sequence analysis
Paired-end V3–V4 regions of 16S rRNA gene sequencing were processed using VSEARCH (v1.9.1) (Rognes et al. 2016). Pairs of reads were merged by FLASH (Magoč and Salzberg 2011). The unoise3 algorithm was used to denoise and remove the chimeras, then operational taxonomic units (OTUs) were identified at a 97% sequence similarity level (Edgar 2010). The taxonomic assignment was performed using the SILVA 132 database. All eukaryotic, chloroplast, archaeal, mitochondrial, unassigned OTUs and single sequence OTUs were removed before the downstream analyses. Each sample was randomly subsampled based on MOTHUR (v.1.44.3) (Schloss et al. 2009). After normalization each sample contained 39,206 sequences and there were 18,847 OTUs in total.
Diversity analyses
The alpha diversity indexes, including Shannon, Simpson, OTU richness, abundance-based coverage estimators (ACE), Chao1 and Pielou’s evenness, were performed in R (v4.0.4) environment using the “vegan” package. Simpson index measures the probability that two individuals, consecutively taking from a community, are classified into same types. Shannon index quantifies the uncertainty in predicting the species identity of an individual that is taken at random from the data set. The greater the value of the above two indexes, the higher the diversity. OTU richness is the count of the number of OTUs present in an area. Chao1 and ACE indexes are theoretical estimators of richness based on observations. Pielou’s evenness index refers to the evenness of a community (Thukral 2017). Principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity was used to investigate the compositional variation of the bacterial community. The contribution of different factors to community composition was tested by permutational multivariate analysis of variance (PERMANOVA) using the “ape” package (R Core Team 2021). Statistical tests were performed using SPSS v20.0 (IBM Corp., USA).
The null model
We analyzed phylogenetic signals in bacterial communities and found that phylogenetic turnover analysis can infer underlying ecological processes (Additional file 1: Fig. S1). Then we quantified the community assembly process using the null model. First, the beta nearest taxon index (βNTI) was calculated. When βNTI was < − 2 or > + 2, the community turnover would be dominated by homogeneous selection and heterogeneous selection, respectively. When |βNTI| was < 2, other ecological processes would affect community turnover. Second, the Bray–Curtis dissimilarity based Raup–Crick metric (RCbray) was calculated. When RCbray was < − 0.95 or > + 0.95, the community turnover would be dominated by homogenizing dispersal and dispersal limitation, respectively. When |RCbray| was < 0.95, it indicated the undominated fraction (Stegen et al. 2013). We used the Mantel test to indicate the relationship between βNTI and pairwise differences in environmental factors among samples.
Relationships between bacterial community and shaping factors
The environmental parameters were log (1 + x) transformed except pH. Because the value of detrended correspondence analysis (DCA) was less than 3, redundancy analysis (RDA) was used (Lepš and Šmilauer 2003). We removed the variance inflation factor (VIF) greater than 20, because these environmental parameters were severely affected by multicollinearity, and then analyzed these filtered VIFs using the forward selection (p < 0.05) (Blanchet et al. 2008). Variation partitioning analysis (VPA) was performed to quantify the relative importance of spatial variables and environmental variables in assembly. The environmental variables were selected according to the previous steps. The spatial variables were calculated using principal coordinates of neighbor matrices (PCNM) procedures and then were selected by the forward selection (p < 0.05) (Griffith and Peres-Neto 2006). We calculated the Levins niche breadth and the average niche breadths of all OTUs in a given habitat (i.e., each station was considered a “habitat”) to represent the niche breadth at the community level (Levins 2020).
Co-occurrence network construction
We removed OTUs that present in less than one-third of the samples and less than 100 sequences. Co-occurrence networks in different seasons were constructed based on Spearman’s rank correlation. Only statistically significant (p < 0.01) correlations were incorporated in the networks. Network visualization was generated with Gephi version 0.9.2. The topological parameters of both real and Erdös–Réyni random networks were compared, which were calculated in the “igraph” R package (R Core Team 2021). Because of continuous high nitrogen concentration in the environment, we focused on nitrogen-related bacteria. The nitrogen-sensitive/nitrogen-tolerant bacterial OTUs in networks were defined as the bacterial OTUs having significant positive/negative Spearman’s rank correlation (p < 0.01) with TN, NH4+–N, NO3−–N or DIN.
