Abstract
Background
The present study aimed to investigate the effects of fish oil supplements compared to corn oil on serum lipid profiles by performing a meta-analysis of randomized controlled trials (RCTs).
Methods
Online databases including PubMed, Web of Science, and Scopus were searched until 30 December 2022. Pooled effect sizes were reported as the weighted mean difference (WMD) with 95% confidence intervals (CI). The Cochrane Collaboration’s risk-of-bias tool was utilized to evaluate the quality of the studies. Lipid parameters, including triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL), were assessed in the meta-analysis.
Results
Overall, 16 eligible trials were included in this systematic review and meta-analysis. The results revealed that the fish oil supplements significantly reduced TG (WMD: − 25.50 mg/dl, 95% CI: − 42.44, − 8.57, P = 0.000) levels compared to corn oil. Also, in this study, fish oil supplements had a positive and significant effect on HDL (WMD: 2.54 mg/dl, 95% CI: 0.55, 4.52). There were no significant changes in TC and LDL.
Conclusions
Our findings showed the effects of fish oil supplements on reducing TG and increasing HDL-c compared to corn oil. Further larger and well-designed RCTs are required to confirm these data.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVDs) are a group of diseases that affect the heart and blood vessels [1]. CVD is the first cause of mortality in the world, responsible for nearly one-third of all deaths [2, 3]. The risk of CVDs is thought to be increased by conditions known as cardiometabolic risk factors (CMR), which include dyslipidemia, hypertension, diabetes, overweight, abdominal obesity, and inflammation [4]. Dyslipidemia is regarded as the most important risk factor that increases the possibility of CVDs. It is described by elevated total cholesterol (TC), plasma triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), and low levels of plasma high-density lipoprotein cholesterol (HDL-c) [5]. Existing evidence indicates that controlling these lipids can effectively reduce the risk of cardiovascular disease [6]. Medication is the usual cure, and lifestyle changes are the important strategies in the management of dyslipidemia. Diet modification can have powerful effects on reducing the need for pharmacologic interventions and their side effects. In particular, oils, due to their various fatty acid compositions, including saturated fatty acids (SFAs), trans fatty acids, monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs), have different effects on the lipid profile and play an important role in the development of CVD [7]. While the fatty acid content of oils may estimate the influence of fats on serum lipid profiles [8], knowing which type of dietary oil has the most effects on lipoproteins still needs further investigation. Recent evidence has revealed that monounsaturated fatty acid (MUFA) intake promotes a healthy blood lipid profile [9], glycemic control [10], and insulin resistance [11]. In addition, the consumption of vegetable and fish oils, which are rich in PUFAs, has been linked to potential cardioprotective, lipid profile, and blood glucose control benefits [12, 13]. Therefore, MUFAs as well as PUFAs have positive effects on human health by lowering the risk of metabolic events. In this regard, the consumption of fish and corn oils, which are rich in MUFAs and PUFAs, respectively, has been recommended. The cardioprotective benefits of these oils have been reported in several studies [14,15,16]. Fish oil is a rich source of omega-3 polyunsaturated fatty acids, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which, compared to types of omega-3 found in plants, are healthier choices for consumption [17]. Fish oil supplementation has been shown to improve multiple risk factors for heart disease [18]. Moreover, it has been associated with some beneficial effects on glycemic control, blood pressure, and the inflammatory response [19]. Hence, there has been increasing attention paid to taking a fish oil supplement in recent years. Corn oil is another oil that has become popular for cooking applications recently. This vegetable oil comprises a high concentration of polyunsaturated omega-6 fat, especially linoleic acid [20]. Compared with other oils, corn oil is one of the richest dietary sources of phytosterols and tocopherols. β-sitosterol (63–70%) and γ-tocopherol (68–89%) are the main types of phytosterol and tocopherol in corn oil, respectively. In some studies, corn oil has been linked to improved plasma lipids, including serum total cholesterol and triglyceride levels, which may be due to the high level of phytosterols in this oil and thus reduce the risk of heart disease. Also, it has been demonstrated that γ-tocopherol displays efficacy against DNA damage, blood pressure, and diabetes [21, 22].
