Abstract
Background
Chronic radiation proctitis (CRP) is a long-term complication of pelvic radiotherapy that manifests as rectal bleeding, diarrhoea, fistula formation and obstruction. Treatments such as endoscopic argon plasma coagulation, hyperbaric oxygen therapy and rectal topical formalin have imposed a significant medical burden on CRP patients. In contrast, oral therapies offer a more accessible and acceptable option for managing CRP. Here, we conducted a systematic review of the efficacy of oral treatments for CRP to assess their potential as an effective and convenient treatment option for this condition.
Methods
We searched the Cochrane Central Register of Controlled Trials, PubMed, Web of Science, China National Knowledge Infrastructure and Chinese VIP in February 2021. We included post-radiotherapy participants with CRP that compared oral medicine alone or in combination with other treatments versus control treatments. The primary outcomes were bleeding, diarrhoea and symptom score. Heterogeneity between studies was checked using Cochrane Q test statistics and I2 test statistics. The Cochrane risk-of-bias tool was used to assess the quality of the included studies.
Results
We included 10 randomised controlled trials (RCTs) and 1 retrospective study with 898 participants. Three placebo-controlled trials evaluated the effects of oral sucralfate on CRP, with meta-analysis showing no significant different with placebo arm. Four trials on TCM demonstrated significant improvement of symptoms, especially for the 3 trials on oral TCM drinks. Retinyl palmitate and high-fibre diet were found to reduce rectal bleeding. The combination of oral pentoxifylline and tocopherol did not significantly change the process of CRP.
Conclusions
Our study implies that oral TCM drinks, retinyl palmitate and a high-fiber diet showed significant improvement in CRP symptoms, but not with the combination of oral pentoxifylline and tocopherol. Further multicentre, larger-scale RCTs are needed to confirm the efficacy and safety of these treatments and optimize treatment strategies, ultimately improving the quality of life for patients with CRP.
Similar content being viewed by others
Background
As the global cancer epidemic continues to rise and post-radiotherapy cancer prognosis improves, an increasing number of pelvic cancer survivors are presenting with chronic radiation proctitis (CRP) and seeking medical treatment [1, 2]. Significant bowel injury occurs in up to 6% of patients receiving pelvic irradiation [3, 4]. This long-term complication of pelvic radiotherapy occurs 3 months to years after pelvic radiotherapy, presenting with diarrhoea, rectal pain and/or haemorrhage in low grade, and 10% of the CRP may become high-grade, severe CRP presented with fistula formation or obstruction, having a substantial impact on patient’s quality of life [1]. And the cost of physical therapy and/or surgery has led to heavy medical burden for CRP patients.
Current standard treatment of CRP includes endoscopic therapies, nonsurgical treatments and surgeries. Nonsurgical treatment for CRP, as one of the late radiation tissue injury, composes of endoscopic argon plasma coagulation, radiofrequency ablation, hyperbaric oxygen therapy and rectal topical formalin, misoprostol or antibiotics [3, 5,6,7]. Oral antibiotics would be used only when systematic bacteraemia or sepsis was about to occur. Oral 5-aminosalicylic acid including olsalazine and mesalazine that are widely applied in chronic colitis has been contraindicated for pelvic radiation therapy. Recent studies began to focus on oral treatments such as for routine CRP treatments [8, 9].
Various medications and supplements have been studied for their potential in treating CRP, with different mechanisms of action and benefits. These include sucralfate, traditional Chinese medicine (TCM) drinks, probiotics, retinyl palmitate, a combination of vitamins C and E and pentoxifylline. Sucralfate binds to various epidermal growth factors, which can help reduce microvascular injury by stimulating angiogenesis. TCM for CRP typically contain multiple herbs, such as Sanguisorbae Radix, Bletilla striata, Phellodendron and Radix Paeoniae Rubra, which are associated with functions like detoxification, haemostasis and analgesia [10, 11]. TCM may also help regulate gut microbiota homeostasis and reduce chronic inflammation [12]. Probiotic supplements containing Lactobacillus spp. have been used to prevent or treat acute radiation proctitis-induced or 5-fluorouracil(5-FU)-based diarrhoea after pelvic cancer therapies [8, 13, 14]. Butyrate, a key metabolite of probiotics, serves as an energy source for colonocytes, and a deficiency in butyrate may lead to mucosal hyperplasia and acute or chronic inflammation [15, 16]. Retinyl palmitate has been demonstrated to promote wound healing by increasing cross-linking of collagen and myofibrils [17]. The combination of vitamins C and E, as antioxidants, reduces bleeding, diarrhoea and urgency [18]. Pentoxifylline, a phosphodiesterase inhibitor, serves as immunomodulator that down-regulates cytokines as well as a fibrogenic reaction mediator after irradiation which eases radiation-induced inflammatory and fibrotic process [19].
