Abstract
Background
The literature is unresolved on whether female receive advanced cardiac life support less than do male and on whether female have a survival advantage over male after cardiopulmonary resuscitation.
Methods
We systematically searched PubMed, Embase and Web of Science databases (from inception to 23-April-2022) for papers reporting outcomes in adult male and female after out-of-hospital cardiac arrest. The main study outcome was the rate of adjusted survival to hospital discharge or 30 days. Secondary outcomes included unadjusted survival to hospital discharge and favourable neurological outcome.
Results
A total of 28 studies were included, involving 1,931,123 patients. Female were older than male, their cardiac arrests were less likely to be witnessed and less likely to present with a shockable rhythm. Unadjusted analysis showed that females had a lower likelihood of survival than males (OR 0.68 [0.62–0.74], I2 = 97%). After adjustment, no significant difference was identified between male and female in survival at hospital discharge/30 days (OR 1.01 [0.93–1.11], I2 = 87%). Data showed that male had a significantly higher likelihood of favorable neurological outcome in unadjusted analysis but this trend disappeared after adjustment. Both the primary outcome (adjusted for several variables) and the secondary outcomes were associated with substantial heterogeneity. The variables examined using meta-regression, subgroup and sensitivity analyses (i.e., study type, location, years, population, quality of adjustment, risk of bias) did not reduce heterogeneity.
Conclusions
The adjusted rate of survival to hospital discharge/30 days was similar for male and female despite an initial seeming survival advantage for male. The validity of this finding is limited by substantial heterogeneity despite in-depth investigation of its causes, which raises concerns regarding latent inequalities in some reports nonetheless. Further study on this topic may require inclusion of factors not reported in the Utstein template and in-depth analysis of decision-making processes.
Similar content being viewed by others
Background
Out-of-hospital cardiac arrest (OHCA), occurs at an incidence of 30.0 to 97.1 individuals per 100,000 people annually [1]. As OHCAs are frequently unexpected yet require immediate treatment, global rates of survival to hospital discharge after OHCA remain dismally low (~ 8.8%) [2]. Patients’ characteristics such as age and co-morbidities affect the outcome [3, 4] but survival is also related to the individual components of the response to OHCA, such as bystander cardiopulmonary resuscitation (CPR) [5,6,7], early defibrillation [8], targeted temperature management [9, 10] and coronary catheterization [11].
The characteristics of OHCA differ in male and female [12]. Female are usually older [13] and have more co-morbidities (e.g., hypertension, diabetes, obesity) [14,15,16]. Female arrest in the privacy of their own home and resultantly their arrests tend to be less witnessed [17]. One meta-analysis showed that 77% of female who arrest do so at home vs. 67% of male. Only 40% of OHCAs among female were witnessed vs. 47% among male [18]. In some studies female receive less bystander CPR [17, 19, 20] but this finding is inconsistent [21]. The interval between emergency medical services (EMS) dispatch to EMS–CPR or first rhythm capture may also be longer in female than in male [22]. Possibly as a result of all these, female present less with shockable rhythms than do male [16,17,18, 23, 24].
It, therefore, seems unsurprising that overall female receive less advanced cardiac life support [25, 26]. Female less frequently undergo defibrillation, receive less epinephrine and are even less likely to undergo endotracheal intubation [18, 22, 27]. There is controversy with regard to whether female are more likely to have return of spontaneous circulation (ROSC) than do male [28] or not [21, 27]. Even when ROSC occurs and the patient is brought to hospital, differences remain in post-resuscitation care as female less frequently undergo coronary angiography [15, 27], percutaneous coronary intervention [16, 26] and other evidence-based interventions [26]. Despite these differences, studies reporting outcomes of male and female after CPR range between better, similar and worse survival outcomes for one sex compared to another.
This systematic review was, therefore, conducted to investigate whether male and female with OHCA have different mortality rates or neurological outcomes despite adjustment for confounding variables.
Methods
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [29, 30] and was registered in the PROSPERO database prior to the study initiation (CRD 42021226050).
PICO question
Do adult female (P) after out-of-hospital cardiac arrest (I) compared to adult male after out-of-hospital cardiac arrest (C) have different survival rates and neurological outcomes (O)?
Search strategy
We systematically searched PubMed, Embase and Web of Science databases (inception to 23-April-2022) for papers reporting outcomes at the time of hospital discharge in adult (age 16 years or older) male and female after out-of-hospital cardiac arrest. The search was performed three times to ensure a full and up-to-date review of the literature. No language restriction was applied during the search, then only studies written in English were included. In brief, we used keywords as exact phrases and subject headings according to database syntaxes with the help of an information specialist. The full search strategy is described in Additional file 1.
