Abstract
Background
Weaning from invasive mechanical ventilation (MV) is a complex and challenging process that involves multiple pathophysiological mechanisms. A combined ultrasound evaluation of the heart, lungs, and diaphragm during the weaning phase can help to identify risk factors and underlying mechanisms for weaning failure. This study aimed to investigate the accuracy of lung ultrasound (LUS), transthoracic echocardiography (TTE), and diaphragm ultrasound for predicting weaning failure in critically ill patients.
Methods
Patients undergoing invasive MV for > 48 h and who were readied for their first spontaneous breathing trial (SBT) were studied. Patients were scheduled for a 2-h SBT using low-level pressure support ventilation. LUS and TTE were performed prospectively before and 30 min after starting the SBT, and diaphragm ultrasound was only performed 30 min after starting the SBT. Weaning failure was defined as failure of SBT, re-intubation, or non-invasive ventilation within 48 h.
Results
Fifty-one patients were included, of whom 15 experienced weaning failure. During the SBT, the global, anterior, and antero-lateral LUS scores were higher in the failed group than in the successful group. Receiver operating characteristic curve analysis showed that the areas under the curves for diaphragm thickening fraction (DTF) and global and antero-lateral LUS scores during the SBT to predict weaning failure were 0.678, 0.719, and 0.721, respectively. There was no correlation between the LUS scores and the average E/e’ ratio during the SBT. Multivariate analysis identified antero-lateral LUS score > 7 and DTF < 31% during the SBT as independent predictors of weaning failure.
Conclusion
LUS and diaphragm ultrasound can help to predict weaning failure in patients undergoing an SBT with low-level pressure support. An antero-lateral LUS score > 7 and DTF < 31% during the SBT were associated with weaning failure.
Graphical Abstract

Similar content being viewed by others
Background
Weaning from invasive mechanical ventilation (MV) is a major challenge in critically ill patients. In a large, prospective, international observational study, only 65% of intensive care unit (ICU) patients receiving more than 2 days of invasive ventilation were successfully weaned at day 90 [1]. Weaning failure is associated with prolonged ICU stay and increased risk of death [2, 3]. The transition from complete ventilatory support to spontaneous breathing is a complex process that places stress on multiple organs [4]. This process has the potential to unmask previously undetected organ dysfunctions, such as loss of lung aeration, ventilator-induced diaphragm dysfunction, and weaning-induced pulmonary edema (WIPO). Consequently, identifying the underlying mechanisms of weaning failure and implementing targeted interventions may thus have important prognostic implications.
Ultrasound is accepted as a reliable tool for monitoring physiological changes in the cardiorespiratory system during various stages of weaning [5,6,7]. Ultrasound assessment of heart, lung, and respiratory muscle functions has improved our understanding of the pathophysiological process of weaning and helped to predict weaning outcomes [8]. However, most published studies have primarily focused on single-organ assessments, overlooking the fact that weaning failure may depend on several factors and the intricate interplay between organs.
Hence, we assumed that a comprehensive ultrasound assessment of the heart, lungs, and diaphragm during the spontaneous breathing trial (SBT) process could help predict weaning outcome. This study aimed to investigate the accuracy of lung ultrasound (LUS), transthoracic echocardiography (TTE), and diaphragm ultrasound for predicting weaning failure and to determine which ultrasound parameters were associated with weaning failure in critically ill patients undergoing an SBT with low-level pressure support.
Methods
Patients
This prospective, observational study was conducted in two mixed intensive care units (ICUs) of tertiary hospitals in China over a 16-month period. This study was conducted according to the tenets of the Declaration of Helsinki. The protocol was approved by the ethics committee of our institution (approval number 2021–69 K). Written informed consent was obtained from each patient’s next of kin prior to participation.