Although previous randomized controlled trials (RCTs) evaluated the impacts of fish oil on lipid profiles and cardiovascular factors, its effects compared to corn oil in the subgroup analysis were still inconsistent. Given the fatty acid content and wide consumption of fish and corn oils, there are many claims that describe their effects on improving blood lipids. It is undoubtedly necessary to conduct a meta-analysis to summarize the evidence and comprehensively compare these two oils, paying attention to the higher percentage of omega-3 in fish oil. To the best of our knowledge, there is no meta-analysis in this area. Therefore, the purpose of this study was to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare the effects of fish oil supplements and corn oils on the lipid profile.
Methods
Search strategy
The current study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. PubMed, Scopus, and Web of Science databases were searched until December 30, 2022. Randomized controlled trials (RCTs) examined the effects of fish oil and corn oil consumption on serum lipid profiles. We used a combination of the following keywords to search the databases: (("low-density lipoprotein") OR (LDL*) OR ("total cholesterol") OR (TC) OR ("high-density lipoprotein") (HDL*) OR (triglyceride*) OR (TG) OR ("polyunsaturated fatty acids") OR (eicosapentaenoic acid) OR (EPA*) OR (docosahexaenoic acid*) OR (DHA*) OR (lipoprotein *) OR ("lipid profile") OR (Lipid*) OR ("cardiovascular disease") OR ("heart disease") OR (hypercholesterolemia*)) AND ((corn oil*) OR ("maize oil") OR ("fish oil") NOT ((rat) OR (mouse) OR (animal*)). The references of related papers were checked to identify additional studies that were not found through online searches.
Study selection and eligibility criteria
The review of literature involved a meticulous examination of the titles, abstracts, and full texts by two independent experts with the aim of identifying potentially relevant articles. Specifically, clinical trials that presented original data on the impacts of fish oil and corn oil interventions on serum lipid factors, including TC, LDL-c, HDL-c, or TG, were taken into consideration. The inclusion criteria established were that the studies must have been published in English, have full text available, feature corn oil as the control group, provide a comparison group, and have been published until December 30, 2022. Conversely, the exclusion criteria comprised studies that had another oil as the control group, studies without complete information or a control group, and studies presented in the form of illegible graphs, animal and in vitro studies, reviews and meta-analyses, and articles with nonclinical trial designs.
Data extraction
After reviewing the articles, all the required information was extracted by two researchers using a predefined screening form. We extracted the data from each selected study, including the first author’s name, year of publication, country, age, sex, baseline body mass index (BMI), study design, sample size, type and dose of intervention and control group, duration of study, and state of health. Moreover, the mean changes of TG, TC, HDL-c, and LDL-c from baseline to the end of the study and their standard deviations (SDs) were also extracted.
Quality assessment
The quality of included studies was assessed independently by two authors, according to the Cochrane Collaboration’s tool [24], which is composed of the following criteria: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, and (5) incomplete outcome data, selective outcome reporting, and other biases based on the domains mentioned, and each study was classified as having a high bias risk, a low bias risk, and an unclear bias risk.
Data synthesis and analysis
The effects of oils on the change in outcome factors were calculated. The weighted mean difference (WMD) with a 95% confidence interval (CI) between intervention and control groups was applied to determine effect sizes. The mean change of variables and the relevant standard deviations (SD) were elicited for analysis. In those studies that did not provide mean and SD changes from baseline, the mean of variables before and after intervention and the SD changes were yielded using a correlation coefficient r (r = 0.5) [25]. Regarding those studies that reported standard error (SE), the following formula was used to calculate SD: SD = SE × √n (n = number of subjects). The reported concentration of outcomes was converted into the usual unit (mg/dl) in the meta-analysis. The heterogeneity of the included studies was assessed by the chi-square (I2) index and the Cochrane Q test with a p < 0.05. An I2 greater than 50% was considered significant heterogeneity. Subgroup analyses based on age, amount of consumed oil, health status, and duration of treatment were conducted to determine the source of heterogeneity between studies. Potential publication bias was also checked through Egger’s funnel plots. Meta-analysis of data was performed using STATA 14.0 (Statistical Software, College Station, TX, USA). Statistical significance was defined by p-values < 0.05.
