Abstract
Background
Painful diabetic peripheral neuropathy (PDPN) is a key concern in clinical practice. In this systematic review and meta-analysis, we compared duloxetine and placebo treatments in terms of their efficacy and safety in patients with PDPN.
Methods
Following the PRISMA guidelines, we searched the Cochrane Library, PubMed, and Embase databases for relevant English articles published before January 11, 2021. Treatment efficacy and safety were assessed in terms of pain improvement, patient-reported health-related performance, and patients’ quality of life.
Results
We reviewed a total of 7 randomized controlled trials. Regarding pain improvement, duloxetine was more efficacious than placebo (mean difference [MD] − 0.89; 95% confidence interval [CI] − 1.09 to − 0.69; P < .00001). Furthermore, duloxetine significantly improved the patients’ quality of life, which was assessed using the Clinical Global Impression severity subscale (MD − 0.48; 95% CI − 0.61 to − 0.36; P < .00001), Patient Global Impression of Improvement scale (MD − 0.50; 95% CI − 0.64 to − 0.37; P < .00001), and European Quality of Life Instrument 5D version (MD 0.04; 95% CI 0.02 to 0.07; P = .0002). Severe adverse events were rare, whereas nausea, somnolence, dizziness, fatigue, constipation, and decreased appetite were common; approximately, 12.6% of all patients dropped out because of the common symptoms.
Conclusions
Duloxetine is more efficacious than placebo treatments in patients with PDPN. The rarity of severe adverse events indicates that duloxetine is safe. When a 60-mg dose is insufficient, 120 mg of duloxetine may improve PDPN symptoms. Our findings may help devise optimal treatment strategies for PDPN.
Systematic review registration
PROSPERO CRD42021225451
Similar content being viewed by others
Background
Painful diabetic peripheral neuropathy (PDPN) is caused by chronic hyperglycemia and characterized by nerve damage and intolerable pain [1]. Approximately 50% of all patients with diabetes develop peripheral neuropathy [2], 10 to 26% of whom experience PDPN [3]. Clinical manifestations of PDPN include the neuropathy of distal lower extremities, which involves tingling, shooting pain, burning pain, allodynia, hyperesthesia, and other unusual sensations. Symptoms often deteriorate at night and affect sleep quality. Some patients may experience mood disorders, such as anxiety and depression [4,5,6].
The precise pathophysiology of PDPN remains debatable. A commonly accepted hypothesis is that distal nerve fiber damage would result in altered peripheral signaling and compensatory changes in the central nervous system, thereby disrupting the mechanisms underlying the inhibition of endogenous pain; this would promote the hyperexcitability and sensitization of pain-transmitting pathways and cause severe and persistent pain [4, 7].
The neurotransmitters serotonin (5-hydroxytryptamine) and norepinephrine help modulate nociceptive transmission by descending pain inhibitory pathways in the brain and spinal cord [8,9,10]. Duloxetine hydrochloride is a potent dual serotonin–norepinephrine reuptake inhibitor (SNRI); it modulates the mechanisms underlying PDPN [11]. SNRI antidepressants increase noradrenaline levels and target α2-adrenergic receptors in the dorsal horn of the spinal cord and the locus coeruleus. These pathways are highly efficacious against allodynia and hyperalgesia, which are associated with neuropathic pain. Serotonin may also enhance the inhibitory effects of noradrenaline in an auxiliary manner [12].