Eligibility and inclusion/exclusion criteria
We included papers describing randomised controlled trials, nonrandomised clinical trials, observational cohort studies or case series of adult humans with OHCA. Case reports, animal models and special populations (children, pregnant female) were excluded. Studies were included if sex differences in outcome were their primary study aim and if they evaluated at least one outcome of interest (survival to hospital discharge or 30-day survival). We used author definitions for OHCA. Only studies published after 1995 were included to account for changes after the 1993 declaration of the Council for International Organizations of Medical Sciences regarding inclusion of women in clinical trials.
Paper selection
The titles and abstracts of all records were screened independently and in duplicate by two of the authors (IL, AN) using the Covidence software tool. The papers selected were downloaded in full and they were reviewed independently and in duplicate by the same two authors to verify fulfilment of inclusion criteria. The reference lists of relevant articles were searched for additional potentially pertinent articles (i.e., snowballing method). All articles rated discrepantly by the two screening authors in the Covidence software were reviewed and discussed one by one by both authors and subsequently included only if both authors agreed on eligibility. In each stage disagreements were resolved by a third author (SE). Among papers reporting completely or partially overlapping data, we selected the paper displaying adjusted data and describing the greatest number of patients. Since many of the included studies presented data from national databases, the risk of overlapping data was high. To avoid such overlap, we excluded any study that presented data from a country whose national database was already being used in another included study on the same period (Additional file 2: Table S1). In this case, the study included was the one with adjusted data, and if more than one study from the same national database had adjusted data, the largest cohort was chosen. Although pre-planned, alternative prioritisation based on clinical sensibility due to poorer adjustment with more patients was never required.
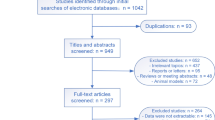
The list of papers with overlapping populations is presented in Additional file 2. We planned to contact the corresponding authors of the screened studies in case questions arose regarding eligibility or data presentation but no such issues arose. The details of the inclusion/exclusion process are shown in the PRISMA diagram (Fig. 1).
Flow chart of the included studies. *A table summarizing the overlapping databases is provided in the Additional file 2: Table S1)
Data extraction
Two authors (IL, AN) extracted the data in duplicate using a standardized data extraction form. Discrepancies in the extracted data were adjudicated by a third author (SE). The data extracted included study characteristics (e.g., source country, study type, single/multicentre), patient demographics (age, sex), medical background, treatments and outcomes. We also collected data on the type of adjusted analyses performed and the variables adjusted for. The final version of the database was validated by all the investigators involved in data collection (IL, SE, AN) and is available as Additional file 3.
Assessment of risk of bias
For the primary outcome, two of the authors (IL, MI) assessed the risk of bias (RoB) of the included studies independently and in duplicate using the ROBINS-I tool. [31] Disagreements over RoB were resolved by consensus or, if necessary, adjudicated by a third author (SE).
Outcomes
The main study outcome was the rate of adjusted survival to hospital discharge or, if not available, at 30 days. Secondary outcomes included the rates of (1) unadjusted survival to hospital discharge (2) favourable neurological outcome at discharge. Favourable neurological outcome at discharge was defined according to Cerebral Performance Category (CPCs) as this is the most commonly used tool for this outcome. CPC 1 and CPC 2 were considered favourable outcomes in our analysis as observed in the studies reporting neurological outcomes.
Certainty of the evidence
The certainty of the evidence (i.e., the overall effect estimates) was assessed for the primary outcome and the secondary outcomes using Grading of Recommendations Assessment, Development and Evaluation (GRADE) [32].
Statistical analysis
The population characteristics were described as weighted means and weighted standard deviation for continuous variables; and weighted means of percentages and weighted standard deviation from percentages for categorical variables. For adjusted analyses we used the generic inverse variance method to pool estimates and standard errors (SEs) as per Cochrane guidance [33, 34]. The results were reported as odds ratios (OR) with their 95% confidence intervals (CI) for dichotomous outcomes. A prediction interval (PI) was calculated for the primary outcome and for any outcome with an OR excluding the value of no difference. Meta-analyses were performed using adjusted estimates from multivariate models or propensity-matched cohorts for the mortality outcomes. ORs and CIs were transformed to natural log and SEs using standard formulas. Random effects models were used for all analyses.
We planned sensitivity analyses based on the RoB of the included studies.
Preplanned subgroup analyses were performed to study possible heterogeneity stemming from the number of centres (multi vs. single), study location (Europe, Asia, North America, other), population denominator (i.e., whether non-survivors to hospital admission were accounted for in the cohort or not), OHCA etiology, quality of adjustment variables.
We added an additional subgroup analysis based on the study timeframe after the literature search due to the broad range of years ultimately studied (31 years). The study timeframe was defined as the year of inclusion of the last patient in the cohort rather than the year of publication, since several studies were published many years after completion of patient inclusion. We also intended to perform an analysis separating before/after the 2015 guidelines, allowing for a 1-year implementation period. However, no study included patients strictly from 2016 onward; those most recent pooled data from both periods.
