Abstract
Background
Medication adherence has a major impact on reducing mortality and healthcare costs related to the treatment of cardiovascular diseases and diabetes mellitus. Selecting the best patient-reported outcome measure (PROM) among the many available for this kind of patient is extremely important. This study aims to critically assess, compare and synthesize the quality of the measurement properties of patient-reported outcome measures to assess medication adherence among patients with cardiovascular diseases and/or type 2 diabetes mellitus.
Methods
This review followed the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) guidelines and was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). The searches were performed in Web of Science, SCOPUS, PubMed, CINAHL, EMBASE, LILACS, PsycINFO, and ProQuest (gray literature).
Results
A total of 110 records encompassing 27 different PROMs were included in the review. The included records were published between 1986 and 2023, most of which reported studies conducted in the United States and were published in English. None of the PROMs were classified in the category “a”, thus being recommended for use due to the quality of its measurement properties. The PROMs that should not be recommended for use (category “c”) are the MTA, GMAS, DMAS-7, MALMAS, ARMS-D, and 5-item questionnaire. The remaining PROMs, e.g., MMAS-8, SMAQ, MEDS, MNPS, ARMS-12, MGT, MTA-OA, MTA-Insulin, LMAS-14, MARS-5, A-14, ARMS-10, IADMAS, MAQ, MMAS-5, ProMAS, ARMS‐7, 3-item questionnaire, AS, 12-item questionnaire, and Mascard were considered as having the potential to be recommended for use (category “b”).
Conclusion
None of the included PROMs met the criteria for being classified as trusted and recommended for use for patients with cardiovascular diseases and/or type 2 diabetes mellitus. However, 21 PROMs have the potential to be recommended for use, but further studies are needed to ensure their quality based on the COSMIN guideline for systematic reviews of PROMs.
Systematic review registration
PROSPERO CRD42019129109
Similar content being viewed by others
Background
Medication adherence has a major impact on reducing mortality and healthcare costs related to the treatment of noncommunicable diseases (NCDs), especially cardiovascular diseases (CVDs) and diabetes mellitus [1,2,3].
Data from 2019 by the World Health Organization (WHO) show that 7 of the top 10 causes of death in the world are noncommunicable diseases (NCDs) [4]. Ischemic heart disease is the leading cause of death and the top 10 causes of death also include stroke, hypertensive heart disease, and diabetes mellitus [5]. The United Nations General Assembly established the reduction of premature mortality from NCDs by one-third as a target for 2030 [6].
Since many patients do not adhere to treatment as prescribed [7, 8] it is paramount to properly measure medication adherence and to take actions that increase patient’s adherence. Medication adherence involves a complex set of behaviors that are influenced by a number of psychosocial determinants such as motivation, self-efficacy, beliefs, and perceived barriers, which makes its measurement particularly challenging [9].
One of the most practical and low-cost ways to assess medication adherence is through the use of measures of patient-reported outcomes (PROs), i.e., any aspect of a patient's health status that is directly assessed by the patient, without interpretation of their response by anyone other than themselves [10]. Patient-Reported Outcome Measures (PROMs) range from simple single-item measures of omitted medication doses to multi-item instruments that aggregate reasons for non-adherence.
The task of selecting the best PROM among the many available for measuring medication adherence in patients with CVDs or type 2 diabetes mellitus (T2DM) [11, 12] requires taking into consideration its conceptual structure and measurement properties.
The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) initiative has recently published a guideline for conducting systematic reviews of studies evaluating the measurement properties of PROMs [13]. This guideline proposes the criteria to assess the methodological quality of studies on measurement properties and the quality of the self-reported measurement itself.
There are systematic reviews evaluating the quality of the measurement properties of medication adherence PROMs in patients with diabetes mellitus using the COSMIN checklist [14,15,16]. However, in these systematic reviews, primary studies using PROMS to measure factors related to medication non-adherence, such as beliefs, self-efficacy, satisfaction, among others, were included. To our knowledge, no systematic review has been conducted according to the COSMIN guidelines to evaluate the quality of the measurement properties of PROMs that exclusively measure medication adherence in patients with CVDs and/or T2DM.
Therefore, this systematic review aims to critically assess, compare, and synthesize the quality of the measurement properties of PROMs for medication adherence among patients with CVDs and/or T2DM.
Methods
Protocol development
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [17] (checklist available in Additional file 1) and the COSMIN guidelines for systematic reviews on PROMs [13]. The protocol of this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42019129109) and published elsewhere [18].
Eligibility criteria
Inclusion criteria:
-
Studies that aimed to develop or culturally adapt a PROM to measure medication adherence among patients aged 18 or older with a CVD and/or T2DM, regardless of the language and date of publication;
-
Studies reporting the assessment of one or more properties of the PROMs.
Exclusion criteria:
-
Studies in which a PROM was used to measure an outcome (e.g., randomized clinical trials);
-
Studies in which a PROM was used to validate another measure;
-
Studies that evaluated the measurement properties of PROMs that aimed to evaluate factors related to medication nonadherence (self-efficacy, beliefs, intention, etc.);
-
Study that did not provide minimally sufficient data on the results of the investigated measurement properties, even after contacting the authors.
Sources and search strategy
The electronic literature searches were performed in July 2020 in the following databases without time limits: Web of Science, Scopus, PubMed (including Medline), CINAHL, EMBASE, LILACS, PsycINFO, and ProQuest (gray literature). Manual searches were performed in the reference lists of the articles in order to complement the main literature. An update of the searches was performed in May 2023 considering the period from 2020 to 2023. The search strategy was based on the second version of the search filter for measurement properties proposed by the COSMIN initiative [19] and also included keywords and MeSH terms related to CVDs, T2DM, PROMs, medication adherence, and measurement properties. The search strategy used in each database was created with the support of an experienced librarian and can be found in Additional file 2. The online software Rayyan QCRI was used for reference management which included the exclusion of duplicates and the evaluation of titles and abstracts [20].
Study selection
The study selection was reported according to the PRISMA flow diagram model [17]. The evaluation of titles and abstracts after the exclusion of duplicates was done independently by three pairs of reviewers (HCO, DH, SDLC, RCLB, MLSN, and CRSA) following a practice set of 50 titles and abstracts to improve inter-reviewer agreement. Inter-reviewer agreement ranged from 96 to 98%, with an overall agreement rate of 94%. Two reviewers independently appraised full-texts for inclusion (HCO and DH). Disagreements were discussed until a consensus was reached. Lastly, the list of references of the included studies was examined to identify other studies that had not been previously identified.
Data extraction
Data were independently extracted by two reviewers (HCO and RCMR) using an adapted version of the extraction form available in the COSMIN manual for systematic reviews of PROMs [21] which included additional fields for other relevant information. The form contains information about the study design, sample size, participants’ demographic and clinical characteristics (gender, age, disease, disease duration, and number of medications in use), response rate, presence of conflicts of interest, funding, setting, country, and language, PROMs’ number of items and domains, mode of administration, recall period, response options, range of scores, original language, available translations, number of studies evaluating the PROM, measurement properties (PROM development, content validity, structural validity, internal consistency, cross-cultural validity/measurement invariance, reliability, measurement error, criterion validity, hypothesis testing for construct validity and responsiveness), interpretability and feasibility and information to assess the studies’ methodological quality.
Methodological quality of the studies: assessment of risk of bias
The methodological quality of the studies was assessed independently by two reviewers (HCO and RCMR) using the COSMIN Risk of Bias checklist for systematic reviews of PROMs [22, 23]. This checklist comprises items that assess the methodological quality of studies that evaluate the measurement properties of PROMs. Disagreements were discussed until a consensus was obtained and a third reviewer (NMCA) was consulted when the reviewers were among the authors of the evaluated paper. According to the COSMIN Risk of Bias checklist, items can be rated as 'very good', 'adequate', 'doubtful', 'inadequate', or 'not applicable' (NA), and each measurement property receives an overall rating based on the worst scored item [21, 23].
Quality of the measurement properties
For each PROM, the quality of each measurement property reported by the included studies was assessed.
These results were assessed independently by two reviewers (HCO and RCMR) based on the quality criteria for good measurement properties proposed by COSMIN [21, 23]. Disagreements were discussed until a consensus was obtained. A third reviewer (NMCA) was consulted when the reviewers were among the authors of the evaluated paper. The quality of the measurement properties of each assessed PROM was classified as sufficient ( +), insufficient (-), inconsistent ( ±), or indeterminate (?), according to the proposed criteria [13, 21,22,23] (Table 1).
When evaluating the exploratory factor analysis (EFA) in structural validity, we established a different criterion from what was previously defined by the COSMIN team [24]. The COSMIN criteria consider a sufficient result in an EFA when the first factor accounts for at least 20% of the variability and the ratio of the variance explained by the first to the second factor is greater than four [24]. Since most PROMs included in our systematic review are one-dimensional, we considered a sufficient result when the total variance explained was at least 60% and when factor loadings were equal to or greater than 0.30 [25]. In addition to the structural features of PROMS, our decision also considered the recommendation of the COSMIN manual that new criteria may be proposed by reviewers if those established by the COSMIN team do not fully meet the evaluation of one or more properties of the measure [21].
When assessing the criterion validity, it was considered that the statistical results obtained in the assessment of the relationship between the PROM and the direct objective measures would be treated as a criterion validity result. Additionally, the statistical results obtained when evaluating the relationship between the PROMs and the direct objective measures of glycosylated hemoglobin and glycaemia (used to assess metabolic control in DM) and the measures of systolic and diastolic blood pressure (used for the control of blood pressure in hypertension) were treated as a result of criterion validity, regardless of how the authors named such validity in the primary studies.
Also in the criterion validity evaluation, when dichotomous variables are evaluated, it is recommended to apply sensitivity and specificity measures, according to the risk of bias checklist, to evaluate these results. However, the guideline does not establish the reference values for the sensitivity and specificity measures for the attribution of the quality of the results. For this reason, the sensitivity and specificity results observed in the primary studies were not considered for assigning the quality ratings of the criterion validity results.