Patients were eligible if they fulfilled the following inclusion criteria: (1) invasive MV for > 48 h; and (2) eligibility for their first SBT according to current guidelines (Electronic Supplementary Material 1) [9]. The exclusion criteria were as follows: (1) age < 18 years; (2) pregnancy; (3) presence of thoracostomy, pneumothorax, or pneumomediastinum; (4) presence of flail chest or rib fractures; (5) pre-existing cervical spinal injury, history or final diagnosis of neuromuscular disorders; (6) tracheostomy; (7) history or new detection of paralysis (no movement) or paradoxical movement of a single hemidiaphragm on diaphragmatic ultrasound; (8) atrial fibrillation, severe mitral valve disease, mitral valve replacement or repair; (9) poor quality of ultrasound images unsuitable for analysis; and (10) patient’s next of kin refused participation.
Weaning trials
Eligible patients underwent an SBT with low-level pressure support (pressure-support level of 8 cmH2O and zero positive end-expiratory pressure) for 2 h, according to current guidelines [10, 11]. SBT was deemed to have failed and was terminated when one of the following signs occurred [9]: (1) acute respiratory distress (respiratory rate > 35 breaths/min); (2) SaO2 < 90% with an FiO2 ≥ 50%; (3) heart rate > 140 beats/min or an increase of ≥ 20%; (4) systolic arterial blood pressure ≥ 180 mmHg or an increase of ≥ 20%; and (5) change in mental status, agitation, or anxiety. If the patient successfully passed the SBT, the physicians in charge decided whether to extubate, independent of the investigators. In the event of SBT failure, the pre-SBT MV settings were resumed.
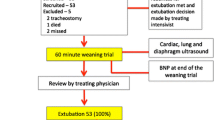
Weaning failure was defined as either a failed SBT, or need for MV (invasive or non-invasive) within 48 h after extubation. The timeline of the study protocol is described in Fig. 1.
Timeline of study protocol. Patients who underwent invasive mechanical ventilation (MV) for > 48 h and who were eligible for their first spontaneous breathing trial (SBT) were included. An integrated ultrasound assessment of the heart, lungs, and diaphragm was performed before and 30 min after starting a 2-h SBT with low-level pressure support. After successfully passing the SBT, the physicians in charge decided whether to extubate, independent of the investigators. Weaning outcome was monitored for 48 h. Weaning failure was defined as failure of SBT, reintubation, or non-invasive ventilation within the following 48 h
Measurements and data collection
Upon inclusion, the following parameters were recorded: demographic data, comorbidities, reasons for ICU admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, albumin, cumulative fluid balance over last 3 days (2 days in case of MV < 3 days before the first SBT), use of diuretics before the SBT, duration of invasive MV, and ventilation parameters. Heart rate, blood pressure, respiratory rate, pulsed oxygen saturation, tidal volume, and blood gas analysis variables were also recorded before and 30 min after starting the SBT.
Ultrasound examinations
All ultrasound examinations were performed using a CX-50 (Philips Healthcare, Amsterdam, The Netherlands) or Venue Go™ (GE Healthcare, Wuxi City, China) ultrasound device. TTE and LUS were performed before and 30 min after starting the SBT, and diaphragm ultrasound was only performed 30 min after starting the SBT. All examinations were performed by two fully trained and experienced operators (XLL and QCL) and reviewed off-line by an independent operator who was blinded to the medical charts (JS). Before this study, three investigators underwent standardized training sessions in cardiac, lung, and diaphragm ultrasound provided by the Chinese Critical Ultrasound Study Group (CCUSG) and obtained qualification certificates.
The techniques and parameters for conducting TTE, LUS, and diaphragm ultrasound evaluations are described in Electronic Supplementary Material 1.