When effect size was attributable to a small number of studies in a subgroup (≤ 5) we calculated a pooled version of τ2 to be used across all subgroups, thereby decreasing reliance on an imprecise estimate of between-study heterogeneity in one subgroup [35].
All P values were two-tailed. P values less than 0.05 were considered statistically significant. Statistical heterogeneity (i.e., chance variation between studies) was sought by visual inspection of forest plots and with the nonparametric Cochran’s Q test and the I2 statistic [34]. Heterogeneity was considered likely if Q > df (degrees of freedom) and was considered confirmed if the P value was 0.10 or less. The possibility of small-study effects was first explored through inspection of funnel plot. The Harbord’s and Peter’s tests were planned to be performed to investigate small-study effects, except in the case of significant heterogeneity between studies, where an arcsine test using Rücker's random effects was to be preferred, assuming no publication bias [36,37,38]. These tests were chosen over the Egger’s test, given the dichotomous nature of the outcome of interest [39, 40].
All analyses were performed using R software (R Core Team 2013, R Foundation for Statistical Computing, Vienna, Austria, URL (http://www.R-project.org/) with the package meta [41].
Results
A total of 5,423 studies were screened, of which 28 were included in the final analysis, corresponding to 1,931,123 patients (1,136,311 male and 794,812 female) (Fig. 1).
Included studies
All the studies identified were observational and all were retrospective analyses of data from cohorts tracked and documented in real time. Five studies were only published as poster presentations [42,43,44,45,46]. The data covered four continents (Europe, Asia, America and Oceania) over a period of 33 years (1988–2021). There were data from single centre studies [43, 45, 47,48,49], national registries [20, 21, 25, 44, 46, 50,51,52,53,54,55,56] or local emergency medical services registries (e.g., comprised of all OHCAs registered in a city, in several countries or in several countries over a period of time) [17, 42, 57,58,59,60,61,62,63,64,65].
All studies included only patients with OHCA. Thirteen studies included only OHCA of cardiac etiology [17, 21, 25, 42, 46, 48, 53, 56,57,58,59, 63, 65] and fifteen studies included OHCA of both cardiac and non-cardiac etiologies [43,44,45, 47, 49,50,51,52, 54, 55, 60,61,62, 64]. Among the latter, seven also included patients whose cardiac arrest was due to trauma [20, 49,50,51,52, 61, 62] and five did not specify whether traumatic cardiac arrests were excluded or not [43,44,45, 54, 55]. Twenty-two studies provided survival data from OHCA to discharge (or to day-30), while six studies excluded patients that did not survive to hospital admission, reporting data from hospital admission to discharge (or to day-30) only (Additional file 2: Table S2) [44, 45, 47, 48, 53, 55].
Baseline data
Female were older than male (weighted means, 71.6 ± 5.1 years vs. 67.4 ± 3.8 years, based on eighteen studies), their arrest was less likely to be witnessed than that of male (weighted means of percentages, 40.5 ± 8.7% vs. 46.0 ± 8.8%, data from 21 studies). Female were more likely to undergo bystander CPR (weighted means of percentages, 46.6 ± 10.7% vs. 40.8 ± 8.9%, data from eighteen studies) but were less likely to present with a shockable rhythm than male (weighted means of percentages, 8.4 ± 4.4% vs. 24.1 ± 12.3%, data from 23 studies).
Less female than male were treated with coronary angiography (weighted means of percentages, 17.8 ± 15.1% vs. 32.3 ± 20.7%, data from four studies), percutaneous coronary intervention (weighted means of percentages, 7.0 ± 7.6% vs. 14.8 ± 7.8%, data from six studies) or targeted temperature management (weighted means of percentages, 12.8 ± 12.2% vs. 17.6 ± 14.6%, data from five studies) (Table 1).
Risk of bias
High RoB was identified predominantly in two domains (Fig. 2); domain 1 which pertains to problems with the adjusted analyses (lack of adjustment and/or lack of important variables in the adjustment) and domain 2 which pertains to selective population inclusion. If only OHCA from cardiac origin or only survivors to hospital admission were included, the domain was rated as moderate at least. The results showed that nine studies were at serious RoB, while the remaining studies were at moderate RoB. The funnel plot suggested publication bias by showing an asymmetry, this was, however, not confirmed by the Rücker’s test (p = 0.058).
Primary outcome
All the studies provided data on survival either to hospital discharge or to day-30 after the occurrence of OHCA. Twenty-one studies provided adjusted data on survival. The variables used for adjustment differed between studies (Table 2).
