Abstract
Background
Due to the rising number of diabetic patients, the burden of diabetic peripheral neuropathy (DPN) is clearly posing a major challenge to the long-term viability of the health-care system. Despite this, most DPN epidemiological research in eastern Africa, including Ethiopia, has so far been limited to survey studies. Thus, we determined the incidence of DPN and its predictors among diabetic patients in tertiary health-care setting of southwest Ethiopia.
Methods
A multicenter retrospective follow-up study was carried out on 567 randomly selected diabetic patients. Data were entered using Epi-Data v4.6 and analyzed using R v4.0.4. The survival curves were estimated using the Kaplan-Meier, and compared using Log-rank test between groups of categorical variables. The PHA were evaluated using the Schoenfeld residuals test. Multivariable Gompertz proportional hazard model was used to examine the predictors of DPN at 5% level of significance.
Results
Overall, of 567 DM patients 119 developed DPN with an incidence rate of 3.75, 95%CI [3.13, 4.49] per 100 PY. About 15.13% and 69% of DPN cases occurred within 2 and 5 years of DM diagnosis, respectively. In the multivariable Gompertz PH model, being female [AHR = 1.47; 95% CI (1.01, 2.15)], T2DM [AHR = 3.49 95% CI (1.82, 6.71)], having diabetic retinopathy [AHR = 1.9 95% CI (1.25, 2.91)], positive proteinuria [AHR = 2.22 95% CI (1.35, 3.65)], being obese [AHR = 3.94 95% CI (1.2, 12.89)] and overweight [AHR = 3.34 95% CI (1.09, 10.25)] significantly predicts the future risk of DPN.
Conclusion
Nearly, 7 in 10 of DPN cases occurred within short period of time (5 year) of DM diagnosis. Being female, T2DM, DR, positive proteinuria, obese and overweight significantly predicts the risk of DPN. Therefore, we recommend screening and early diagnosis of diabetes with its complication. While doing so, attention should be given for DM patients with DR and positive proteinuria at baseline.
Similar content being viewed by others
Text box 1. Contributions to the literature |
|---|
• Given the increasing prevalence of T2DM, its complications, particularly DPN, continue to present serious challenges to healthcare system in developing countries. |
• Despite this fact, there are few studies, and the literature that is currently available is limited to survey studies in Ethiopia. |
• Furthermore, to our knowledge, no previous investigation of DPN has used advanced survival analysis (Gompertz survival analysis). |
• The Identification of predictive factors and timing of DPN development would enable early detection of the condition, reducing the financial loss related to complications like diabetic foot ulcers and amputation brought on by DPN. |
Introduction
Despite the fact that diabetes mellitus (DM) has been the focus of most international organizations, the disease’s burden and complications remain a global issue [1]. According to the 2021 international diabetic federation (IDF), there were an estimated 537 million people living with DM and 6.7 million deaths due DM and its complications which corresponds to one death for every five seconds [2]. Because of its complications, such as Diabetic peripheral neuropathy (DPN), DM is a serious condition [3]. DPN is one the most common vascular complication of DM and is estimated to affect around half of the people with diabetes [4,5,6]. One prospective cohort study revealed an increase of DPN after five years from 13.2 to 34.2%, representing a 2.6-fold increase [7].
Clinically, DPN is defined as the presence of symptoms or signs of peripheral nerve dysfunction in people with diabetes after other possible causes have been excluded [8]. DPN mainly alters the symmetrical sensory function causing abnormal feelings and numbness [9]. Studies showed that the prevalence of DPN ranges from 6 to 87% in developed countries [10,11,12] and 46% pooled prevalence in Africa with a higher proportion in West (49.4%) and Central (35.9%) Africa [13]. In Ethiopia, 32.8% of DM patients develop diabetes-related complications [14] with the prevalence of DPN ranging from 29.5 to 52.2% [15]. Other study found the pooled prevalence of 22% in Ethiopia, with a higher proportion in Oromia 27%, and 16% in southern nation nationalities and peoples region [16].
DPN is a major cause of disability, leading to foot ulcers, amputations, urinary inconsistence and sexual dysfunction [17]. For example DPN raises the global burden of diabetic foot ulcers, with a rate of 7.2% in Africa [18]. Similarly, 25–90% of amputations are thought to be attributable to the combination of DPN and other infection due to diabetes [19]. Not only the above consequences, painful DPN which affects 20–30% of patients, has been linked to severe physical and psychological distress leading to poor patients quality of life [20]. All these consequences of DPN will unavoidably end in the reduction in the quality of life and significant economic burden both to the patients and society.