Results
Environmental factors
Environmental conditions showed temporal variations rather than spatial ones. The important environmental parameters from two depths in the same season were almost identical (Additional file 1: Fig. S2). The Chl-a concentrations were higher in spring and summer than in autumn and winter (Additional file 1: Fig. S2, Table S1). In autumn and winter, the mean concentrations of phosphate and nitrogen reached eutrophication levels. In spring and summer, the mean concentrations were at P-limited potential eutrophication levels because of phytoplankton growth (Additional file 1: Fig. S2, Tables S1 and S2).
Alpha and beta diversity
The rarefaction curves demonstrated that the bacterial community richness for the total and seasonal samples was close to saturation (Additional file 1: Fig. S3). Alpha diversity indexes showed a significant seasonal difference (p < 0.05). By contrast, there was no significant difference in alpha diversity indexes for the surface and bottom water samples in spring, autumn, and winter (p > 0.05, Table 1). The community richness and evenness indexes were the highest in summer (Additional file 1: Fig. S4).
PCoA showed a good clustering effect of different seasons. However, the separation between two depths was difficult to distinguish (Fig. 2). PERMANOVA demonstrated that the season (R2 = 0.719, p < 0.001) explained the most variation of community composition, compared with the depth (R2 = 0.012, p < 0.001) and interaction of these two factors (R2 = 0.017, p < 0.01). Based on the above results, we focused on bacterial community variation across seasons instead of vertical depth. The taxonomy of composition in summer was quite different from those in other seasons, and the composition in autumn and winter was more similar (Additional file 1: Fig. S5a, b).
Bacterial community assembly
Both the stochastic and deterministic processes increased the similarity of bacterial communities in spring, autumn, and winter. However, the assembly process in summer was more complex. The community homogeneity dominates in three seasons. Homogeneous selection was the primary driver of community assembly in spring. In autumn and winter, the community was mainly driven by homogenizing dispersal. In summer, the proportions of homogenizing dispersal and variable selection were close. Dispersal limitation had a slight effect on community assembly processes in all seasons (Fig. 3).
Community assembly processes of the bacterial community in different seasons. The assembly processes across seasons tended to homogenize the bacterial community. The proportion of homogenous selection was the highest in spring and decreased in the following order: spring > winter > autumn > summer. The assembly process in summer was most complex. Homogenizing dispersal dominated in autumn and winter. Dispersal limitation had little impact in all seasons
Shaping factors and assembly mechanisms
Mantel test and RDA results showed that water temperature was always significantly correlated with community variation in different seasons (p < 0.05) (Additional file 1: Table S3, Fig. 4a). The niche breadths of bacterial community (Bcom) had a significant difference among different seasons (p < 0.05). The values of Bcom in different seasons from high to low were: winter, autumn, spring, summer (Fig. 4b). VPA indicated that drivers of community variation were different among seasons. Pure spatial variables dominated community assembly in autumn and winter. Pure environmental variables dominated community assembly in spring and summer (Fig. 5). We further explored the relationship between environmental factors and βNTI in summer. The depth had a strong relationship with βNTI (p < 0.001, r = 0.44) (Additional file 1: Fig. S6), while other factors were not (p < 0.001).
A Redundancy analysis (RDA) indicating relationships between environmental factors and seasonal dynamics of bacterial community. Only significant environmental factors are plotted (p < 0.05). Temp: temperature; TP: total phosphorus; Sal: salinity; DSi: active silicon; PO43−–P: phosphate phosphorus. B Boxplots illustrating the average niche breadths of all OTUs at the community level (Bcom) in different seasons. Horizontal black lines show the median values. The values of Bcom have a significant difference among different seasons (Kruskal–Wallis test, p < 0.05). Letters refer to pairwise comparison results after the Kruskal–Wallis test and the absence of the same letter means a significant difference between the two niche breadths (p < 0.05)
Variation partitioning analysis (VPA) quantifying the relative important of spatial variables and environmental variables in bacterial community assembly. Values include pure variables explained, shared explained, and unexplained percentage. Values < 0 are shown as 0. Env: environmental variables; Spatial: spatial variables. ***p < 0.001, **p < 0.01, ·p < 0.1
Co-occurrence networks
The network topological properties among different seasons were different and the summer network had the most degrees and edges (Additional file 1: Table S4, Fig. 6). The real networks showed scale-free and small-world properties compared with their corresponding random networks (Additional file 1: Table S4, Fig. S7). The proportion of nodes related to nitrogen was more than 1/3 in the networks (Additional file 1: Fig. S8). This reflected the advantage of nitrogen-related bacteria in network construction under the environmental selection of high nitrogen concentration. The ratios of degrees and weights for nitrogen-sensitive bacteria to nitrogen-tolerant bacteria were highest in the summer network (Fig. 7). The importance of nitrogen-sensitive bacteria increased in the community interaction in summer.