While unadjusted data showed a lower likelihood of survival in females than in males, with an OR 0.68 [0.62–0.74], PI [0.43; 1.05] (I2 = 97%), adjusted aggregated data showed no difference in survival between male and female OR 0.98 [0.92–1.05], PI [0.76–1.35] (Fig. 3). As heterogeneity was high for our primary outcome (I2 = 86%, Fig. 3), sub-group and meta-regression analyses were performed as preplanned. Subgroup meta-analyses based on the quality of the variables adjusted for, geographical location, etiology of OHCA, type of cohort, number of centres and population denominator (Additional file 4) and meta-regression analyses for the same variables (Additional file 4) did not reduce heterogeneity. The quality of the variables adjusted for was added as a post-hoc analysis, as the latter was very uneven. Sensitivity analysis omitting studies identified as outliers most affecting heterogeneity, studies that had included traumatic arrest as the cause of OHCA or omitting studies assessed as having high RoB (Additional file 4) also did not reduce heterogeneity. We also tried to omit studies displaying data in a non-Utstein style, with no satisfactory results. Publication bias was also not found (Additional file 4). In other words, survival to hospital discharge or 30 days was not significantly associated with any of the variables that could be adjusted for when using published data (Table 3).
The pre-planned strategy was to refrain from aggregating data if heterogeneity was high. After seeking clinical heterogeneity that might have explained the levels of statistical heterogeneity, we concluded that the latter was stemming from latent variables and we decided to share the meta-analyses as supplemental data to show our honest reasoning and approach to the reader.
Secondary outcomes
The meta-analyses performed for the secondary outcomes are reported in Additional file 4. Females had a lower likelihood of unadjusted survival than males with an OR 0.68 [0.62–0.74], PI [0.43; 1.05] (I2 = 97%), and females also had a significantly lower likelihood of favourable neurological outcome than males with an OR 0.56 [0.49–0.66], PI [0.32; 0.98], (I2 = 95%). This trend disappeared when the data were adjusted, without difference between male in female in adjusted neurologically intact survival with an OR 0.96 [0.83–1.10], PI [0.63; 1.46] (I2 = 84%).
We found high heterogeneity (ranging from I2 = 98% to I2 = 83%) for unadjusted survival to discharge/30 days, and both adjusted and unadjusted favourable neurological outcome, precluding any interpretation.
Certainty of the evidence
The results of the GRADE assessment with regard to primary and secondary outcomes are reported in e-Table 3. Certainty was rated as low for the estimated rates of adjusted and unadjusted survival to hospital discharge (or 30-day survival), and low for the adjusted rates of neurological outcomes.
Discussion
This analysis identified no difference between male and female in the adjusted rate of survival to hospital discharge or 30 days after OHCA. This finding is striking when compared to the major survival advantage for male in our unadjusted analysis. Some of the unadjusted difference in the outcomes of male and female may be explained by baseline factors less conducive to a successful outcome (i.e., older age, greater comorbidity, less witnessed arrests and less shockable rhythms). This finding is in line with the "gender paradox" described by Bougouin et al., wherein females have similar survival outcomes despite worse prognostic factors than males [18]. Adjustment for many of these factors seems to have corrected the initial imbalance. However, the ongoing heterogeneity in our meta-analyses for both the primary and secondary outcomes suggests prudence is still required before equal outcomes are assumed. Had some of our adjusted analyses shown less heterogeneity, this would have served as proof that ultimate survival is related to the factors studied. Our failure to eliminate or even reduce heterogeneity suggests the presence of latent factors that could still tilt the final balance in favor of better survival for either male or female. This latent factors could include disparities in post resuscitation care, such as access to CAG, PCI and TTM as suggested in our data. These elements were not included as variables for adjustment in many studies and may relate to upstream factors or inequities in the system of care. Post-resuscitation management has been reported to be more conservative in women than in men (less referral for cardiac interventions in particular) [12]. We found no sex differences in survival despite the fact that females have worse prognostic factors than males. Therefore, the sex-related risk–benefit ratios of specific treatments should undergo thoughtful reconsideration as there may be unwarranted inequalities in the care we provide to our patients.
Three metanalyses have been published on the topic of sex differences in outcomes following OHCA. One was published in 2015 and, therefore, required an update [18]. We identified and included fifteen papers published after the last search date of this study. The second was published more recently but suffers from several major flaws; its protocol was not registered, sex differences as primary and secondary outcomes were pooled, there was no division of single centre, national registry and emergency medical services data, changes over time were not studied and, most importantly, adjusted and unadjusted outcomes were not separated [66]. The third meta-analysis was performed on adjusted data, but it too was not registered and its main focus was on age as a confounder [13]. All of these meta-analyses reported a high degree of heterogeneity as do we. The authors of one of these meta-analyses have put forward that differences in survival of male and female may be “a matter of education" (i.e., poorer outcomes in locations with greater inequality for female) [66]. We sought such effects in the subgroup analysis by geographical location and found none. Put together, all of these findings suggest the need to study variables outside those reported in the Utstein template. Our failure to elucidate the causes of heterogeneity suggests the presence of unstudied factors (e.g., in-hospital treatment and/or decision making). Ignoring this finding may perpetuate latent inequalities, perhaps even unrelated to sex.