Several epidemiological researches around the world identified older age [21,22,23], smoking [22, 24], high low density lipoprotein cholesterol (LDL-C), triglycerides, and lower high density lipoprotein cholesterol (HDL-C) [21, 22] as a risk factors for DPN. Dyslipidemia [23, 25], the presence of cardiovascular disease (CVD), diabetic retinopathy (DR) was also significantly associated with DPN [24, 25]. Other studies found that, female gender were the strongest predictors followed by longer duration of DM [23,24,25,26]. Higher levels of Body Mass Index (BMI) at baseline were also significantly increases the risk of incident DPN [21].
DPN is poorly managed because of its insidious onset and delayed diagnosis [27]. The identification of predictive factors and the time when DPN occurs after DM diagnosis would facilitate early screening for DPN and DPN-related symptoms. Physicians’ care for patients with DPN might also benefit from a greater understanding of the factors that predict DPN. Despite this fact most of previous studies in Ethiopia focused on survey data using crossectional study design which cannot solve the above problems. Thus, the current study determined the incidence of diabetic peripheral neuropathy and its predictors among diabetic patients in tertiary health-care setting of southwest Ethiopia.
Methods
Study design, periods and settings
The current study is a, retrospective follow up study done at Jimma University Medical center (JUMC) and Mettu Karl Comprehensive and Specialized Hospital (MKCSH) to assess the incidence DPN and its predictors among T2DM patients from September 9, 2013 to February march, 2021. JUMC and MKCSH are the largest tertiary health-care setting that found in Jimma zone and Ilu Ababor zone respectively, Oromia regional state, southwest Ethiopia. JUMC is found at about 352 KM and MKCSH at 600KM from Addis Ababa, the capital city of Ethiopia. In both hospitals, follows-up service for chronic disease is being provided and diabetic clinic is found separated from other chronic disease.
Population
The source population for the current study was all type 1 DM (T1DM) and type 2 DM (T2DM) patients (age of 15 and above years old) who had follow-up at JUMC and MKCSH. We included all newly diagnosed with T1DM and T2DM patients during the follow-up visits from September 2013 to August 2015 at JUMC and MKCSH and those patients were followed until March 2021. Once diagnosed for DM, follow up was started immediately for all patient. Diabetic patients with DPN at the date of diagnosis and no baseline records at baseline were excluded from the study. In addition, diabetic patients with unknown date of DM and DPN diagnosis were excluded from the study.
Sample size and sampling procedure
The final sample size was determined based on the power method by using the Schoenfeld formula using Stata version 14 [28]. We assumed 80% of power, 10% of withdrawal probability and 95% confidence level. Considering 1.82 adjusted hazard ratio (AHR) of other DM complication and 16.6% probability of events from previous study done in Ethiopia [29] the final sample size was calculated to be 587. Then subjects were selected using simple random sampling technique after proportionally allocated to both hospital (Fig. 1) by collecting the identification number of DM patients from the registration book.
Measurement of variables and operational definition
The primary outcome of the current study was time to DPN and the event of interest was the development of DPN within the follow-up period. DPN can be either small fiber or large fiber neuropathy. Pain, tingling, and paraesthesia are symptoms of small fiber neuropathy, which diagnosed with a pinprick and temperature examination. Numb feet and gait ataxia are symptoms of large fiber neuropathy, which are confirmed by touch sensitivity with a 10 g monofilament, vibration sensibility with a biothesiometer, and ankle reflex [8, 30]. When a participant had more than one endpoint, the initial event was utilized to determine when DPN had started. This remark implies that a pinprick and temperature are used to identify small fiber DPN. While 10 g monofilament touch sensitivity, biothesiometer vibration sensitivity, and ankle reflex are used to diagnose big fiber DPN. Sociodemographic, clinical factors, and physiologic characteristics were among our independent variables. Time to DPN was defined by the time it takes between being diagnosed with DM to developing DPN. Patients who did not acquire diabetic neuropathy during the research period, or who died, lost follow-up, or transferred out before developing DPN were considered as censoring. DR is a microvascular consequence of diabetes that is diagnosed by ophthalmologists through clinical examination or indirect ophthalmoscopy [31]. Hypertension (HTN) is defined as a systolic/diastolic blood pressure of 140/90 mmHg or above on two or more separate days, with the measurement taken from a medical record review [32].