A Proportions of degree for nitrogen-tolerant/nitrogen-sensitive bacteria in different seasons. B Proportions of weight for nitrogen-tolerant/nitrogen-sensitive bacteria in different seasons. Ratios of degree/weight for nitrogen-sensitive bacteria to nitrogen-tolerant bacteria are indicated at the top of the bar
Discussion
The vertical variability of community composition and structure is not significant
Seasonal environmental changes have been found to have a greater effect than biogeography on marine bacterial communities (Pinhassi et al. 2006; Ward et al. 2017; Wu et al. 2017). There was no significant vertical variability on community composition and structure, except for summer (Fig. 2, Table 1). In this work, the vertical distance between the surface and bottom sampling locations ranged mostly from 6 to 30 m (Additional file 1: Table S1). The short distance made the vertical transport of bacteria by mixing processes easier. The physical and chemical conditions at the surface and bottom in small spatial scales are more similar by mixing (Bunse and Pinhassi 2017; Fortunato et al. 2012; Salmaso et al. 2018). It could explain that the seasonal variation in community composition and structure eclipses the vertical variability.
The temperature was the key environmental factor that influenced the community composition (Fig. 4a, Additional file 1: Table S3). The growth and metabolic processes in bacteria were related to the temperature and marine bacteria displayed distinct ecological preferences based on the temperature (Yung et al. 2015). At the phylotype level, some specific taxa showed a correlation between distribution and temperature, indicating that taxa with distinct temperature preferences underlie apparent clustering (Wang et al. 2019). When the bacterial metabolism was not limited by inorganic nutrients, responses of aquatic bacterial communities to temperature variations were stronger (Berggren et al. 2010).
Homogeneous selection does not dominate the assembly across seasons
Changes in microbial community composition are influenced by historical environmental conditions (Evans and Wallenstein 2012; Fierer et al. 2003). Repeated and predictable environmental conditions cause homogeneous selection (Aguilar and Sommaruga 2020). Community characteristics show similarities by ongoing eutrophication acting as an environmental filter, which preserves species with fitting biological traits (Louca et al. 2016; Stegen et al. 2012; Zeglin 2015). A study in semi-enclosed bays found that moderate eutrophication induced selectivity and stability of bacterioplankton communities, thereby strengthening community structure and enhancing deterministic processes (Dai et al. 2017). In a study focusing on bacterial communities in coastal areas influenced by surface runoff, hydrocarbonoclastic bacteria played a central role and homogeneous selection was the dominant ecological process (Wu et al. 2021). This advantage of homogenous selection was interpreted as the selection of appropriate genomic architecture and metabolic strategies in a eutrophic environment (Grzymski and Dussaq 2012; Lauro et al. 2009; Louca et al. 2016).
In Sansha Bay, homogeneous selection did not dominate the assembly across seasons, although ongoing eutrophication made an important contribution to homogenizing the bacterial community. Our study suggested that the proportion of different ecological processes in community assembly was affected by seasonally changing habitats. The relative importance of homogeneous selection decreased in the following order: spring > winter > autumn > summer. The proportion of homogenous selection reached the highest in spring (77.93%), then suddenly dropped in summer (18.62%) (Fig. 3). The relative importance of environmental filtering depends on the stabilized duration of the primary selection condition (eutrophication) in community assembly (Berga et al. 2012; Gibbons et al. 2016; Santillan et al. 2019; Shade et al. 2011). When the environment changes, the escalatory effect of other disturbances will disrupt this steady state (Langenheder and Lindström 2019). The differences of seasonal physicochemical properties and artificial aquaculture activities in Sansha Bay interfered with the steady state maintained by long-term eutrophication conditions and changed the bacterial community assembly process. The effects of disturbance were most obvious in summer, because changes in environmental conditions were most complex. There is an association between the specific bacteria and nutrient levels, and some bacterial communities have served as in situ environmental indicators to reflect environmental disturbances (Fodelianakis et al. 2014; Olsen et al. 2017; Xiong et al. 2015). When other environmental disturbances weakened the intense stress of eutrophication, the specific bacteria changed accordingly and affected the interspecific interactions they participated in (Dai et al. 2017; Langenheder and Lindström 2019; Xiong et al. 2015). We found that the importance of nitrogen-sensitive bacteria in the summer network suddenly increased (Fig. 7). One possibility was that environmental disturbances made more nitrogen-sensitive bacteria participate in community construction even in a high nitrogen environment. As the environment stabilizes in autumn and winter, nitrogen-tolerant bacteria re-dominated the communities.