We did find important selection bias introduced by use of a population denominator of survivors to hospital admission. While this bias seems to contribute little to the heterogeneity observed in relation to our PICO question, it may affect other studies related to survival outcomes. The omission of some cases is often understandable in emergency circumstances. However, our finding suggests there is a need to establish a reporting template that highlights inclusion bias when reporting outcomes after cardiopulmonary resuscitation.
The differences observed between males and females in this analysis may be explained by multiple factors. Females clearly have worse predisposing factors than males at the time of cardiac arrest. In some places poor female education may lead to neglect of risk factors and even late referral if symptoms occur pre-arrest. There may be intrinsic differences in physiological responses to ischemia–reperfusion mechanisms which require elucidation. Finally post-resuscitation care may differ based on family request, professional concerns regarding potential risk/benefit ratio and more.
Our study has several strengths. We prospectively registered the study protocol, performed analysis of adjusted data, assessed the RoB of the included studies and applied GRADE to assess the certainty of the evidence. However, any meta-analysis is only as good as the studies it includes. Most of the studies included in this review were judged at high RoB and neither randomization nor blinding are an option with regard to the study question at hand. Analysis of retrospective studies (regardless of real time data collection) carries an inherent risk of confounding due to study design. Consequently, GRADE, which depends on the lowest quality of evidence for the clinically meaningful outcomes being studied, yielded only low certainty for our conclusion. This is further compounded by the fact that our strategy to handle statistical heterogeneity was unsuccessful. The internal and external validity of the studies included are low and the study populations are also very heterogeneous. Finally, although we studied adjusted data, the variables adjusted for differed between studies. Some studies did adjust their data on care management variables (speed of intervention, PCI, TTM), and there was a possibility of collinearity between these variables and sex, as differences in care themselves can be related to sex leading to a risk of overfitting in our analysis. Sensitivity analyses excluding those studies did not lead to a significant change in results.
Conclusions
No difference was found between male and female in the adjusted rate of survival to hospital discharge or at 30 days after OHCA. Substantial heterogeneity and failure to elucidate its causes suggest the existence of undetermined factors that were not reported in the Utstein template or the underestimation of factors, such as post-resuscitation care. This systematic review calls for additional further investigation for possible latent inequalities in some reports. Future studies should include data on post-resuscitation care (such as provision of CAG, PCI and TTM) as well as information that is currently no included in the Utstein template such as end of life decisions and in-depth analysis of decision-making processes with regard to provision of organ and life support.
Availability of data and materials
Data are available in the literature corpus and can be find using the search strategy defined in the manuscript.
References
Kiguchi T, Okubo M, Nishiyama C, Maconochie I, Ong MEH, Kern KB, et al. Out-of-hospital cardiac arrest across the World: first report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation. 2020;152:39–49.
Yan S, Gan Y, Jiang N, Wang R, Chen Y, Luo Z, et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24:61.
Weisfeldt ML, Sitlani CM, Ornato JP, Rea T, Aufderheide TP, Davis D, et al. Survival after application of automatic external defibrillators before arrival of the emergency medical system. J Am Coll Cardiol. 2010;55:1713–20.
Kim SH, Choi SP, Park KN, Lee SJ, Lee KW, Jeong TO, et al. Association of blood glucose at admission with outcomes in patients treated with therapeutic hypothermia after cardiac arrest. Am J Emerg Med. 2014;32:900–4.
Sasson C, Rogers MAM, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81.
Ritter G, Wolfe RA, Goldstein S, Landis JR, Vasu CM, Acheson A, et al. The effect of bystander CPR on survival of out-of-hospital cardiac arrest victims. Am Heart J. 1985;110:932–7.
Sasson C, Haukoos JS, Ben-Youssef L, Ramirez L, Bull S, Eigel B, et al. Barriers to calling 911 and learning and performing cardiopulmonary resuscitation for residents of primarily Latino, high-risk neighborhoods in Denver. Colorado Ann Emerg Med. 2015;65:545-552.e2.
Hollenberg J, Riva G, Bohm K, Nordberg P, Larsen R, Herlitz J, et al. Dual dispatch early defibrillation in out-of-hospital cardiac arrest: the SALSA-pilot. Eur Heart J. 2009;30:1781–9.