Data synthesis
Meta-analysis was performed to pool the results of internal consistency of the PROMs, estimated by Cronbach's alpha coefficient [26]. The analysis was performed considering a random effects model and a significance level of 5%. At first, the Cronbach's alphas values of each study were transformed to Fisher's ɀ values according to the following equation [26]:

The next step was to calculate the average of ɀ weighting according to the sample size of the studies (nj) [26]:

The final step was the conversion of the average weighted ɀ to the estimated value of the pooled Cronbach's alpha coefficient [26]:

These calculations were performed in the software SPSS 23 using an SPSS Meta-Analysis Macro [27]. The heterogeneity was evaluated using the Chi-squared test and the I2 coefficient.
Quality of evidence: adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) by COSMIN
The overall quality of evidence of all studies was assessed using an adapted version of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) proposed by the COSMIN initiative [13]. The quality of the evidence was classified as high, moderate, low, or very low based on the following factors: risk of bias, inconsistency (of the results of the studies), imprecision (related to the sample size of the studies), and indirect results (the evidence comes from the studies that were performed in a population or context other than the ones defined in the review) [21].
The risk of bias could be classified as serious, very serious, or extremely serious, resulting in the downgrade of 1 to 3 levels, respectively (Table 2) [21].
Regarding the inconsistency, when the reviewers could not find an explanation for inconsistent results observed across the studies, these results are considered inconsistent. Consequently, the quality of evidence is not applicable. Concerning the imprecision, when the total sample size of the summarized studies is lower than 100 (serious), one level must be downgraded and when it is lower than 50 (very serious) two levels must be downgraded. For indirectness, the reviewers can downgrade the level of evidence by one or two levels.
Thus, according to the GRADE approach, it was initially assumed that the summarized results were of high quality and, subsequently, downgraded by one or two levels per factor, considering the following aspects: risk of bias, inconsistency, imprecision, or indirect results. When the evidence was based on only one inadequate study (extremely serious risk of bias) quality of evidence was downgraded by three levels [21].
The results were assessed independently by two reviewers (HCO and RCMR). Disagreements were discussed until a consensus was obtained and a third reviewer (NMCA) was consulted when the reviewers were among the authors of the evaluated paper.
Recommendations for selecting a PROM
The review's final stage was the establishment of recommendations to select the most appropriate PROM. PROMs were classified into three categories:
-
(a)
PROMs that presented sufficient content validity and at least low quality of evidence for sufficient internal consistency;
-
(b)
PROMs that are not classified in categories (a) or (c);
-
(c)
PROMs that presented high-quality evidence for an insufficient measurement property.
A PROM that falls under category (a) means it is reliable and can be recommended. A PROM that falls under category (b) means it has the potential to be recommended, though further studies are needed to ensure its quality. A PROM classified under category (c) should not be recommended.
Results
Study selection and data extraction
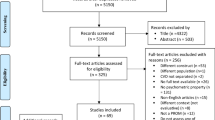
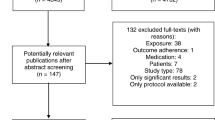
The results of the selection and data extraction of the studies are presented in the PRISMA flow diagram (Fig. 1). The searches done in July 2020 resulted in a total of 41,886 records published between 1973 and June of 2020 were considered potentially eligible and retrieved from eight databases. A total of 14,826 duplicates were removed. The titles and abstracts of 27,060 records were peer-reviewed by three pairs of peer reviewers, who evaluated 9,020 records each. A total of 336 records were identified for full-text assessment and 84 records were included. Eight additional relevant records were added after manually searching the lists of references from the included studies.
PRISMA flow diagram. Note: ARMS = Adherence to Refills and Medication Scale; AS = Adherence Scale; DMAS-7 = 7-item Diabetes Medication Adherence Scale; GMAS = General Medication Adherence Scale; IADMAS = Iraqi Anti-Diabetic Medication Adherence Scale; LMAS-14 = Fourteen-item Lebanese Medication Adherence Scale; MALMAS = Malaysian Medication Adherence Scale; MAQ = Medication Adherence Questionnaire; MARS-5 = 5-item Medication Adherence Report Scale; Mascard = Medication Adherence Scale in Cardiovascular disorders; MEDS = Medication Adherence Estimation and Differentiation Scale; MGT = Morisky-Green test; MMAS-5 = 5-item adapted Morisky Medication Adherence Scale; MMAS-8 = 8-item Morisky Medication Adherence Scale; MNPS = Medication Non-persistence Scale; MTA = Measurement of Treatment Adherence; MTA-Insulin = Measurement of Treatment Adherence—Insulin; MTA-OA = Measurement of Treatment Adherence—Oral Antidiabetics; PROM = Patient-reported outcome measures; ProMAS = Probabilistic Medication Adherence Scale; SMAQ = Simplified Medication Adherence Questionnaire
The update done in May 2023 resulted in 11,538 records published between 2020 and 2023 where 4,370 duplicates were removed. Out of 52 records assessed for full-text, 18 records were included resulting in a total of 110 records and 27 PROMs included in the systematic review (Fig. 1).
Study and PROMs characteristics
The included studies were published between 1986 and 2023, and most of them were conducted in the United States in the English language (n = 19). The sample size of included studies ranged from 30 to 6,261 participants. The percentage of females ranged from 17.0% to 79.3% and the mean age ranged from 43.1 to 81.9 years. About half of the studies were conducted in hospital settings (n = 54) and observed an average disease duration of 9.8 years (n = 26) and an average of 4.5 medications in use (n = 29).
Most of the 27 PROMs included in the review are one-dimensional and composed of items with a Likert-type scale response. A total of 39 studies [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] conducted with patients who only had TD2M, another 47 studies [67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] with patients who only had CVD, and the remaining 24 studies with patients having both T2DM and/or CVD [114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137]. The most prevalent original language of the 27 PROMs included in this review was English (n = 15). In addition to the original versions, translated versions of the PROMs were also included in the review. Of 27 PROMs included, 10 had been translated into at least another language. Regarding the application characteristics, for the majority of PROMs (n = 20) it was not clear what the recall period was (Table 3). It was not possible to describe the time to complete the PROMs because the majority of the studies did not present this information.
A total of 38 studies reported a response rate for PROMs that ranged from 21.1% to 100.0%. Out of the 110 records, only 63.6% of the studies presented information about conflict of interests, and 68.2% informed if the research had any source of funding (Additional file 3).
The two reviewers (HCO and RCMR) are authors of one of the included studies [120]. The analysis of this article was done by a third reviewer (NMCA).
Evidence synthesis
The summary of findings of measurement properties of the PROMs is presented in Table 4. The summary of the assessment of risk of bias can be found in the Additional files 4 and 5. The results based on each measurement property of the PROMs are presented below.
Content validity
The content validity resulted in overall ratings per PROM for relevance, comprehensiveness and comprehensibility, and overall content validity of the PROM. The indeterminate ratings for development or content validity studies were ignored in the overall rating assignment (n = 30). All the PROM development studies were classified as having inadequate methodological quality except the studies that developed the PROMs ProMAS [130]. and Mascard [113]. which were classified as having doubtful methodological quality. Very few studies assessed the target population's comprehensibility of the developed items through cognitive interviews or debriefing [38, 123]. According to the COSMIN, the criteria for recommending the use of a PROM is based on a sufficient content validity and at least low quality of evidence for internal consistency. The content validity encompasses the evaluation of aspects such as relevance, comprehensiveness, and comprehensibility of the PROM. The comprehensibility was the most often evaluated aspect in the records, but even studies that evaluated it had done so incompletely. Most of the studies assessed comprehensibility of the items [28, 41, 47, 57, 67, 68, 73, 76, 86, 88, 91, 93, 100, 104, 108, 113, 132, 134, 136], but only in a few of the studies participants were asked about the comprehensibility of response options or recall periods [28, 44, 91, 105, 106]. Relevance and comprehensiveness of PROMS were rarely evaluated among patients [32, 52, 67, 105] and expertise committee [32, 52, 61, 66, 105, 113, 114, 128]. In some aspects the PROMs, overall rating was based only on the rating of the reviewers. The evaluation of the risk of bias of the development and content validity studies resulted in studies being rated as doubtful or inadequate because some of the criteria evaluated in the COSMIN checklist were not clearly described in the records.
The PROM MGT showed moderate-quality evidence for sufficient content validity and the PROMs MMAS-8, MTA-OA, MTA – Insulin, MARS-5, ARMS-12, MTA, ARMS-7, MEDS, IADMAS, GMAS, ProMAS, A-14, 12-item questionnaire, and Mascard showed showed inconsistent content validity. The remaining PROMs included in the review did not have their content validity evaluated in the selected papers.
Structural validity
The EFA was the most commonly applied statistical method in the evaluation of structural validity of the PROMs (ARMS-7, ARMS-10, ARMS-12, ARMS-D, DMAS-7, GMAS, LMAS-14, MARS-5, MGT, MMAS-8, SMAQ, and Mascard), [29, 31, 35, 37, 39, 40, 44, 46, 47, 50,51,52, 55, 57, 61, 62, 67, 68, 71, 75, 79, 81, 86,87,88, 93, 100, 106, 111, 113, 117, 119, 121, 123, 125, 127,128,129, 132, 133] followed by the confirmatory factor analysis (ARMS-7, ARMS-12, GMAS, MGT, MEDS, MMAS-8, MNPS, MTA, and MARS-5), [46, 52, 55, 57, 65, 72, 73, 80, 87, 88, 108, 114, 115, 119, 128, 129, 132,133,134] and the item response theory (MARS-5, GMAS, MMAS-8, and ProMAS) [28, 35, 92, 130, 135].
Regarding the assessment of the methodological quality of EFA, some studies were classified as having doubtful quality, since they did not report the rotation method used in the analysis [29, 39, 81, 86, 117].
In the evaluation of the EFA, some studies were classified as indeterminate because they did not report the percentage of variance explained [29, 35, 39, 40, 63, 81, 93, 117, 133] or the factor loadings [35, 81, 121]. One study did not report the results of the indices used to evaluate the confirmatory factor analysis [88] and another study [35] did not present the results of the indices of the item response theory analysis.
The PROMs MEDS, MNPS, GMAS, ProMAS, and ARMS-7 showed high-quality evidence for sufficient structural validity. The PROMs DMAS-7, MARS-5, ARMS-D, and ARMS-10 showed moderate-quality evidence for sufficient structural validity. Moderate and high-quality evidence for insufficient structural validity was observed for the Mascard and MTA, respectively.