Results
Study selection
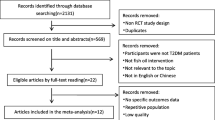
The flow diagram of the article selection process is presented in Fig. 1. In the primary search, 2095 potential records were identified from the literature search, and 648 articles were excluded because of duplication. After screening titles and abstracts and removing irrelevant studies, 31 articles were retained for further assessment. Of these articles, 15 were removed for the following reasons: animal studies (n = 2), studies that did not have a control group (2), studies in which the control group was other than corn oil (n = 6), articles that used the effects of fish oil in combination with other oils and/or interventions (n = 3), and without complete information about the mean and standard deviation of the control and/or intervention groups (n = 2). Finally, 16 articles were included in the meta-analysis.
Study characteristics
The main characteristics of the included studies are displayed in Table 1. A total of 491 participants in the 16 randomized clinical trials examined the effects of fish oil on the serum lipid profile compared with corn oil as a control. Overall, the age range of participants and the intervention duration were 14 to 65 years and 28 to 180 days, respectively. In terms of the health status of the participants, in one study, there were healthy subjects [25], five studies involved patients with lipid disorders [26,27,28,29,30], four studies involved type 2 diabetes mellitus [31,32,33,34], two studies included patients with kidney disorders [35, 36], and other trials included patients with heart disease [37], fatty liver disorder [38], and high blood pressure [39]. All the articles were published between 2007 and 2019. These trials were conducted in different countries, including Iran [36], China [32, 38, 39], the USA [30, 34, 35], Israel [26], Norway [37], Australia [27], Germany [28], Canada [25], Denmark [31, 33, 40], and England [29]. Out of 16 studies, 16 RCTs investigated the intervention’s efficacy on TC, 15 on TG, 16 on HDL-c, and 13 on LDL-c.
Quality assessment
The quality assessment of included studies is shown in Fig. 2. As shown in Fig. 2, seven studies were assessed as having a low risk of bias in the random sequence generation [25, 26, 29, 31, 32, 34, 39], and other studies showed an unclear risk of bias [27, 28, 30, 33, 35,36,37,38, 40]. Five trials showed a high risk of bias for allocation concealment [25, 28, 30, 31, 36], nine did not provide specific methods for this operation [26, 27, 29, 31, 32, 34, 35, 37, 38], and two had a low risk of bias [39, 40]. There were four studies [29, 34, 36, 37] using a double-blind design that were classified as having an unclear risk of bias for the blinding of the participants and personnel, and the others showed a low risk of bias [25,26,27,28, 30,31,32,33, 35, 38,39,40]. Regarding blinding of outcome assessment, 4 studies showed a low risk of bias [30, 37, 38, 40], 10 had an unclear risk [25,26,27,28,29, 31,32,33, 35, 36], and only 2 showed a high risk of bias [34, 39]. All studies provided complete outcome data for analysis [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Also, all studies were judged to have a low risk of bias in selective reporting [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Four studies did not report other measures of quality, such as dose [27], or had imprecisions [25, 28, 34] (Fig. 2).
Meta-analysis
TC
The combined results of the random-effects model showed no significant reduction in TC level following fish oil intake compared to corn oil (WMD: 1.39 mg/dl, 95% CI: − 4.08, 6.87, P = 0.001), which was identified by significant heterogeneity (I2 = 61.1%; p = 0.001) (Fig. 2). Subgroup analysis indicated no potential source of heterogeneity based on age, duration, health condition, or dose (Table 2). The sensitivity analysis for TC revealed that no individual study had a great impact on the overall effect size. Visual inspection of the funnel plot showed no evidence of publication bias (Fig. 3). Moreover, Egger’s test confirmed the same result (P = 0.253).