Statistical analysis
The sample size was calculated by considering an area under the receiver operating characteristic (ROC) curve > 0.80 as acceptable diagnostic accuracy. According to previous study [12], a weaning failure rate of 25% was assumed. We planned to include 48 patients, with a Type I error of 0.05 and a Type II error of 0.10 (power is 90%). Given an expected dropout rate of approximately 10% due to technical problems, we estimated that the sample size of 52 patients. The results are expressed as the median (interquartile range) for quantitative variables and the number and percentage for categorical variables. The patients were divided into weaning success and weaning failure groups according to the weaning outcome. Numerical data were compared between groups using a Mann–Whitney U test, and categorical data were compared using χ2 or Fisher’s exact tests. Data before and 30 min after starting the SBT were compared using a paired-samples Wilcoxon’s test. ROC curves were constructed to evaluate the performance of nine indices to predict weaning failure: global LUS score, anterior LUS score, antero-lateral LUS score, E/A, septal E/e’, lateral E/e’, average E/e’, left ventricular ejection fraction (LVEF) and diaphragm thickening fraction (DTF) 30 min after starting the SBT. The sensitivities, specificities, positive predictive values, and negative predictive values of the parameters were calculated. The best diagnostic threshold for each index was defined as the value providing the greatest Youden index (sensitivity + specificity − 1). Patients were grouped according to cutoff values derived from ROC curve analysis, and potential independent variables affecting the weaning outcome were then identified by univariate regression analysis. All parameters with a p value ≤ 0.1 in univariate analysis were included in a multivariable binary logistic regression model to identify independent factors of weaning failure after adjusting for potential confounding factors such as age, sex, and APACHE II score. A two-tailed p value ≤ 0.05 was considered statistically significant. All data were analyzed using R version 4.0.2.
Results
During the study period, a total of 136 patients received invasive MV for > 48 h and were ready to undergo their first SBT. Fifty-one patients were finally included in the study (38 males; median age 72 years (55–82); APACHE II: 19 (16–22); SOFA: 6 (4–8)). At the time of inclusion, the median duration of MV was 6 (3–8) days. Of the 51 patients, 15 (29.4%) failed weaning, including nine (17.6%) who failed SBT and six (11.8%) who required non-invasive or invasive ventilation within 48 h of extubation (4 reintubated, 2 received non-invasive ventilation). A flowchart of the study is shown in Fig. 2. The demographic characteristics, laboratory findings, cumulative fluid balance over the last 3 days, ventilator parameters prior to SBT, length of ICU stay, and ICU mortality did not differ significantly between the weaning success and failure groups (Table 1).
There were no significant differences between the groups in terms of clinical, blood gas analysis, and echocardiographic variables before and during the SBT (Electronic Supplementary Material 2 and Table 2). Notably however, the antero-lateral LUS score before the SBT and all LUS scores during the SBT differed significantly between the two groups (Fig. 3), and the DTF was significantly higher in the weaning success group than in the weaning failure group (p = 0.048).
ROC curve analysis was conducted to assess the diagnostic accuracy of ultrasound variables for predicting weaning failure (Fig. 4). The best cutoff value and diagnostic performance for each variable are shown in Table 3. There was no correlation between any of the LUS scores and average E/e′ during the SBT.
Multivariate logistic regression analysis identified antero-lateral LUS score > 7 and DTF < 31% during the SBT as independent predictors of weaning failure after adjusting for age, sex, and APACHE II score (Table 4).
Discussion
In this prospective study, we conducted a comprehensive ultrasound assessment of the heart, lungs, and diaphragm in a general ICU population before and during the SBT with low-level pressure support. The main results are as follows: (1) During the SBT, patients who failed to wean had higher LUS scores and lower DTF, as compared to patients who were successfully weaned. (2) No significant difference was found between patients who were successfully weaned and those who failed in echocardiographic parameters either before or during the SBT. (3) LUS scores and DTF demonstrate moderate diagnostic performance in predicting weaning failure, and an antero-lateral LUS score > 7 and DTF < 31% during the SBT were independent predictors of weaning failure.