Currently, duloxetine and pregabalin are the only drugs approved by the US Food and Drug Administration (FDA) for treating PDPN. Pregabalin and duloxetine have been recommended as the first-line therapy in a total of 5 and 4 relevant guidelines, respectively. The 4 guidelines recommending duloxetine are the Neuropathic Pain Special Interest Group guideline (International Association for the Study of Pain), European Federation of Neurological Societies guideline, National Institute for Health and Care Excellence guideline (UK), and Canadian Pain Society guideline [13,14,15,16,17]. The additional guideline recommending pregabalin is the American Academy of Neurology guideline; the aforementioned difference between the 2 drugs in terms of recommendation stems from the fact that a limited number of duloxetine trials were graded as class I, and thus, data were regarded to be insufficient for level A recommendation for this drug [18, 19]. Nevertheless, duloxetine is not inferior to pregabalin in treating PDPN [20, 21]. To the best of our knowledge, no meta-analysis conducted after 2015 has evaluated the role of duloxetine in the treatment of PDPN [22]. Moreover, our knowledge regarding the clinical utility of duloxetine, different efficacies of its prescribed dose, and severity of associated adverse events in patients with PDPN remains limited. Therefore, in the present updated systematic review and meta-analysis of randomized-controlled trials (RCT; placebo-controlled), we evaluated the efficacy and safety of duloxetine in the treatment of PDPN. We extracted data from earlier studies in which relevant scales and questionnaires were used to investigate the optimal dose for treatment, the drug’s efficacy in improving pain and patients’ quality of life and the severity and occurrence of adverse events.
Methods
Study design and inclusion criteria
This systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [23]. The study protocol was developed and registered in the PROSPERO database (CRD42021225451; January 9, 2021). The selection criteria were defined before the literature search and included the PICO components—problem/population, intervention, comparison, and outcome. We included studies on the comparison between duloxetine and placebo treatments in adult patients with PDPN due to diabetic polyneuropathy who presented with daily pain for > 6 months, in whom the symptoms started from distal extremities bilaterally, and whose weekly average visual analog scale pain scores were > 4. All doses and treatment durations of duloxetine were reviewed. The primary outcome measure was a reduction in patients’ weekly mean pain scores, assessed using an 11-point Likert-type scale, for 24-h average pain. The secondary outcomes included 24-h worst pain and night pain; numbers of patients with 30% and 50% reductions in 24-h pain scores [24]; and scores on the Brief Pain Inventory (BPI) scale (severity and interference) [25], Short Form-36 (SF-36) questionnaire [26], Patient Global Impression of Improvement (PGI-I) scale [27], Clinical Global Impression scale (CGI; severity subscale) [28], European Quality of Life Instrument 5D version (EQ-5D) [29], and Short-Form McGill Pain Questionnaire (SF-MPQ; sensory component) [30]. Adverse events and compliance were assessed in terms of the number of patients discontinued because of adverse events, number of patients with at least one adverse event or severe adverse event, and incidence rate of common adverse events. Non-RCTs, case reports, reviews, animal studies, letters to editors, and conference abstracts were excluded from our review. No restrictions related to trial duration were applied.
Search strategy
We searched the Cochrane Library, PubMed, and EMBASE databases for relevant RCTs published in English before January 11, 2021. Keywords used for the search in each database were as follows: (((DM or “diabetes mellitus” or diabet*) and (neuropathy or neuropath* or neurolog* or neuralgia)) or ((PDPN or “painful diabetic peripheral neuropath*” or DPNP or “diabetic peripheral neuropathic pain” or DPN or “diabetic peripheral neuropath*” or PDN or “peripheral diabetic neuropath*” or DSPN or “distal symmetric polyneuropath*”) or (“diabetic polyneuropath*” or “diabetic sensorimotor polyneuropath*” or “distal symmetric sensorimotor polyneuropath*” or “diabetic distal sensorimotor polyneuropath*”) or (“diabetic focal neuropath*” or “diabetic multifocal neuropath*” or “diabetic amyotroph*” or “diabetic autonomic neuropath*” or “symmetric diabetic proximal motor neuropath*” or “diabetic mononeuropath*”))) and (duloxetin* or cymbalta or irenka or “drizalma sprinkle” or ariclaim or xeristar or yentreve). In addition, the reference lists of the included studies were manually searched for additional eligible studies.