Oral treatments may provide advantages for CRP as it is claimed to be effective in improving CRP sign and symptom with more accessibility and lower medical burdens. However, the oral treatment options for CRP have not been clearly defined, and systematic review focusing on CRP oral drug is absent. Here, we sought out to assess the benefits and harms of oral treatments for CRP.
Methods
Search strategy and selection criteria
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting standards for systematic reviews and meta-analyses [20]. All randomised controlled trials (RCTs) or historic/retrospective control group study in both English and Chinese, irrespective of publication status, which compared any oral intervention for CRP to no intervention, placebo or any other intervention was eligible for inclusion. Any person who had been treated with pelvic radiotherapy after more than 3 months, with or without chemotherapy, subsequently developed CRP of any grade. We accepted any oral treatment for CRP, including traditional Chinese medicine (TCM), sulfasalazine, glutamine, probiotics and vitamins in any dosage, and no intervention, placebo or any other nonsurgical intervention as control. We considered signs and symptoms scoring systems, including urgency, diarrhoea, rectal pain, hemorrhage, fistula formation and obstruction as primary outcomes. We determined the mortality, morbidity and quality of life (QoL) as secondary outcomes. Scoring systems of QoL involved Karnofsky performance status (KPS) as secondary outcomes.
In February 2021, we conducted a search for relevant studies in the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Web of Science, China National Knowledge Infrastructure (CNKI) and Chinese VIP. No restrictions were placed on language or publication type (Additional files 1, 2, 3, 4, and 5 for the search strategies used for each database). In addition, we searched the Chinese Trial Registry (www.chictr.org) using the keywords “proctitis” or “proctopathy” and the prospective trial register (ClinicalTrials.gov) using the keywords (proctitis OR proctitides OR proctopathy OR proctocolitis OR proctosigmoiditis OR rectitis OR rectocolitis OR rectocolitides OR rectosigmoiditis) AND (radiation OR radiotherapy). Furthermore, we searched reference lists to identify unpublished trials, ongoing trials, confidential reports and raw data from published trials. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions [21].
Selection of studies and data management
One review author (L. Z. L.) was responsible for handsearching and identification of appropriate studies for consideration and entered all possibly relevant studies into a bibliographic software package Reference Manager (RefMan 5). Three review authors (L. Z. L., J. J. L. and N. N. X.) examined the electronic search results and independently reviewed the studies. Randomized controlled trials (RCTs) and retrospective studies that investigated the effectiveness of various oral medications and supplements for treating CRP were included. We retained studies when one or more review authors identified them as appropriate. We resolved any disagreements through discussion or, if required, by consulting a third review author (J. J. L.). We excluded trials that failed to meet our inclusion criteria, and the reasons are listed in the “Characteristics of excluded studies table” (supplementary document). The review authors all had content expertise in clinical practice, two had content expertise in medicine (L. Z. L. and N. N. X.) and one (J. J. L.) is an expertise in clinical surgery.
One review author (L. Z. L.) extracted relevant population and intervention characteristics using a standard data extraction template. Another author (J. J. L.) resolved any disagreements by discussion. We planned to solve all relevant missing information about the trials from the original authors of the articles. We resolved disagreements through discussion. Two review authors (L. Z. L., N. N. X.) independently assessed the risk of bias of all included studies according to the Cochrane Handbook for Systematic Reviews of Interventions [21]. We resolved disagreements through consensus. We allocated the level of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [22]. RefMan 5 was used to assess the quality of evidence in accordance with selection bias, performance bias, detection bias, attrition bias and reporting bias. Evidence qualities were evaluated as high quality, medium quality, low quality or very low quality. The ROBINS-I tool was used to assess included non-randomized observational studies. Each domain was judged among the following options: low, moderate, serious, critical or no information. A final judgment with the same options was then made for the entire study based on the findings from each domain.
