Abstract
Background
Rapid consumption of fossil fuels as well as rising environmental deterioration caused by extreme CO2 emissions has become crucial in searching for a clean and renewable energy source such as biodiesel. The current work is an attempt to produce biodiesel from a potential non-edible feedstock of Aphanamixis polystachya, locally known as ‘Royna’ seed oil in Bangladesh.
Methods
Royna oil was extracted from the seed by Soxhlet extraction method. Biodiesel was synthesized by a three-step process: saponification of oil, followed by acidification of the soap, and esterification of the free fatty acid (FFA).
Results
The result presented showed that royna seed was found to be rich in oil with a maximum yield of 51% (w/w). Several reaction parameters were optimized during biodiesel production in their percentage proportion of oil to a catalyst (1:2), soap to HCl (1:1.5), FFA to an alcohol molar ratio (1:7), and catalyst (1 wt%). As a result, the highest yield of 97% was obtained from 7.5 wt% FFA content oil at 70 °C for 90-min reaction time. ASTM verified standard methods were employed to analyze the physicochemical properties of the as-prepared biodiesel. The structural and surface properties of the royna oil and as-prepared biodiesel were determined by 1H NMR and FTIR spectroscopic methods indicating a complete conversion of oil to biodiesel.
Conclusions
The study investigated the promising viability of royna oil to biodiesel using a three-step conversion route along with the heterogeneous catalysis system to circumvent the current environmental issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
The depressing scenario of fossil fuel depletion and the strict environmental legislation accompanied with growing energy demand has become a significant concern in recent years. Presently, non-renewable fossil fuels meet up to 80% of the world’s energy demand. Unfortunately, the world's total energy consumption has been predicted to decrease by 28% by 2040 with an estimation of 736 quadrillions Btu, but only 575 quadrillions (BTU) were in achieved in 2015 [1]. So, there is an urgent need to find alternative, eco-friendly, and renewable resources to fulfill the world's energy demand. In this context, biodiesel can contribute to future energy needs, especially, in the transportation sector.
Biodiesel has the potential to contribute to global sustainable development with regard to the economy, society, and environment. Biodiesel development is complex as it carries risks of exogenous problems that may indeed undermine sustainable development. Energy plays a crucial role in the development of every country, and biodiesel is one practical option that countries may add to their energy portfolios, as part of their sustainable development strategy. Production and adoption of biodiesel as an alternative fuel may also create positive socioeconomic impacts that can eventually lead to sustainable development. Agricultural sectors and rural communities, in particular, can gain socioeconomic benefits from biodiesel development. Perhaps the most recognized potential contribution towards sustainable development of biodiesel is its environmental prospects. In the use phase, biodiesel generally produces cleaner emissions than regular diesel, and in this way it contributes to a more environmentally friendly whole lifecycle of biodiesel. Biodiesel should not be simply be promoted because it could substitute conventional diesel. Instead, it should be promoted with the aim of leading society towards economic advancement, poverty reduction, energy security, carbon mitigation, biodiversity preservation, and climate change adaptation. With this perspective, one can realize more ways in which biodiesel can direct a society to sustainable development.
Biodiesel refers to fatty acid alkyl esters, particularly, fatty acid methyl esters (FAMEs) consisting of C14–C22 chain length, which has great potential as an alternative renewable fuel [2]. Usually, biodiesel is non-toxic, sulfur-free, biodegradable, oxygen-rich, and a low greenhouse gas emission fuel [3,4,5]. Biodiesel has a higher cetane rating and flashpoint with better lubricating properties that significantly enhance its fuel quality compared to petroleum diesel [6]. In addition, the calorific value of biodiesel (~ 37.27 MJ/kg) is only 9% lower than that of a typical diesel [7]. As a result, many countries, e.g., Indonesia, Thailand, and Colombia, compete in biodiesel production, among which Malaysia provided about 0.17 million tons from 2.7 million tons based upon 23 biodiesel resources in 2011 [8]. However, biodiesel from the vast feedstock category has become a new essential hub for the energy sector due to its attractive features [9, 10]. The feedstock selection is a vital concern of biodiesel and is preferred provided it is based on local conveniences, cost-effectiveness, industrial feasibilities, and technical viabilities [11].
Fat or oil from animals and plants is considered the most dominant feedstock. There are about 350 oil-bearing plants, among which rapeseed (Brassica rapa), soybean (Glycine max), canola (Brassica napus), coconut (Cocos nucifera), etc., as edible oils [12] and pithraj (Aphanamixis polystachya), jatropha (Jatropha curcus), mahua (Madhuca indica), sea mango (Cerbera odollam), Karanja (Pongamia pinnata), etc., as non-edible oils are remarkable for biodiesel generation [13]. Edible oils as first-generation feedstock are not economically feasible and may create food versus fuel controversy [14]. However, these difficulties of edible oils have forced the experts to focus on non-edible oils as second-generation feedstock. Hence, the synthesis of biodiesel from non-edible oils increased significantly due to their easy accessibility, low cultivation cost, and unsuitability as a source for human consumption [14, 15].