Homogenizing dispersal dominates the assembly in autumn and winter
It is generally accepted that deterministic and stochastic processes jointly influence bacterial community assembly, but there are still uncertainties about their relative importance (Aguilar and Sommaruga 2020; Heino et al. 2015; Nemergut et al. 2013; Zhou and Ning 2017). Deterministic processes usually play a more important role than stochastic processes in eutrophic aquatic ecosystems (Dai et al. 2017; Jiao et al. 2021; Li et al. 2020; Wu et al. 2021). However, this study showed that homogenizing dispersal was the most important assembly mechanism in autumn and winter (Fig. 3). VPA showed that the pure spatial variables explained more on community variation in autumn and winter, while the pure environmental variables explained less in these two seasons (Fig. 5). In a frequently disturbed environment, microbial inhabitants may have evolved to endure such dynamics and be less responsive to deterministic factors (Dai et al. 2017; Langenheder et al. 2012; Santillan et al. 2019). Homogenizing dispersal occurs in communities with small and inhabiting relatively stable environments (Fodelianakis et al. 2019; König et al. 2018). Thus, the bacterial community that experienced complex environmental disturbances in summer gained relatively high resistance. In addition, it allowed the stochastic assembly process advantageous in autumn and winter when the environment was more stable. In addition to the above reasons, we found that communities in autumn and winter had wider niche breadths than other seasons (Fig. 4b). The bacteria need to survive the conditions encountered during dispersal to a new location and wider niche breadths increase the probability of successful colonization (Comte et al. 2014; Martiny et al. 2006). In a recent study, the construction processes of archaea communities with wider niche breadths were more influenced by homogenizing dispersal compared with bacterial communities (Wang et al. 2020b).
Dispersal limitation had little impact on community assembly in all seasons (Fig. 3). Because our studying region was small (< 20 km), the small scale amplified the homogenizing dispersal effect (Fodelianakis et al. 2019; Fuhrman et al. 2015). It has been shown that the bacterial community from the mesotrophic water was more dispersed compared with that of the oligotrophic one (Aguilar and Sommaruga 2020). It is possible for marine bacteria to overcome physical barriers easily and disperse passively through living vectors, hydrological changes (e.g., ocean currents and tides) and other ways (Grossart et al. 2010; Lindström and Langenheder 2012; Müller et al. 2014).
The composition and assembly of bacterial community in summer are most complex
There were significant differences in bacterial community composition at different depths in summer. The community assembly process and network were the most complex. The limited physical mixing between the surface and bottom water explained this phenomenon. In Sansha Bay, the water was vertically stratified near the surface (1.5–10 m) in summer (Wang et al. 2018). The depth was the key factor mediating the balance between deterministic and stochastic assembly processes (Additional file 1: Fig. S6). Different selective pressures from heterogeneous environments (surface and bottom water) acted on microbial communities, which produced great composition differences and enhanced the relative importance of variable selection. It has been reported that bacterial communities between May and September became progressively less similar because of water stratification (Shade et al. 2007).
Explanation of pure environmental variables to community assembly was highest in summer (Fig. 5). Compared with other seasons, the community variation was significantly correlated with more environmental factors (e.g., different types of nutrients) in summer (Additional file 1: Table S3). The complex changes of the community were caused by various environmental factors. Previous studies have found that narrow niches are most vulnerable to environmental factors (Pandit et al. 2009; Qiao et al. 2016). The bacterial communities in summer had the narrowest niche breadths among four seasons (Fig. 4b). The subtle environmental changes among different stations reflected more in community heterogeneity. The relative importance of niche segregation enhanced and the community structure was more variable in summer (Ren et al. 2019).