Data analysis
We reported dichotomous data as risk ratios (RR) with 95% confidence intervals (CI), while for events with low probability, we utilized the Peto odds ratio (OR). Continuous variables were presented as mean differences (MD) with 95% CI. We assessed statistical heterogeneity using both the χ2 and I2 statistics, and clinical heterogeneity was evaluated in subgroup analysis. We considered P < 0.1 as evidence of statistical heterogeneity and an I2 value greater than 50% as indicative of significant statistical heterogeneity [21]. To account for expected clinical and methodological heterogeneity among the included trials, we used a random-effects model for meta-analysis of outcome measures from individual trials. The collected studies were categorized based on the type of oral medication or supplement being studied.
Our statistical analyses were conducted in accordance with the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions. We summarized data that was sufficiently similar and of sufficient quality, with both event (dichotomous) data and continuous data. Dichotomous data was expressed as RR, while for low event rates, we utilized the Peto OR. Continuous data was presented as MD. When using RR or MD, we calculated overall results based on a fixed-effects model. Results from clinically comparable trials were reported separately.
Results
Characteristics of included studies
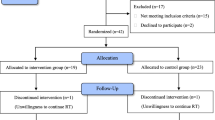
The bibliographic research generated a total of 675 references. After deduplication, 606 unique records were suitable for title and abstract screening. After title and abstract screening, 53 references remained for full-text screening. Ongoing studies and finished study without full-text report were not included. At the end of full-text review, a total of 11 references fully met our inclusion criteria and were considered eligible for data extraction. Ten randomised control trials (RCTs) and 1 retrospective study were included for qualitative and quantitative analysis (Fig. 1). No multicentre clinical trial was found. Four clinical control trials were conducted with placebo [17, 23,24,25]. Six reports were published in English and 5 published in Chinese. Four trials evaluated the effect of oral TCM [12, 26,27,28], 3 trials focused on the oral application of sucralfate [23,24,25] and a trial tested retinyl palmitate in radiation-induced intestinal inflammation [17]. Other studies on the application pentoxifylline [29], butyrate [19] and high-fibre diet [30] were also documented.
Eight-hundred ninety-eight patients in total were included in all 11 trials. Three studies included post-irradiation for various of disease including prostate, uterine, cervical, rectal or vaginal cancer [23, 24, 30]. Amoung these trails, some studies on Gynecological tumors included female specifically [27, 31], while some focus on prostate cancers which only occurs in male [25]. Four studies did not point out specific original cancer site [12, 17, 19, 29]. Detailed information of included studies and corresponding risk of bias was listed in Supplementary Table series S1.
Risk of bias
Seven studies clearly indicated patients were randomly assigned [23,24,25, 27, 30, 31]. As for random sequence generation, the use of computer or the Doll’s randomization list was considered as low risk of selection bias. Hille’s study was retrospective and therefore categorized in high risks of selection bias. Five studies suggested that double blind was performed in their studies [17, 23,24,25, 31], which were scored in low risk of performance and detection bias. We cannot draw conclusion whether outcome data of Hille et al. were completed with confidence as they are retrospective studies. No missing data including drop-outs were documented by the end of the studies, and outcomes were clearly described in other trials. Report data were consistent with protocol provided by Chruscielewska et al., and this study is considered as low risk [23]. Other included studies presented available protocols, and we judged them as having an unclear risk of bias for this domain. We defined Hille, Christiansen, Pradier, Hermann, Siekmeyer and Weiss with moderate bias on confounding, no information in selection of participants, low bias on classification of interventions, deviations from the originally stated intervention, missing data, measurement of outcomes and selection of the reported result.
Three studies involving oral application of Chinese medicine may be identical in terms of smell and taste, and blinding was not clearly announced in the report. We consider these papers as high risk of performance bias [12, 27, 30]. We cannot draw solid conclusion whether report of Hille et al. was complete [29]. Very small population (< 20 participants in total) did Ehrenpreis include in their studies, and we cannot exclude the possible systematic bias. We did not identify any other potential bias, and we rated this domain at low risk of bias (Fig. 2).