TG
Pooling data from 15 studies, a significant reduction in TG levels (WMD: − 25.50 mg/dl, 95% CI: − 42.44, − 8.57, P = 0.000) was found after fish oil consumption than the controls. There was high heterogeneity between studies (I2 = 90.9%; P = 0.000) (Fig. 4). Age, dose, duration of intervention, and health status of subjects were considered as possible sources of heterogeneity. The reduction of TG was significant in studies done in participants aged ≤ 50 years (WMD: − 36.15 mg/dl, 95% CI: − 52.49, − 19.81), with dosage ≥ 4 g/day (WMD: − 34.36 mg/dl, 95% CI: − 52.83, − 15.89), duration > 9 weeks (WMD: − 25.39 mg/dl, 95% CI: − 47.76, − 3.01), and conducted among those with dyslipidemia (WMD: − 40.14 mg/dl, 95% CI: − 58.87, − 21.52). Overall meta-analysis result for LDL-c was not sensitive to individual studies. Assessment of publication bias by visual inspection of a funnel plot illustrated no sign of publication bias (Fig. 2). Egger’s test demonstrated no publication bias (P = 0.16).
HDL-c
The pooled estimate from the random-effect model performed on 15 studies showed that fish oil supplementation had a significant positive effect on the serum level of HDL-c in comparison to corn oil (WMD: 2.54 mg/dl, 95% CI: 0.55, 4.52), with significant heterogeneity (I2 = 81.5%; P = 0.000) (Fig. 5). However, subgroup analysis showed that age, dose, health status, and duration were potential sources of heterogeneity (Table 2). The sensitivity analysis revealed that no study had a significant impact on the overall effect size of HDL-c. Evaluation of publication bias by visual inspection of a funnel plot displayed no evidence of publication bias among the included studies (Fig. 2). The same finding was also concluded by Egger’s regression test (P = 0.19).
LDL-c
The effect of fish oil supplements on LDL-c concentrations was examined in 13 clinical trials. The analysis revealed no significant reduction in the LDL-c concentration following fish oil consumption compared to control groups (WMD: 3.98 mg/dl, 95% CI: − 0.51, 8.46) (Fig. 6). There was high heterogeneity between the effect sizes of the included studies (I2 = 60.1%; P = 0.003). The sensitivity analysis provided no evidence of the impact of an individual study on the overall result. Subgroup analysis indicated no potential source of heterogeneity based on age, dosage, health condition, or study duration (Table 2). Visual inspection of the funnel plot (Fig. 2) and further assessment using the Egger test did not suggest evidence of publication bias (Egger test: P = 0.301).
Discussion
This systematic review and meta-analysis evaluated 16 clinical controlled trials that examined the effects of fish oil supplements on lipid profiles compared to corn oil. Our meta-analysis results proved that fish oil significantly decreased TG and increased HDL-c levels compared to corn oil. However, no significant associations were found in other lipids, including TC and LDL-c. Our findings are similar to the results of a previous meta-analysis on the effect of fish oil supplementation on lipid levels among patients with type 2 diabetes. Gao et al. (2020) found that fish oil supplementation reduced TG level by − 35.39 (95% CI: − 46.89, − 24.77, I2 = 0%, p < 0.05) and increased HDL-c level by 8.10 (95% CI: 1.93, 14.28, I2 = 37.1%, p < 0.05), whereas TC and LDL-c were not significantly affected [41]. Almost in line with our study, the meta-analysis carried out by Wu et al. (2021) which included 12 RCTs reported a significant decrease in serum triglyceride (WMD: − 21.23 mg/dl, 95% CI: − 35.39 to − 7.07, P = 0.004) but had no significant effects on TC, LDL-c, or HDL-c in subjects with overweight after taking fish oil compared to the control group [42]. Similar to our result, another meta-analysis based on 13 RCTs evaluating the effects of fish oil supplementation on serum lipid profile in dialysis patients showed a significant improvement in TG, TC, and HDL-c levels compared with the control group, while no changes were seen in LDL-c levels. In contrast, they reported a decrease in TC concentration [43]. The different control groups that were included in the study of Zhu et al. (2014) without subgroup analysis but were excluded in the present study can be considered a reason for the significance of the TC results compared to our work. Another reason may be the absence of subgroup analyses based on specific oils that were included in the meta-analysis as a control group in order to know more about the effects of fish oil on the lipid profile. Subgroup analysis was conducted based on age, dose, and duration of the articles related to fish oil and corn oil, unlike the meta-analysis by Zhu et al. (2014) and Wu et al. (2021), in which subgroup analysis was performed on articles related to fish oil supplementation with patients receiving different control oils. Moreover, in the current study, unlike the mentioned meta-analyses, the age and different health statuses of participants have also been calculated.