Data collection and quality control
This secondary data was acquired from the study participants’ medical records by professional nurses using a pre-approved standardized checklist. The consistency and completeness of the checklist were checked. Any missing data was double-checked and clarified. From the time of enrolment (September 9, 2013) until the end of the study (March, 2021), we monitored the subjects retrospectively.
Data processing and statistical analysis
Epi-Data version 4.6 was used to enter data, which was subsequently exported to R version 4.0.4 for further cleaning and analysis. The study population was described using descriptive statistics such as mean (standard deviation) normally distributed for continuous variables or median (inter-quartile range) for non-normally distributed continuous variables. Tables and frequencies with percent were used for categorical variables. The baseline categorical variables of the cohort were compared using the Pearson’s chi-square test based on the status of DPN during follow-up.
The number of new cases (DPN) per total original study population was used to determine cumulative incidence, and the incidence rate was derived as the number of new cases per patient-years at risk. The multivariable regression included a variable with p value less than 0.2 in bivariable analysis. The pseudo-Variance Inflation Factor (VIF) was used to check multicollinearity within independent variables. The survival curves were estimated using the Kaplan-Meier approach, and the Log-rank test was used to evaluate survival time between groups of categorical variables. The proportional hazard assumptions (PHA) were evaluated using the Schoenfeld residuals method and Akakie Information Criterion (AIC) was used to choose a parsimonious survival model. The model’s quality of fit was determined using the Cox Snell residual plot. Finally, the Gompertz proportional hazard model were fitted, and variables with a significance level of 0.05 were identified as a predictors of incident DPN.
Results
Socio-demographic and clinical characteristics
Of the initial cohort of 587 enrolled participants, 20 (3.75%) records with incomplete information were excluded. We included the remaining 567 participants in the final analysis. Male patients comprised 60.49% of the study’s participants, while urban inhabitants thought up 58.2%.
The baseline socio-demographic and clinical characteristics of study participants by incident DPN status are shown in Table 1. The number of incidents were higher in those who were older and took oral hypoglycemic medications more often at baseline. Those with nephropathy, Acute Kidney Injury (AKI), HTN, positive proteinuria, T2DM, family history of DM, or CVD at baseline also had a greater percent of incident DPN.
Physiologic characteristics
Physiological characteristics and comparisons based on DPN status during follow-up are presented in Table 2. More incident DPN was observed in those obese DM patients at baseline. Similarly, those with greater baseline TC, TG, LDL-C, SBP, DBP, and lower HDL-C levels, as well as lower HDL-C levels, had a larger percentage of new DPN.
Incidence of DPN and survival probability
After diagnosis T1DM and T2DM, subjects were followed for a total of 3174.78 PY, with a median follow-up period of 5.79 years (minimum of.09 and maximum of 7.48 years). The cumulative incidence of DPN was 21% (95% CI: [17.82, 24.55]). The total incidence rate was found to be 3.75, with a 95%CI of [3.13, 4.49] per 100 PY. T1DM had an incidence rate of 1.14; 95% CI: [0.63, 2.06] and T2DM had an incidence rate of 4.89; 95% CI: [4.04, 5.90] per 100 PY.
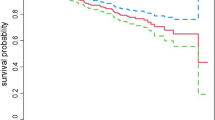
The cumulative probability of event free survival was found to be 0.966, 0.878, 0.80 and 0.696 at 2-year, 4-year, 6-year and the end of the follow-up period, respectively (Fig. 2). Because the study ended before half of the participants developed DPN, the median survival time was not met. Furthermore, a Kaplan–Meier survival estimate revealed that individuals with T2DM (Fig. 3) and positive proteinuria (Fig. 4) at baseline had an increased risk of developing DPN than their counterparts.
Predictors of diabetic peripheral neuropathy
As variable selection precedes model diagnostics, 11 factors significantly associated with incident DPN in the univariate analysis at p values less than 0.2 were included in the multivariable survival model. We used the Gompertz proportional hazard model, which has the lowest AIC when compared to other models, to examine the predictors of new-onset DPN (Table 3). The pseudo VIF values ranged from 1.04 to 1.62, showing that the independent variables were not multicollinear. PHA was met because the Schoenfeld residual to test the PHA was not significant (P-values for each variable ranged from 0.462 to 0.949, with a global P-value of 0.624). Furthermore, the Cox Snell residual plot revealed that the model’s goodness of fit was fulfilled because the cumulative hazard plot follows a 45 degree or a straight line through the origin with slope one. In the multivariable Gompertz proportional hazard model, there are five independent variables (female sex, positive proteinuria, DR, T2DM, and high BMI) that significantly predicts the likelihood of DPN in diabetic patients after controlled for other variables (Table 4).