However, the structural validity of the MMAS-8, MGT, LMAS-14, and the ARMS-12 were classified as inconsistent. The MMAS-8 presented results with one or two-factor solutions and also sufficient, insufficient, and indeterminate ratings. Similarly, the ARMS-12 presented sufficient and insufficient results in two or three-factor solutions, while the MGT presented only one-dimensional solution, but with sufficient, insufficient, and indeterminate ratings. LMAS-14 presented three or four -factor solutions. An overall rating indeterminate was attributed to SMAQ, since the included studies for this PROM were classified as indeterminate [29, 63]. The remaining PROMs included in the systematic review did not have their structural validity evaluated in the selected records.
Internal consistency
Regarding the analysis of the internal consistency property, the original factor structure of the PROM was considered in order to evaluate if Cronbach's alpha should be calculated for the total scale and or subscales or domains. One included study [91] of the PROM MMAS-8 was classified as of doubtful methodological quality, since the authors excluded four items from the PROM because of the low Cronbach's alpha coefficient obtained, without considering other reliability or validity estimates. In two studies for the PROM A-14 [74, 131] it was not clear the number of the subscales of the PROM and in another three studies [29, 65, 75] that used the PROMs ARMS-10, SMAQ, and GMAS, the Cronbach’s alpha was not calculated for each of the subscales of the PROMs.
Four PROMs (MEDS, MNPS, ARMS-D, and ProMAS) showed high-quality evidence for sufficient internal consistency. However, very low-quality evidence for sufficient internal consistency for the ARMS-10 it was observed, while the PROMs DMAS-7 and ARMS-7 showed moderate quality evidence for insufficient internal consistency. Also GMAS showed low quality evidence for insufficient internal consistency. The internal consistency of the 15 PROMs (MMAS-8, SMAQ, ARMS-12, MGT, MTA-OA, MTA-Insulin, LMAS-14, MTA, A-14, MALMAS, IADMAS, MAQ, AS, 12-item questionnaire, and Mascard) were classified as indeterminate. The PROM MARS-5 had its internal consistency classified as inconsistent. The remaining PROMs included in the review did not have their internal consistency evaluated in the selected papers.
Reliability
All included studies that evaluated the reliability of the PROMs (ARMS-7, ARMS-10, ARMS-12, GMAS, IADMAS, MALMAS, MAQ, MARS-5, MGT, and MMAS-8) were classified as of doubtful or inadequate methodological quality [33, 34, 38, 41, 45,46,47, 50, 52, 54, 55, 61, 62, 69, 75,76,77, 79, 82, 86,87,88, 93, 94, 97, 104, 117, 119, 123, 127,128,129, 132, 136] and did not provide enough data to address items 4 (“Did the professional(s) administer the measurement without knowledge of scores or values of other repeated measurement(s) in the same patients?”) and 5 (“Did the professional(s) assign scores or determine values without knowledge of the scores or values of other repeated measurement(s) in the same patients?”) of the risk of bias checklist [138]. The other included studies [46, 47, 52, 60, 86,87,88, 93, 117, 127] were classified as inadequate, since the evaluation of the item 3 of the risk of bias checklist (“Were the measurement conditions similar for the repeated measurements – except for the condition being evaluated as a source of variation?”) was considered inadequate.
Considering that the statistical analyses recommended to estimate reliability were intraclass correlation coefficient (ICC) and kappa, the results of some studies [33, 34, 38, 41, 45, 52, 54, 69, 76, 77, 79, 86, 117, 119, 123, 128, 136] were classified as indeterminate because Spearman's or Person's correlation coefficients were used to estimate the reliability of PROMs.
Regarding the best evidence of the reliability, the PROMs ARMS-10, ARMS-12, and MMAS-8 showed low-quality evidence for sufficient reliability, while the PROMs MAQ and ARMS-7 presented very low-quality evidence for sufficient reliability. The PROM GMAS showed moderate-quality evidence for insufficient reliability. The reliability of the PROMs MGT, MARS-5, MALMAS, and IADMAS were classified as indeterminate.
A meta-analysis to the reliability results was not performed, because the included studies did not show good methodological quality, according to the COSMIN guideline.
Criterion validity
The analyses applied in the evaluation of the criterion validity were area under the curve, sensitivity, specificity, and some hypothesis tests. Some of the included studies that did not report sensitivity and specificity analyses of the PROMs ARMS-12 [44, 106, 123], GMAS [128, 129], MARS-5 [117], MMAS-8 [43, 121], and SMAQ [29], and one study regarding the PROM LMAS-14 [71] that did not provide the area under the curve analysis were classified as of inadequate methodological quality.
The PROMs DMAS-7, MTA, MALMAS, ARMS-D, and 5-item questionnaire showed high-quality evidence for insufficient criterion validity. MMAS-8 and MGT showed low-quality evidence for insufficient criterion validity. IADMAS and SMAQ presented moderate and very low-quality evidence for insufficient criterion validity, respectively. ARMS-12 and MARS-5 presented inconsistent results and MEDS, MNPS, LMAS-14, GMAS, 3-item questionnaire, and Mascard had its criterion validity classified as indeterminate. The criterion validity was not evaluated in the included papers regarding the remaining PROMs included in the review.
Hypotheses testing
There were four included studies in which the methodological quality regarding the convergent validity of the PROMs was considered inadequate. Two of them that used the MMAS-8 [50, 103] were rated as inadequate because the comparator instrument had insufficient measurement properties. In the other two studies [32, 71] that used the PROMs LMAS-14, MTA-OA, and MTA-Insulin, the statistical tests applied were not optimal or appropriate. One study that used MALMAS was classified as having indeterminate quality, because the PROMs being correlated were not applied to the same participants [33].
Concerning the known‐groups validity, there was one included study for the PROM GMAS [128] that did not provide a description of the important characteristics of the groups being compared.
The analysis applied by the included studies were mainly correlation coefficients, regression models, and comparison and association tests.
The PROMs MEDS, DMAS-7, ARMS-12, A-14, ARMS-D, ProMAS, 5-item questionnaire, and AS showed high-quality evidence for sufficient construct validity. IADMAS and GMAS showed moderate-quality evidence for sufficient construct validity. LMAS-14 and MALMAS showed low-quality evidence for sufficient construct validity. MTA-OA and MTA-Insulin presented very low-quality evidence for sufficient construct validity. The PROMs MMAS-8, MGT, MTA, and MARS-5 showed inconsistent construct validity. The remaining PROMs included in the review did not have their construct validity evaluated in the selected papers.
Responsiveness
Responsiveness was evaluated only for an adapted version of MMAS-8 composed by 5 items, nominated in this systematic review as MMAS-5, in a single study [98]. The study reported very good methodological quality and high-quality evidence for sufficient responsiveness.
Meta-analysis
The meta-analysis was performed to pool the results of Cronbach’s Alpha of the included studies. Considering a Cronbach's Alpha equal to or higher than 0.7 to be satisfactory [13], the PROM MARS-5 showed high-quality evidence for sufficient internal consistency. GMAS showed high-quality evidence for insufficient internal consistency. The PROMs MMAS-8, ARMS-12, MTA, and MGT were classified as indeterminate because their structural validity was classified as inconsistent. The MALMAS was classified as indeterminate because it did not have its structural validity evaluated in the included studies. Values of I2 equal or higher than 50% and 75% indicates the presence of moderate and high heterogeneity, respectively [139]. Moderate or high heterogeneity was observed in the PROMs MMAS-8, ARMS-12, MGT, MARS-5, and GMAS (Table 5).
The graphical representation of the pooled Alpha results for each of the included PROM in the meta-analyzes are shown in the Fig. 2.
Pooled Cronbach's alpha estimates of the PROMs included in the meta-analyses. Note: ARMS = Adherence to Refills and Medication Scale; GMAS = General Medication Adherence Scale; MALMAS = Malaysian Medication Adherence Scale; MARS-5 = 5-item Medication Adherence Report Scale; MGT = Morisky-Green test; MMAS-8 = 8-item Morisky Medication Adherence Scale; MTA = Measurement of Treatment Adherence; PROM = Patient-reported outcome measures
Interpretability and Feasibility
It was not possible to identify the information needed to evaluate the interpretability and feasibility in most of the included records. Considering that the evaluation of these aspects would be incomplete because of the lack of information, the reviewers decided not to evaluate these aspects.
Recommendations for selecting a PROM
According to the results of our systematic review, none of the evaluated PROMs reached the criteria of category “a”, i.e., the results obtained across the studies can be trusted and the PROM can be recommended for use.
The PROMs MTA, GMAS, DMAS-7, MALMAS, ARMS-D, and 5-item questionnaire were categorized as not recommended for use (category “c”), because they presented high-quality evidence for at least one insufficient measurement property.
The remaining PROMs, i.e., MMAS-8, SMAQ, MEDS, MNPS, ARMS-12, MGT, MTA-OA, MTA-Insulin, LMAS-14, MARS-5, A-14, ARMS-10, IADMAS, MAQ, MMAS-5, ProMAS, ARMS‐7, 3-item questionnaire, AS, 12-item questionnaire, and Mascard were considered as having the potential to be recommended for use (category “b”) because they did not reach the criteria of the categories “a” or “c”.
Discussion
The objective of this systematic review was to critically assess, compare, and synthesize the quality of the psychometric properties of PROMs for the assessment of medication adherence among patients with CVDs and/or T2DM.
To our knowledge, this is the first systematic review to assess the quality of the measurement properties of instruments that exclusively measure medication adherence using the COSMIN guideline. The results obtained allowed the identification of which instruments presented the best measurement properties in the population considered in this review. According to the COSMIN guidelines used in this systematic review, of the 27 PROMs extracted from the 110 studies included, none of the PROMs were recommended for use, 21 PROMs were considered to have the potential to be recommended and 6 PROMs were not recommended for use, as they did not meet the minimum criteria, i.e., demonstrated sufficient content validity and at least low quality of evidence for internal consistency. The summarized results of the meta-analysis and quality of evidence of internal consistency showed that, based on the results of three studies, only the MARS-5 has a high-quality evidence for a sufficient internal consistency.