Study selection and quality assessment
After removing duplicate studies, 2 reviewers (CS Wu and YR Huang) independently reviewed the titles and abstracts of the eligible studies. Non-RCTs and non-eligible studies were excluded (upon reviewer consensus) from further analyses. Subsequently, the full texts of the included articles were analyzed. Both reviewers independently assessed the risk of bias by using the Cochrane risk-of-bias tool for RCTs (RoB 2.0) [31]. Any disagreement between the 2 reviewers was mitigated by a third reviewer.
Data collection and analysis
Odds ratio (OR) and 95% confidence interval (CI) values were calculated using the Mantel–Haenszel method for dichotomous outcome data, whereas the mean difference (MD) and 95% CI values were calculated through inverse variance weighting for continuous data. Outcome data, such as sample size, mean, and standard deviation (SD), were extracted for each treatment. If the included articles reported only standard error values, the corresponding SD values were calculated using relevant software. Heterogeneity was investigated using the I2 statistic, and 25%, 50%, and 75% values indicated low, moderate, and high degrees of heterogeneity, respectively [32]. The pooled estimates of the MD and OR values were calculated using random-effects models. In addition to reporting the overall effects of duloxetine, we also have done subgroup analyses of the effects of different doses of duloxetine on primary outcomes of reduction in patients’ weekly mean pain scores. Statistical significance was set at P < 0.05. The meta-analysis was performed using RevMan (version 5.4) [33].
Results
Included articles
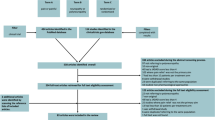
Our search returned a total of 1862 relevant articles; of them, 1475 were excluded after the removal of duplicates. Next, 794 studies were excluded after the titles and abstracts were screened. The full texts of the remaining 681 articles were reviewed, and 674 of them were eliminated on the basis of the exclusion criteria. Finally, a total of 7 eligible RCTs [34,35,36,37,38,39,40] were included in our meta-analysis. Figure 1 depicts the flowchart of study selection.
Study characteristics
The 7 included studies comprised a total 2205 patients (men, 1246 [56.5%]; mean age, 60.2 years). Patients received 20, 40, 60, and 120 mg of duloxetine per day. Six studies had a follow-up duration of 12 weeks, and one study had a follow-up duration of 8 weeks [39]. Reduction of 24-h weekly mean pain score (daily scores were recorded in a diary) was reported as the primary outcome in 6 studies and the reduction in 24-h average BPI pain score (scores recorded during weekly visits) in one study. Dropout rates were between 13.6% and 25.7%, which were not sufficiently high to affect the statistical power. Table 1 presents a summary of the characteristics of the included studies.
Outcomes
Pain improvement
A total of six studies reported improvements in weekly mean pain scores. Duloxetine was more efficacious than placebo, with low to moderate heterogeneity noted across studies (MD = − 0.95, 95% CI = − 1.18 to − 0.72, Z = 7.79, P < 0.00001, and I2 = 36%; Fig. 2A). Subgroup analysis of different doses of duloxetine indicated significant improvements with doses of 40, 60, and 120 mg but not with that of 20 mg (MD = − 0.45, 95% CI = − 1.05 to 0.15, and P = 0.14). Head-to-head comparisons between the effective doses revealed no significant differences.
Pain. A Mean improvement in the weekly average of patients’ 24-h pain scores on an 11-point Likert-type scale at ≤ 12 weeks. B Mean improvement in pain severity assessed using the Brief Pain Inventory scale: average pain scores at ≤ 12 weeks. C Number of patients with ≥ 50% improvement in the weekly average of 24-h pain scores on the 11-point Likert-type scale at ≤ 12 weeks. D Number of patients with ≥ 30% improvement in the weekly average of 24-h pain scores on the 11-point Likert-type scale at ≤ 12 weeks. E Mean improvement in patients’ night pain scores on the 11-point Likert-type scale at ≤ 12 weeks. F Mean improvement in patients’ worst pain scores on the 11-point Likert-type scale at ≤ 12 weeks
Six studies reported improvements in BPI average pain scores, and overall, duloxetine was significantly more efficacious than placebo, with low heterogeneity observed across studies (MD = − 0.88, 95% CI = − 1.08 to − 0.68, Z = 8.54, P < 0.00001, and I2 = 14%; Fig. 2B).