Aphanamixis polystachya, a non-edible oil plant which is native to Bangladesh, India, Malaysia, Singapore, Taiwan, and the Philippines, is regarded as a potential and cheap feedstock for biodiesel production. In Bangladesh, Aphanamixis polystachya, locally known as ‘Royna’ or ‘Pithraj’ grows plentifully due to the fertile land of Bangladesh and favorable climatic conditions. Moreover, the plant seed has a relatively high oil content (40–45 wt%) compared to some other non-edible oil resources, which stimulates its probability to contribute to the bioenergy sector [16, 17]. However, the most critical challenge to commercializing biodiesel largely depends on the preparation method. Therefore, selecting an appropriate preparation method is essential to improve the final yields at low operating costs [18].
There are various methods associated with biodiesel production: one of which is transesterification catalyzed with acid or base [19], carried out in a two-step method [20, 21], or three-step method [22]. Nevertheless, all preparation methods are not feasible for non-edible oils, especially those containing high free fatty acids (e.g., Aphanamixis polystachya). Several studies dealt with a two-step esterification of royna oil followed by a conversion of FFA into FAME [21, 23, 24]. Usually, this process is tricky to reduce the FFA effectively for raw oils with a high acid value, as a high amount of water is produced during the reaction. However, in the case of a three-step method, water removal is essential after each step to obtain a high-quality biodiesel fuel [22]. As a result, the three-step method can be a better choice for royna biodiesel, as non-edible oils have high acid values.
In this study, we explore the potentiality of royna seed oil as an available, low-cost, and non-edible raw material for biodiesel production. Here, we used a three-step process consisting of saponification, acidification, and finally, esterification, involving a homogeneous catalysis system to achieve the effective conversion of FAME from FFA. In addition, the reaction conditions were optimized to obtain a higher yield. Fuel properties, e.g., cloud point, flash point, pour point, kinematic viscosity, density, iodine value, and saponification value of the as-prepared biodiesel were studied following ASTM standard methods and their comparison with available databases.
Methods
Materials and chemicals
Royna seed was procured from the local area of Jashore, Bangladesh. The analytical reagent grade chemicals such as methanol (99.8%), calcium oxide (95%), ethanol (99%), sulfuric acid (95–98%), hydrochloric acid (37%), hexane (97–99%), diethyl ether (96–98%), bromine and potassium dichromate were purchased from MERCK (Germany), whereas potassium hydroxide (97%) and sodium hydroxide (97%) were purchased from MERCK (India). Phenolphthalein (Reagent Grade pH 8.2–9.8) was supplied by Losa Chemical Limited, India.
Oil extraction
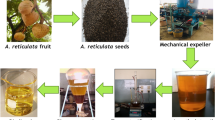
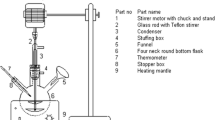
Firstly, royna seed (250 gm for each run) was ground by a mechanical grinder, and oil was collected using a Soxhlet extraction apparatus as shown in Fig. 1. Hexane (99%) was used as a solvent and initially it was vaporized by heating by means of a heating mantle. The vapor went upward through the distillation arm and cooled down by the condenser. The hot condensate was added continuously to the seed, kept in the thimble filter chamber. The condenser was fixed to ensure that the condensed solvent directly dropped back to the chamber and came in contact with the seed. The chamber was slowly filled with hot solvent to dissolve the oil into the solvent from the seed. When the Soxhlet chamber was entirely filled with the condensed solvent, it was emptied by the siphon process. In this process, the solvent was again returned to the distillation tank. The thimble filter keeps the seed collected and stops entering the siphon process. This cycle of vaporization and condensed returning to the distillation flask is allowed for several hours to days to obtain the desired compound. After around 4 h, the oil was concentrated in the solvent tank. After completing the extraction process, the solvent was removed from the distillation tank and separated from the oil using a vacuum rotary evaporator. Then the desired raw oil was found as a residue in the evaporation flask. The extracted oil was kept under sunlight for a couple of days to settle down unwanted foreign particles and remove foul odor and microorganisms. After that, it was filtered in the laboratory, and the oil content was found to be about 51% (w/w).
Production of biodiesel
A three-step method was used to produce biodiesel: saponification, acidification, and finally, esterification. In the case of the saponification process, the required amount of raw oil was placed in a 500 ml three-necked round-bottom flask which was connected to a condenser. A thermometer was placed over the hot plate. Additionally, various amounts of the aqueous solution of sodium hydroxide were mixed with the oil. The oil and sodium hydroxide mixtures were then heated to around 100 °C under reflux with continuous stirring for various reaction times. Finally, the reaction volume was cooled to stop the reaction.