As mentioned before, no systematic review was found in the literature evaluating the quality of the measurement properties of medication adherence PROMs, according to COSMIN guidelines specifically in patients with CVDs and/or T2DM. A recent study [140] analyzed systematic reviews in order to assess scope, validity, and reporting of PROMS of medication adherence in patients with T2DM. However, as previously noted, it included systematic reviews that do not specifically assess medication adherence and included studies that assessed factors related to medication adherence (self-efficacy, for an example), and reviews that used the PROM to evaluate interventions to promote medication adherence and did not apply a robust tool such as the COSMIN initiative guidelines for evaluating studies assessing the measurement properties of PROMs.
The main result of this review of reviews [140] was identifying that many PROMs have been translated into other languages without first presenting minimally adequate measurement properties in previous validation studies. It also pointed out that in some studies, the PROM was applied to a population without having been translated into the language of the country. The authors suggested that translated and adapted versions of PROMs that might in some way affect their items and/or subscales should be categorized separately from PROMs in their original format. In our review, studies of adapted versions of the PROMs were included, but they were not categorized separately. This may be a topic for future investigation of the PROMs evaluated in this review.
The findings of this systematic review about summarizing the data on the content validity of medication adherence PROMs showed that there were deficits and high heterogeneity of data in the included studies that investigated this property of the measure. The checklist for evaluation of methodological quality and the criteria for evaluation of the results related to the content validity of the PROMs proposed by the COSMIN initiative [141] are detailed in a large set of items, which were not covered by most of the studies included in the review. The reporting and data on the content validity of the included PROMs were extremely brief, with little information about the procedures performed, which hindered an adequate evaluation of this measurement property.
One difficulty observed in the evaluation of content validity was the absence of detailed information about the evaluation of relevance and comprehensiveness by the Expert Committee, as well as the comprehensibility by the target population in primary studies. Most of the included studies did not inform which one was investigated, and when they informed it, there was great heterogeneity in the way they were evaluated, implying inconsistent content validity. Our findings are congruent with a previous systematic review and meta-analysis that used the COSMIN guidelines to evaluate the evidence on measurement properties of the Hip disability and Osteoarthritis Outcome Score—Physical function Shortform (HOOS-P) and the Knee Injury and Osteoarthritis Outcome Score Physical function Shortform (KOOS-PS) [142], in which aspects such as the appropriateness of the response options and recall period and the relevance of the construct and context of the use were not evaluated in the primary studies, as in our review.
The internal consistency is an important measurement property that was included as an essential criterion to determine the recommendation for use of the PROM. However, the internal consistency of 15 PROMs in the included studies was rated as indeterminate. These results can be explained by the absence of at least low evidence for sufficient structural validity for these PROMS or because the structural validity of these measures was not performed in the included studies. The results of the meta-analysis and quality of evidence of internal consistency of the seven PROMS included in this analysis showed that only the MARS-5 has high-quality evidence for sufficient internal consistency, GMAS showed high-quality evidence for insufficient internal consistency and the other five PROMs showed indeterminate results. The meta-analysis resulted in many indeterminate results because of the structural validity results of the PROMs ARMS-12, MALMAS, MAT, MGT, and MMAS-8. The PROM ARMS-12 presented a pooled alpha that would result in a sufficient overall rating, but the structural validity limited this evaluation.
Regarding the evaluation of the structural validity, the PROMs MMAS-8, ARMS-12, MGT, and LMAS-14 were rated as inconsistent. This rating was attributed because different factor solutions were observed, i.e., different numbers of factors across the included records. Furthermore, four PROMs (MGT, MARS-5, MALMAS, and IADMAS) had their reliability rated as inconsistent because the results of the included records applied different coefficients (e.g. Pearson correlation coefficient) from the ones considered in the criteria stablished by the COSMIN initiative, i.e., ICC or Kappa.
As previously described, in the evaluation of the criterion validity, the review team considered that the obtained statistical results in the assessment of the relation between the PROMs and objective measures should be treated as a result of criterion validity, despite how the authors considered it in the primary studies. The reviewers considered that this change in the evaluation of the results would be beneficial as it would produce a standardization in the assessments. The findings showed that most of the evaluated PROMs presented an insufficient criterion validity and a sufficient overall rating in the hypothesis testing evaluation.
Another measurement property that had its evaluation compromised was the reliability. As previously mentioned, some items of the checklist for evaluation of the methodological quality were not described in the included studies, which resulted in studies being rated as having doubtful or inadequate methodological quality.
The evaluation of the measurement properties of the PROM’s included in this review indicated that none of the included PROMs could be considered trusted and recommended for use according to the criteria proposed by the COSMIN initiative. These results can be explained by the complexity of the medication adherence construct itself, which has made it challenging for researchers to obtain a PROM with good measurement properties [9]. The second aspect refers to the number of included studies in which each selected PROM was used. The evaluation of the measurement properties of a given PROM in several studies included in this review contributed to some measurement properties being rated as inconsistent because of the observed heterogeneity in the results of that PROM. The MMAS-8, for example, was the PROM for which the measurement properties were evaluated in 37 studies included in this review. Therefore with this large number of studies, heterogeneous results were likely for MMAS-8, which contributed to this PROM not being classified in the "a" category of recommendations. The other aspect that had a huge impact on the results was the evaluation of content validity, since this was one of the major gaps in the measurement properties of the medication adherence instruments, due to the lack of details of the data and analysis in primary studies, as previously mentioned. In addition to these issues identified when assessing PROM development and content validity, the fact that the methodological quality score of the measure properties considered the worst score of the COSMIN checklist may have contributed to downgrading the overall rating of the properties of the measure evaluated, as highlighted in previous studies [15, 143]. According to the COSMIN guideline, it is recommended to consider the worst score assigned to one of the assessment items of all COSMIN boxes, since methodological aspects considered poor in primary studies cannot be compensated by aspects considered to be good. In the guideline was highlighted that only significant flaws in study design or statistical analysis should be classified with the worst score [141]. Although, for some standars of the boxes, the worst possible response option was defined by COSMIN as "doubtful" or "adequate" rather than "inadequate" by the guideline, in order to reduce the impact of these assessments on the risk of bias, our results showed the influence of this criteria on the risk of bias assessment. Another point was the absence of a criteria for the evaluation of sensitivity and specificity results, when testing the criterion validity of PROMs. Furthermore, most of the studies included in the review were developed before the release of the guideline proposed by the COSMIN initiative, which may justify the fact that many of the assessment criteria proposed by the initiative were not performed or presented in the expected way in the primary studies evaluated. Thus, since the COSMIN guideline has not yet been widely used, future studies are recommended to refine its suitability, acceptability and quality.
The findings of our systematic review have implications for clinical practice, since it contributes to improve the evidence-based selection of PROMs in research and practice. Considering the perception of the patient about their adherence to medication treatment contributes to promoting a person-centered model of care, whose results are known to be promising in the management of chronic diseases. Therefore, the knowledge about which PROMs are evidence-based, recommended, or potentially recommended for use in clinical practice is crucial for positively impacting human health. The use of PROMs with high quality of evidence contributes to improving implementation science, because they have the best properties to measure the behavior, to evaluating the effect of interventions to optimize medication adherence, and to positive changes in chronic disease management and clinical practice.
Strengths and limitations
One of the strengths of this systematic review was the careful application of the methodology proposed by the COSMIN initiative for the conduction of the systematic reviews in the evaluation of measurement properties of PROMs [21]. Another strength is the number of databases included in the literature searches which contributed to a more complete result. The review team should also be highlighted because it was composed of professionals from different areas, including nurses, dietitians, statistician, and researchers with expertise in research methodology and the use of PROMs. The different expertises of the team allowed for contributions related to the evaluation of the methodological rigor and statistical analyses of the studies, as well as those related to the evaluation of the pertinence and clinical relevance of different PROMS in measuring medication adherence.
A limitation of our study was the lack of detailed data in many studies to evaluate some of the measurement properties, especially content validity. This characteristic observed in the studies hampered the evaluation of many PROMs which resulted in many of them being poorly evaluated.
Another limitation was the high heterogeneity observed in the meta-analyses performed. Even if models with random effects were applied, the presence of high heterogeneity may bring limitations to the estimates obtained.
Conclusions
The conclusion of this systematic review none of the evaluated PROMs could be considered trusted and recommended for use for patients with cardiovascular diseases and/or type 2 diabetes mellitus. However, another 21 PROMs have the potential to be recommended for use but need further studies to ensure their quality, according to the COSMIN guidelines for systematic reviews of PROMs. Furthermore, the findings showed that it is key to improve the reporting of results in PROM validation studies, especially with regard to content validity.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARMS:
-
Adherence to Refills and Medication Scale
- AS:
-
Adherence Scale
- AUC:
-
Area under the curve
- CFA:
-
Confirmatory factor analysis
- CINAHL:
-
Cumulative index to nursing and allied health literature
- COSMIN:
-
Consensus-based standards for the selection of health measurement instruments
- CVDs:
-
Cardiovascular diseases
- DMAS-7:
-
7-item Diabetes Medication Adherence Scale
- EFA:
-
Exploratory factor analysis
- EMBASE:
-
Excerpta medica database
- GMAS:
-
General Medication Adherence Scale
- GRADE:
-
Grading of recommendations assessment, development, and evaluation
- HOOS-P:
-
Hip disability and osteoarthritis outcome score - physical function shortform
- IADMAS:
-
Iraqi Anti-Diabetic Medication Adherence Scale
- ICC:
-
Intraclass correlation coefficient
- IRT:
-
Item response theory
- KOOS-PS:
-
Knee injury and osteoarthritis outcome score physical function shortform
- LILACS:
-
Literatura latino-americana e do Caribe em ciências da saúde
- LMAS-14:
-
Fourteen-item Lebanese Medication Adherence Scale
- MALMAS:
-
Malaysian Medication Adherence Scale
- Mascard:
-
Medication Adherence Scale in Cardiovascular disorders
- MAQ:
-
Medication Adherence Questionnaire
- MARS-5:
-
5-item Medication Adherence Report Scale
- MEDS:
-
Medication Adherence Estimation and Differentiation Scale
- MGT:
-
Morisky-Green test
- MMAS-5:
-
5-item adapted Morisky Medication Adherence Scale
- MMAS-8:
-
8-item Morisky Medication Adherence Scale
- MNPS:
-
Medication Non-persistence Scale
- MTA:
-
Measurement of Treatment Adherence
- MTA-Insulin:
-
Measurement of Treatment Adherence - Insulin
- MTA-OA:
-
MTA-Oral Antidiabetics
- NCDs:
-
Noncommunicable diseases
- NPV:
-
Negative predictive value
- ProMAS:
-
Probabilistic Medication Adherence Scale
- PPV:
-
Positive predictive value
- PRISMA:
-
Preferred reporting items for systematic review and meta-analyses
- PROMs:
-
Patient-reported outcome measures
- PROSPERO:
-
International prospective register of systematic reviews
- SMAQ:
-
Simplified Medication Adherence Questionnaire
- T2DM:
-
Type 2 diabetes mellitus
- WHO:
-
World Health Organization
References
Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–307. https://doi.org/10.2147/PPA.S106821.