In total, 6 studies reported the numbers of patients with a 50% pain reduction. Overall, the efficacy of duloxetine was higher than that of a placebo, with low heterogeneity noted across studies (OR = 2.06, 95% CI = 1.67 to 2.54, Z = 6.72, P < 0.00001, and I2 = 25%; Fig. 2C).
A total of 6 studies reported a number of patients with a 30% pain reduction. Duloxetine was significantly more efficacious than placebo, with low heterogeneity observed across studies (OR = 2.25, 95% CI = 1.86 to 2.72, Z = 8.42, P < 0.00001, and I2 = 0%; Fig. 2D).
Improvements in night pain scores were reported in a total of 5 studies. The efficacy of duloxetine was higher than that of a placebo, with low heterogeneity noted across studies (MD = − 0.89, 95% CI = − 1.09 to − 0.69, Z = 8.70, P < 0.00001, and I2 = 0%; Fig. 2E).
In total, 5 studies reported improvements in worst pain scores. Duloxetine was significantly more efficacious than placebo, with low to moderate heterogeneity noted across studies (MD = − 1.05, 95% CI = − 1.31 to − 0.80, Z = 8.12, P < 0.00001, and I2 = 31%; Fig. 2F).
Patient-reported health performance and quality of life
Improvements in the physical functioning, mental health, and bodily pain domains of the SF-36 questionnaire were reported by a total of 3, 3, and 2 studies, respectively. For all domains, duloxetine exhibited higher levels of efficacy than did placebo, with low heterogeneity across studies for all domains (physical functioning: MD = 2.75, 95% CI = 1.77 to 3.72, Z = 5.53, P < 0.00001, and I2 = 0% (Fig. 3A); mental health: MD = 1.60, 95% CI = 0.56 to 2.63, Z = 3.03, P = 0.002, and I2 = 0% (Fig. 3B); and bodily pain: MD = 6.88, 95% CI = 4.15 to 9.60, Z = 4.95, P < 0.00001, and I2 = 0% (Fig. 3C)).
Quality of life. A Mean improvement in patients’ scores on the Short Form-36 (SF-36) physical functioning domain at ≤ 12 weeks. B Mean improvement in patients’ scores on the SF-36 mental health domain at ≤ 12 weeks. C Mean improvement in patients’ scores on SF-36 bodily pain domain at ≤ 12 weeks. D Patients’ scores on the Patient Global Impression of Improvement scale at ≤ 12 weeks. E Mean improvement in patients’ scores on the Brief Pain Inventory (interference) scale: average of the scores on 7 items at ≤ 12 weeks. F Mean improvement in patients’ scores on the Clinical Global Impression severity subscale at ≤ 12 weeks. G Mean improvement in patients’ scores on the European Quality of Life Instrument 5D version at ≤ 12 weeks. H Mean improvement in patients’ scores on the Short-Form McGill Pain Questionnaire sensory component at ≤ 12 weeks
Improvements in patients’ scores on the PGI-I, BPI (interference subscale), CGI (severity subscale), EQ-5D, and SF-MPQ (sensory component) tools were reported by 7, 7, 5, 4, and 4 studies, respectively. Overall, the efficacy of duloxetine in improving the aforementioned scores was significantly higher than that of the placebo, with low to moderate heterogeneity observed among studies (PGI-I: MD = − 0.50, 95% CI = − 0.64 to − 0.37, Z = 7.31, P < 0.00001, and I2 = 44% (Fig. 3D); BPI: MD = − 0.69, 95% CI = − 0.85 to − 0.53, Z = 8.39, P < 0.00001, and I2 = 0% (Fig. 3E); CGI: MD = − 0.48, 95% CI = − 0.61 to − 0.36, Z = 7.64, P < 0.00001, and I2 = 21% (Fig. 3F); EQ-4D: MD = 0.04, 95% CI = 0.02 to 0.07, Z = 3.75, P = 0.0002, and I2 = 0% (Fig. 3G); and SF-MPQ: MD = − 2.97, 95% CI = − 3.68 to − 2.27, Z = 8.22, P < 0.00001, and I2 = 0% (Fig. 3H)).