Afterward, the acidification step is followed where the different stoichiometric amounts of hydrochloric acid were mixed with the soap solution at a temperature range from 60 to 70 °C under intense stirring. The solution was taken in a separating funnel after the reaction, and the fatty acid content was separated. The mineral acid was removed from the fatty acid layer by a hot water wash. The separated free fatty acid (FFA) was dried at a temperature of 100 °C in a vacuum drier for about 30 min and determined by using a titration.
Finally, the esterification process was conducted by using a suitable catalyst that helps converting the FFA to biodiesel. The esterification process was carried out in the same three-necked flask system employed earlier. The FFA was charged in the flask followed by the reaction of various stoichiometric ratios with alcohol under intense stirring at 70 °C for 1 h. All the results obtained from the three-step method are graphically presented with their standard deviations.
Purification of biodiesel
The produced biodiesel usually contains excess alcohol, catalyst, or soap as residues and glycerol as a byproduct. When the reaction was finished, the obtained product was transferred to a separating funnel at least until two distinct aqueous layers of biodiesel, and dense glycerol appeared. Next, the glycerol layer was removed, and the remained biodiesel was washed by distilled water to remove other residues. The water layer was then separated and the washing process was repeated several times until the water turned clear. The as-prepared biodiesel was then dried at 100 °C for 60 min. Finally, various properties of the obtained product were measured.
Characterization methods
To determine the FFA value of raw oil and biodiesel, 1 gm of each sample was weighed and dispersed in a 5 ml diethyl-ether solution and then titrated against 0.1 N KOH [25]. The percentage of moisture content in the samples was also calculated. Under wet conditions, 1 g of each sample was weighed after drying at 115 °C to measure the final weight and moisture content. The iodine value of the extracted oil was determined according to the American Oil Chemist’s Society method [26], where titration was carried out using a 0.01 N solution of sodium thiosulfate with the sample and chemical reagents until the blue color disappeared. The following equation is used for iodine value (IV) calculation:
where S and B are the amounts of sodium thiosulfate blank sample and titrate for the test, respectively. N defines the molar concentration of sodium thiosulfate (mol/L) and W is the weight (g) of the tested samples.
The saponification value (SV) of the sample was determined according to the method described by Leonard et al. in [27]. Briefly, around 2 mg of the sample was heated at 65 °C with 50 ml of alcoholic KOH for around 30 min under vigorous stirring. Then the solution was titrated with 0.5 M HCl to achieve the SV value. The physical properties of royna oil-derived biodiesel were determined by using the standard methods described by the American Society for Testing Materials (ASTM). The moisture content, density, and color identification were followed by ASTM D 240, ASTM D 1500, ASTM D 1480/81, and ASTM D 1744 methods. The viscosity of the samples was measured using the Ostwald viscometer following ASTM D 445 at 25 °C. The flashpoint, pour point, and cloud point were evaluated by means of the methods ASTM D 93, ASTM D 2500, and ASTM D 97, respectively. The disappearance of the triglyceride backbone from the oil to the biodiesel (fatty acid methyl ester) sample indicated the complete conversion, which was also confirmed by 1H NMR (Proton Nuclear Magnetic Resonance) spectroscopy. The NMR spectra of the produced biodiesel were determined in D2O using an NMR spectrometer (Varian UNITY INOVA 400NB) at 400 MHz. Tetramethylsilanen was used as an internal reference during the measurement of 1H chemical shifts. Additionally, the different functional groups present in the royna seed-derived biodiesel were identified using FTIR (Fourier transform infrared) Spectroscopy. The FTIR spectra of royna oil and its biodiesel were recorded in KBr using an IRPrestige-21 FTIR Spectrophotometer (Shimadzu, Japan). The measuring frequency region was maintained from 400 to 4000 cm–1 to obtain suitable results for the sample.
Results
Characterization of royna seed oil
The physical and chemical properties of extracted royna oil were measured to justify its potential to produce biodiesel. The characteristics of royna oil, for instance, FFA content, viscosity, specific gravity, saponification value, molecular weight of the oil, etc., are represented in Table 1. The color of the obtained oil appeared to be dark green, and its specific gravity (0.92) is lighter than water. The obtained FFA content was found to be significantly lower (7.5%) than that of some other seed oils such as palm oil, jatropha oil, etc. [28, 29]. The iodine value was 74.5 g I2/g oil pointing to a level of unsaturation in the form of a double bond and to the oxidation stability of the oil. Similarly, the saponification value and cetane number of the royna seed-derived oil are 233.7 and 55.3, respectively.
Three-step method of biodiesel production
Saponification and acidification: oil to FFA
The saponification process followed by acidification played a crucial role in preparing free fatty acid from the royna seed oil. Saponification was carried out using a different stoichiometric amount of aqueous potassium hydroxide (KOH) at different reaction times. Figure 2 shows the effect of reaction time on the conversion of % FFA with standard deviations. The conversion of oil to FFA increased with time, and almost half of the FFA conversion was completed in the first 20 min. Similarly, over 80% of the oil was converted to FFA within 60 min of reaction time. About 95% FFA was obtained after 120 min of reaction time at a 1:2 oil to KOH molar ratio at 100 °C temperature. Kakati et al. investigated the effect of NaOH concentration on biodiesel production from Amari tree seed oil (ATSO), using almost similar reaction conditions; however, it required more time (2.5 h) at 60 °C [30]. After saponification, the potassium salts of free fatty acids were converted to FFA by the acidification reaction. In this step, the 1:1.5 molar ratio of soap to HCl was considered to be the optimum, and the reaction time amounted to 60 min at 60 °C.