Overall, 3 randomised, double-blinded, placebo-controlled trials on the effect of sucralfate; 4 randomized controlled trials on TCM; 3 trials on daily supplements including vitamin A, butyrate and high-fibre diet; and 1 trial which combined pentoxifylline and tocopherol were analysis. Most of the trials made use of comprehensive scoring system for evaluation and data presentation, such as RTOG/EORTC toxicity grades [25, 27, 29], KPS score [27, 31] and Vienna rectoscopic score [27]. Some studies applied self-developed scaling systems [17, 19, 24, 31]. These scaling systems commonly involve diarrhoea frequency, bleeding, pain and surgery possibility. And we grouped specific outcomes dichotomously by the conclusive determination from each article.
Effects of interventions
Three randomised, double-blinded, placebo-controlled trials on the effect of sucralfate have been reported [23,24,25]. In total, 235 and 251 participants were included throughout the three studies in sucralfate arm and placebo arm, respectively. Outcome documented in the three studies were commonly based on the overall score of clinical symptoms involving diarrhoea score and bleeding score. Low heterogeneity was found in the three studies in terms of diarrhoea score (χ2 = 0.32, df = 1, P = 0.57; I2 = 0%) or bleeding score (χ2 = 0.06, df = 1, P = 0.81; I2 = 0%). No significant difference between the effect of sucralfate or placebo was found in the three studies regarding diarrhoea (OR = 0.81, 95% CI = [0.47, 1.41]) or bleeding (OR = 0.81, 95% CI = [0.47, 1.41]). One RCT with 198 participants weighted 83% of the meta-analysis, reported each of the RTOG toxicity grades, number of worse bleeding and frequency and showed no significant difference between two arms in all aspects. Chruscielewska et al. showed no change in diarrhoea score or bleeding score from subacute phase (week 8) to chronic phase (week 52, Fig. 3, Table 1).
Four randomised controlled trials were conducted to evaluate the effect of TCM on totally 232 participants [12, 26, 27, 31]. The four trials did not show high heterogeneity (χ2 = 2.52, df = 4, P = 0.64; I2 = 0%). Meta-analysis result shows that TCM is in favour for reducing symptom of CRP (OR = 0.18, 95% CI = [0.10, 0.34]; Z = 5.35, P < 0.00001, Fig. 4).
Combination of oral TCM therapies and probiotics was applied in these studies. In Chen et al., oral treatment and enema with dexamethasone, gentamicin and lidocaine were compared with Entrocoordinatibiogen, a Bacillus licheniformis capsule; the addition of TCM showed advantages in reducing diarrhoea, hematochezia and serum TNF-α, IL-6, IL-8 and IL-10. Oral smectite was used by Xiao et al. The combination of bifid triple viable, probiotics composed of enterococcus, Lactobacillus acidophilus and Bifidobacterium improved symptoms of CRP but did not significantly altered quality of life (OR = 0.24, 95% CI = [−3.89, 4.37]).
We classified the application of retinyl palmitate [17], butyrate [19] and high-fibre diet (HFD, [30]) as supplements and compared their effect on radiation proctitis using meta-analysis. Ehrenpreis and Wang’s studies are heterogeneity, and we used random effect model instead for evaluation (Tau2 = 6.34; χ2 = 2.89, df = 1, P = 0.09; I2 = 65%). The supplementary of both VitA and high-fibre diet resulted in reduction of bleeding and increase in haemoglobin (Z = 2.76, P = 0.006, MD = 6.02, 95% CI = [1.75, 10.30], Fig. 5). Effect of VitA was estimated by the scale developed by the author, the Radiation Proctopathy System Assessments Scale (RPSAS). And Wang demonstrated that the effects of HFD on CRP, including change in haemoglobin level and inflammation factors level, were not relying on the change of BMI (P = 0.74). It should be noted that HFD was claimed prescribed before and during radiotherapy, but Wang did not clearly indicate the time line of data collection. The improvement mediated by HFD may be transient, but the authorship did not precisely recommend the duration of HFD for CRP. Mete, Assisi and Casale conducted endoscopic for 70 participants, and those who received butyrate showed better condition under endoscope (Z = 2.51, P = 0.01), in terms of telangiectasia, adjacent mucosa, ulcers, stenosis or necrosis. Since the data symptoms were significantly different at T0 (before treatment), we did not include this study in meta-analysis.