Keeping other variables constant, the hazard of developing DPN for female was 47% higher than males [AHR = 1.47; 95% CI (1.01, 2.15)]. Similarly, the hazard of DPN was increased by 3.49 among newly diagnosed T2DM [AHR = 3.49 95% CI (1.82, 6.71)] compared with T1DM. Holding other variables constant, the risk of developing DPN was increased by 1.90 [AHR = 1.9 95% CI (1.25, 2.91)] and 2.22 [AHR = 2.22 95% CI (1.35, 3.65)] among participants who had DR and proteinuria respectively. Adjusting for other variables, the risk of developing DPN was increased by 3.34 [AHR = 3.34 95% CI (1.09, 1.025)] and 3.94 [AHR = 3.94 95% CI (1.2, 12.89)] times among obese and overweight when compared to their counter parts respectively.
Discussion
DPN is a global life-threatening disease that has a significant impact on patients’ quality of life which present a sizable financial burden to patients and health care system. Thus, this study determined the incidence and predictors of DPN among newly diagnosed T1DM and T2DM patients in tertiary health care setting of southwest Ethiopia. A total of five factors were identified to significantly predict the incidence of DPN and these factors include: Female sex, T2DM, DR, positive proteinuria, and high BMI.
The findings of this study showed that the cumulative incidence of DPN was 21% [17.82, 24.55] while incidence rate was 3.75, with a 95%CI of [3.13, 4.49] per 100 PY. The incidence of the current study is in line with a prospective cohort study conducted in Austria [7]. However, the finding is higher than studies conducted in Denmark and North West Ethiopia which was 10% and 16.63%, respectively [21, 29]. This discrepancy might be due to the different populations included in those studies and shorter follow up compared with our study for those conducted in Denmark. The other possible reason could be due to higher percentage of obesity and overweight in our study population compared to those conducted in North west Ethiopia. The incidence of DPN in the current study is lower than previous studies conducted in Jordan 39.5%, in Germany 40.3%, and 29.4% in Uganda [11, 33, 34]. This might be owing to the fact that, typically, DPN is often diagnosed clinically with little further laboratory investigations to confirm the diagnosis or poorly captured in patients’ records [35]. In a study by Day et al. [36], more than 40% of diabetic patients in general practice had no biochemical evaluation. The wide geographical variations in incidence of DPN highlight the need for a global multicenter study in which the selection criteria for diabetic patients are unified to the finest detail.
According to this study, being female significantly predicts the risk of DPN. The hazard of developing DPN was increased by 47% among females compared with males. This result agrees with studies done in Jordan and Japan which showed that the female has about six times more likely to develop DPN compared with male [37, 38]. However, The results of the study are in stark contrast to those of most studies in the literature [39, 40] whereas another study found no significant association and variation in gender [41]. This discrepancy may be a result of differences in the tests used to measure neuropathic changes, the duration of diabetes, and the level of glycemic control for the subjects. These inconsistent findings might show that further high-standard research is needed to put evidence on the association between gender and developing DPN.
The risk of developing DPN was twice among participants who had positive proteinuria than those with negative proteinuria. Albuminuria has been proposed to be an independent predictor of increasing levels of microvascular and macrovascular diseases in DM patients [42]. However, the number of published studies with the specific aim of investigating the correlation between albuminuria and DPN are very limited, although the results of some studies from Iran [43] and Taiwan [44] and Vietnam [45] may indirectly reflect this association. High serum cystatin C levels were linked to DPN in Chinese research [46], suggesting that this molecule could be used as a biomarker for DPN in DM patients. Callaghan et al. [47] suggested in their review that neuropathy can be particularly severe in diabetic chronic kidney disease. To date, the mechanisms of neurotoxicity in DM patients with renal impairment due to proteinuria remains unclear. However, the experimental evidence indicates that renal impairment result in alteration in membrane excitability which consequently, leading to an accumulation of extracellular K + that causes depolarization [48]. Disruption of these various ionic gradients may affect the Na+/Ca2 + exchanger, leading to increased levels of intracellular Ca2 + and axonal loss [49]. In addition, previous research as impaired renal function results in endothelial injury [50] which eventually, leads to neuropathy due to impaired nerve blood flow, and reduced nerve oxygen tension.