Esterification: FFA to biodiesel
The obtained FFA was used as a feedstock for the esterification step to produce biodiesel. Esterification was performed with ethanol in the presence of KOH as a base catalyst. The effect of reaction time, alcohol molar ratio, catalyst concentration, etc., plays a vital role in converting FFA to biodiesel, which was investigated at a temperature of 70 °C under vigorous stirring.
Effect of ethanol on the FFA molar ratio
The molar ratio of ethanol to FFA has a significant impact on the conversion of FFA to biodiesel. It is evident from the stoichiometric reaction that one mole of ethanol is required for one mole of FFA to convert the biodiesel. Moreover, the reaction is reversible, as excess alcohol is used to avoid the reverse reaction as well as to speed-up the process [31, 32]. From Fig. 3, it is evident that the conversion of biodiesel increases with the alcohol molar ratio. The figure with standard deviation also indicates that the molar ratio of ethanol to FFA also affected the FFA conversion. The conversion increased steadily until it reached the conversion peak of 97% at a 1:7 ethanol to FFA ratio. However, as the ratio of ethanol to FFA increased further, the rate of biodiesel production started to decrease. Therefore, the optimum value of the molar ratio of ethanol to FFAis considered to be 1:7.
Effect of the catalysts
The effect the catalyst on FFA to FAME is demonstrated in Figs. 4 and 5 with standard deviations. Generally, 1 wt% of each catalyst was taken for the reaction at 70 °C for 90 min. According to Fig. 4, the esterification reaction without any catalyst showed of an FFA conversion of around 84%. However, the conversion of FFA to biodiesel was increased dramatically under the catalysis system. It is clear from Fig. 4 that the KOH catalyst showed a 94% fatty acid conversion. In contrast, using CuO as a catalyst improves the conversion further to around 97%, which is the highest conversion compared to other conditions. Although the higher conversion was achieved from the CuO catalyst, KOH can also be considered a suitable catalyst because of its easy accessibility and low costs. Additionally, Fig. 5 with standard deviation describes the effect of the presence and absence of the catalysts in the esterification reaction at different alcohol molar ratios. The reaction was slow until 84% of FFA conversion, and then the reaction remained constant in the case of a system without catalyst. However, the catalytic esterification reaction increased steadily at an around 97% conversion. Therefore, it was found that the use of catalysts enhances the conversion rate in esterification reactions compared to those without using any catalyst [30, 31]. However, excess catalysts may cause undesirable side reactions and reduce the desired product. Moreover, glycerol recovery became difficult due to soap formation. Therefore, increased saponification with left-over base catalysts is also described in several literature sources [33,34,35].
Effect of reaction time
The reaction rate and % conversion of FFA to biodiesel significantly depend on reaction time. The impact of reaction time on biodiesel production by converting FFA is shown in Fig. 6. The reaction was conducted by using 1 wt% of catalyst at 70 °C. Initially, the esterification reaction was increased sharply with an increase in reaction time. Figure 6 demonstrates that ~ 80% (with standard deviations) of FFA was converted to biodiesel within half an hour and continued until the highest point (97%) after 90 min. After this time, the reaction rate started to decrease slightly. Therefore, 90 min was considered as the optimum reaction time for this study. Here, the effect of reaction time on biodiesel preparation was comparable to other studies [30, 31].
Optimization of reaction parameters
The saponification, acidification and esterification extensively depend on several reaction parameters such as the ratio of oil to KOH, of soap to HCl, of FFA to alcohol of catalyst wt% reaction time as shown in Table 2. As described above, the oil to the KOH ratio was found to be 1:2 and 120 min was proved to be the optimum time for the saponification reaction. Furthermore, the ratio of soap to HCl and the optimum reaction time for acidification were 1:1.5 and 60 min, respectively. The esterification was carried out by using 1 wt% of catalyst for 90 min while the conversion of FFA to biodiesel reached around 97%. The reactions were carried out at a temperature of 70 °C. Some reaction parameters of FAME preparation from some non-edible oils were also compared to these results [36,37,38].
Fuel properties of biodiesel
The physicochemical characteristics of royna oil-derived methyl ester (biodiesel) were investigated using the American Standard Methods (ASTM) displayed in Table 3. The color of the biodiesel was reddish-brown. The respective specific gravity and viscosity of the biodiesel corresponded to 0.88 and 2.1 mm2/s which are close to those of the commercially available biodiesel [24, 30]. The percentage of FFA significantly decreased from 7.5 to 0.82, pointing to a proper conversion of FFA to biodiesel. The cetane index (48–60) indicates the fuel quality, which is slightly higher than that of commercial diesel. Additionally, the obtained calorific value amounted to 42.1 MJ/kg. The cloud formation was checked at 3 °C, and the pour point at 0 °C. Therefore, the flash point of the biodiesel was higher (150 °C) than that of standard diesel (145 °C). A high flash point is always advantageous as it minimizes the dangers during storage and handling.