We consider the combination therapy of pentoxifylline and tocopherol (PT) as a separated treatment. Despite tocopherol (VitE) serves as a daily supplementary, pentoxifylline is a prescription which is not over the counter. The effect of PT was evaluated by RTOG/EORTC toxicity criteria, and the PT arm demonstrated no worsening, but the improvement was not statically significant (OR = 5.00, 95% CI = [0.93, 26.79], Fig. 6). It should be noted that 6 patients who were treated with pentoxifylline and tocopherol received additional symptomatic therapy with smectite (n = 1), laxatives (n = 1), short-chain fatty acids (n = 3) or misoprostol (n = 1). The improvement in RTOG grading may not be solely result from PT treatment [29].
Discussion
This systematic review and meta-analysis of 11 studies found that oral TCM drinks, but not sucralfate, demonstrated significant symptom improvement in CRP. In addition, probiotics and retinyl palmitate may have potential benefits in reducing inflammation and improving symptoms. To our knowledge, this is the first systematic review summarizing oral treatment for CRP. Oral TCM seems to be useful for increasing haemoglobin, reducing CRP symptom and improving overall quality of life. But the complexity nature of herbs and the combination of several herbs together with the use of smectite and other treatment raise the concern of heterogeneity in general application. VitA and high-fibre diet remitted rectal bleeding, but few participants (17 and 63 participants) were included.
The present study is in line with previous findings. Wetering et al. reviewed a series of nonsurgical interventions for late rectal problems after pelvic radiotherapy including enema, hyperbaric oxygen therapy and argon plasma coagulation. Updated version in 2016 included some of outcome data of sucralfate trials without meta-analysis, and 1 TCM trial consists of Shen Ling Bai Zhu powders administered through anorectal [7]. Zhou et. al systematically compared the therapeutic effect with and without combination of TCM on acute radiation proctitis which also showed significant benefit [32].
TCM seems to be useful to reduce rectal bleeding without severe side effect on the liver or kidney [12, 33]. But the dosage and herbs composition variated between trials, decreasing the confidence of meta-analysis and the applicability worldwide. Well-designed, large-scale, multicentre placebo-controlled trial should be conducted in the future for validation. Neither 6 g/day nor 12 g/day sucralfate may be efficient to improve symptom of CRP. The application of daily supplements may relieve CRP, but more evidence should be provided. The combination of 800-mg pentoxifylline and 1000-mg tocopherol daily did not significantly improve CRP symptom. The sole effect of pentoxifylline or tocopherol remains unclear [29, 34,35,36].
This evidence implies that TCM, probiotics and prebiotics are potential treatment options for CRP. Coptidis, Glycyrrhiza and Pulsatilla were commonly used in TCM treatment, and the active ingredients of these herbs may be found to promote a uniform prescription. Animal studies have shown that an array of TCM treatments may protect colorectal tissue from fibrosis, apoptosis or inflammation, indicating the potential for future clinical application. Probiotics have also been applied to improve symptoms of bowel diseases such as inflammatory bowel disease (IBD). Although the effect of butyrate as a metabolite of probiotics was not statistically significant, probiotics have been demonstrated to relieve diarrhoea in acute radiation proctitis. Vitamin A, vitamin E and butyrate have also been supplemented for CRP treatment, and a double-blind placebo-controlled trial is needed to confirm the benefit. Finally, a specific scaling system should be developed to uniformly assess the severity of CRP symptoms, including diarrhoea, rectal bleeding, ulcers and quality of life.
Variated, comprehensive outcomes scoring systems in these trials make it difficult to be interpretated. Radiation Therapy Oncology Group toxicity scale [37,38,39], Karnofsky Performance Scale [40, 41], rectoscopic score and self-developed score were found to be used. A specific scaling system shall be developed to uniform the outcome assessment for CRP documenting the severity of diarrhoea, rectal bleeding, ulcer and the quality of life. The meta-analysis of sucralfate was based on Chutkan and Gilinski scale, RTOG toxicity sore and self-developed diarrhoea score.