According to the findings, the hazards of developing DPN were 90% higher for those participants with DR at baseline compared with their counterparts. This finding is in line with the previous studies conducted in Germany, Libya and Vietnam which showed that the likelihood of DPN was almost twice among patients with DR [11, 45, 51]. A meta-analysis strongly supported the significant association between DPN and DR by showing a similar increase in risk of developing DPN in the patients who have complications [52]. The coexistence of diverse diabetic problems could be explained by the fact that the majority of diabetic complications have comparable risk factors, particularly poor glycemic control. The other reason could be that both complications have the same pathophysiology, and hence the existence of one complication could indicate the presence of the other. Diabetes-induced hyperglycemia has a role in the pathogenesis of DPN and retinopathy, with a link between the buildup of advanced glycation end products and the activation of the polyol pathway, protein kinase C, and free radicals, resulting in biomolecular damage in the affected tissues [53].
Having T2DM increases the hazard of DPN by more than 3-fold when compared to T1DM participants. This finding is consistent with the cohort study conducted among youth in the USA which showed youth with T2DM were four times more likely to develop DPN compared with T1DM [22, 54]. Similarly, other systemic review study showed a higher percentage of T2DM patients with symptoms of neuropathy compared with T1DM patients [55]. The possible explanation for this could be that sensory nervous impairment occurs earlier in type 2 diabetes than in T1DM [56]. This could be due to the physiology of the somatosensory pathways, and it’s interesting to note that, while traveling through separate sensory pathways, both superficial and deep sensitivity mainly degrade in T2DM [57]. The other possible reason could be the currently decreasing age of onset of T2DM allowing enough time for the development of DM complication [58].
The BMI is a standard unit for quantifying overweight and obesity. DPN was also more in overweight and obese than in nonobese/normal cases ‑ 48.8%, 37.5% and 12.9%, respectively. Results of the current study also supports this evidence as being obese and overweight increases the hazard of DPN by approximately four and three folds, respectively. This is in line with previous studies that revealed the obese group was more likely to have DPN than the non-obese group [22, 59]. Five recent epidemiological studies two from China [60, 61], and one from Denmark [21], one from Netherlands [62] and one from Germany [63] also implicate obesity as the second most influential metabolic risk factor for neuropathy after diabetes. The possible explanation for this might be due to high risk of overweight and obesity for different chronic conditions in human beings which consistently applicable in developing DPN.
Conclusion and recommendation
The incidence of DPN was relatively high compared with previous studies. Nearly, 7 in 10 of DPN cases occurred within a short period (5 years) of DM diagnosis. Being female, T2DM, DR, positive proteinuria, being obese and overweight significantly predicts the risk of DPN. Therefore, we recommend screening and early diagnosis of diabetes and its complications. Given the significancy of obesity as a DPN risk factor, educating DM patients on a healthy lifestyle and the benefit of regular checkups to reduce obesity is recommended. While doing so, attention should be given for DM patients with DR and positive proteinuria. Further prospective study on the topic is recommended by including some missed variables. Since female sex is contradictory whether used as a protective or risk for DPN in DM patient, we recommend further research on this issue.
Limitation of the study
In spite of its strength, we acknowledge several limitations in our study. The nature of study design as a retrospective study limits the accurate assessment of the association between DPN and risk factors. Due to the retrospective nature of data, we have not considered other potential predictors such as exercise, adherence to treatment, smoking, alcohol drinking and diet due to their unavailability in the patients’ card. Study assumed all the DPN were caused by DM and relying only on clinical signs and symptoms to confirm the diagnosis of DPN may underestimate its incidence in the study.
Data availability
Data will be available from the corresponding author upon request.
Abbreviations
- AKI:
-
Acute Kidney Injury
- AHR:
-
Adjusted Hazard Ratio
- AIC:
-
Akakie Information Criterion
- BMI:
-
Body Mass Index
- CVD:
-
Cardiovascular Disease
- DM:
-
Diabetes Mellitus
- DPN:
-
Diabetic Peripheral Neuropathy
- DR:
-
Diabetic Retinopathy
- HDL-C:
-
High Density Lipoprotein Cholesterol
- IDF:
-
International Diabetic Federation
- HTN:
-
Hypertension
- JUMC:
-
Jimma University Medical center
- LDL-C:
-
Low Density Lipoprotein Cholesterol
- MKCSH:
-
Mettu Karl Comprehensive and Specialized Hospital
- PHA:
-
Proportional Hazard Assumptions
- PY:
-
Person Years
- T1DM:
-
Type one DM
- T2DM:
-
Type two DM
References
Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in Diabetes Complications: a review of current evidence. Diabetologia. 2019;62(1):3–16.