Lascarrou J-B, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al. Targeted temperature management for cardiac arrest with Nonshockable Rhythm. N Engl J Med. 2019;381:2327–37.
Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullén S, et al. Hypothermia versus Normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384:2283–94.
Camuglia AC, Randhawa VK, Lavi S, Walters DL. Cardiac catheterization is associated with superior outcomes for survivors of out of hospital cardiac arrest: Review and meta-analysis. Resuscitation. 2014;85:1533–40.
Helviz Y, Ong M, Einav S. Cardiac arrest, gender and resuscitation outcomes. Intensive Care Med. 2019;45:278–81.
Feng D, Li C, Yang X, Wang L. Gender differences and survival after an out-of-hospital cardiac arrest: a systematic review and meta-analysis. Intern Emerg Med. 2021;16:765–75.
Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SAE. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241:211–8.
Zandecki L, Sadowski M, Janion M, Gierlotka M, Gasior M, Polonski L. Trends in sex differences in clinical characteristics, treatment strategies, and mortality in patients with ST-elevation myocardial infarction in Poland from 2005 to 2011. Coron Artery Dis. 2017;28:417–25.
Ko DT, Qiu F, Koh M, Dorian P, Cheskes S, Austin PC, et al. Factors associated with out-of-hospital cardiac arrest with pulseless electric activity: a population-based study. Am Heart J. 2016;177:129–37.
Safdar B, Stolz U, Stiell IG, Cone DC, Bobrow BJ, deBoehr M, et al. Differential survival for men and women from out-of-hospital cardiac arrest varies by age: results from the OPALS study. Acad Emerg Med. 2014;21:1503–11.
Bougouin W, Mustafic H, Marijon E, Murad MH, Dumas F, Barbouttis A, et al. Gender and survival after sudden cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2015;94:55–60.
Perers E, Abrahamsson P, Bång A, Engdahl J, Karlson BW, Lindqvist J, et al. Outcomes of patients hospitalized after out-of-hospital cardiac arrest in relation to sex. Coron Artery Dis. 1999;10:509–14.
Hubert H, Jaeger D, Baert V, Vilhelm C, Genin M, Manzo-Silberman S, et al. Effect of gender on out-of-hospital cardiac arrest survival: a registry-based study. Eur J Emerg Med. 2021;28:50–7.
Ng YY, Wah W, Liu N, Zhou SA, Ho AFW, Pek PP, et al. Associations between gender and cardiac arrest outcomes in Pan-Asian out-of-hospital cardiac arrest patients. Resuscitation. 2016;102:116–21.
Mumma BE, Umarov T. Sex differences in the prehospital management of out-of-hospital cardiac arrest. Resuscitation. 2016;105:161–4.
Wigginton JG, Pepe PE, Bedolla JP, DeTamble LA, Atkins JM. Sex-related differences in the presentation and outcome of out-of-hospital cardiopulmonary arrest: A multiyear, prospective, population-based study. Crit Care Med. 2002;30:S131–6.
Goodwin G, Picache D, Gaeto N, Louie BJ, Zeid T, Aung PP, et al. Gender Disparities in Out-of-hospital Cardiac Arrests. Cureus. 2018. https://www.cureus.com/articles/13946-gender-disparities-in-out-of-hospital-cardiac-arrests. Accessed Oct 3 2021.
Ahn KO, Shin SD, Hwang SS. Sex disparity in resuscitation efforts and outcomes in out-of-hospital cardiac arrest. Am J Emerg Med. 2012;30:1810–6.
Jarman AF, Mumma BE, Perman SM, Kotini-Shah P, McGregor AJ. When the female heart stops: sex and gender differences in out-of-hospital cardiac arrest epidemiology and resuscitation. Clin Ther. 2019;41:1013–9.
Winther-Jensen M, Hassager C, Kjaergaard J, Bro-Jeppesen J, Thomsen JH, Lippert FK, et al. Women have a worse prognosis and undergo fewer coronary angiographies after out-of-hospital cardiac arrest than men. Eur Heart J Acute Cardiovasc Care. 2018;7:414–22.
Sauter TC, Iten N, Schwab PR, Hautz WE, Ricklin ME, Exadaktylos AK. Out-of-hospital cardiac arrests in Switzerland: Predictors for emergency department mortality in patients with ROSC or on-going CPR on admission to the emergency department. PLoS ONE. 2017;12:e0188180.
PRISMA-P Group, Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement. Syst Rev. 2015. https://doi.org/10.1186/2046-4053-4-1.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/bmj.n71.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Higgins J, Deeks J, editors. Chapter 6: Choosing effect measures and computing estimates of effect. Higgins JPT Thomas J Chandl J Cumpston M Li T Page MJ Welch VA Ed Cochrane Handb Syst Rev Interv Version 62 Updat Febr 2021. Cochrane. www.training.cochrane.org/handbook. Accessed 14 Dec 2022.