Future trial is needed to test the contribution of novel oral therapies on CRP, such as probiotics, protein supplements and targeted medicine. Probiotics have been applied for improving symptoms of bowel diseases such as inflammatory bowel disease (IBD), Although effect of butyrate as a metabolite of probiotic was not statistically significant, probiotics have been demonstrated to relief acute diarrhoea. Delia et al. investigated the efficacy of a high-potency probiotic preparation on prevention of radiation-induced diarrhoea in 490 patients [13]. The combination of Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis was also utilized by Yuan et al. in CRP [31]. But RCT on probiotics for CRP was not identified in this systematic review. Enteral and parenteral nutrition have been widely applied for an array of IBD and protein/amino acids supplementary treatment for cancer [42, 43]. More evidence is expected to investigate the effect of CRP-induced malnutrition correction on adjusting anaemia and enhancing recovery. Recent study on molecular mechanism of CRP implied that platelet-derived growth factor C signaling is a potential therapeutic target in animal model [44]. Targeted medicine may be developed as another promising oral treatment for CRP.
Conclusion
Pelvic cancer survivors are suffering from CRP, presenting with prolong diarrhoea, rectal bleeding, ulcers or even fistula, which requires surgery. Oral drugs are convenience for daily application, and some have been reported improved symptoms of CRP. However, the treatment strategy of CRP remains controversial. We systematically reviewed the efficacy of oral treatment for CRP and found that TCM, daily supplement with high-fibre diet and vitamin A contributed to reduce rectal bleeding and diarrhoea. In spite of oral sucralfate, current oral treatments should be suggested for relieving symptom of CRP. The effect of enteral nutrition, probiotics, smectite and other commonly used oral CRP treatment may be evaluated in future.
Availability of data and materials
Not applicable. This is a systematic review of published literature.
Abbreviations
- CRP:
-
Chronic radiation proctitis
- RCTs:
-
Randomized control trials
- CI:
-
Confidence intervals
- OR:
-
Odds ratio
- MD:
-
Mean difference
- HFD:
-
High-fibre diet
- TCM:
-
Traditional Chinese medicine
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
References
Grodsky MB, Sidani SM. Radiation proctopathy. Clin Colon Rectal Surg. 2015;28(2):103–11 . [cited 2021 Feb 4].
Babb RR. Radiation proctitis: a review. Am J Gastroenterol. 1996;91(7):1309–11.
Rustagi T, Mashimo H. Endoscopic management of chronic radiation proctitis. World J Gastroenterol. 2011;17:4554–62.
Ashcraft KA, Miles D, Sunday ME, Choudhury KR, Young KH, Palmer GM, et al. Development and preliminary evaluation of a murine model of chronic radiation-induced proctitis. Int J Radiat Oncol Biol Phys. 2018;101(5):1194–201. https://doi.org/10.1016/j.ijrobp.2018.04.061.
Zinicola R, Rutter MD, Falasco G, Brooker JC, Cennamo V, Contini S, et al. Haemorrhagic radiation proctitis: endoscopic severity may be useful to guide therapy. Int J Colorectal Dis. 2003;18(5):439–44.
Gibson RJ, Keefe DMK, Lalla RV, Bateman E, Blijlevens N, Fijlstra M, et al. Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer. 2013;21:313–26.
van de Wetering FT, Verleye L, Andreyev HJN, Maher J, Vlayen J, Pieters BR, et al. Non-surgical interventions for late rectal problems (proctopathy) of radiotherapy in people who have received radiotherapy to the pelvis. Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd; 2016;2016
Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat Oncol. 2010;5(1):31. https://doi.org/10.1186/1748-717X-5-31.
Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096.
Yang JH, Yoo JM, Cho WK, Ma JY. Anti-inflammatory effects of Sanguisorbae Radix water extract on the suppression of mast cell degranulation and STAT-1/Jak-2 activation in BMMCs and HaCaT keratinocytes. BMC Complement Altern Med. 2016;16(1):347.