Inernational. Diabetes federation DIABETES ATLAS, Tenth edition 2021.
Association AD. 2. Classification and diagnosis of Diabetes: standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Supplement1):14–S31.
Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, et al. Diabetic Peripheral Neuropathy: Epidemiology, diagnosis, and Pharmacotherapy. Clin Ther. 2018;40(6):828–49.
Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):473.
Young M, Boulton A, MacLeod A, Williams D, Sonksen P. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–4.
Walter-Höliner I, Barbarini DS, Lütschg J, Blassnig-Ezeh A, Zanier U, Saely CH, et al. High prevalence and incidence of diabetic peripheral neuropathy in children and adolescents with type 1 Diabetes Mellitus: results from a five-year prospective cohort study. Pediatr Neurol. 2018;80:51–60.
Boulton A, Gries F, Jervell J. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15(6):508–14.
Federation ID. IDF Diabetes atlas ninth. Dunia: IDF; 2019.
Alleman CJ, Westerhout KY, Hensen M, Chambers C, Stoker M, Long S, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract. 2015;109(2):215–25.
Pfannkuche A, Alhajjar A, Ming A, Walter I, Piehler C, Mertens PR. Prevalence and risk factors of diabetic peripheral neuropathy in a diabetics cohort: Register initiative Diabetes and nerves. Endocr Metabolic Sci. 2020;1(1–2):100053.
Sobhani S, Asayesh H, Sharifi F, Djalalinia S, Baradaran HR, Arzaghi SM, et al. Prevalence of diabetic peripheral neuropathy in Iran: a systematic review and meta-analysis. J Diabetes Metabolic Disorders. 2014;13(1):1–7.
Shiferaw WS, Akalu TY, Work Y, Aynalem YA. Prevalence of diabetic peripheral neuropathy in Africa: a systematic review and meta-analysis. BMC Endocr Disorders. 2020;20:1–9.
Gudina EK, Amade ST, Tesfamichael FA, Ram R. Assessment of quality of care given to diabetic patients at Jimma University Specialized Hospital Diabetes follow-up clinic, Jimma, Ethiopia. BMC Endocr Disorders. 2011;11(1):1–9.
Worku D, Hamza L, Woldemichael K. Patterns of diabetic Complications at jimma university specialized hospital, southwest Ethiopia. Ethiop J Health Sci. 2010;20(1).
Abdissa D, Hamba N, Kene K, Bedane DA, Etana G, Muleta D et al. Prevalence and Determinants of peripheral neuropathy among type 2 adult diabetes patients attending jimma university medical center, southwest ethiopia, 2019, an institutional-based cross-sectional study. Journal of diabetes research. 2020;2020.
Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Research. 2016;5.
Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49(2):106–16.
Connolly V, Unwin N, Sherriff P, Bilous R, Kelly W. Diabetes prevalence and socioeconomic status: a population based study showing increased prevalence of type 2 Diabetes Mellitus in deprived areas. J Epidemiol Community Health. 2000;54(3):173–7.
Degu H, Wondimagegnehu A, Yifru YM, Belachew A. Is health related quality of life influenced by diabetic neuropathic pain among type II Diabetes Mellitus patients in Ethiopia? PLoS ONE. 2019;14(2):e0211449.
Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, et al. Risk factors for Incident Diabetic Polyneuropathy in a Cohort with screen-detected type 2 Diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care. 2018;41(5):1068–75.
Jaiswal M, Divers J, Dabelea D, Isom S, Bell RA, Martin CL, et al. Prevalence of and risk factors for Diabetic Peripheral Neuropathy in Youth with Type 1 and type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care. 2017;40(9):1226–32.
Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 Diabetes Mellitus in a tertiary care setting. J Diabetes Invest. 2014;5(6):714–21.
Katulanda P, Ranasinghe P, Jayawardena R, Constantine GR, Sheriff MR, Matthews DR. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol Metab Syndr. 2012;4(1):1–8.
Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM. The prevalence and risk factors of peripheral neuropathy among patients with type 2 Diabetes Mellitus; the case of Jordan. Diabetol Metab Syndr. 2018;10:8.