Deeks J, Higgins J, Altman D (editors). Chapter 10: Analysing data and undertaking meta-analyses. Higgins JPT Thomas J Chandl J Cumpston M Li T Page MJ Welch VA Ed Cochrane Handb Syst Rev Interv Version 62 Updat Febr 2021. Cochrane. www.training.cochrane.org/handbook. Accessed 14 Dec 2022.
Rosenblad A. Introduction to meta-analysis by Michael Borenstein, Larry V. Hedges, Julian P.T. Higgins, Hannah R. Rothstein. Int Stat Rev. 2009;77:478–9. https://doi.org/10.1111/j.1751-5823.2009.00095_15.x.
Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med. 2008;27:746–63.
Peters JL. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676.
Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ British Med J Publ Gr. 1997;315:629–34.
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002–d4002.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60.
Allan KS, Morrison LJ, Tu JV, Pinter A, Aves T, McGillion M, et al. The effect of gender on survival from sudden cardiac death in the young. Can J Cardiol. 2016;32:S213–4.
Arabi A, Patel A, Alsuwaidi J, Singh R, Albinali H. Gender differences in patients with out-of-hospital cardiac arrest over a 20-year period: a middle eastern perspective. J Am Coll Cardiol. 2013;61:E13.
Castro Y, Kim G, Lansky A. TCT-524 sex differences in survival from out-of-hospital cardiac arrest: insights from the national inpatient sample. J Am Coll Cardiol. 2019;74:B517.
Nagraj S, Narvel H, Mathai SV, Kharawala A, Varrias D, Thachil R. Racial and sex differences in outcomes after out-of-hospital cardiac arrest: a glimpse of the largest municipal healthcare system in the United States. J Am Coll Cardiol. 2022;79:9.
Shin SD. Abstract 56: gender difference in treatment options and outcomes in patients with out of hospital cardiac arrest. Circulation. 2010. https://doi.org/10.1161/circ.122.suppl_21.A56.
Bougouin W, Dumas F, Marijon E, Geri G, Champigneulle B, Chiche J-D, et al. Gender differences in early invasive strategy after cardiac arrest: Insights from the PROCAT registry. Resuscitation. 2017;114:7–13.
Arrich J, Sterz F, Fleischhackl R, Uray T, Losert H, Kliegel A, et al. Gender modifies the influence of age on outcome after successfully resuscitated cardiac arrest: a retrospective cohort study. Medicine (Baltimore). 2006;85:288–94.
Rob D, Kavalkova P, Smalcova J, Franek O, Smid O, Komarek A, et al. Gender differences and survival after out of hospital cardiac arrest. Am J Emerg Med. 2022;55:27–31.
Akahane M, Ogawa T, Koike S, Tanabe S, Horiguchi H, Mizoguchi T, et al. The effects of Sex on out-of-hospital cardiac arrest outcomes. Am J Med. 2011;124:325–33.
Al-Dury N, Ravn-Fischer A, Hollenberg J, Israelsson J, Nordberg P, Strömsöe A, et al. Identifying the relative importance of predictors of survival in out of hospital cardiac arrest: a machine learning study. Scand J Trauma Resusc Emerg Med. 2020;28:60.
Goto Y, Funada A, Maeda T, Okada H, Goto Y. Sex-specific differences in survival after out-of-hospital cardiac arrest: a nationwide, population-based observational study. Crit Care. 2019;23:263.
Jeong JS, Kong SY, Shin SD, Ro YS, Song KJ, Hong KJ, et al. Gender disparities in percutaneous coronary intervention in out-of-hospital cardiac arrest. Am J Emerg Med. 2019;37:632–8.
Pell J. Sex differences in outcome following community-based cardiopulmonary arrest. Eur Heart J. 2000;21:239–44.
Perman SM, Siry BJ, Ginde AA, Grossestreuer AV, Abella BS, Daugherty SL, et al. Sex differences in “Do Not Attempt Resuscitation” orders after out-of-hospital cardiac arrest and the relationship to critical hospital interventions. Clin Ther. 2019;41:1029–37.
Wissenberg M, Hansen CM, Folke F, Lippert FK, Weeke P, Karlsson L, et al. Survival after out-of-hospital cardiac arrest in relation to sex: a nationwide registry-based study. Resuscitation. 2014;85:1212–8.
Auricchio A, Caputo ML, Baldi E, Klersy C, Benvenuti C, Cianella R, et al. Gender-specific differences in return-to-spontaneous circulation and outcome after out-of-hospital cardiac arrest: results of sixteen-year-state-wide initiatives. Resusc Plus. 2020;4: 100038.