Characterization of the oil converted to biodiesel
The NMR analysis of the royna seed oil and of prepared biodiesel was carried out from 0.0–8.0 ppm. The presence of glyceride of the triglyceride was confirmed by comparing the 1H NMR spectrum of royna oil and biodiesel. Figures 7 and 8 display the 1H NMR spectra of the raw oil and the produced biodiesel, respectively. The peak at approximately 3.7 ppm reveals the protons from methyl ester moiety, and the signals at around 2.3 ppm result from α-carbonyl methylene groups [39]. The peak at 2.72 ppm represents the presence of polyunsaturated fatty acids. These bonds generally come from the methylene group of higher unsaturated fatty acid or linoleic acid chains [40]. Moreover, Fig. 7 shows that the peaks of royna oil between 4.1 and 4.5 ppm are generally obtained from the glyceride protons of triglycerides; however, these peaks were unavailable in the spectrum of produced biodiesel. The absence of these short peaks (Fig. 8) represents the fundamental difference between biodiesel and commercially available diesel, as glyceride protons are only available in commercial diesel. In contrast, aliphatic hydrogen is present in standard diesel and biodiesel [41]. Therefore, the absence of glyceride of triglyceride in the spectrum of biodiesel samples ensures a proper conversion of royna oil to biodiesel.
FTIR spectroscopy was employed to determine the functional group compositions of raw oil and the produced biodiesel. Figure 9 shows the FTIR spectrum of the royna seed oil and its biodiesel, respectively. Similarly, Table 4 describes the possible functional groups according to the peak position of the Figure. Resulting from this Figure, in the lower wave number region (250 to 1000 cm–1), small peaks are present pointing to limited functional groups. In the region 1100–1500 cm–1, there are several peaks in both royna oil and produced biodiesel, indicating the functional groups of ether, amine, nitro compound, and alcohol. However, royna seed-derived biodiesel shows peaks representing the ester and carboxylic acid (C–O) in the 1100–1300 cm–1 region as biodiesel is mainly monoalkyl ester. The characteristic peaks at 2850–2960 cm−1 are due to C–H stretching vibration, and the peaks at 800–840 cm–1 represent the trisubstituted alkanes by C–H weak rocking. The intense C=O stretching band at around 1746 cm–1 is due to the carbonyl groups of the triglycerides (C=O) [42]. Similarly, a weaker peak at 1711 cm–1 was obtained for royna oil, representing the presence of fatty acids [43, 44]. The peaks at 2975–3080 cm–1 indicate the characteristic peaks for (–C=C–) alkenes. The earlier results confirmed the conversion of triglycerides (TG) to fatty acid methyl ester (FAME).
Discussion
Biodiesel has been accepted globally as a potential renewable energy source to substitute petro-diesel. However, the fuel shows some demerits with immense merits towards practical application. Particular fuel properties of the as-prepared biodiesel are discussed earlier and compared to the corresponding ASTM and EN standards. Here some other fuel properties which need to be considered to overcome the challenges are described as follows:
-
1.
Carbon residue of the as-prepared FAME is a crucial factor that measures the tendency of a fuel sample to produce carbon deposits on the injector tips, valve seats, and walls of the combustion chamber. The maximum allowable value of the carbon residue is 0.3 wt% as per the EN 14,214:2003 standard. A high carbon residue value implies high concentrations of free fatty acids, glycerides, polyunsaturated FAME, and polymeric materials [45]. Therefore, a value higher than the acceptable limit possibly causes severe engine fouling.
-
2.
The vapor pressure is an additional factor indicating the volatility of liquid fuel, and it depends on fatty acid composition of the biodiesel. Vapor pressure is a function of atomization, ignition and fuel evaporation [46]. Biodiesel usually has low vapor pressure than petro-diesel (0.05 kg/cm2).
-
3.
Distillation characteristics are important because they measure a liquid fuel's tendency to produce potentially explosive vapor and proper air–fuel mixture for combustion. Commercial biodiesel should have a boiling range of 68–388 °C, which is a function of the types of hydrocarbons (short/long-chain) in FAME [47]. However, blending biodiesel with diesel could be a solution to this problem, and in that case, the distillation characteristics would be comparable to that of petro-diesel.
-
4.
The sulfur content is one of the significant advantages, where biodiesel has a low value compared to conventional diesel fuel (340 ppm). Hence, diesel engines fueled with biodiesel and its blends produce less emission of harmful sulfur oxides and sulfate particulates and low operational cost. Therefore, biodiesel and its blending could be a good choice in this regard.
-
5.