Safety and compliance
A total of 7 studies reported a number of patients who dropped out because of adverse events. The risk associated with duloxetine was significantly higher than that associated with placebo, with low heterogeneity noted across studies (OR = 3.00, 95% CI = 2.18 to 4.13, Z = 6.72, P < 0.00001, and I2 = 0%; Fig. 4A).
In total, 6 studies reported a number of patients with at least 1 adverse event. Duloxetine was associated with significantly higher levels of risk than was placebo, with low heterogeneity observed across studies (OR = 1.80, 95% CI = 1.47 to 2.21, Z = 5.62, P < 0.00001, and I2 = 0%; Fig. 4B).
A total of 5 studies reported the numbers of all adverse events. We analyzed the 6 most common adverse events associated with duloxetine (Table 2). Nausea had the highest incidence rate (20.21%; 10.4 to 30.2%), followed by somnolence (12.73%; 8.4 to 21.6%), dizziness (10.10%; 5.8 to 15.1%), fatigue/malaise (8.14%; 5.0 to 12.4%), constipation (8.01%; 5.0 to 12.8%), and decreased appetite (2.89%; 5.4 to 10.4%). The numbers of serious adverse events, defined as events leading to prolonged hospitalization, life-threatening experience, severe disability, or death during the study, reported in the 7 included studies were also recorded (Table 1). No significant differences were observed between duloxetine and placebo; moreover, the studies reported no common severe adverse event, except hyperglycemia, which was reported by a total of 3 studies [36, 37, 40] and electrolyte imbalance, which was reported by a total of 2 studies [35, 39].
Study quality
The quality of the included studies was assessed using RoB 2.0; the details are presented in Fig. 5 (ROB) and the supplementary information file. RoB 2.0 is an outcome-based instrument, but since each study’s outcome measures do not have different potential risks of bias (they all have the same assessment process and are all subjective scales), the evaluation is presented within a single study-based figure. A total of 4 studies were classified as low risk and 3 studies (conducted by Goldstein et al. [37], Yasuda et al. [34], and Gao et al. [40]) indicated some concerns. The study by Goldstein et al. had some concerns in RoB 2.0 domains 2 and 3, which was based on a high dropout rate of 24.7% and significant differences between the treatment (duloxetine: 60 mg/day, 13.2%; 120 mg/day, 19.5%) and placebo (5.2%) groups (P < 0.001). The study by Yasuda 2011 had some concerns in domains 1 and 2 because allocation concealment was not explained clearly. The study conducted by Gao et al. incompletely described the processes of randomization sequence generation and allocation concealment, leading to some concerns in domains 1 and 2.
Discussion
The efficacy of duloxetine in treating PDPN has been demonstrated in a total of 4 RCTs conducted between 2005 and 2011 [34,35,36,37]. In total, 3 sequential 52-week-long phase studies have revealed that duloxetine is superior to routine care in the long-term management of PDPN [41,42,43]. By contrast, another 52-week-long study reported no significant difference between PDPN and routine care in terms of their efficacy in pain control; nevertheless, the findings confirmed the long-term safety of duloxetine [44]. Other medications for PDPN include antiepileptic agents, pregabalin, and gabapentin and certain antidepressants (including tricyclic antidepressants) [45]. However, most of these medications are only partially effective for PDPN; moreover, they are frequently discontinued because of their associated adverse events, which limit their clinical utility.