Al-Ganmi AH, Perry L, Gholizadeh L, Alotaibi AM. Cardiovascular medication adherence among patients with cardiac disease: a systematic review. J Adv Nurs. 2016;72(12):3001–14. https://doi.org/10.1111/jan.13062.
Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8(1):e016982. https://doi.org/10.1136/bmjopen-2017-016982.
World Health Organization. 2020. https://www.who.int/data/stories/leading-causes-of-death-and-disability-2000-2019-a-visual-summary. Accessed 10 Jan 2022.
World Health Organization. 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 10 Jan 2022.
The Global Goals for Sustainable Development. 2015. https://www.globalgoals.org/3-good-health-and-well-being. Accessed 10 Jan 2022
Kim S, Shin DW, Yun JM, Hwang Y, Park SK, Ko YJ, et al. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension. 2016;67(3):506–12. https://doi.org/10.1161/HYPERTENSIONAHA.115.06731.
World Health Organization. Adherence to long term therapies: evidence for action. 2003. https://apps.who.int/iris/handle/10665/42682. Accessed 20 Jan 2023.
Nguyen TMU, La Caze A, Cottrell N. What are validated self-report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. 2014;77(3):427–45. https://doi.org/10.1111/bcp.12194.
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. https://doi.org/10.1186/1477-7525-4-79.
Lu Y, Xu J, Zhao W, Han HR. Measuring self-care in persons with type 2 diabetes: a systematic review. Eval Health Prof. 2016;39(2):131–84. https://doi.org/10.1177/0163278715588927.
Pareja-Martínez E, Esquivel-Prados E, Martínez-Martínez F, García-Corpas JP. Questionnaires on adherence to antihypertensive treatment: a systematic review of published questionnaires and their psychometric properties. Int J Clin Pharm. 2020;42(2):355–65. https://doi.org/10.1007/s11096-020-00981-x.
Prinsen CAC, Mokkink LB, Bouter LM, Alonso J, Patrick DL, de Vet HCW, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–57. https://doi.org/10.1007/s11136-018-1798-3.
Wee PJL, Kwan YH, Loh DHF, Phang JK, Puar TH, Østbye T, et al. Measurement properties of patient-reported outcome measures for diabetes: systematic review. J Med Internet Res. 2021;23(8):e25002. https://doi.org/10.2196/25002.
Kim CJ, Schlenk EA, Ahn JA, Kim M, Park E, Park J. Evaluation of the measurement properties of self-reported medication adherence instruments among people at risk for metabolic syndrome: a systematic review. Diabetes Educ. 2016;42(5):618–34. https://doi.org/10.1177/0145721716655400.
Kwan YH, Weng SD, Loh DHF, Phang JK, Oo LJY, Blalock DV, et al. Measurement properties of existing patient-reported outcome measures on medication adherence: systematic review. J Med Internet Res. 2020;22(10):e19179. https://doi.org/10.2196/19179.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. https://doi.org/10.1186/s13643-021-01626-4.
Oliveira HC, Neto DH, Carvalho SDL, de Cássia Lopes Barros R, Luzia Dos Santos Neves M, Andrechuk CRS, et al. Psychometric properties of medication adherence instruments in cardiovascular diseases and type 2 diabetes mellitus: systematic review protocol. Syst Rev. 2021;10(1):202. https://doi.org/10.1186/s13643-021-01755-w.
Terwee CB, Jansma EP, Riphagen II, de Vet HC. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res. 2009;18(8):1115–23. https://doi.org/10.1007/s11136-009-9528-5.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Mokkink LB, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, de Vet HCW, et al. COSMIN Methodology for Systematic Reviews of Patient-Reported Outcome Measures (PROMs) User Manual. COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN). 2018. https://www.cosmin.nl/wp-content/uploads/COSMIN-syst-review-for-PROMs-manual_version-1_feb-2018-1.pdf. Accessed 30 Jun 2020.
Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, et al. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res. 2018;27(5):1171–9. https://doi.org/10.1007/s11136-017-1765-4.
Terwee CB, Prinsen CAC, Chiarotto A, Westerman MJ, Patrick DL, Alonso J, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. 2018;27(5):1159–70. https://doi.org/10.1007/s11136-018-1829-0.
Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” - a practical guideline. Trials. 2016;17(1):449. https://doi.org/10.1186/s13063-016-1555-2.
Hair JF, Black WC, B.J., Anderson RE. Multivariate data analysis. 9th ed. Hampshire: Cengage Learning; 2019.
Feldt LS, Charter RA. Averaging internal consistency reliability coefficients. Educ Psychol Meas. 2006;66(2):215e27. https://doi.org/10.1177/0013164404273947.
Wilson DB. SPSS Meta-Analysis Macro. Available at http://mason.gmu.edu/~dwilsonb/MetaAnal.html under a Creative Commons Attribution-Noncommercial-Share Alike 4.0 International. Full terms at https://creativecommons.org/licenses/by-nc-sa/4.0/legalcode.
Al Abboud SA, Ahmad S, Bidin MB, Ismail NE. Validation of Malaysian versions of Perceived Diabetes Self-Management Scale (PDSMS), Medication Understanding and Use Self-Efficacy Scale (MUSE) and 8-Morisky Medication Adherence Scale (MMAS-8) using Partial Credit Rasch Model. J Clin Diagn Res. 2016;10(11):LC01–5. https://doi.org/10.7860/JCDR/2016/15079.8845.
Al Matari RA, Maneno MK, Daftary MN, Wingate LM, Ettienne EB. Development and validation of Amharic version of the Simplified Medication Adherence Questionnaire among literate Amharic speaking persons an urban teaching hospital in Washington DC region. Int J Case Rep Short Rev. 2019;5(1):001–5.
Ashur ST, Shamsuddin K, Shah SA, Bosseri S, Morisky DE. Reliability and known-group validity of the Arabic version of the 8-item Morisky Medication Adherence Scale among type 2 diabetes mellitus patients. East Mediterr Health J. 2015;21(10):722–8. https://doi.org/10.26719/2015.21.10.722.
Ayoub D, Mroueh L, El-Hajj M, Awada S, Rachidi S, Zein S, et al. Evaluation of antidiabetic medication adherence in the Lebanese population: development of the Lebanese Diabetes Medication Adherence Scale. Int J Pharm Pract. 2019;27(5):468–76. https://doi.org/10.1111/ijpp.12558.
Boas LC, Lima ML, Pace AE. Adherence to treatment for diabetes mellitus: validation of instruments for oral antidiabetics and insulin. Rev Lat Am Enfermagem. 2014;22(1):11–8. https://doi.org/10.1590/0104-1169.3155.2386.
Chua SS, Lai PSM, Tan CH, Chan SP, Chung WW, Morisky DE. The development and validation of the Malaysian medication adherence scale (MALMAS) among patients with 2 type diabetes in Malaysia. Int J Pharm Pharm Sci. 2013;5(3):790–4.
Chung WW, Chua SS, Lai PS, Morisky DE. The Malaysian Medication Adherence Scale (MALMAS): concurrent validity using a clinical measure among people with type 2 diabetes in Malaysia. PLoS One. 2015;10(4):e0124275. https://doi.org/10.1371/journal.pone.0124275.
DiBonaventura M, Wintfeld N, Huang J, Goren A. The association between nonadherence and glycated hemoglobin among type 2 diabetes patients using basal insulin analogs. Patient Prefer Adherence. 2014;8:873–82. https://doi.org/10.2147/PPA.S55550.
Mallah Z, Hammoud Y, Awada S, Rachidi S, Zein S, Ballout H, et al. Validation of diabetes medication adherence scale in the Lebanese population. Diabetes Res Clin Pract. 2019;156:107837. https://doi.org/10.1016/j.diabres.2019.107837.
Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract. 2013;102(2):96–104. https://doi.org/10.1016/j.diabres.2013.09.010.
Mikhael EM, Hussain SA, Shawky N, Hassali MA. Validity and reliability of anti-diabetic medication adherence scale among patients with diabetes in Baghdad, Iraq: a pilot study. BMJ Open Diabetes Res Care. 2019;7(1):e000658. https://doi.org/10.1136/bmjdrc-2019-000658.
Osborn CY, Gonzalez JS. Measuring insulin adherence among adults with type 2 diabetes. J Behav Med. 2016;39(4):633–41. https://doi.org/10.1007/s10865-016-9741-y. (Epub 2016 Apr 9. Erratum in: J Behav Med. 2016;39(4):733).
Zongo A, Guénette L, Moisan J, Guillaumie L, Lauzier S, Grégoire JP. Revisiting the internal consistency and factorial validity of the 8-item Morisky Medication Adherence Scale. SAGE Open Med. 2016;4:2050312116674850. https://doi.org/10.1177/2050312116674850.Erratum.In:SAGEOpenMed.2018;5:2050312117723969.
Al-Qazaz HKh, Hassali MA, Shafie AA, Sulaiman SA, Sundram S, Morisky DE. The eight-item Morisky Medication Adherence Scale MMAS: translation and validation of the Malaysian version. Diabetes Res Clin Pract. 2010;90(2):216–21. https://doi.org/10.1016/j.diabres.2010.08.012.
de Araújo MFM, de Freitas RWJF, Marinho NBP, Alencar AMPG, Damasceno MMC, Zanetti ML. Validation of two methods to evaluate adherence to oral anti-diabetic medication. J Nurs Health Chronic Illn. 2011;3:275–82. https://doi.org/10.1111/j.1752-9824.2011.01099.x.