Zhang X, Tian R, Zhao C, Birch S, Lee JA, Alraek T, et al. The use of pattern differentiation in WHO-registered traditional Chinese medicine trials – a systematic review. Eur J Integr Med. 2019;1(30): 100945.
Chen B, Liang J. Clinical study on treatment of radiation proctitis with Bupi-Qingchang recipe combined with Chinese herbal enema. Int J Tradit Chinese Med. 2019;41(6):580–4.
Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, et al. Prevention of radiation-induced diarrhea with the use of VSL#3, a new high-potency probiotic preparation. Am J Gastroenterol. 2002;97(8):2150–2.
Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61(6):829–38.
Liu W, Zhang J, Wu C, Cai S, Huang W, Chen J, et al. Unique features of ethnic Mongolian gut microbiome revealed by metagenomic analysis. Sci Rep. 2016;6:6.
Hibberd AA, Lyra A, Ouwehand AC, Rolny P, Lindegren H, Cedgård L, et al. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017;4(1):e000145.
Ehrenpreis ED, Jani A, Levitsky J, Ahn J, Hong J. A prospective, randomized, double-blind, placebo-controlled trial of retinol palmitate (vitamin A) for symptomatic chronic radiation proctopathy. Dis Colon Rectum. 2005;48(1):1–8.
Krichevsky AM, Kosik KS, Aakalu G, Smith WB, Nguyen N, Jiang C, et al. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32(4):683–96.
Mete LS, Assisi D, Casale V. Efficacy of butyrate on rectal toxicity of radiotherapy in prostate cancer patients. Dig Liver Dis Suppl. 2007;1:23–6.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Sterne JAC, Higgins JPT RB on B of the DG for A-N, Sterne J, Higgins J, Reeves B, on behalf of the development group for ACROBAT-NRSI. A Cochrane risk of bias assessment tool: for non-randomized studies of interventions (ACROBAT-NRSI). Version 100. 2014;
The Cochrane Collaboration. GRADEpro GDT. Cochrane Community. 2017.
Chruscielewska-Kiliszek MR, Regula J, Polkowski M, Rupinski M, Kraszewska E, Pachlewski J, et al. Sucralfate or placebo following argon plasma coagulation for chronic radiation proctitis: a randomized double blind trial. Color Dis. 2013;15(1):e48–55.
Henriksson R, Franzén L, Littbrand B. Prevention and therapy of radiation-induced bowel discomfort. Scand J Gastroenterol Suppl. 1992;191:7–11.
Kneebone A, Mameghan H, Bolin T, Berry M, Turner S, Kearsley J, et al. Effect of oral sucralfate on late rectal injury associated with radiotherapy for prostate cancer: a double-blind, randomized trial. Int J Radiat Oncol Biol Phys. 2004;60(4):1088–97.
Jiang W, Lu J, Xin L, Wang J, Shen Z. Clinical study on the treatment of radiation enteritis by Hongyu decoction. SHAANXI J Tradit CHINESE Med. 2018;39(08):1008–10.
Xiao C. The evaluation of the moxibustion reduce the chronic radiation proctitis in the radiotherapy of cervical carcinoma. 2019.
Wang Z, Wang Q, Wang X, Zhu L, Chen J, Zhang B, et al. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J Cell Mol Med. 2019;23(5):3747–56.
Hille A, Christiansen H, Pradier O, Hermann RM, Siekmeyer B, Weiss E, et al. Effect of pentoxifylline and tocopherol on radiation proctitis/enteritis. Strahlenther Onkol. 2005;181(9):606–14.
Wang Y. Effect of dietary fiber on nutritional status and immune function in patients with pelvic radiotherapy. 2019.
Yuan J. Clinical observation of Fuzheng Qingchang decoction combined with probiotics in the treatment of chronic radiotherapy proctitis after radiotherapy for cervical cancer (Rehan cuoza zheng). 2019.
Zhou Y. Meta-analysis of the efficacy of integrated traditional Chinese and Wetern medicine in the treatment of radiation proctitis. 2019.
Tan YQ, Chen HW, Li J, Wu QJ. Efficacy, chemical constituents, and pharmacological actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front Pharmacol. Frontiers Media S.A.; 2020;11.