The environmental disturbance caused by aquaculture activities could be an indirect factor. In Sansha Bay, different species of macroalgae are alternatively cultured within 1 year in the same area (Ji et al. 2021). Given that the chlorophyll-a concentration was high in summer, phytoplankton–bacteria interactions could play an important role. Bacteria are stimulated by secreted allelopathic substances of phytoplankton. The diversity of phytoplankton and the special metabolism of different bacteria determine the type of interactions between them (Cirri and Pohnert 2019; Ramanan et al. 2016; Seymour et al. 2017). Recruitment of specific bacterial taxa by interactions is an important process shaping communities.
Conclusions
We analyzed the variation of bacterial community composition and assembly processes in the eutrophic enclosed bay. Our results support that the temperature plays a key role in the seasonal variation of community composition. We also find that long-term eutrophication is not sufficient to overwhelm the influence of seasonal variation in community assembly processes. Though eutrophication increases the importance of homogeneous selection, its proportion suddenly drops in summer due to seasonally changing habitats. The most complex community assembly in summer could be driven by the stratification, the changes in the community niche breadth, and the interaction between the bacteria and phytoplankton. In general, homogeneous selection does not dominate the assembly processes among seasons. The seasonal differences interfered with the steady state maintained by ongoing eutrophication and changed the community assembly processes. Our results can help a better understanding of community seasonal patterns in the eutrophic enclosed bay.
Availability of data and materials
16S rRNA gene sequences have been submitted to the NCBI Sequence Read Archive (SRA) database under the BioProject number PRJNA747131 and the Accession number SRP328863.
Abbreviations
- ACE:
-
Abundance-based coverage estimators
- Chl-a :
-
Chlorophyll-a
- DCA:
-
Detrended correspondence analysis
- DIN:
-
Dissolved inorganic nitrogen
- DSi:
-
Active silicon
- NH4 +–N:
-
Ammonium nitrogen
- NO3 −–N:
-
Nitrate nitrogen
- OTU:
-
Operational taxonomic unit
- PCoA:
-
Principal coordinate analysis
- PERMANOVA:
-
Permutational multivariate analysis of variance
- PO4 3−–P:
-
Phosphate phosphorus
- RCbray :
-
Bray–Curtis dissimilarity based Raup–Crick metric
- RDA :
-
Redundancy analysis
- TN:
-
Total nitrogen
- TP:
-
Total phosphorus
- VIF:
-
Variance inflation factor
- VPA:
-
Variation partitioning analysis
- βNTI:
-
Beta nearest taxon index
References
Aguilar P, Sommaruga R (2020) The balance between deterministic and stochastic processes in structuring lake bacterioplankton community over time. Mol Ecol 29:3117–3130
Berga M, Székely AJ, Langenheder S (2012) Effects of disturbance intensity and frequency on bacterial community composition and function. PLoS ONE 7:e36959
Berggren M, Laudon H, Jonsson A, Jansson M (2010) Nutrient constraints on metabolism affect the temperature regulation of aquatic bacterial growth efficiency. Microb Ecol 60:894–902
Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89:2623–2632
Bunse C, Pinhassi J (2017) Marine bacterioplankton seasonal succession dynamics. Trends Microbiol 25:494–505
Chave J (2004) Neutral theory and community ecology. Ecol Lett 7:241–253
Chen Y, Wang Y, Xie T, Zhang M (2019) Analysis for the change of aquaculture area and water quality in Sansha Bay during 2010–2018. In: IEEE international symposium on geoscience and remote sensing IGARSS, pp 8264–8267
Cirri E, Pohnert G (2019) Algae–bacteria interactions that balance the planktonic microbiome. New Phytol 223:100–106
Comte J, Lindström ES, Eiler A, Langenheder S (2014) Can marine bacteria be recruited from freshwater sources and the air? ISME J 8:2423–2430
Dai W, Zhang J, Tu Q, Deng Y, Qiu Q, Xiong J (2017) Bacterioplankton assembly and interspecies interaction indicating increasing coastal eutrophication. Chemosphere 177:317–325
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Evans SE, Wallenstein MD (2012) Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry 109:101–116