The weaning process induces significant physiological changes in multiple organs, which can result in decreased aeration of the pulmonary parenchyma, such as alveolar decruitment, pulmonary edema, and atelectasis, ultimately leading to weaning failure [4, 8, 13, 14]. LUS has emerged as a valuable technique for assessing lung aeration in critically ill patients due to its accuracy, ease of use, and noninvasive nature [15, 16], and thus offers several advantages in detecting lung issues during the weaning process. Bouhemad et al. recently conducted a prospective observational study involving 40 elderly patients at high risk of weaning or extubation failure and found that patients who experienced weaning or extubation failure had significantly higher LUS scores at the end of the SBT. Both global and antero-lateral LUS scores at the end of the SBT were identified as independent risk factors for weaning or extubation failure [17]. Our study yielded similar findings in a general population of critically ill patients at their first attempt to wean from MV. These findings highlight the utility of LUS as a bedside tool for identifying patients at risk of weaning failure and accurately predicting weaning outcomes.
The transition from invasive MV to spontaneous breathing is associated with increased cardiac preload and afterload, potentially triggering cardiogenic pulmonary edema in cases of volume overload or left ventricular (LV) systolic or diastolic dysfunction [18, 19]. Echocardiography is a useful tool for assessing cardiac performance during the weaning process [20, 21]. Several studies have examined the value of echocardiographic parameters for predicting weaning outcomes [22,23,24,25]. A recent meta-analysis highlighted the pivotal roles of LV diastolic function dysfunction (E/e′, e′ wave, E wave) and increased LV filling pressure (E/e′) in weaning failure, while the influence of LV systolic dysfunction remains ambiguous [5]. In contrast to previous studies, the present results showed that echocardiographic parameters of diastolic function were not associated with weaning failure. A plausible explanation might be that the effects of therapeutic interventions. Up to 63% of our patients had received diuretics before SBT, while the reduction in cardiac preload induced by diuretics may lead to soften the effects of SBT on LV filling pressure [26, 27]. This postulate was more or less supported by the fact that the E/e′, as a routine indicator of LV filling pressure, was not significantly increased during the SBT. Such findings indicate that patients may adapt better to SBT-induced hemodynamic changes after diuretic therapy and a more negative fluid balance. Similarly, no significant difference in LVEF was found between the weaning success and weaning failure groups. This could be attributed to the relatively low incidence of impaired LV systolic function in our cohort. Only 10% of our patients had an LVEF lower than 50%.
WIPO is a common cause of weaning failure. LUS is considered to be an accurate and useful diagnostic tool for detecting WIPO [6]. B-lines detected by LUS are strongly correlated with increased extravascular lung water [28, 29], while multiple B-lines in bilateral lung regions are considered to be characteristic of cardiogenic pulmonary edema [30]. Ferre et al. recently found that an increase in the number of B-lines ≥ 6 on four anterior thoracic quadrants during SBT allowed the diagnosis of WIPO with the best accuracy [6]. Nevertheless, our study found no correlation between LUS scores and LV filling pressure (E/e′), suggesting that WIPO might not be the main mechanism of lung aeration loss in our study population. There are many possible causes of lung aeration loss during weaning, including alveolar decruitment secondary to loss of positive end-expiratory pressure, diaphragm dysfunction, atelectasis, and pulmonary edema. An elevated LUS score does not necessarily equate to a rise in LV filling pressure and cardiogenic pulmonary edema. Similar results were obtained in a previous study involving an elderly population [17]. The complex pathophysiological changes that affect lung aeration during weaning highlight the need for an integrative ultrasound assessment.
The diaphragm acts as the primary inspiratory muscle and plays a crucial role in ventilation. The existing evidence suggests that 44% of patients experience a decrease in diaphragm thickness in the first 72 h of MV, and this atrophy was associated with low diaphragm contractile activity [31]. The diaphragm atrophy may contribute to weaning failure and prolongation of invasive mechanical ventilation [32]. Therefore, assessing diaphragm function could help predict the capacity of the respiratory muscles to cope with the increased respiratory load. However, in the present study, DTF is a more accurate parameter of diaphragm contractile activity than DE during a low-level PSV. Actually, during assisted ventilation, DE is the result of the combined forces of active diaphragm contraction and passive displacement caused by the pressure from the ventilator [33]. In this case, there is no means to distinguish which part of displacement is passive, and which is active. Moreover, DE varies depending on the patient’s position, breathing pattern and abdominal and/or thoracic pressure [34]. Consequently, DE may not be a reliable parameter for monitoring diaphragm contractile activity during PSV. In contrast, DTF is primarily influenced by active diaphragm contraction rather than passive insufflation during PSV [35]. Umbrello et al. demonstrated a significant correlation between DTF and classic parameters of inspiratory muscle effort, such as esophageal pressure-time product per breath (PTPes) and diaphragmatic pressure-time product per breath (PTPdi) [36], indicating that DTF serves as a dependable indicator of respiratory effort in PSV.