Pareek P, Samdariya S, Sharma A, Gupta N, Shekhar S, Kirubakaran R. Pentoxifylline and vitamin E alone or in combination for preventing and treating side effects of radiation therapy and concomitant chemoradiotherapy. Cochrane Database of Systematic Reviews. 2016;2016(3):CD012117.
Venkitaraman R, Coffey J, Norman AR, James FV, Huddart RA, Horwich A, et al. Pentoxifylline to treat radiation proctitis: a small and inconclusive randomised trial. Clin Oncol. 2008;20(4):288–92.
EUCTR2012-004211-31-GB. PPALM - palm oil and pentoxifylline against late morbidity. 2014.
Lund JÅ, Kaasa S, Wibe A, Widmark A, Fransson P. Late radiation effects to the rectum and anus after treatment for prostate cancer; validity of the LENT/SOMA score. Acta Oncol. 2013;52(4):727–35.
Syndikus I, Morgan RC, Sydes MR, Graham JD, Dearnaley DP. Late gastrointestinal toxicity after dose-escalated conformal radiotherapy for early prostate cancer: results from the UK Medical Research Council RT01 trial (ISRCTN47772397). Int J Radiat Oncol Biol Phys. 2010;77(3 CC-Gut CC-Urology):773–83.
Esco R, Valencia J, Coronel P, Carceller JA, Gimeno M, Bascón N. Efficacy of orgotein in prevention of late side effects of pelvic irradiation: a randomized study. Int J Radiat Oncol Biol Phys. 2004;60(4 CC-Colorectal CC-Pain, Palliative and Supportive Care CC-Gynaecological, Neuro-oncology and Orphan Cancer):1211‐1219.
Sidik S, Hardjodisastro D, Setiabudy R, Gondowiardjo S. Does hyperbaric oxygen administration decrease side effect and improve quality of life after pelvic radiation? Acta Med Indones. 2007;39(4 CC-Complementary Medicine CC-Gynaecological, Neuro-oncology and Orphan Cancer CC-Gut):169‐173.
EUCTR2007-002082-13-DE. Randomised, double-blind, placebo-controlled, multicentre, comparative phase II pilot study on the efficacy and tolerability of an 8-week rectal treatment with 2 mg budesonide or placebo for the prevention of acute radiation proctitis - budesonide foam ve. 2009.
Altomare R, Damiano G, Abruzzo A, Palumbo VD, Tomasello G, Buscemi S, et al. Enteral nutrition support to treat malnutrition in inflammatory bowel disease. Nutrients. MDPI AG; 2015;7:2125–33. Available from: /pmc/articles/PMC4425135/
Virizuela JA, Camblor-Álvarez M, Luengo-Pérez LM, Grande E, Álvarez-Hernández J, Sendrós-Madroño MJ, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Transl Oncol. 2018;20(5):619–29.
Lu W, Xie Y, Huang B, Ma T, Wang H, Deng B, et al. Platelet-derived growth factor C signaling is a potential therapeutic target for radiation proctopathy. Sci Transl Med. 2021;13(582):eabc2344.
Acknowledgements
We thank NMPA Key Laboratory for Technology Research and Evaluation of Pharmacovigilance, the Guangzhou Traditional Chinese medicine Core and Characteristic Clinical Diagnosis and Treatment Technology Construction Project and Guangdong Medical Research Foundation for supporting this research. We thank Dr. Yang Li for his contribution on administration issue.
Funding
This article is independent research funded by NMPA Key Laboratory for Technology Research and Evaluation of Pharmacovigilance (2022ZDZ06), the Guangzhou Traditional Chinese medicine Core and Characteristic Clinical Diagnosis and Treatment Technology Construction Project (No. 10) and Guangdong Medical Research Foundation (B2023149).
Author information
Authors and Affiliations
Contributions
LZL extracted relevant population and intervention characteristics using a standard data extraction template. JJL resolved any disagreements by discussion. LZL and NNX independently assessed the risk of bias of all included studies according to the Cochrane Handbook for Systematic Reviews of Interventions.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, L., Xiao, N. & Liang, J. Comparative efficacy of oral drugs for chronic radiation proctitis — a systematic review. Syst Rev 12, 146 (2023). https://doi.org/10.1186/s13643-023-02294-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02294-2