As mentioned, duloxetine and pregabalin are the only 2 FDA-approved drugs for PDPN. Some studies have reported that the efficacy of duloxetine in PDPN pain relief is non-inferior [46, 47] to or even better than that of pregabalin [48, 49]. Only 3 recent systemic reviews and meta-analyses included RCTs on duloxetine for PDPN. Of them, 2 studies included a total of 5 studies [50, 51] and 1 study included a total of 6 studies [22]. Unfortunately, no relevant meta-analyses have been conducted since 2017. In addition to assessing pain severity and PGI scores, which were analyzed in an earlier meta-analysis [43], in the present study, we assessed patients’ health performance and quality of life in terms of night pain, worst pain, and SF-36 (physical functioning, mental health, and bodily pain domains), CGI, BPI (7-item interference), EQ-5D, and SF-MPQ (sensory component) scores. To the best of our knowledge, the present study is the first meta-analysis to explore the incidence rates of common adverse events.
Compared with placebo, duloxetine significantly improved patients’ pain scores on every item; the total weighted mean (TWM) of the reduction in the weekly mean of 24-h pain scores was − 2.62 (MD − 0.89; P < 0.00001). The TWM values of the reductions in the weekly mean values of average pain scores on BPI, night pain scores, and 24-h worst pain scores were –2.64 (MD − 0.84 and P < 0.00001), − 2.80 (MD − 0.82 and P < 0.00001), and − 3.02 (MD − 1.00 and P < 0.00001), respectively. Furthermore, 45.6% and 64.5% of all patients experienced 50% and 30% pain reduction, respectively, which highlights the clinical efficacy of duloxetine in the treatment of PDPN. Duloxetine considerably improved the physical and mental health and the quality of life of patients with PDPN, as evident from their scores on the following tools: CGI (TWM = − 1.39), PGI-I (TWM = 2.47), EQ-5D (TWM = 0.13), SF-36 (physical functioning and mental health domains, respectively: TWM = 6.06 and 1.01), and SF-MPQ (TWM = − 7.80). (All these analyses included the 20 mg and 40 mg subgroups data).
A total of 4 doses of duloxetine were compared in the present study. The 20-mg dose improved only the weekly worst pain and CGI scores of patients with PDPN; this dose exhibited the lowest efficacy in the aforementioned 2 items. This finding implies that the clinical value of the 20-mg dose is low. By contrast, the 40-mg dose markedly improved the patients’ scores on all pain relief items and PGI-I; in terms of efficacy, this dose was non-inferior to the 60- and 120-mg doses. The 40-mg dose achieved 50% pain reduction in most patients. Although only a single study included the 40-mg dose, the aforementioned finding suggests that the 40-mg dose of duloxetine can serve as an alternative to the 60- and 120-mg doses. The 60- and 120-mg doses exhibited significant efficacy in almost all items (except for the low efficacy of the 60-mg dose in improving patients’ SF-36 mental health and EQ-5D scores), and no significant differences were observed between them. However, the 120-mg dose was associated with numerically better outcomes than the 60-mg dose in terms of pain reduction (weekly mean of 24-h pain, BPI average pain, night pain, and worst pain), and health performance, and quality of life. Thus, the 120-mg dose of duloxetine may be useful when the 60-mg dose fails to ensure adequate relief in patients with PDPN.
Duloxetine was found to be safe in the treatment of PDPN: the incidence of severe adverse events was low, not higher than that associated with placebo treatments. However, complications such as nausea, somnolence, dizziness, fatigue, constipation, and decreased appetite were common; because of these common adverse events, approximately 12.6% of patients dropped out of the studies. Approximately, 71.3% of all patients experienced at least 1 adverse event. Therefore, when administering duloxetine, patients must be informed about these adverse effects, and they must be closely monitored for the incidence and severity of common adverse events. Symptomatic interventions should be administered as required.