Fierer N, Schimel JP, Holden PA (2003) Influence of drying–rewetting frequency on soil bacterial community structure. Microb Ecol 45:63–71
Fodelianakis S, Papageorgiou N, Pitta P, Kasapidis P, Karakassis I, Ladoukakis Emmanuel D, Kostka JE (2014) The pattern of change in the abundances of specific bacterioplankton groups is consistent across different nutrient-enriched habitats in Crete. Appl Environ Microbiol 80:3784–3792
Fodelianakis S, Lorz A, Valenzuela-Cuevas A, Barozzi A, Booth JM, Daffonchio D (2019) Dispersal homogenizes communities via immigration even at low rates in a simplified synthetic bacterial metacommunity. Nat Commun 10:1314
Fortunato CS, Herfort L, Zuber P, Baptista AM, Crump BC (2012) Spatial variability overwhelms seasonal patterns in bacterioplankton communities across a river to ocean gradient. ISME J 6:554–563
Fuhrman JA, Cram JA, Needham DM (2015) Marine microbial community dynamics and their ecological interpretation. Nat Rev Microbiol 13:133–146
Gibbons SM, Scholz M, Hutchison AL, Dinner AR, Gilbert JA, Coleman ML (2016) Disturbance regimes predictably alter diversity in an ecologically complex bacterial system. mBio 7:e01372
Gilbert JA, Steele JA, Caporaso JG, Steinbrück L, Reeder J, Temperton B, Huse S, McHardy AC, Knight R, Joint I (2012) Defining seasonal marine microbial community dynamics. ISME J 6:298–308
Griffith DA, Peres-Neto PR (2006) Spatial modeling in ecology: the flexibility of eigenfunction spatial analyses. Ecology 87:2603–2613
Grossart H-P, Dziallas C, Leunert F, Tang KW (2010) Bacteria dispersal by hitchhiking on zooplankton. Proc Natl Acad Sci USA 107:11959–11964
Grzymski JJ, Dussaq AM (2012) The significance of nitrogen cost minimization in proteomes of marine microorganisms. ISME J 6:71–80
Guo W, Zhang X, Yang Y, Hu M (1998) Evaluation of the potential eutrophication of China’s offshore waters. Taiwan Strait 1:64–70
Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JBH (2012) Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10:497–506
Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM (2015) Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw Biol 60:845–869
Help CHR, Herman PMJ, Soetaert K (1998) Indices of diversity and evenness. Oceanis 24:61–87
Hughes JB, Hellmann JJ, Ricketts TH, Bohannan BJM (2001) Counting the uncountable: statistical approaches to estimating microbial diversity. Appl Environ Microbiol 67:4399–4406
Ji W, Yokoyama H, Fu J, Zhou J (2021) Effects of intensive fish farming on sediments of a temperate bay characterised by polyculture and strong currents. Aquac Rep 19:100579
Jiao C, Zhao D, Huang R, He F, Yu Z (2021) Habitats and seasons differentiate the assembly of bacterial communities along a trophic gradient of freshwater lakes. Freshw Biol 66:1515–1529
König S, Worrich A, Banitz T, Centler F, Harms H, Kästner M, Miltner A, Wick LY, Thullner M, Frank K (2018) Spatiotemporal disturbance characteristics determine functional stability and collapse risk of simulated microbial ecosystems. Sci Rep 8:9488
Langenheder S, Lindström ES (2019) Factors influencing aquatic and terrestrial bacterial community assembly. Environ Microbiol Rep 11:306–315
LangenhederS BM, Östman Ö, Székely AJ (2012) Temporal variation of β-diversity and assembly mechanisms in a bacterial metacommunity. ISME J 6:1107–1114
Lauro FM, McDougald D, Thomas T, Williams TJ, Egan S, Rice S, DeMaere MZ, Ting L, Ertan H, Johnson J (2009) The genomic basis of trophic strategy in marine bacteria. Proc Natl Acad Sci USA 106:15527–15533
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Levins R (2020) Evolution in changing environments. Princeton University Press, Princeton
Li Y, Gao Y, Zhang W, Wang C, Wang P, Niu L, Wu H (2019) Homogeneous selection dominates the microbial community assembly in the sediment of the Three Gorges Reservoir. Sci Total Environ 690:50–60
Li N, Chen X, Zhao H, Tang J, Jiang G, Li Z, Li X, Chen S, Zou S, Dong K, Xu Q (2020) Spatial distribution and functional profile of the bacterial community in response to eutrophication in the subtropical Beibu Gulf, China. Mar Pollut Bull 161:111742
Lindström ES, Langenheder S (2012) Local and regional factors influencing bacterial community assembly. Environ Microbiol Rep 4:1–9