The copper strip corrosion characterizes the tendency of a fuel to corrode the components of an engine fuel system made up of copper, zinc, and bronze. Its corrosion value for FAME should not exceed the limit of maximum 3 (ASTM D 6751 standard).
-
6.
Biodiesel is usually hygroscopic and has the affinity to absorb water due to its polar characteristic. This could be the reason for the higher water content in FAME. The limit of water content in biodiesel is 0.05 vol% (ASTM D 6751-07b standard). However, the problem of higher water content with biodiesel could be overcome through blending with conventional diesel.
-
7.
The composition of ester in FAME affects the property of biodiesel, and the average value of the ester content should be a minimum of 96.5% (EN14103 standard). Several components, e.g., monoglyceride, diglyceride, and triglyceride, are present in the biodiesel required to be controlled as minute as [48].
-
8.
Ash content is an added property that shows the presence of inorganic contaminants in a fuel sample that form ash during combustion. These ash particles contribute to engine wear and accumulate on emission control components (e.g., diesel particulate filters). The maximum permissible limit of ash content of the FAME could be 0.02 wt% (ASTM D6751 standard).
Conclusions
The ability of Aphanamixis polystachya, a viable non-edible feedstock with maximum oil content of 51% (w/w) for clean biodiesel production has proven. Three crucial steps were practiced for biodiesel synthesis, e.g., saponification, acidification, and esterification; and various reaction parameters such as the molar ratio of oil to ethanol (1:7), the amount of catalysts (1 wt%), and the reaction time (1½ h) were successfully optimized. The FFA content of royna oil was found to be about 7.5 wt%; however, it was reduced to only 0.82 wt% for as-prepared biodiesel, indicating the suitable conversion. The maximum yield of FFA to biodiesel was found to be 97% for the CuO catalyst and 80% for the non-catalysis system. In addition, the standard methods of the American Society for Testing Materials (ASTM) were followed effectively to analyze numerous physical and chemical properties of the clean fuel. Particularly, the calorific value and viscosity of the obtained biodiesel which corresponded to 42.1 MJ/kg and 2.1 mm2/s at 25 °C are very close to commercial diesel. Finally, the biodiesel formation was confirmed by means of 1H NMR and FTIR spectroscopic analyses of raw oil and as-prepared biodiesel. Therefore, royna oil-derived biodiesel can play an integral role as a suitable substitute for petro-diesel to run diesel engines.
Availability of data and materials
The main data sets on which the results of the manuscript based are presented in the main paper.
References
Khan HM, Ali CH, Iqbal T, Yasin S, Sulaiman M, Mahmood H, Mu B (2019) Current scenario and potential of biodiesel production from waste cooking oil in Pakistan: an overview. Chin J Chem Eng 27(10):2238–2250. https://doi.org/10.1016/j.cjche.2018.12.010
Anwar M, Rasul M, Ashwath N (2019) Optimization of biodiesel production from stone fruit kernel oil. Energy Procedia 160(2018):268–276. https://doi.org/10.1016/j.egypro.2019.02.146
Vicente G, Martínez M, Aracil J (2007) Optimization of integrated biodiesel production. Part I. A study of the biodiesel purity and yield. Bioresour Technol 98(9):1724–1733. https://doi.org/10.1016/j.biortech.2006.07.024
Rafati A, Tahvildari K, Nozari M (2019) Production of biodiesel by electrolysis method from waste cooking oil using heterogeneous MgO–NaOH nanocatalyst. Energy Sources A: Recovery Util Environ Eff 41(9):1062–1074. https://doi.org/10.1080/15567036.2018.1539139
Rahman MM, Rasul M, Hassan NMS, Hyde J (2016) Prospects of biodiesel production from macadamia oil as an alternative fuel for diesel engines. Energies. https://doi.org/10.3390/en9060403
Ibeto CN, Ofoefule AU, Ezugwu HC (2011) Analytical methods for quality assessment of biodiesel from animal and vegetable oils. Trends Appl Sci Res 6(6):537. https://doi.org/10.3923/tasr.2011.537.553
Mishra VK, Goswami R (2018) A review of the production, properties, and advantages of biodiesel. Biofuels 9(2):273–289. https://doi.org/10.1080/17597269.2017.1336350
Nomanbhay S, Hussein R, Ong MY (2018) Sustainability of biodiesel production in Malaysia by production of bio–oil from crude glycerol using microwave pyrolysis: a review. Green Chem Lett Rev 11(2):135–157. https://doi.org/10.1080/17518253.2018.1444795