Fish oil is derived from the tissues of cold-water oily fish, such as mackerel, tuna, herring, sardines, and salmon [44]. It has become one of the most widely consumed supplements. Fish oil is a rich source of omega-3 PUFAs, mainly EPA and DHA [45]. On the other hand, corn oil is a kind of refined vegetable oil that is valued for its cooking properties, and its content, especially linoleic acid and phytosterols, is responsible for its health benefits. In addition, it has been demonstrated that, despite having a high PUFA content, there is a lower amount of SFAs in corn oil than in other vegetable oils, including soybean and cottonseed oil [46]. Ghobadi et al. (2019) reported that a diet rich in PUFA and low in SFA can improve lipid profiles [47]. Many mechanisms of action of PUFAs have been proposed for their effects on blood lipids. Fatty acid composition, such as EPA and DHA, is one of the underlying mechanisms for the effect of fish oil supplements on blood lipids [48]. Furthermore, available research evidence from studies shows that omega-3 PUFA alters the function of cellular phospholipids and lowers triglycerides by inhibiting the phosphatidic acid phosphatase [49]. Previous studies have shown that the prescription of fish oil supplements containing omega-3 fatty acids can reduce serum TG levels. For instance, Tummala et al. (2019) reported that omega-3 fatty acid esters at high doses reduced fatty acid synthesis from carbohydrates and lowered triglycerides, therefore leading to a decrease in cardiovascular diseases [50]. Another study conducted by Shearer et al. (2012) demonstrated that n-3 FA has contributed to a reduction in triacylglycerol synthesis due to decreased diacyl-glycerol acyltransferase activity, the necessary enzyme associated with the production of triglyceride-rich lipoproteins in the liver [51]. Additionally, n-3 FA treatment may inhibit the assembly of apolipoprotein-B100 and VLDL-c particles and consequently cause a reduction in TG levels [52, 53]. In our study, the serum content of TG was reduced following the intervention of fish oil supplements compared to corn oil.
This meta-analysis has some advantages. The current study is the first meta-analysis on the comparison of fish oil supplements and corn oil consumption on the serum lipid profile. The comprehensive evidence search was so precious and performed without language or time restrictions to find all relevant publications. On the one hand, only randomized controlled trials were included in the analysis. Also, the statistical test for the determination of publication bias was non-significant. However, this study has some limitations. Participants in the included studies had different health statuses, including T2DM, diabetes, hypertension, dyslipidemia, HIV, kidney failure, overweight, and healthy subjects. Therefore, the extracted data showed different baseline levels of lipid profiles; however, we conducted a subgroup analysis. Moreover, finding the sources of heterogeneity is another concern that was not completely resolved by subgroup and sensitivity analyses.
Conclusion
In summary, compared with the control group, combined findings from 16 eligible clinical trials showed that dietary intake of fish oil supplements containing n-3 FA significantly decreased and increased TG and HDL-c, respectively. However, no significant effect was observed on TC and LDL-c variables, which are CVD risk factors. Thus, fish oil supplements might be efficient in reducing the mentioned serum lipids and preventing heart disease. Additional studies with larger sample sizes and different doses are needed to confirm our findings.
References
Ros E, Martínez-González MA, Estruch R, Salas-Salvadó J, Fitó M, Martínez JA, Corella D. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr. 2014;5(3):330S-S336. https://doi.org/10.3945/an.113.005389.
Schwingshackl L, Bogensberger B, Benčič A, Knüppel S, Boeing H, Hoffmann G. Effects of oils and solid fats on blood lipids: a systematic review and network meta-analysis. J Lipid Res. 2018;59(9):1771–82. https://doi.org/10.1194/jlr.P085522.
Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, Brauer M, Kutty VR, Gupta R, Wielgosz A, AlHabib KF. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. https://doi.org/10.1016/S0140-6736(19)32008-2.
Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006;113(25):2943–6. https://doi.org/10.2337/dc06-9911.
Heshmat-Ghahdarijani K, Mashayekhiasl N, Amerizadeh A, Teimouri Jervekani Z, Sadeghi M. Effect of fenugreek consumption on serum lipid profile: a systematic review and meta-analysis. Phytother res. 2020;34(9):2230–45. https://doi.org/10.1002/ptr.6690.
Davis A, Meyerson BE, Aghaulor B, Brown K, Watson A, Muessig KE, Yang L, Tucker JD. Barriers to health service access among female migrant Ugandan sex workers in Guangzhou, China. Int J Equity Health. 2016;15(1):1–8. https://doi.org/10.1186/s12939-016-0453-2.
Pourrajab B, Sohouli MH, Amirinejad A, Fatahi S, Găman MA, Shidfar F. The impact of rice bran oil consumption on the serum lipid profile in adults: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2022;62(22):6005–15. https://doi.org/10.1080/10408398.2021.1895062.
Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–55. https://doi.org/10.1093/ajcn/77.5.1146.
DiNicolantonio JJ, O’Keefe JH. Effects of dietary fats on blood lipids: a review of direct comparison trials. Open heart. 2018;5(2):e000871. https://doi.org/10.1136/openhrt-2018-000871.
Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13(7):e1002087. https://doi.org/10.1371/journal.pmed.1002087.
Jebb SA, Lovegrove JA, Griffin BA, Frost GS, Moore CS, Chatfield MD, Bluck LJ, Williams CM, Sanders TA. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (reading, Imperial, Surrey, Cambridge, and kings) trial. Am J Clin Nutr. 2010;92(4):748–58. https://doi.org/10.3945/ajcn.2009.29096.
Funtikova AN, Navarro E, Bawaked RA, Fíto M, Schröder H. Impact of diet on cardiometabolic health in children and adolescents. Nutr J. 2015;14:1–1. https://doi.org/10.1186/s12937-015-0107-z.
Ogawa S, Abe T, Nako K, Okamura M, Senda M, Sakamoto T, Ito S, DIMS Study Group. Eicosapentaenoic acid improves glycemic control in elderly bedridden patients with type 2 diabetes. Tohoku J Exp Med. 2013;231(1):63–74. https://doi.org/10.1620/tjem.231.63.
Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ. n− 3 fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr. 2008;87(6):2003S-S2009. https://doi.org/10.1093/ajcn/87.6.2003S.
Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009;204(2):476–82. https://doi.org/10.1016/j.atherosclerosis.2008.09.020.
Maki KC, Lawless AL, Kelley KM, Kaden VN, Geiger CJ, Palacios OM, Dicklin MR. Corn oil intake favorably impacts lipoprotein cholesterol, apolipoprotein and lipoprotein particle levels compared with extra-virgin olive oil. Eur J Clin Nutr. 2017;71(1):33–8. https://doi.org/10.1038/ejcn.2016.169.
Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n− 3 fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary-and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84(1):5–17. https://doi.org/10.1093/ajcn/84.1.5.
Roth EM, Harris WS. Fish oil for primary and secondary prevention of coronary heart disease. Curr Atheroscler Rep. 2010;12:66–72. https://doi.org/10.1007/s11883-009-0079-6.
Dasgupta A, Klein K. Herbal and other dietary supplements that are antioxidants. Antioxidants in food, vitamins and supplements, 1st edn. Amsterdam, Boston: Elsevier. 2014; 295–315. https://doi.org/10.1186/s12903-021-01711-z
Barrera-Arellano D, Badan-Ribeiro AP, Serna-Saldivar SO. Corn oil: composition, 628 processing, and utilization, in: Serna-Saldivar, S.O. (Ed.), Corn chemistry and technology. AACC 629 International, Duxford. 2019. pp. 593–613. https://doi.org/10.1016/B978-0-12-811971-6.00021-8
Ghazani SM, Marangoni AG. In Reference module in food science (2nd ed.). Elsevier Ltd. 2016. https://doi.org/10.1016/b978-0-08-100596-5.00100-1
Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–78. https://doi.org/10.1161/CIRCULATIONAHA.114.010236.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65. https://doi.org/10.7326/0003-4819-151-4-200908180-00136.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. Wiley. 2019
Ramprasath VR, Eyal I, Zchut S, Jones PJ. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013;12(1):1–1. https://doi.org/10.1186/1476-511X-12-178.