Louca S, Parfrey LW, Doebeli M (2016) Decoupling function and taxonomy in the global ocean microbiome. Science 353:1272–1277
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112
Mo Y, Zhang W, Yang J, Lin Y, Yu Z, Lin S (2018) Biogeographic patterns of abundant and rare bacterioplankton in three subtropical bays resulting from selective and neutral processes. ISME J 12:2198–2210
Müller AL, de Rezende JR, Hubert CRJ, Kjeldsen KU, Lagkouvardos I, Berry D, Jørgensen BB, Loy A (2014) Endospores of thermophilic bacteria as tracers of microbial dispersal by ocean currents. ISME J 8:1153–1165
Nemergut DR, Schmidt SK, Fukami T, O'Neill SP, Bilinski TM, Stanish LF, Knelman JE, Darcy JL, Lynch RC, Wickey P, Ferrenberg S (2013) Patterns and processes of microbial community assembly. Microbiol Mol Biol Rev 77:342–356
Niu S-J, Li M-T, Tong M, Liu X-Q, Lin M-D, Yin G-y (2021) Responses of distribution of water quality in the Sansha Bay on the land discharge and aquaculture. Mar Environ Sci 40:41–49
Nyirabuhoro P, Liu M, Xiao P, Liu L, Yu Z, Wang L, Yang J (2020) Seasonal variability of conditionally rare taxa in the water column bacterioplankton community of subtropical reservoirs in China. Microb Ecol 80:14–263
Office of the State Oceanic Administration (2006) Comprehensive survey and assessment technique regulations in offshore area of China. Marine chemistry survey technique regulations. Ocean Press, Beijing
Olsen LM, Hernández KL, Van Ardelan M, Iriarte JL, Bizsel KC, Olsen Y (2017) Responses in bacterial community structure to waste nutrients from aquaculture: an in situ microcosm experiment in a Chilean fjord. Aquac Environ Interact 9:21–32
Pandit SN, Kolasa J, Cottenie K (2009) Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90:2253–2262
Pinhassi J, Gómez-Consarnau L, Alonso-Sáez L, Sala MM, Vidal M, Pedrós-Alió C, Gasol JM (2006) Seasonal changes in bacterioplankton nutrient limitation and their effects on bacterial community composition in the NW Mediterranean Sea. Aquat Microb Ecol 44:241–252
Qiao H, Saupe EE, Soberón J, Peterson AT, Myers CE (2016) Impacts of niche breadth and dispersal ability on macroevolutionary patterns. Am Nat 188:149–162
R Core Team (2021) R: a language and environment for statistical computing
Ramanan R, Kim B-H, Cho D-H, Oh H-M, Kim H-S (2016) Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv 34:14–29
Ren L, Song XY, He D, Wang J, Tan M, Xia X, Li G, Tan Y, Wu QL (2019) Bacterioplankton metacommunity processes across thermal gradients: weaker species sorting but stronger niche segregation in summer than in winter in a subtropical bay. Appl Environ Microbiol 85:e02088-e2118
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Salmaso N, Albanese D, Capelli C, Boscaini A, Pindo M, Donati C (2018) Diversity and cyclical seasonal transitions in the bacterial community in a large and deep Perialpine Lake. Microb Ecol 76:125–143
Santillan E, Seshan H, Constancias F, Drautz-Moses DI, Wuertz S (2019) Frequency of disturbance alters diversity, function, and underlying assembly mechanisms of complex bacterial communities. Npj Biofilms Microbiomes 5:8
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Seymour JR, Amin SA, Raina J-B, Stocker R (2017) Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nat Microbiol 2:17065
Shade A, Kent AD, Jones SE, Newton RJ, Triplett EW, McMahon KD (2007) Interannual dynamics and phenology of bacterial communities in a eutrophic lake. Limnol Oceanogr 52:487–494
Shade A, Read JS, Welkie DG, Kratz TK, Wu CH, McMahon KD (2011) Resistance, resilience and recovery: aquatic bacterial dynamics after water column disturbance. Environ Microbiol 13:2752–2767
Stegen JC, Lin X, Konopka AE, Fredrickson JK (2012) Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J 6:1653–1664
Stegen JC, Lin X, Fredrickson JK, Chen X, Kennedy DW, Murray CJ, Rockhold ML, Konopka A (2013) Quantifying community assembly processes and identifying features that impose them. ISME J 7:2069–2079
Stegen JC, Lin X, Fredrickson JK, Konopka AE (2015) Estimating and mapping ecological processes influencing microbial community assembly. Front Microbiol 6:370