Gebabo TF, Zewdie TH, Shagaro SS, Haile F. Determinants of peripheral neuropathy among diabetic patients under follow-up in chronic care clinics of public hospitals at Gamo and Gofa zones, southern Ethiopia. PLoS ONE. 2021;16(2):e0246722.
Javed S, Hayat T, Menon L, Alam U, Malik RA. Diabetic peripheral neuropathy in people with type 2 Diabetes: too little too late. Diabet Med. 2020;37(4):573–9.
Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983:499–503.
Kebede SA, Tusa BS, Weldesenbet AB, Tessema ZT, Ayele TA. Time to diabetic neuropathy and its predictors among newly diagnosed type 2 Diabetes Mellitus patients in Northwest Ethiopia. Egypt J Neurol Psychiatry Neurosurg. 2021;57(1):1–7.
Gylfadottir SS, Weeracharoenkul D, Andersen ST, Niruthisard S, Suwanwalaikorn S, Jensen TS. Painful and non-painful diabetic polyneuropathy: clinical characteristics and diagnostic issues. J Diabetes Invest. 2019;10(5):1148–57.
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema Disease severity scales. Ophthalmology. 2003;110(9):1677–82.
WHO. Regional Office for the Western Pacific. (2017). Noncommunicable disease education manual for primary health care professionals and patients. Manila: WHO Regional Office for the Western Pacific. 2017 [Available from: https://apps.who.int/iris/handle/10665/254746.
Kisozi T, Mutebi E, Kisekka M, Lhatoo S, Sajatovic M, Kaddumukasa M, et al. Prevalence, severity and factors associated with peripheral neuropathy among newly diagnosed diabetic patients attending Mulago hospital: a cross-sectional study. Afr Health Sci. 2017;17(2):463–73.
Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM. The prevalence and risk factors of peripheral neuropathy among patients with type 2 Diabetes Mellitus; the case of Jordan. Diabetol Metab Syndr. 2018;10(1):1–10.
Aleidan FAS, Ahmad BA, Alotaibi FA, Aleesa DH, Alhefdhi NA, Badri M, et al. Prevalence and risk factors for Diabetic Peripheral Neuropathy among Saudi Hospitalized Diabetic patients: a nested case-control study. Int J Gen Med. 2020;13:881–9.
Yuan D, Hussain T, Tan B, Liu Y, Ji P, Yin Y. The evaluation of antioxidant and anti-inflammatory effects of Eucommia ulmoides flavones using diquat-challenged piglet models. Oxidative Medicine and Cellular Longevity. 2017;2017.
Katulanda P, Ranasinghe P, Jayawardena R, Constantine GR, Sheriff M, Matthews DR. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol Metab Syndr. 2012;4(1):1–8.
Yokoyama H, Tsuji T, Hayashi S, Kabata D, Shintani A. Factors associated with diabetic polyneuropathy-related sensory symptoms and signs in patients with polyneuropathy: a cross-sectional Japanese study (JDDM 52) using a non-linear model. J Diabetes Investig. 2020;11(2):450–7.
Group DR. Factors in development of diabetic neuropathy: baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). Diabetes. 1988;37(4):476–81.
D’Souza M, Kulkarni V, Unnikrishnan Bhaskaran HA, Naimish H, Prakash A, Tabreez S et al. Diabetic peripheral neuropathy and its determinants among patients attending a tertiary health care centre in Mangalore, India. J Public Health Res. 2015;4(2).
Shrestha H, Katwal P. Prevalence and risk factors of Diabetic Peripheral Neuropathy in T2DM patient presenting to Commnity Hospital in Nepal. Kathmandu Univ Med J. 2017;58(2):146–9.
Rosenson R, Fioretto P, Dodson P. Does microvascular Disease predict macrovascular events in type 2. Diabetes? Atherosclerosis. 2011;218(1):13–8.
Janghorbani M, Rezvanian H, Kachooei A, Ghorbani A, Chitsaz A, Izadi F, et al. Peripheral neuropathy in type 2 Diabetes Mellitus in Isfahan, Iran: prevalence and risk factors. Acta Neurol Scand. 2006;114(6):384–91.
Pai Y-w, Lin C-H, Lee I-T, Chang M-H. Prevalence and biochemical risk factors of diabetic peripheral neuropathy with or without neuropathic pain in Taiwanese adults with type 2 diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2018;12(2):111-6.
