Abstract
Background
Chronic obstructive pulmonary disease (COPD) exacerbation and protective mechanical ventilation of acute respiratory distress syndrome (ARDS) patients induce hypercapnic respiratory acidosis.
Main text
Extracorporeal carbon dioxide removal (ECCO2R) aims to eliminate blood CO2 to fight against the adverse effects of hypercapnia and related acidosis. Hypercapnia has deleterious extrapulmonary consequences, particularly for the brain. In addition, in the lung, hypercapnia leads to: lower pH, pulmonary vasoconstriction, increases in right ventricular afterload, acute cor pulmonale. Moreover, hypercapnic acidosis may further damage the lungs by increasing both nitric oxide production and inflammation and altering alveolar epithelial cells. During an exacerbation of COPD, relieving the native lungs of at least a portion of the CO2 could potentially reduce the patient's respiratory work, Instead of mechanically increasing alveolar ventilation with MV in an already hyperinflated lung to increase CO2 removal, the use of ECCO2R may allow a decrease in respiratory volume and respiratory rate, resulting in improvement of lung mechanic. Thus, the use of ECCO2R may prevent noninvasive ventilation failure and allow intubated patients to be weaned off mechanical ventilation. In ARDS patients, ECCO2R may be used to promote an ultraprotective ventilation in allowing to lower tidal volume, plateau (Pplat) and driving pressures, parameters that have identified as a major risk factors for mortality. However, although ECCO2R appears to be effective in improving gas exchange and possibly in reducing the rate of endotracheal intubation and allowing more protective ventilation, its use may have pulmonary and hemodynamic consequences and may be associated with complications.
Conclusion
In selected patients, ECCO2R may be a promising adjunctive therapeutic strategy for the management of patients with severe COPD exacerbation and for the establishment of protective or ultraprotective ventilation in patients with ARDS without prognosis-threatening hypoxemia.
Similar content being viewed by others
Background
Extracorporeal carbon dioxide removal (ECCO2R) is a technique whose objective is the decarboxylation of blood and thus to correct hypercapnia and respiratory acidosis [1, 2]. ECCO2R is similar to extracorporeal membrane oxygenation (ECMO) but uses lower blood flow, usually less than 1500 mL/min. Therefore, this technique has little or no impact on blood oxygenation. Initially, ECCO2R was developed in the treatment of patients with acute respiratory distress syndrome (ARDS) [3], but because of the progressive improvement of this technique and its use in hospitals, ECCO2R could be proposed as a therapeutic option in cases of hypercapnic respiratory insufficiency, either during acute and severe decompensation of chronic obstructive pulmonary disease (COPD) [4] or in ARDS to achieve less invasive mechanical ventilation (IMV) [5]. In this review of the literature, we will discuss the current knowledge on the pathophysiology related to hypercapnic respiratory failure, the principles of the ECCO2R technique, and its place in the treatment of ARDS and acute and severe decompensations of COPD.
ECCO2R: from applied physiology to clinical studies
Pathophysiological rationale of the use of ECCO 2 R in COPD exacerbations
The amount of CO2 in the blood is higher than that of oxygen. CO2 is mainly present in blood as bicarbonates and to a lesser extent in dissolved form, whereas O2 is mainly linked to hemoglobin. Small variations in the partial pressure of CO2 (PaCO2) cause significant variations in the level of CO2 in the blood, unlike the relationship between the O2 partial pressure and O2 blood content. Therefore, extracorporeal CO2 removal can be realized with lower blood flow rates than requires extracorporeal oxygenation but with enough fresh gas flow sweeping the exchange membrane [6].
ECCO2R aims to eliminate blood CO2 to fight against potential adverse effects of hypercapnia and related acidosis. Hypercapnia has deleterious extrapulmonary consequences, particularly on the brain, by increasing cerebral blood flow and therefore intracranial pressure [7]. In addition, in the lungs, hypercapnia leads to pulmonary vasoconstriction, increases right ventricular afterload, and decreases myocardial contractility with consequent right heart failure [8]. Moreover, hypercapnic acidosis may further damage the lungs by increasing both nitric oxide production and inflammation and altering alveolar epithelial cells [9]. Finally, because of its immunosuppressive properties, hypercapnic acidosis may exacerbate lung damage by exacerbating pulmonary bacterial infections [9].