One of the strengths of our study was the comprehensive bedside ultrasound assessment of cardiac, lungs, and diaphragm functions with multiple parameters. Weaning from MV is a complex process, involving pathophysiological changes in multiple organs as well as cross-talk between organs [13]; assessing a single organ may thus overlook potential risk factors for weaning failure. Moreover, dynamic ultrasound assessments were conducted both before and during the SBT. An integrative, dynamic, and comprehensive ultrasound assessment of the heart, lungs, and diaphragm not only enables clinicians to evaluate the risk of weaning failure more accurately, but also helps identify underlying mechanisms and guide further treatments in patients with weaning failure.
Our study also had some limitations. First, the sample size was small. We only enrolled patients from two centers and only included patients undergoing their first SBT. This may limit the generalizability of our findings. Second, SBT was performed with low-level pressure support, which is not as challenging for the heart as T-tube. The current results may thus not translate to patients undergoing SBT with T-tube. Third, our study did not classify diastolic dysfunction according to the American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) Recommendations [37]. Since the E/e’ is accepted as a surrogate parameter of LV filling pressure, we used E/e’ as an index of diastolic function in this study. Fourth, ultrasound examination is operator-dependent. Inter- and intra-operator reproducibility were not assessed. However, in the present study, all ultrasound examinations were performed by experienced physicians with advanced-level training and confirmed off-line by an independent operator, which may reduce the intra- and inter-operator variability to some extent. Finally, ultrasound examination may be difficult in cases with a poor viewing window due to obesity, subcutaneous emphysema, and/or thoracic dressings.
Conclusion
In conclusion, the current study showed that LUS and diaphragm ultrasound can help to predict weaning failure in critically ill patients undergoing an SBT with low-level pressure support. Among the ultrasound parameters, an antero-lateral LUS score > 7 and DTF < 31% during the SBT were independent predictors of weaning failure. Further studies should focus on optimizing the protocol of combined cardiac, lung, and diaphragm ultrasound assessment. In addition, interventional studies are required to validate the effectiveness of an ultrasound-guided strategy for MV weaning in a broader population.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MV:
-
mechanical ventilation
- LUS:
-
lung ultrasound
- TTE:
-
transthoracic echocardiography
- SBT:
-
spontaneous breathing trial
- DTF:
-
diaphragm thickening fraction
- WIPO:
-
weaning-induced pulmonary edema
- ICU:
-
intensive care units
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- SOFA:
-
Sequential Organ Failure Assessment
- ROC:
-
receiver operating characteristic
- DE:
-
diaphragm excursion
- LVEF:
-
left ventricular ejection fraction
References
Pham T, Heunks L, Bellani G, Madotto F, Aragao I, Beduneau G, et al. Weaning from mechanical ventilation in intensive care units across 50 countries (WEAN SAFE): a multicentre, prospective, observational cohort study. Lancet Respir Med. 2023;11:465–76.
Béduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, et al. Epidemiology of Weaning Outcome according to a New Definition. The WIND study. Am J Respir Crit Care Med. 2017;195:772–83.
Burns KEA, Rizvi L, Cook DJ, Lebovic G, Dodek P, Villar J, et al. Ventilator Weaning and Discontinuation practices for critically ill patients. JAMA. 2021;325:1173–84.