Bitzur R, Cohen H, Cohen T, Dror TW, Herzog Y, Lifshitz Y, Lubish T, Harats D, Rubinstein A. The metabolic effects of omega-3 plant sterol esters in mixed hyperlipidemic subjects. Cardiovasc Drugs Ther. 2010;24:429–37. https://doi.org/10.1007/s10557-010-6249-5.
Chan DC, Watts GF, Mori TA, Barrett PH, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n–3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr. 2003;77(2):300–7. https://doi.org/10.1093/ajcn/77.2.300.
Schmidt S, Stahl F, Mutz KO, Scheper T, Hahn A, Schuchardt JP. Different gene expression profiles in normo-and dyslipidemic men after fish oil supplementation: results from a randomized controlled trial. Lipids Health Dis. 2012;11:1–5. https://doi.org/10.1186/1476-511X-11-105.
Borthwick LJ. The effects of an omega-3 ethyl ester concentrate on blood lipid concentrations in patients with hyperlipidaemia. Clinic Drug Invest. 1998;15:397–404. https://doi.org/10.2165/00044011-199815050-00004.
Gidding SS, Prospero C, Hossain J, Zappalla F, Balagopal PB, Falkner B, Kwiterovich P. A double-blind randomized trial of fish oil to lower triglycerides and improve cardiometabolic risk in adolescents. J Pediatr. 2014;165(3):497–503. https://doi.org/10.1016/j.jpeds.2014.05.039.
Petersen M, Pedersen H, Major-Pedersen A, Jensen T, Marckmann P. Effect of fish oil versus corn oil supplementation on LDL and HDL subclasses in type 2 diabetic patients. Diabetes Care. 2002;25(10):1704–8. https://doi.org/10.2337/diacare.25.10.1704.
Wang F, Wang Y, Zhu Y, Liu X, Xia H, Yang X, Sun G. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: a randomized, double-blind, placebo-controlled trial. Eur J Nutr. 2017;56:2415–22. https://doi.org/10.1007/s00394-016-1352-4.
Pedersen H, Petersen M, Major-Pedersen A, Jensen T, Nielsen NS, Lauridsen ST, Marckmann P. Influence of fish oil supplementation on in vivo and in vitro oxidation resistance of low-density lipoprotein in type 2 diabetes. Eur J Clin Nutr. 2003;57(5):713–20. https://doi.org/10.1038/sj.ejcn.1601602.
Lee TC, Ivester P, Hester AG, Sergeant S, Case LD, Morgan T, Kouba EO, Chilton FH. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014;13(1):1–1. https://doi.org/10.1186/1476-511X-13-196.
Bowden RG, Jitomir J, Wilson RL, Gentile M. Effects of omega-3 fatty acid supplementation on lipid levels in endstage renal disease patients. J Renal Nutr. 2009;19(4):259–66. https://doi.org/10.1053/j.jrn.2009.01.030.
Khajehdehi P. Lipid-lowering effect of polyunsaturated fatty acids in hemodialysis patients. J Ren Nutr. 2000;10(4):191–5. https://doi.org/10.1053/jren.2000.16326.
Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high-dose concentrate of n− 3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr. 2001;74(1):50–6. https://doi.org/10.1093/ajcn/74.1.50.
Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, Wu Y, Chen JL, Kang C, Shu FR, Zhang QY. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS ONE. 2015;10(7):e0133496. https://doi.org/10.1371/journal.pone.0133496.
Yang B, Shi MQ, Li ZH, Shi L, Wang AM, Guo XJ, Li D. Effects of n-3 fatty acid supplements on cardiometabolic profiles in hypertensive patients with abdominal obesity in Inner Mongolia: a randomized controlled trial. Food funct. 2019;10(3):1661–70. https://doi.org/10.1039/C8FO01707G.