Blom MT, Oving I, Berdowski J, van Valkengoed IGM, Bardai A, Tan HL. Women have lower chances than men to be resuscitated and survive out-of-hospital cardiac arrest. Eur Heart J. 2019;40:3824–34.
Bray JE, Stub D, Bernard S, Smith K. Exploring gender differences and the “oestrogen effect” in an Australian out-of-hospital cardiac arrest population. Resuscitation. 2013;84:957–63.
Cline SL, von Der Lohe E, Newman MM, Groh WJ. Factors associated with poor survival in women experiencing cardiac arrest in a rural setting. Heart Rhythm. 2005;2:492–6.
Dicker B, Conaglen K, Howie G. Gender and survival from out-of-hospital cardiac arrest: a New Zealand registry study. Emerg Med J. 2018;35:367–71.
Herlitz J, Engdahl J, Svensson L, Young M, Ängquist K-A, Holmberg S. Is female sex associated with increased survival after out-of-hospital cardiac arrest? Resuscitation. 2004;60:197–203.
Johnson MA, Haukoos JS, Larabee TM, Daugherty S, Chan PS, McNally B, et al. Females of childbearing age have a survival benefit after out-of-hospital cardiac arrest. Resuscitation. 2013;84:639–44.
Kim C, Fahrenbruch CE, Cobb LA, Eisenberg MS. Out-of-Hospital cardiac arrest in men and women. Circulation. 2001;104:2699–703.
Mahapatra S. 2538 gender differences in survival and quality of life after an out of hospital cardiac arrest with early defibrillation in a population-based study. Eur Heart J. 2003;24:478.
Lei H, Hu J, Liu L, Xu D. Sex differences in survival after out-of-hospital cardiac arrest: a meta-analysis. Crit Care. 2020;24:613.
Acknowledgements
Not applicable.
Funding
The GERAR (Groupe Education Recherche en Anesthesie Reanimation) funded the use of the Covidence software.
Author information
Authors and Affiliations
Contributions
Conceptualization, SE and IL; methodology, SE, IL, MI.; software, IL, LD; validation, SE; formal analysis, IL, PT, LD; investigation, IL, AN; data curation, IL, AN.; writing—original draft preparation, IL, AN.; writing—review and editing, SE, MI, ML; visualization, IL.; supervision, SE; project administration, IL. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Marc Leone reports a relationship with LFB that includes: speaking and lecture fees, with Ambu that includes: speaking and lecture fees and with Gilead that includes: speaking and lecture fees.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Full search strategy.
Additional file 2: Table S1. Overlapping studies:
To avoid overlap, we excluded any study that presented data from a country whose national database was already being used in another included study on the same period. In this case, the study included was the one with adjusted data, and if more than one study from the same national database had adjusted data, the largest cohort was chosen. Table S2. Description of the population according to the inclusion and exclusion criteria of each study. Table S3. Certainty estimates of different outcomes using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods. Table S4 Meta-regression results.
Additional file 3:
Database.
Additional file 4: Figure S3.
Quality of the variables used for adjustment: subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA. Figure S3. BIS. Quality of the variables used for adjustment: subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA (with the pooled τ2). Figure S4. Geographical location: subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA. Figure S4 BIS. Geographical location: subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA (with the pooled τ2). Figure S5. OHCA etiology: subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA. Figure S6. Type of cohort and number of centres: subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA. Figure S6. BIS Type of cohort and number of centres: subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA (with the pooled τ2). Figure S7. Population denominator (whether non-survivors to hospital admission were included or not): subgroup analysis of adjusted data on men and women survival to discharge or to 30 days after OHCA. Figure S8. Scatter plot showing the results of the regression of time vs. the log(OR) for adjusted survival to discharge or to 30 days. A log(OR) below 0 favors men and a log(OR) above 0 favors women. Figure S9. A Baujat plot showing the contribution of each study to the overall heterogeneity. B Same plot zoomed in Studies are numbered in alphabetical order. Figure S10. Sensitivity analysis omitting studies rated identified as outliers in the Baujat plot (see Fig. 9)—adjusted survival data. Figure S11. Sensitivity analysis omitting studies including traumatic etiology for OHCA—adjusted survival data. Figure S12. Sensitivity analysis omitting studies assessed as having a high risk of bias. Figure S13. Forest plot of unadjusted data on men and women survival to discharge or to 30 days after OHCA. Figure S14. Funnel plot of the included studies. Figure S15. Forest plot of unadjusted data on men and women neurologically intact survival. Figure S16. Forest plot of adjusted data on men and women favourable neurological outcome in survival
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lakbar, I., Ippolito, M., Nassiri, A. et al. Sex and out-of-hospital cardiac arrest survival: a systematic review. Ann. Intensive Care 12, 114 (2022). https://doi.org/10.1186/s13613-022-01091-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-01091-9