Dinh Le T, Phi Thi Nguyen N, Thanh Thi Tran H, Luong Cong T, Ho Thi Nguyen L, Do Nhu B, et al. Diabetic Peripheral Neuropathy Associated with Cardiovascular Risk factors and Glucagon-Like Peptide-1 concentrations among newly diagnosed patients with type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2022;15:35–44.
Hu Y, Liu F, Shen J, Zeng H, Li L, Zhao J, et al. Association between serum cystatin C and diabetic peripheral neuropathy: a cross-sectional study of a Chinese type 2 diabetic population. Eur J Endocrinol. 2014;171(5):641–8.
Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA. 2015;314(20):2172–81.
Wang C-S, Pai Y-W, Lin C-H, Lee I-T, Chang M-H. Renal impairment is one of appropriate predictors of future diabetic peripheral neuropathy: a hospital-based 6-year follow-up study. Sci Rep. 2022;12(1):1–9.
Craner MJ, Lo AC, Black JA, Waxman SG. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain. 2003;126(7):1552–61.
Malyszko J. Mechanism of endothelial dysfunction in chronic Kidney Disease. Clin Chim Acta. 2010;411(19–20):1412–20.
Elbarsha A, Hamedh MA, Elsaeiti M. Prevalence and risk factors of diabetic peripheral neuropathy in patients with type 2 Diabetes Mellitus. Ibnosina J Med Biomedical Sci. 2019;11(1):25.
Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS ONE. 2019;14(2):e0212574.
Ahmed N. Advanced glycation endproducts—role in pathology of diabetic Complications. Diabetes Res Clin Pract. 2005;67(1):3–21.
Sempere-Bigorra M, Julian-Rochina I, Cauli O. Differences and similarities in Neuropathy in Type 1 and 2 Diabetes: a systematic review. J Pers Med. 2021;11(3).
Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):1–11.
Orlando G, Balducci S, Bazzucchi I, Pugliese G, Sacchetti M. Neuromuscular dysfunction in type 2 Diabetes: underlying mechanisms and effect of resistance training. Diab/Metab Res Rev. 2016;32(1):40–50.
Silverthorn D, Johnson B, Ober W, Garrison C, Silverthorn A. The central nervous system. Human Physiology: An Integrated Approach 6th ed Boston, MA: Pearson Education. 2013:288–324.
Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, et al. Long-term Complications and mortality in young-onset Diabetes: type 2 Diabetes is more hazardous and lethal than type 1 Diabetes. Diabetes Care. 2013;36(12):3863–9.
Ylitalo KR, Sowers M, Heeringa S. Peripheral vascular Disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity: National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2011;34(7):1642–7.
Callaghan BC, Gao L, Li Y, Zhou X, Reynolds E, Banerjee M, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397–405.
Lu B, Hu J, Wen J, Zhang Z, Zhou L, Li Y, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with Diabetes and pre-diabetes–ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). PLoS ONE. 2013;8(4):e61053.
Hanewinckel R, Drenthen J, Ligthart S, Dehghan A, Franco OH, Hofman A, et al. Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population-based cohort study. J Neurol Neurosurg Psychiatry. 2016;87(12):1336–42.
Schlesinger S, Herder C, Kannenberg JM, Huth C, Carstensen-Kirberg M, Rathmann W, et al. General and abdominal obesity and incident distal sensorimotor polyneuropathy: insights into inflammatory biomarkers as potential mediators in the KORA F4/FF4 cohort. Diabetes Care. 2019;42(2):240–7.
Acknowledgements
Our heartfelt gratitude goes to the Mattu University for support by all necessary services. Additionally, we appreciate the support from Hospital’s administrations and data collector.
Funding
No any fund was taken for this specific study.
Author information
Authors and Affiliations
Contributions
GRD, BTK and SA designed the study, supervised data collection, performed data analysis and interpretation, and drafted the manuscript. All others assisted in designing the study, did data analysis and interpretation, and revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for all the contents of the work in the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Ethical Review Board of Mattu University and permission or waiver to review data from medical record was obtained from the medical directors of MKCSH and JUMC. The need for consent was waived by the ethics committee of Mattu University. Data confidentiality was kept during all phases of research activities and held on secured password protected system.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Debele, G.R., Kuse, S.A., Kefeni, B.T. et al. Why too soon? Predictors of time to diabetic peripheral neuropathy among newly diagnosed diabetes mellitus patients: a multicenter follow-up study at health-care setting of Ethiopia. Arch Public Health 81, 186 (2023). https://doi.org/10.1186/s13690-023-01202-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-023-01202-3