Kelly K, Grau-Sepulveda MV, Goldstein BA, Spratt SE, Wolfley A, Hatfield V, et al. The agreement of patient-reported versus observed medication adherence in type 2 diabetes mellitus (T2DM). BMJ Open Diabetes Res Care. 2016;4(1):e000182. https://doi.org/10.1136/bmjdrc-2015-000182.
Kim CJ, Park E, Schlenk EA, Kim M, Kim DJ. Psychometric evaluation of a Korean version of the Adherence to Refills and Medications Scale (ARMS) in adults with type 2 diabetes. Diabetes Educ. 2016;42(2):188–98. https://doi.org/10.1177/0145721716632062.
Lai PSM, Sellappans R, Chua SS. Reliability and validity of the M-MALMAS instrument to assess medication adherence in Malay-speaking patients with type 2 diabetes. Pharmaceut Med. 2020;34(3):201–7. https://doi.org/10.1007/s40290-020-00335-y.
Lee WY, Ahn J, Kim JH, Hong YP, Hong SK, Kim YT, et al. Reliability and validity of a self-reported measure of medication adherence in patients with type 2 diabetes mellitus in Korea. J Int Med Res. 2013;41(4):1098–110. https://doi.org/10.1177/0300060513484433.
Sakthong P, Chabunthom R, Charoenvisuthiwongs R. Psychometric properties of the Thai version of the 8-item Morisky Medication Adherence Scale in patients with type 2 diabetes. Ann Pharmacother. 2009;43(5):950–7. https://doi.org/10.1345/aph.1L453.
Tandon S, Chew M, Eklu-Gadegbeku CK, Shermock KM, Morisky DE. Validation and psychometric properties of the 8-item Morisky Medication Adherence Scale (MMAS-8) in type 2 diabetes patients in sub-Saharan Africa. Diabetes Res Clin Pract. 2015;110(2):129–36. https://doi.org/10.1016/j.diabres.2015.10.001.
Vluggen S, Hoving C, Schaper NC, De Vries H. Psychological predictors of adherence to oral hypoglycaemic agents: an application of the ProMAS questionnaire. Psychol Health. 2020;35(4):387–404. https://doi.org/10.1080/08870446.2019.1672873.
Wang J, Bian R, Mo Y. Validation of the Chinese version of the eight-item Morisky medication adherence scale in patients with type 2 diabetes mellitus. J Clin Gerontol Geriatr. 2013;4:119–22. https://doi.org/10.1016/j.jcgg.2013.06.002.
Wang Y, Lee J, Toh MP, Tang WE, Ko Y. Validity and reliability of a self-reported measure of medication adherence in patients with type 2 diabetes mellitus in Singapore. Diabet Med. 2012;29(9):e338–44. https://doi.org/10.1111/j.1464-5491.2012.03733.x.
Yesilbalkan OU, Gencer E. The validity and reliability study of the Self-reported Measure of Medication Adherence Scale in patients taking oral antidiabetic treatment. Int J Caring Sci. 2019;12(2):917–24.
Zongo A, Guénette L, Moisan J, Grégoire JP. Predictive validity of Self-Reported Measures of Adherence to Noninsulin Antidiabetes Medication against control of glycated hemoglobin levels. Can J Diabetes. 2016;40(1):58–65. https://doi.org/10.1016/j.jcjd.2015.06.008.
Kristina SA, Putri LR, Riani DA, Ikawati Z, Endarti D. Validity of self-reported measure of medication adherence among diabetic patients in Indonesia. Int Res J Pharm. 2019;10(7):144–8. https://doi.org/10.7897/2230-8407.1007234.
Nakhaeizadeh M, Khalooei A. Psychometric properties of Persian version of the 8-item Morisky Medication Adherence Scale in type 2 diabetes atients. J Clin Diagn Res. 2019;13(8):14–8. https://doi.org/10.7860/JCDR/2019/41408.13076.
Piñeiro F, Gil V, Donis M, Orozco D, Pastor R, Merino J. Validez de seis métodos indirectos para valorar el cumplimiento del tratamiento farmacológico en la diabetes no insulinodependiente. Rev Clin Esp. 1997;197(8):555–9.
Sayiner ZA, Savaş E, Kul S, Morisky DE. Validity and reliability of the Turkish Version of the 8-Item Morisky Medication Adherence Scale in patients with type 2 diabetes. Eur J Ther. 2020;26(1):47–52. https://doi.org/10.5152/eurjther.2020.19132.
Allela O, Mohammed Salih H, Haji AI. Adherence to medication and glucose control in diabetic patients in Duhok Iraq. Pharmacia. 2022;69(3):673–9. https://doi.org/10.3897/pharmacia.69.e86649.
Gumilas NSA, Harini IM, Samodro P, Ernawati DA. MMAS-8 score assessment of therapy adherence to glycemic control of patients with type 2 diabetes mellitus, Tanjung Purwokerto, Java, Indonesia (october 2018). Southeast Asian J Trop Med Public Health. 2021;52(3):359–70.
Iranpour A, Sarmadi V, Alian Mofrad A, Mousavinezhad SA, Mousavinezhad SM, Mohammad Alizadeh F, et al. The Persian version of the 8-item Morisky Medication Adherence Scale (MMAS-8): can we trust it? J Diabetes Metab Disord. 2022;21(1):835–40. https://doi.org/10.1007/s40200-022-01047-7.
Laghousi D, Rezaie F, Alizadeh M, Asghari JM. The eight-item Morisky Medication Adherence Scale: validation of its Persian version in diabetic adults. Caspian J Intern Med. 2021;12(1):77–83. https://doi.org/10.22088/cjim.12.1.77.
Martinez-Perez P, Orozco-Beltrán D, Pomares-Gomez F, Hernández-Rizo JL, Borras-Gallen A, Gil-Guillen VF, et al. Validation and psychometric properties of the 8-item Morisky Medication Adherence Scale (MMAS-8) in type 2 diabetes patients in Spain. Aten Primaria. 2021;53(2):101942. https://doi.org/10.1016/j.aprim.2020.09.007.
Saeed N. Translation and validation of a simplified medication adherence questionnaire and factors affecting chronic diseases medication adherence among arab americans [thesis on the Internet]. Washington (USA): Howard University; 2020. Available from: https://www.proquest.com/docview/2451414723?pq-origsite=gscholar&fromopenview=true.
Wu J, Tao Z, Gong H, Shen J, Song Z. ARMS in evaluating the medication adherence in elderly patients with type 2 diabetes mellitus. Fudan Univ J Med Sci. 2020;47(5):686–93. https://doi.org/10.3969/j.issn.1672-8467.2020.05.007.
Mahmoud MA, Islam MA, Ahmed M, Bashir R, Ibrahim R, Al-Nemiri S, et al. Validation of the arabic version of General Medication Adherence Scale (GMAS) in sudanese patients with diabetes mellitus. Risk Manag Healthc Policy. 2021;14:4235–41. https://doi.org/10.2147/RMHP.S325184.
Anuradha HV, Prabhu PS, Kalra P. Development and validation of a questionnaire for assessing medication adherence in type 2 diabetes mellitus in India. Biomed Pharmacol J. 2022;15(1):363–7. https://doi.org/10.13005/bpj/2375.
Arnet I, Metaxas C, Walter PN, Morisky DE, Hersberger KE. The 8-item Morisky Medication Adherence Scale translated in German and validated against objective and subjective polypharmacy adherence measures in cardiovascular patients. J Eval Clin Pract. 2015;21(2):271–7. https://doi.org/10.1111/jep.12303.
Hacıhasanoğlu Aşılar R, Gözüm S, Çapık C, Morisky DE. Reliability and validity of the Turkish form of the eight-item Morisky medication adherence scale in hypertensive patients. Anadolu Kardiyol Derg. 2014;14(8):692–700. https://doi.org/10.5152/akd.2014.4982.
Ben AJ, Neumann CR, Mengue SS. The Brief Medication Questionnaire and Morisky-Green test to evaluate medication adherence. Rev Saúde Pública. 2012;46(2):279–89. https://doi.org/10.1590/s0034-89102012005000013.
Bloch KV, Melo AN, Nogueira AR. Prevalência da adesão ao tratamento anti-hipertensivo em hipertensos resistentes e validação de três métodos indiretos de avaliação da adesão [Prevalence of anti-hypertensive treatment adherence in patients with resistant hypertension and validation of three indirect methods for assessing treatment adherence]. Cad Saude Publica. 2008;24(12):2979–84. https://doi.org/10.1590/s0102-311x2008001200030.
Bou Serhal R, Salameh P, Wakim N, Issa C, Kassem B, Abou Jaoude L, et al. A new Lebanese Medication Adherence Scale: validation in Lebanese hypertensive adults. Int J Hypertens. 2018;2018:3934296. https://doi.org/10.1155/2018/3934296.
Cabral AC, Moura-Ramos M, Castel-Branco M, Caramona M, Fernandez-Llimos F, Figueiredo IV. Influence of the mode of administration on the results of medication adherence questionnaires. J Eval Clin Pract. 2017;23(6):1252–7. https://doi.org/10.1111/jep.12773.
Cabral AC, Moura-Ramos M, Castel-Branco M, Fernandez-Llimos F, Figueiredo IV. Cross-cultural adaptation and validation of a European Portuguese version of the 8-item Morisky medication adherence scale. Rev Port Cardiol (Engl Ed). 2018;37(4):297–303. https://doi.org/10.1016/j.repc.2017.09.017.
Chatziefstratiou A, Giakoumidakis K, Fotos NV, Baltopoulos G, Brokalaki H. Scales for assessing medication adherence in patients with hypertension. Br J Nurs. 2019;28(21):1388–92. https://doi.org/10.12968/bjon.2019.28.21.1388.
Chen YJ, Chang J, Yang SY. Psychometric evaluation of Chinese version of Adherence to Refills and Medications Scale (ARMS) and blood-pressure control among elderly with hypertension. Patient Prefer Adherence. 2020;14:213–20. https://doi.org/10.2147/PPA.S236268.
de Oliveira-Filho AD, Morisky DE, Neves SJ, Costa FA, de Lyra DP, Jr. The 8-item Morisky Medication Adherence Scale: validation of a Brazilian-Portuguese version in hypertensive adults. Res Social Adm Pharm. 2014;10(3):554–61. https://doi.org/10.1016/j.sapharm.2013.10.006.