Thusgaard M, Christensen JH, Mørn B, Andersen TS, Vige R, Arildsen H, Schmidt EB, Nielsen H. Effect of fish oil (n-3 polyunsaturated fatty acids) on plasma lipids, lipoproteins and inflammatory markers in HIV-infected patients treated with antiretroviral therapy: a randomized, double-blind, controlled study. Scand J Infect Dis. 2009;41(10):760–6. https://doi.org/10.1080/00365540903168056.
Gao C, Liu Y, Gan Y, Bao W, Peng X, Xing Q, Gao H, Lai J, Liu L, Wang Z, Yang Y. Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2020;19:1. https://doi.org/10.1186/s12944-020-01214-w.
Wu S, Zhu C, Wang Z, Wang S, Yuan P, Song T, Hou X, Lei Z. Effects of fish oil supplementation on cardiometabolic risk factors in overweight or obese children and adolescents: a meta-analysis of randomized controlled trials. Front Pediatr. 2021;9:604469. https://doi.org/10.3389/fped.2021.604469.
Zhu W, Dong C, Du H, Zhang H, Chen J, Hu X, Hu F. Effects of fish oil on serum lipid profile in dialysis patients: a systematic review and meta-analysis of randomized controlled trials. Lipids health and dis. 2014;13(1):1–1. https://doi.org/10.1186/1476-511X-13-127.
Weinberg RL, Brook RD, Rubenfire M, Eagle KA. Cardiovascular impact of nutritional supplementation with omega-3 fatty acids: JACC focus seminar. J Am Coll Cardiol. 2021;77(5):593–608. https://doi.org/10.1016/j.jacc.2020.11.060.
Suzan AJ, Garcia PH, Furlan CP, Barba FC, Franco YE, Longato GB, Contesini FJ, de Oliveira CP. Oxidative stability of fish oil dietary supplements and their cytotoxic effect on cultured human keratinocytes. Nfs J. 2022;29:1–7. https://doi.org/10.1016/j.nfs.2022.09.002.
Bai G, Ma CG, Chen XW. Effect of unsaturation of free fatty acids and phytosterols on the formation of esterified phytosterols during deodorization of corn oil. J Sci Food Agri. 2021;101(7):2736–43. https://doi.org/10.1002/jsfa.10900.
Ghobadi S, Hassanzadeh-Rostami Z, Mohammadian F, Nikfetrat A, Ghasemifard N, Raeisi Dehkordi H, Faghih S. Comparison of blood lipid-lowering effects of olive oil and other plant oils: a systematic review and meta-analysis of 27 randomized controlled clinical trials. Crit Rev Food Sci Nutr. 2019;59(13):2110–24. https://doi.org/10.1080/10408398.2018.1438349.
Sherratt SC, Mason RP. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by X-ray diffraction. Chem physics lipids. 2018;212:73–9. https://doi.org/10.1016/j.chemphyslip.2018.01.002.
Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–67. https://doi.org/10.1016/j.jacc.2011.06.063.
Tummala R, Ghosh RK, Jain V, Devanabanda AR, Bandyopadhyay D, Deedwania P, Aronow WS. Fish oil and cardiometabolic diseases: recent updates and controversies. Am J Med. 2019;132(10):1153–9. https://doi.org/10.1016/j.amjmed.2019.04.027.
Shearer GC, Savinova OV. Harris WS (2012) Fish oil—how does it reduce plasma triglycerides? Biochim Biophys Acta Mol Cell Biol Lipids. 1821;5:843–51. https://doi.org/10.1016/j.bbalip.2011.10.011.
Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17(4):387–93. https://doi.org/10.1097/01.mol.0000236363.63840.16.
Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. 2001;131(4):1129–32. https://doi.org/10.1093/jn/131.4.1129.
Funding
The authors reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Contributions
AM, conceptualization, investigation, and supervision. PS, methodology, writing — review & editing, and resources. GB, methodology, data collection, formal analysis, and writing — review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Safaei, P., Bayat, G. & Mohajer, A. Comparison of fish oil supplements and corn oil effects on serum lipid profile: a systematic review and meta-analysis of randomized controlled trials. Syst Rev 13, 54 (2024). https://doi.org/10.1186/s13643-023-02426-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02426-8