Encinar JM, González JF, Rodríguez JJ, Tejedor A (2002) Biodiesel fuels from vegetable oils: transesterification of Cynara cardunculus L. oils with ethanol. Energy Fuels 16(2):443–450. https://doi.org/10.1021/ef010174h
Venkanna BK, Venkataramana CR (2009) Biodiesel production and optimization from Calophyllum inophyllum linn oil (honne oil)–a three–stage method. Bioresour Technol 100(21):5122–5125. https://doi.org/10.1016/j.biortech.2009.05.023
Lim S, Teong LK (2010) Recent trends, opportunities, and challenges of biodiesel in Malaysia: an overview. Renew Sust Energ Rev 14(3):938–954. https://doi.org/10.1016/j.rser.2009.10.027
Mofijur M, Masjuki HH, Kalam MA, Atabani AE, Fattah IMR, Mobarak HM (2014) Comparative evaluation of performance and emission characteristics of Moringa oleifera and Palm oil–based biodiesel in a diesel engine. Ind Crops Prod 53:78–84. https://doi.org/10.1016/j.indcrop.2013.12.011
Sharma YC, Singh B, Korstad J (2010) High yield and conversion of biodiesel from a nonedible feedstock (Pongamia pinnata). J Agric Food Chem 58(1):242–247. https://doi.org/10.1021/jf903227e
Demirbas A, Bafail A, Ahmad W, Sheikh M (2016) Biodiesel production from non–edible plant oils. Energy Explor Exploit 34(2):290–318. https://doi.org/10.1177/0144598716630166
Bhuiya MMK, Rasul MG, Khan MMK, Ashwath N, Azad AK (2016) Prospects of 2nd generation biodiesel as a sustainable fuel—part: 1 selection of feedstock’s, oil extraction techniques, and conversion technologies. Renew Sust Energ Rev 55:1109–1128. https://doi.org/10.1016/j.rser.2015.04.163
Gui MM, Lee KT, Bhatia S (2008) Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 33(11):1646–1653. https://doi.org/10.1016/j.energy.2008.06.002
Banik S, Rouf M, Khanam M, Islam M, Rabeya T, Afrose F, Saha D (2015) Production of bio–diesel from Pithraj (Aphanamixis polystachya) seed oil. Bangladesh J Sci Ind Res 50(2):135–142. https://doi.org/10.3329/bjsir.v50i2.24354
Ojumu TV, Madzimbamuto TN, Ikhu-Omoregbe DIO, Aransiola EF, Oyekola O (2013) A review of current technology for biodiesel production: state of the art. Biomass Bioenergy 61:276–297. https://doi.org/10.1016/j.biombioe.2013.11.014
Musa IA (2016) The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt J Pet 25(1):21–31. https://doi.org/10.1016/j.ejpe.2015.06.007
Zullaikah S, Lai CC, Vali SR, Ju YH (2005) A two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresour Technol 96(17):1889–1896. https://doi.org/10.1016/j.biortech.2005.01.028
Palash SM, Masjuki HH, Kalam MA, Atabani AE, Rizwanul Fattah IM, Sanjid A (2015) Biodiesel production, characterization, diesel engine performance, and emission characteristics of methyl esters from Aphanamixis polystachya oil of Bangladesh. Energy Convers Manag 91:149–157. https://doi.org/10.1016/j.enconman.2014.12.009
Morshed M, Ferdous K, Khan MR, Mazumder MSI, Islam MA, Uddin MT (2011) Rubber seed oil as a potential source for biodiesel production in Bangladesh. Fuel 90(10):2981–2986. https://doi.org/10.1016/j.fuel.2011.05.020
Ifteqar S, Sultana R, Banik S, Rahman AM (2020) Production and characterization of biodiesel from Aphanamixis polystachya seed oil. Dhaka Univ J Sci 68(2):129–136. https://doi.org/10.3329/dujs.v68i2.54610
Ferdous K, Deb A, Ferdous J, Uddin MR, Khan MR, Islam MA (2013) Aphanamixis polystachya: a potential non–edible source of biodiesel in Bangladesh. J Chem Eng 28(1):45–49. https://doi.org/10.3329/jce.v28i1.18111
Der Land BO, Er F (1997) Official methods and recommended practices of the American Oil Chemists’ Society. 5th edn, AOCS–Press, Champaign, AOCS. Book Reviews. p. 197
Lin CY, Lin HA, Hung LB (2006) Fuel structure and properties of biodiesel produced by the peroxidation process. Fuel 85(12–13):1743–1749. https://doi.org/10.1016/j.fuel.2006.03.010
Leonard M (1990) Vogel’s textbook of quantitative chemical analysis. Endeavour 14(2):100. https://doi.org/10.1016/0160-9327(90)90087-8
Chhetri AB, Tango MS, Budge SM, Watts KC, Islam MR (2008) Non–edible plant oils as new sources for biodiesel production. Int J Mol Sci 9(2):169–180. https://doi.org/10.3390/ijms9020169
Khan MA, Yusup S, Ahmad MM (2010) Acid esterification of a high free fatty acid crude palm oil and crude rubber seed oil blend: optimization and parametric analysis. Biomass Bioenergy 34(12):1751–1756. https://doi.org/10.1016/j.biombioe.2010.07.006
Kakati J, Gogoi TK, Pakshirajan K (2017) Production of biodiesel from Amari (Amoora Wallichii King) tree seeds using optimum process parameters and its characterization. Energy Convers Manag 135:281–290. https://doi.org/10.1016/j.enconman.2016.12.087