Mahler C, Hermann K, Horne R, Ludt S, Haefeli WE, Szecsenyi J, et al. Assessing reported adherence to pharmacological treatment recommendations. Translation and evaluation of the Medication Adherence Report Scale (MARS) in Germany. J Eval Clin Pract. 2010;16(3):574–9. https://doi.org/10.1111/j.1365-2753.2009.01169.x.
Contreras EM, Marín CG, Jerez CJ, Rubio CF, Sánchez CB, Bonilla RR. Observancia terapéutica en la hipertensión arterial. Validación de métodos indirectos que valoran el cumplimiento terapéutico. Atención Primaria. 1995;16(8):496–500.
Moharamzad Y, Saadat H, Nakhjavan Shahraki B, Rai A, Saadat Z, Aerab-Sheibani H, et al. Validation of the Persian version of the 8-Item Morisky Medication Adherence Scale (MMAS-8) in Iranian hypertensive patients. Glob J Health Sci. 2015;7(4):173–83. https://doi.org/10.5539/gjhs.v7n4p173.
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348–54. https://doi.org/10.1111/j.1751-7176.2008.07572.x.
Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. https://doi.org/10.1097/00005650-198601000-00007.
Muntner P, Joyce C, Holt E, He J, Morisky D, Webber LS, et al. Defining the minimal detectable change in scores on the eight-item Morisky Medication Adherence Scale. Ann Pharmacother. 2011;45(5):569–75. https://doi.org/10.1345/aph.1P677.
Nobles BM, Erickson SR. Variations of a Commonly Used Medication Adherence Assessment Scale: Do Changes in Scale Change Structure Results? J Pharm Technol. 2018;34(6):252–8. https://doi.org/10.1177/8755122518796586.
Pandey A, Raza F, Velasco A, Brinker S, Ayers C, Das SR, et al. Comparison of Morisky Medication Adherence Scale with therapeutic drug monitoring in apparent treatment-resistant hypertension. J Am Soc Hypertens. 2015;9(6):420–426.e2. https://doi.org/10.1016/j.jash.2015.04.004.
Gil VF, Belda J, Muñoz C, Martínez JL, Soriano JE, Merino J. Validez de cuatro métodos indirectos que valoran el cumplimiento terapéutico en la hipertensión arterial [Validity of four indirect methods which evaluate therapeutic compliance for arterial hypertension]. Rev Clin Esp. 1993;193(7):363–7.
Jankowska-Polanska B, Uchmanowicz I, Chudiak A, Dudek K, Morisky DE, Szymanska-Chabowska A. Psychometric properties of the Polish version of the eight-item Morisky Medication Adherence Scale in hypertensive adults. Patient Prefer Adherence. 2016;10:1759–66. https://doi.org/10.2147/PPA.S101904.
Kim JH, Lee WY, Hong YP, Ryu WS, Lee KJ, Lee WS, et al. Psychometric properties of a short self-reported measure of medication adherence among patients with hypertension treated in a busy clinical setting in Korea. J Epidemiol. 2014;24(2):132–40. https://doi.org/10.2188/jea.je20130064.
Korb-Savoldelli V, Gillaizeau F, Pouchot J, Lenain E, Postel-Vinay N, Plouin PF, et al. Validation of a French version of the 8-item Morisky medication adherence scale in hypertensive adults. J Clin Hypertens (Greenwich). 2012;14(7):429–34. https://doi.org/10.1111/j.1751-7176.2012.00634.x.
Koschack J, Marx G, Schnakenberg J, Kochen MM, Himmel W. Comparison of two self-rating instruments for medication adherence assessment in hypertension revealed insufficient psychometric properties. J Clin Epidemiol. 2010;63(3):299–306. https://doi.org/10.1016/j.jclinepi.2009.06.011.
Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15(1):59–66.
Li WW, Stewart AL, Stotts NA, Froelicher ES. Cultural factors and medication compliance in Chinese immigrants who are taking antihypertensive medications: instrument development. J Nurs Meas. 2005;13(3):231–52. https://doi.org/10.1891/jnum.13.3.231.
Lin CY, Ou HT, Nikoobakht M, Broström A, Årestedt K, Pakpour AH. Validation of the 5-Item Medication Adherence Report Scale in older stroke patients in Iran. J Cardiovasc Nurs. 2018;33(6):536–43. https://doi.org/10.1097/JCN.0000000000000488.
Okello S, Nasasira B, Muiru AN, Muyingo A. Validity and Reliability of a Self-Reported Measure of Antihypertensive Medication Adherence in Uganda. PLoS One. 2016;11(7):e0158499. https://doi.org/10.1371/journal.pone.0158499. (Erratum. In: PLoS One. 2017;12 (10): e0187620).
Peersen K, Munkhaugen J, Gullestad L, Dammen T, Moum T, Otterstad JE. Reproducibility of an extensive self-report questionnaire used in secondary coronary prevention. Scand J Public Health. 2017;45(3):269–76. https://doi.org/10.1177/1403494816688375.
Piñeiro F, Gil V, Donis M, Orozco D, Pastor R, Merino J. Validez de 6 métodos indirectos para valorar el cumplimiento del tratamiento farmacológico en la hipertensión arterial [The validity of 6 indirect methods for assessing drug treatment compliance in arterial hypertension]. Aten Primaria. 1997;19(7):372–4, 376.
Prado JC Jr, Kupek E, Mion D Jr. Validity of four indirect methods to measure adherence in primary care hypertensives. J Hum Hypertens. 2007;21(7):579–84. https://doi.org/10.1038/sj.jhh.1002196.
Sadakathulla I, Mateti UV, Kellarai A, Bhat K. Adhering to antihypertensive treatment is vitally important. Salud(i)Ciencia. 2019;23:314–24. https://doi.org/10.21840/siic/157368.
Shaw R, Bosworth HB. Baseline medication adherence and blood pressure in a 24-month longitudinal hypertension study. J Clin Nurs. 2012;21(9–10):1401–6. https://doi.org/10.1111/j.1365-2702.2011.03859.x.
Shilbayeh SAR, Almutairi WA, Alyahya SA, Alshammari NH, Shaheen E, Adam A. Validation of knowledge and adherence assessment tools among patients on warfarin therapy in a Saudi hospital anticoagulant clinic. Int J Clin Pharm. 2018;40(1):56–66. https://doi.org/10.1007/s11096-017-0569-5.
Shin DS, Kim CJ. Psychometric evaluation of a Korean version of the 8-item Medication Adherence Scale in rural older adults with hypertension. Aust J Rural Health. 2013;21(6):336–42. https://doi.org/10.1111/ajr.12070.
Valencia-Monsalvez F, Mendoza-Parra S, Luengo-Machuca L. Evaluación de la escala Morisky de adherencia a la medicación (mmas-8) en adultos mayores de un centro de atención primaria en Chile [Evaluation of Morisky medication adherence scale (mmas-8) in older adults of a primary health care center in Chile]. Rev Peru Med Exp Salud Publica. 2017;34(2):245–9. https://doi.org/10.17843/rpmesp.2017.342.2206.
van de Steeg N, Sielk M, Pentzek M, Bakx C, Altiner A. Drug-adherence questionnaires not valid for patients taking blood-pressure-lowering drugs in a primary health care setting. J Eval Clin Pract. 2009;15(3):468–72. https://doi.org/10.1111/j.1365-2753.2008.01038.x.
Wang Y, Kong MC, Ko Y. Comparison of three medication adherence measures in patients taking warfarin. J Thromb Thrombolysis. 2013;36(4):416–21. https://doi.org/10.1007/s11239-013-0872-5.
Yan J, You LM, Yang Q, Liu B, Jin S, Zhou J, et al. Translation and validation of a Chinese version of the 8-item Morisky medication adherence scale in myocardial infarction patients. J Eval Clin Pract. 2014;20(4):311–7. https://doi.org/10.1111/jep.12125.
Da Silva Carvalho AR, Dantas RA, Pelegrino FM, Corbi IS. Adaptation and validation of an oral anticoagulation measurement of treatment adherence instrument. Rev Lat Am Enfermagem. 2010;18(3):301–8. https://doi.org/10.1590/s0104-11692010000300002.
Lomper K, Chabowski M, Chudiak A, Białoszewski A, Dudek K, Jankowska-Polańska B. Psychometric evaluation of the Polish version of the Adherence to Refills and Medications Scale (ARMS) in adults with hypertension. Patient Prefer Adherence. 2018;12:2661–70. https://doi.org/10.2147/PPA.S185305.
Val Jiménez A, Amorós Ballestero G, Martínez Visa P, Fernández Ferré ML, León SM. Estudio descriptivo del cumplimiento del tratamiento farmacológico antihipertensivo y validación del test de Morisky y Green [Descriptive study of patient compliance in pharmacologic antihypertensive treatment and validation of the Morisky and Green test]. Aten Primaria. 1992;10(5):767–70.
Wang Y, Kong MC, Ko Y. Psychometric properties of the 8-item Morisky Medication Adherence Scale in patients taking warfarin. Thromb Haemost. 2012;108(4):789–95. https://doi.org/10.1160/TH12-05-0368.
Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43(3):413–22. https://doi.org/10.1345/aph.1L496.
Grégoire JP, Guibert R, Archambault A, Contandriopoulos AP. Medication compliance in a family practice: testing a self-report questionnaire in a primary care setting. Can Fam Physician. 1992;38:2333–7.
Sakr F, Dabbous M, Akel M, Salameh P, Hosseini H. Adherence to post-stroke pharmacotherapy: scale validation and correlates among a sample of stroke survivors. Medicina (Kaunas). 2022;58(8):1109. https://doi.org/10.3390/medicina58081109.
Lukina YV, Kutishenko NP, Martsevich SY, Drapkina OM. The Questionnaire survey method in medicine on the example of treatment adherence scales. Ration Pharmacother Cardiol. 2021;17(4):576–83. https://doi.org/10.20996/1819-6446-2021-08-02.
Martin-Latry K, Latry P, Pucheu Y, Couffinhal T. Validation d’une échelle de mesure de l’adhésion médicamenteuse cardiovasculaire utilisable en contexte hospitalier [Hospital medication adherence scale development in cardiovascular disorders]. Ann Pharm Fr. 2021;79(4):457–64. https://doi.org/10.1016/j.pharma.2020.11.008. (French).