Santangelo E, Mongodi S, Bouhemad B, Mojoli F. The weaning from mechanical ventilation: a comprehensive ultrasound approach. Curr Opin Crit Care. 2022;28:322–30.
Sanfilippo F, Di Falco D, Noto A, Santonocito C, Morelli A, Bignami E, et al. Association of weaning failure from mechanical ventilation with transthoracic echocardiography parameters: a systematic review and meta-analysis. Br J Anaesth. 2021;126:319–30.
Ferré A, Guillot M, Lichtenstein D, Mezière G, Richard C, Teboul J-L, et al. Lung ultrasound allows the diagnosis of weaning-induced pulmonary oedema. Intensive Care Med. 2019;45:601–8.
Haaksma ME, Smit JM, Heldeweg ML, Nooitgedacht JS, Atmowihardjo LN, Jonkman AH, et al. Holistic ultrasound to Predict Extubation failure in clinical practice. Respir Care. 2021;66:994–1003.
Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42:1107–17.
Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56.
Schmidt GA, Girard TD, Kress JP, Morris PE, Ouellette DR, Alhazzani W, et al. Liberation from mechanical ventilation in critically ill adults: executive summary of an official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline. Chest. 2017;151:160–5.
Thille AW, Gacouin A, Coudroy R, Ehrmann S, Quenot J-P, Nay M-A, et al. Spontaneous-breathing trials with pressure-support ventilation or a T-Piece. N Engl J Med. 2022;387:1843–54.
Subirà C, Hernández G, Vázquez A, Rodríguez-García R, González-Castro A, García C, et al. Effect of pressure support vs T-Piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a Randomized Clinical Trial. JAMA. 2019;321:2175.
Silva S, Ait Aissa D, Cocquet P, Hoarau L, Ruiz J, Ferre F, et al. Combined thoracic Ultrasound Assessment during a successful Weaning Trial predicts Postextubation Distress. Anesthesiology. 2017;127:666–74.
Haji K, Haji D, Canty DJ, Royse AG, Green C, Royse CF. The impact of heart, lung and diaphragmatic ultrasound on prediction of failed extubation from mechanical ventilation in critically ill patients: a prospective observational pilot study. Crit Ultrasound J. 2018;10:13.
Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199:701–14.
Chiumello D, Mongodi S, Algieri I, Vergani GL, Orlando A, Via G, et al. Assessment of Lung Aeration and Recruitment by CT scan and Ultrasound in Acute Respiratory Distress Syndrome patients. Crit Care Med. 2018;46:1761–8.
Bouhemad B, Mojoli F, Nowobilski N, Hussain A, Rouquette I, Guinot P-G, et al. Use of combined cardiac and lung ultrasound to predict weaning failure in elderly, high-risk cardiac patients: a pilot study. Intensive Care Med. 2020;46:475–84.
Routsi C, Stanopoulos I, Kokkoris S, Sideris A, Zakynthinos S. Weaning failure of cardiovascular origin: how to suspect, detect and treat—a review of the literature. Ann Intensive Care. 2019;9:6.
Teboul J-L. Weaning-induced cardiac dysfunction: where are we today? Intensive Care Med. 2014;40:1069–79.
Papanikolaou J, Makris D, Saranteas T, Karakitsos D, Zintzaras E, Karabinis A, et al. New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med. 2011;37:1976–85.
De Meirelles Almeida CA, Nedel WL, Morais VD, Boniatti MM, De Almeida-Filho OC. Diastolic dysfunction as a predictor of weaning failure: a systematic review and meta-analysis. J Crit Care. 2016;34:135–41.
Tongyoo S, Thomrongpairoj P, Permpikul C. Efficacy of echocardiography during spontaneous breathing trial with low-level pressure support for predicting weaning failure among medical critically ill patients. Echocardiography. 2019;36:659–65.
Moschietto S, Doyen D, Grech L, Dellamonica J, Hyvernat H, Bernardin G. Transthoracic Echocardiography with Doppler Tissue Imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care. 2012;16:R81.