The present study has some limitations. We found only 1 additional eligible study [40] compared with the last relevant meta-analysis [22]. Nevertheless, because the sample size of the additional RCT was high (second largest among the 7 included studies; n = 405) and comprehensive data on pain relief were reported by the RCT, we could provide considerable additional and updated data regarding the efficacy and safety of duloxetine in the treatment of PDPN. Of the studies included in the present study, the study by Gao et al. (2010) [29] reported variable dosage schedules for duloxetine depending on clinical responses (60 or 120 mg); this made it challenging for us to perform subgroup analyses. Hence, we performed the analyses for benefit considering that all patients received the higher dose (120 mg) and for harm considering that if all patients received the lower dose (60 mg). We further performed sensitivity analysis by excluding the data of the aforementioned study from each item, and the results remained significant. All 7 studies had similar cohorts and good uniformity in terms of the use of assessment tools. Although recent studies have demonstrated a high efficacy of duloxetine, further studies are warranted to evaluate the effects of various duloxetine doses, particularly the 40-mg dose. Furthermore, head-to-head RCTs on pregabalin, gabapentin, and SNRI drugs are necessary to identify the optimal treatment option for patients with PDPN.
Conclusions
The findings of our systematic review and meta-analysis suggest that duloxetine is more efficacious than placebo treatments in terms of pain relief and improvements in the quality of life of patients with PDPN. Duloxetine is safe, as indicated by the rarity of severe adverse events; however, patients receiving this drug should be monitored for some common complications, such as nausea, somnolence, dizziness, fatigue, constipation, and decreased appetite. This study may serve as a reference for future studies aimed at developing suitable interventions and optimal treatment strategies for patients with PDPN.
Availability of data and materials
The data sets produced and analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- PDPN:
-
Painful diabetic peripheral neuropathy
- SNRI:
-
Serotonin–norepinephrine reuptake inhibitor
- BPI:
-
Brief Pain Inventory
- SF-36:
-
Short form-36
- PGI-I:
-
Patient’s Global Impression of Improvement
- CGI:
-
Clinical Global Impression scale
- EQ-5D:
-
European Quality of Life Instrument 5D version
- SF-MPQ:
-
Short-Form McGill Pain Questionnaire
- RCT:
-
Randomized controlled trial
- MD:
-
Mean difference
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- SE:
-
Standard error
- TWM:
-
Total weighted mean
- AE:
-
Adverse event
References
Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–9.
Boulton AJ, Malik RA, Arezzo JC, et al. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–86.
Poncelet AN. Diabetic polyneuropathy. Risk factors, patterns of presentation, diagnosis, and treatment. Geriatrics. 2003;58:16–8, 24-15, 30.
Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36:2456–65.
Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14.
Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–8.
Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci. 1997;20:404–19; discussion 435-513.
Zuo H, Shi Z, Hussain A. Prevalence, trends and risk factors for the diabetes epidemic in China: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;104:63–72.
Clark FM, Proudfit HK. The projections of noradrenergic neurons in the A5 catecholamine cell group to the spinal cord in the rat: anatomical evidence that A5 neurons modulate nociception. Brain Res. 1993;616:200–10.
Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38.
Wong DT, Bymaster FP. Dual serotonin and noradrenaline uptake inhibitor class of antidepressants potential for greater efficacy or just hype? Prog Drug Res. 2002;58:169–222.
Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci. 2017;18:2483.
Cruccu G, Truini A. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther. 2017;6:35–42.
Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27.
Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113-e1188.
Centre for Clinical Practice at N. National institute for health and care excellence: clinical guidelines, in neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings. London: National Institute for Health and Care Excellence, (UK) Copyright © 2013, National Institute for Health and Care Excellence; 2013.
Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–35.
Price R, Smith D, Franklin G, et al. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary. Neurology. 2022;98:31.
Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–65.
Tanenberg RJ, Irving GA, Risser RC, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clin Proc. 2011;86:615–26.
Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the american diabetes association. Diabetes Care. 2017;40:136.
Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115. https://doi.org/10.1002/14651858.CD007115.pub3.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123-130.
Farrar JT, Young JP Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58.
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–38.