Athavale AS, Bentley JP, Banahan BF 3rd, McCaffrey DJ 3rd, Pace PF, Vorhies DW. Development of the Medication Adherence Estimation and Differentiation Scale (MEDS). Curr Med Res Opin. 2019;35(4):577–85. https://doi.org/10.1080/03007995.2018.1512478.
Athavale AS. Development of the Medication Non-Adherence Scale (MNAS) and the Medication Non-Persistence Scale (MNPS) [dissertation on the Internet]. Mississippi: The University of Mississippi; 2015. Available from: https://www.proquest.com/docview/1696074498/4DCF52DED2546B4PQ/1?accountid=8113.
Baruel Okumura PC, Okumura LM, Reis WC, Godoy RR, Cata-Preta BO, de Souza TT, et al. Comparing medication adherence tools scores and number of controlled diseases among low literacy patients discharged from a Brazilian cardiology ward. Int J Clin Pharm. 2016;38(6):1362–6. https://doi.org/10.1007/s11096-016-0390-6.
Chan AHY, Horne R, Hankins M, Chisari C. The Medication Adherence Report Scale: a measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol. 2020;86(7):1281–8. https://doi.org/10.1111/bcp.14193.
Cook CL, Wade WE, Martin BC, Perri M 3rd. Concordance among three self-reported measures of medication adherence and pharmacy refill records. J Am Pharm Assoc (2003). 2005;45(2):151–9. https://doi.org/10.1331/1544345053623573.
Naqvi AA, AlShayban DM, Ghori SA, Mahmoud MA, Haseeb A, Faidah HS, et al. Validation of the General Medication Adherence Scale in Saudi patients with chronic diseases. Front Pharmacol. 2019;10:633. https://doi.org/10.3389/fphar.2019.00633.
Oliveira-Kumakura ARS, Pacheco I, de Oliveira HC, Rodrigues RCM. Relationship between anticoagulant medication adherence and satisfaction in patients with Stroke. J Neurosci Nurs. 2019;51(5):229–34. https://doi.org/10.1097/JNN.0000000000000463.
Martínez EP, Prados EE, Trigo LF, García-Corpas JP. Adherence to antihypertensive therapy in community pharmacy: evaluating the psychometric properties of the Morisky Medication Adherence Scale (MMAS-8) translated into Spanish. Pilot study Lat Am J Pharm. 2015;34(1):86–93.
Jerant A, DiMatteo R, Arnsten J, Moore-Hill M, Franks P. Self-report adherence measures in chronic illness: retest reliability and predictive validity. Med Care. 2008;46(11):1134–9. https://doi.org/10.1097/MLR.0b013e31817924e4.
Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–23. https://doi.org/10.1111/j.1524-4733.2008.00400.x.
Anbazhagan S, Fathima FN, Agrawal T, Misquith D. Psychometric properties of Morisky Medication Adherence Scale (MMAS) in known diabetic and hypertensive patients in a rural population of Kolar District. Karnataka Indian J Public Health. 2016;7(2):250–6. https://doi.org/10.5958/0976-5506.2016.00102.9.
Beyhaghi H, Reeve BB, Rodgers JE, Stearns SC. Psychometric properties of the four-item Morisky Green Levine Medication Adherence Scale among Atherosclerosis Risk in Communities (ARIC) study participants. Value Health. 2016;19(8):996–1001. https://doi.org/10.1016/j.jval.2016.07.001.
Delgado AB, Lima ML. Contributo para a validação concorrente de uma medida de adesão aos tratamentos. Psicologia, saúde & doenças. 2001;2(2):81–100.
Gökdoğan F, Kes D. Validity and reliability of the Turkish Adherence to Refills and Medications Scale. Int J Nurs Pract. 2017;23(5). https://doi.org/10.1111/ijn.12566.
Naqvi AA, Hassali MA, Rizvi M, Zehra A, Iffat W, Haseeb A, et al. Development and validation of a novel General Medication Adherence Scale (GMAS) for chronic illness patients in Pakistan. Front Pharmacol. 2018;9:1124. https://doi.org/10.3389/fphar.2018.01124.
Naqvi AA, Hassali MA, Jahangir A, Nadir MN, Kachela B. Translation and validation of the English version of the general medication adherence scale (GMAS) in patients with chronic illnesses. J Drug Assess. 2019;8(1):36–42. https://doi.org/10.1080/21556660.2019.1579729.
Kleppe M, Lacroix J, Ham J, Midden C. The development of the ProMAS: a Probabilistic Medication Adherence Scale. Patient Prefer Adherence. 2015;9:355–67. https://doi.org/10.2147/PPA.S76749.
Jank S, Bertsche T, Schellberg D, Herzog W, Haefeli WE. The A14-scale: development and evaluation of a questionnaire for assessment of adherence and individual barriers. Pharm World Sci. 2009;31(4):426–31. https://doi.org/10.1007/s11096-009-9296-x.
Alammari G, Alhazzani H, AlRajhi N, Sales I, Jamal A, Almigbal TH, et al. Validation of an arabic version of the Adherence to Refills and Medications Scale (ARMS). Healthcare (Basel). 2021;9(11):1430. https://doi.org/10.3390/healthcare9111430.
Al-Qerem W, Al Bawab AQ, Abusara O, Alkhatib N, Horne R. Validation of the Arabic version of medication adherence report scale questionnaire and beliefs about medication -specific questionnaire: A factor analysis study. PLoS One. 2022;17(4):e0266606. https://doi.org/10.1371/journal.pone.0266606.
Naqvi AA, Mahmoud MA, AlShayban DM, Alharbi FA, Alolayan SO, Althagfan S, et al. Translation and validation of the Arabic version of the General Medication Adherence Scale (GMAS) in Saudi patients with chronic illnesses. Saudi Pharm J. 2020;28(9):1055–61. https://doi.org/10.1016/j.jsps.2020.07.005.
Meng X, Li S, Shen W, Li D, Lv Q, Wang X, et al. Exploration of the psychometric properties of the novel General Medication Adherence Scale (GMAS) for chronic illness patients. Curr Med Res Opin. 2023;39(5):671–9. https://doi.org/10.1080/03007995.2023.2196219.
Nguyen TH, Truong HV, Vi MT, Taxis K, Nguyen T, Nguyen KT. Vietnamese version of the General Medication Adherence Scale (GMAS): translation, adaptation, and validation. Healthcare (Basel). 2021;9(11):1471. https://doi.org/10.3390/healthcare9111471.
Lauffenburger JC, Fontanet CP, Isaac T, Gopalakrishnan C, Sequist TD, Gagne JJ, et al. Comparison of a new 3-item self-reported measure of adherence to medication with pharmacy claims data in patients with cardiometabolic disease. Am Heart J. 2020;228:36–43. https://doi.org/10.1016/j.ahj.2020.06.012.
Mokkink LB, Boers M, Vleuten C, Patrick DL, Alonso J, Bouter LM, et al. COSMIN Risk of Bias tool to assess the quality of studies on reliability and measurement error of outcome measurement instrument User Manual. 2021. https://www.cosmin.nl/wp-content/uploads/user-manual-COSMIN-Risk-of-Bias-tool_v4_JAN_final.pdf. Accessed 27 Mar 2021.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Wells J, Crilly P, Kayyali R. A systematic analysis of reviews exploring the scope, validity, and reporting of patient-reported outcomes measures of medication adherence in type 2 diabetes. Patient Prefer Adherence. 2022;16:1941–54. https://doi.org/10.2147/PPA.S375745.
Terwee CB, Prinsen CAC, Chiarotto A, de Vet HCW, Bouter LM, Alonso J, et al. COSMIN methodology for assessing the content validity of PROMs User Manual. 2018. https://cosmin.nl/wp-content/uploads/COSMIN-methodology-for-content-validity-user-manual-v1.pdf. Accessed 27 Mar 2021.
Braaksma C, Wolterbeek N, Veen MR, Prinsen CAC, Ostelo RWJG. Systematic review and meta-analysis of measurement properties of the Hip disability and Osteoarthritis Outcome Score - Physical Function Shortform (HOOS-PS) and the Knee Injury and Osteoarthritis Outcome Score - Physical Function Shortform (KOOS-PS). Osteoarthritis Cartilage. 2020;28(12):1525–38. https://doi.org/10.1016/j.joca.2020.08.004.
Lee J, Lee EH, Kim CJ, Moon SH. Diabetes-related emotional distress instruments: a systematic review of measurement properties. Int J Nurs Stud. 2015;52(12):1868–78. https://doi.org/10.1016/j.ijnurstu.2015.07.004.
Acknowledgements
We would like to thank the Fundo de Apoio ao Ensino, Pesquisa e Extensão—FAEPEX, University of Campinas – Unicamp, Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001—PrInt—Programa Institucional de Internacionalização for the funding support to this research.
Funding
The authors acknowledge the following financial support: Fundo de Apoio ao Ensino, Pesquisa e Extensão—FAEPEX, University of Campinas – Unicamp, São Paulo, Brazil (grant: 3056/18; No. 519.292), Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (grant No. 312367/2017–1), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001—PrInt—Programa Institucional de Internacionalização (Process number: 8881.311294/201800).
Author information
Authors and Affiliations
Contributions
The authors HCO, NMCA and RCMR conceived and developed this research. PABR contributed to the methodological design, analysis, and review of the manuscript. HCO, DHN, SDLC, RCLB, CRSA and MLSN evaluated the titles and abstracts. Full-text assessment was done by HCO and DH. Data extraction, risk of bias and results assessment were done by HCO and RCMR. All authors contributed to the improvement, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA Checklist.
Additional file 2:
Search strategies.
Additional file 3.
Studies’ characteristics.
Additional file 4.
Quality of studies on the PROM development and content validity.
Additional file 5.
Quality of studies on measurement properties.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Oliveira, H.C., Hayashi, D., Carvalho, S.D.L. et al. Quality of measurement properties of medication adherence instruments in cardiovascular diseases and type 2 diabetes mellitus: a systematic review and meta-analysis. Syst Rev 12, 222 (2023). https://doi.org/10.1186/s13643-023-02340-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02340-z