Sun P, Yu G, Chen Z, Hu J, Liu G, Xu D (2015) Diagnostic model construction and example analysis of habitat degradation in enclosed bay: III. Sansha Bay habitat restoration strategy. Chin J Oceanol Limnol 33:477–489
Thukral AK (2017) A review on measurement of alpha diversity in biology. Agric Res J 54:1–10
Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85:183–206
Wang J, Shen JI, Wu Y, Tu C, Soininen J, Stegen JC, He J, Liu X, Zhang L, Zhang E (2013) Phylogenetic beta diversity in bacterial assemblages across ecosystems: deterministic versus stochastic processes. ISME J 7:1310–1321
Wang G, Han A, Chen L, Tan E, Lin H (2018) Fluxes of dissolved organic carbon and nutrients via submarine groundwater discharge into subtropical Sansha Bay, China. Estuar Coast Shelf Sci 207:269–282
Wang Z, Juarez DL, Pan J-H, Blinebry SK, Gronniger J, Clark JS, Johnson ZI, Hunt DE (2019) Microbial communities across nearshore to offshore coastal transects are primarily shaped by distance and temperature. Environ Microbiol 21:3862–3872
Wang J, Beusen AHW, Liu X, Bouwman AF (2020a) Aquaculture production is a large, spatially concentrated source of nutrients in Chinese freshwater and coastal seas. Environ Sci Technol 54:1464–1474
Wang K, Yan H, Peng X, Hu H, Zhang H, Hou D, Chen W, Qian P, Liu J, Cai J, Chai X, Zhang D (2020b) Community assembly of bacteria and archaea in coastal waters governed by contrasting mechanisms: a seasonal perspective. Mol Ecol 29:3762–3776
Ward CS, Yung C-M, Davis KM, Blinebry SK, Williams TC, Johnson ZI, Hunt DE (2017) Annual community patterns are driven by seasonal switching between closely related marine bacteria. ISME J 11:1412–1422
Wu W, Logares R, Huang B, Hsieh Ch (2017) Abundant and rare picoeukaryotic sub-communities present contrasting patterns in the epipelagic waters of marginal seas in the northwestern Pacific Ocean. Environ Microbiol 19:287–300
Wu W, Xu Z, Dai M, Gan J, Liu H (2021) Homogeneous selection shapes free-living and particle-associated bacterial communities in subtropical coastal waters. Divers Distrib 27:1904–1917
Xie B, Huang J, Huang C, Wang Y, Shi S, Huang L (2020) Stable isotopic signatures (δ13C and δ15N) of suspended particulate organic matter as indicators for fish cage culture pollution in Sansha Bay, China. Aquaculture 522:735081
Xiong J, Chen H, Hu C, Ye X, Kong D, Zhang D (2015) Evidence of bacterioplankton community adaptation in response to long-term mariculture disturbance. Sci Rep 5:15274
Yung C-M, Vereen MK, Herbert A, Davis KM, Yang J, Kantorowska A, Ward CS, Wernegreen JJ, Johnson ZI, Hunt DE (2015) Thermally adaptive tradeoffs in closely related marine bacterial strains. Environ Microbiol 17:2421–2429
Zeglin LH (2015) Stream microbial diversity in response to environmental changes: review and synthesis of existing research. Front Microbiol 6:454
Zhou J, Ning D (2017) Stochastic community assembly: does it matter in microbial ecology? Microbiol Mol Biol Rev 81:e00002-17
Acknowledgements
The authors would like to thank Dr. Yuanyuan Mo for constructive comments on the manuscript, Prof. Jianyu Hu for providing CTD data and Dr. Peng Xiao for the data processing.
Funding
This work was funded by the National Natural Science Foundation of China (42176147), the Natural Science Foundation of Fujian Province of China (2021J01025) and the National Key Research and Development Program of China (2018YFC1406306).
Author information
Authors and Affiliations
Contributions
JZ has made a significant contribution to the data analysis, interpretation of the results, and wrote the manuscript. YM has contributed to sample collection and experiments. LH has organized the cruises and sample collection. WZ has made a significant contribution to the experiment design, sample collection, data analysis and writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional Figures S1–S8 and Tables S1–S5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, J., Ma, Y., Huang, L. et al. Homogeneous selection is not always important in bacterial community in the eutrophic enclosed bay. Ecol Process 11, 27 (2022). https://doi.org/10.1186/s13717-022-00373-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13717-022-00373-1