Ware JE, Snow KK, Kosinski M. SF-36 Health survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993.
Guy W. ECDEU assessment manual for psychopharmacology, revised, 1976. Rockville, MD US Department of Health: Education, and Welfare; 1976.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont (Pa : Township)). 2007;4:28–37.
Kind P. EuroQoL instrument: an index of health-related quality of life. In: Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Lippincott–Raven: Philadelphia; 1996.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Collaboration TC. Review Manager (RevMan) [Computer program] Version 5.4. 2020.
Yasuda H, Hotta N, Nakao K, et al. Superiority of duloxetine to placebo in improving diabetic neuropathic pain: results of a randomized controlled trial in Japan. J Diabetes Investig. 2011;2:132–9.
Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67:1411–20.
Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6:346–56.
Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–18.
Gao Y, Ning G, Jia WP, et al. Duloxetine versus placebo in the treatment of patients with diabetic neuropathic pain in China. Chin Med J (Engl). 2010;123:3184–92.
Rowbotham MC, Arslanian A, Nothaft W, et al. Efficacy and safety of the α4β2 neuronal nicotinic receptor agonist ABT-894 in patients with diabetic peripheral neuropathic pain. Pain. 2012;153:862–8.
Gao Y, Guo X, Han P, et al. Treatment of patients with diabetic peripheral neuropathic pain in China: a double-blind randomised trial of duloxetine vs. placebo. Int J Clin Pract. 2015;69:957–66.
Wernicke JF, Raskin J, Rosen A, et al. Duloxetine in the long-term management of diabetic peripheral neuropathic pain: an open-label, 52-week extension of a randomized controlled clinical trial. Curr Ther Res Clin Exp. 2006;67:283–304.
Wernicke JF, Wang F, Pritchett YL, et al. An open-label 52-week clinical extension comparing duloxetine with routine care in patients with diabetic peripheral neuropathic pain. Pain Med. 2007;8:503–13.
Yasuda H, Hotta N, Kasuga M, et al. Efficacy and safety of 40 mg or 60 mg duloxetine in Japanese adults with diabetic neuropathic pain: results from a randomized, 52-week, open-label study. J Diabetes Investig. 2016;7:100–8.
Raskin J, Smith TR, Wong K, et al. Duloxetine versus routine care in the long-term management of diabetic peripheral neuropathic pain. J Palliat Med. 2006;9:29–40.
Yang H, Sloan G, Ye Y, et al. New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol (Lausanne). 2019;10:929.
Devi P, Madhu K, Ganapathy B, et al. Evaluation of efficacy and safety of gabapentin, duloxetine, and pregabalin in patients with painful diabetic peripheral neuropathy. Indian J Pharmacol. 2012;44:51–6.
Boyle J, Eriksson ME, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35:2451–8.
Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”–a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154:2616–25.
Shahid W, Kumar R, Shaikh A, et al. Comparison of the efficacy of duloxetine and pregabalin in pain relief associated with diabetic neuropathy. Cureus. 2019;11:e5293.
Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161:639–49.
van Nooten F, Treur M, Pantiri K, et al. Capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy: a systematic literature review and network meta-analysis. Clin Ther. 2017;39:787-803.e718.
Acknowledgements
We gratefully acknowledge technical support from the Laboratory Animal Center at Taipei Medical University, overall support from Taipei Medical University Hospital (109TMUH-H-07), and language support from Wallace Academic Editing.
Funding
This study received no funding support.
Author information
Authors and Affiliations
Contributions
CSW and YCK were involved in data extraction, software manipulation, and original draft preparation. YJH and CHL conceptualized and supervised the study and performed data extraction and calculation. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, CS., Huang, YJ., Ko, YC. et al. Efficacy and safety of duloxetine in painful diabetic peripheral neuropathy: a systematic review and meta-analysis of randomized controlled trials. Syst Rev 12, 53 (2023). https://doi.org/10.1186/s13643-023-02185-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02185-6