Lamia B, Maizel J, Ochagavia A, Chemla D, Osman D, Richard C, et al. Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med. 2009;37:1696–701.
Bedet A, Tomberli F, Prat G, Bailly P, Kouatchet A, Mortaza S, et al. Myocardial ischemia during ventilator weaning: a prospective multicenter cohort study. Crit Care. 2019;23:321.
Liu J, Shen F, Teboul J-L, Anguel N, Beurton A, Bezaz N, et al. Cardiac dysfunction induced by weaning from mechanical ventilation: incidence, risk factors, and effects of fluid removal. Crit Care. 2016;20:369.
Goudelin M, Champy P, Amiel J-B, Evrard B, Fedou A-L, Daix T, et al. Left ventricular overloading identified by critical care echocardiography is key in weaning-induced pulmonary edema. Intensive Care Med. 2020;46:1371–81.
Mayr U, Lukas M, Habenicht L, Wiessner J, Heilmaier M, Ulrich J, et al. B-Lines scores derived from lung ultrasound provide Accurate Prediction of Extravascular Lung Water Index: an observational study in critically ill patients. J Intensive Care Med. 2022;37:21–31.
Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19:36.
Lichtenstein DA, Mezière GA. Relevance of Lung Ultrasound in the diagnosis of Acute Respiratory failure: the BLUE Protocol. Chest. 2008;134:117–25.
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of Diaphragm thickness during mechanical ventilation. Impact of Inspiratory Effort. Am J Respir Crit Care Med. 2015;192:1080–8.
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced Diaphragm Atrophy strongly impacts Clinical outcomes. Am J Respir Crit Care Med. 2018;197:204–13.
Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39:801–10.
Song J, Qian Z, Zhang H, Wang M, Yu Y, Ye C, et al. Diaphragmatic ultrasonography-based rapid shallow breathing index for predicting weaning outcome during a pressure support ventilation spontaneous breathing trial. BMC Pulm Med. 2022;22:337.
Grassi A, Ferlicca D, Lupieri E, Calcinati S, Francesconi S, Sala V, et al. Assisted mechanical ventilation promotes recovery of diaphragmatic thickness in critically ill patients: a prospective observational study. Crit Care. 2020;24:85.
Umbrello M, Formenti P, Longhi D, Galimberti A, Piva I, Pezzi A, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19:161.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by Echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314.
Acknowledgements
We would like to express our gratitude to all of the nursing and medical staff from the ICU at Zhejiang Hospital (Zhejiang, China) and Gongli Hospital (Shanghai, China) for their contributions to this project.
Funding
This work was supported by the scientific research fund of the Zhejiang Medical and Health Science and Technology Plan Project (grant number 2020KY382; WKJ-ZJ-1601; 2023KY004), Shanghai Pudong New Area Health System Discipline Leader Training Project (grant number: PWRd2022-14), and Discipline Construction Project of Pudong New Area Health Commission (grant number: PWZzb2022-21).
Author information
Authors and Affiliations
Contributions
SG and CH conceived and designed the study. SJ, QL, and XL collected the ultrasound data. YK and GC collected the clinical data. SJ, LQ and WC analyzed and interpreted the data. SJ and QL drafted the manuscript. WH, YY, and MW contributed to manuscript revision for critical intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Zhejiang Hospital (protocol number: 2021–69 K). All participants provided written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1:
The eligibility criteria for the spontaneous breathing trial (SBT) and the acquisition of images and measurement of parameters for transthoracic echocardiography (TTE), lung ultrasound (LUS), and diaphragm ultrasound.
Supplementary Material 2: Table S1.
Clinical and blood gas analysis variables before and during the spontaneous breathing trial (SBT).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, J., Luo, Q., Lai, X. et al. Combined cardiac, lung, and diaphragm ultrasound for predicting weaning failure during spontaneous breathing trial. Ann. Intensive Care 14, 60 (2024). https://doi.org/10.1186/s13613-024-01294-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01294-2