Deb A, Ferdous J, Kaniz Ferdous M, Uddin R, Khan MR, Wasikur Rahman Md (2017) Prospect of castor oil biodiesel in Bangladesh: process development and optimization study. Int J Green Energy. https://doi.org/10.1080/15435075.2017.1357558
Freedman BEHP, Pryde EH, Mounts TL (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc 61(10):1638–1643. https://doi.org/10.1007/BF02541649
Cao L, Zhang S (2015) Production and characterization of biodiesel derived from Hodgsonia macrocarpa seed oil. Appl Energy 146:135–140. https://doi.org/10.1016/j.apenergy.2015.02.062
Chakraborty M, Baruah DC (2013) Production and characterization of biodiesel obtained from Sapindus mukorossi kernel oil. Energy 60:159–167. https://doi.org/10.1016/j.energy.2013.07.065
Kafuku G, Mbarawa M (2010) Alkaline catalyzed biodiesel production from Moringa oleifera oil with optimized production parameters. Appl Energy 87(8):2561–2565. https://doi.org/10.1016/j.apenergy.2010.02.026
Agarwal D, Agarwal AK (2007) Performance and emissions characteristics of Jatropha oil (preheated and blends) in a direct injection compression ignition engine. Appl Therm Eng 27(13):2314–2323. https://doi.org/10.1016/j.applthermaleng.2007.01.009
Pramanik K (2003) Properties and use of Jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew Energy 28(2):239–248. https://doi.org/10.1016/S0960-1481(02)00027-7
Lakshmi Narayana Rao G, Durga Prasad B, Sampath S, Rajagopal K (2007) Combustion analysis of diesel engine fueled with jatropha oil methyl ester–diesel blends. Int J Green Energy 4(6):645–658. https://doi.org/10.1080/15435070701665446
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83(10):823–833. https://doi.org/10.1007/s11746-006-5033-y
Morgenstern M, Cline J, Meyer S, Cataldo S (2006) Determination of the kinetics of biodiesel production using proton nuclear magnetic resonance spectroscopy (1H NMR). Energy Fuels 20(4):1350–1353. https://doi.org/10.1021/ef0503764
Portela NA, Oliveira EC, Neto AC, Rodrigues RR, Silva SR, Castro EV, Filgueiras PR (2016) Quantification of biodiesel in petroleum diesel by 1H NMR: evaluation of univariate and multivariate approaches. Fuel 166:12–18. https://doi.org/10.1016/j.fuel.2015.10.091
Vlachos N, Skopelitis Y, Psaroudaki M, Konstantinidou V, Chatzilazarou A, Tegou E (2006) Applications of Fourier transform-infrared spectroscopy to edible oils. Anal Chim Acta 573–574:459–465. https://doi.org/10.1016/j.aca.2006.05.034
Moharam MA, Abbas LM (2010) A study on the effect of microwave heating on the properties of edible oils using FTIR spectroscopy. Afr J Microbiol Res 4(19):1921–1927. https://doi.org/10.5897/AJMR.9000011
Jovic O, Smolic T, Jurišic Z, Meic Z, Hrenar T (2013) Chemometric analysis of Croatian extra virgin olive oils from Central Dalmatia Region. Croat Chem Acta 86(3):335–344. https://doi.org/10.5562/cca2377
Černoch M, Hájek M, Skopal F (2010) Relationships among flash point, carbon residue, viscosity and some impurities in biodiesel after ethanolysis of rapeseed oil. Bioresour Technol 101(19):7397–7401. https://doi.org/10.1016/j.biortech.2010.05.003
Freitas SV, Oliveira MB, Lima AS, Coutinho JA (2012) Measurement and prediction of biodiesel volatility. Energy fuels 26(5):3048–3053. https://doi.org/10.1021/ef3004174
Sarma AK, Konwer D, Bordoloi PK (2005) A comprehensive analysis of fuel properties of biodiesel from Koroch seed oil. Energy fuels 19(2):656–657. https://doi.org/10.1021/ef049754f
Kumar R, Prasanna KT, Gowda B (2012) Evaluation of Aphanamixis polystachya (Wall.) R. Parker as a potential source of biodiesel. J Biochem Technol 3:128–133
Acknowledgements
The authors would like to acknowledge the technical support of MENTECH Labs of Jashore University of Science and Technology, Bangladesh.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MWR designed the experiment and directed the creative work. AKM and MSH carried out the extraction part of the work and were involved in the biodiesel production and analysis of the biodiesel. MS was also involved in the research work and prepared the manuscript. All authors worked together to achieve the optimum outcome of this research work. Finally, All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rahman, M.W., Mondal, A.K., Hasan, M.S. et al. Biodiesel production from a non-edible source of royna (Aphanamixis polystachya) oil. Energ Sustain Soc 12, 33 (2022). https://doi.org/10.1186/s13705-022-00360-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13705-022-00360-6