During exacerbations of COPD, the volume of CO2 removed by the lungs is reduced due to worsening dynamic overdistension and the gap between ventilation and perfusion [10], accompanied by severe hypercapnia. In addition, in patients with COPD exacerbation, CO2 production is estimated to be 23% higher than the normal value of 200 to 250 mL/min due to increased respiratory muscle work and metabolism [10].
Therefore, during an exacerbation of COPD, relieving the native lungs of at least a portion of the CO2 could potentially improve the acid–base balance and reduce the patient's respiratory work, resulting in a reduced respiratory rate and alveolar ventilation [11]. Instead of mechanically increasing alveolar ventilation with IMV in an already hyperinflated lung to increase CO2 removal, the use of ECCO2R may allow a decrease in respiratory volume and respiratory rate, resulting in longer expiratory time that is better adapted to the high expiratory time constant of the respiratory system. Through these physiological mechanisms, ECCO2R can neutralize the vicious cycle of dynamic hyperinflation and its harmful respiratory and cardiovascular consequences. Beneficial effects derived from respiratory mechanics, ventilatory muscle efficiency, respiration, and cardiovascular function can improve gas exchange and relieve dyspnea, potentially preventing the failure of NIV or facilitating weaning from IMV [10,11,12]. The pathophysiological rationale for the use of ECCO2R in COPD exacerbation is presented in Fig. 1 (Pathophysiology of respiratory acidosis is presented in Additional file 1 and pathophysiology of COPD is presented in Additional files 1 and 2 (Figure S1)).
Pathophysiological rationale of the use of ECCO 2 R in ARDS
In recent decades, very important progress has been made in the understanding of the pathophysiology of ARDS. The recognition of ventilatory-induced lung injury (VILI) has led to drastic changes in the ventilatory management of these patients [13, 14]. The historical trial conducted by the ARDSNet group demonstrated that the ventilation of ARDS patients with a low tidal volume (VT) of 6 mL/kg (vs. 12 mL/kg) significantly reduced mortality [15]. However, recent results have shown that pulmonary hyperinflation still occurs in approximately 30% of ARDS patients despite this so-called “protective” ventilation [16]. This analysis suggests a beneficial effect of VT reduction, even in patients already at a plateau pressure (Pplat) < 30 cm H2O [17]. The decrease in the VT and Pplat will also decrease the driving pressure, which has recently been identified as a major risk factor for mortality in ARDS patients [18]. A reduction in VT to less than 6 mL/kg to reach a low Pplat level may induce severe hypercapnia that may increase intracranial pressure, causes pulmonary hypertension, decreases myocardial contractility, reduce renal blood flow, and releases endogenous catecholamines [19, 20]. In a recent multicenter study on 35 ARDS patients with PaO2/FiO2 ≤ 150 mmHg, Richard et al. reduced VT to 4 mL/kg and further adjusted respiratory rate (RR) to keep pH ≥ 7.20. RR was augmented up to 40 breaths/min. On day 2, VT decreased from 6.0 [5.9–6.1] to 4.1 [4.0–4.7] ml/kg leading to a significant decrease in driving pressure from 12 [9–15] to 8 [6–11] cmH2O. They concluded that ultra-low tidal volume ventilation may be applied in approximately 2/3 of moderately severe-to-severe ARDS patients while 2 patients (6%) developed acute cor pulmonale and 11 patients (32%) developed transient severe acidosis with pH < 7.15. A 4 cmH2O median reduction in driving pressure has been reached, at the price of transient episodes of severe acidosis [21]. This strategy is therefore not feasible for most ARDS patients with conventional IMV [22]. Therefore, ECCO2R could be used to achieve a VT < 6 mL/kg, thus lowering the Pplat, driving pressure and mechanical power [23,24,25,26,27] while maintaining PaCO2 and pH in physiological standards.
Technical principles
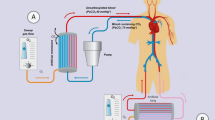
Catheters or cannulas are needed to implement this technique. There are two categories of ECCO2R. The first category is the so-called arteriovenous technique, where the removal of CO2 is possible without a pump. A femoro-femoral approach is used. This technique requires arterial and venous cannulation with 15 French cannulas. The blood flow inside the device depends exclusively on the cardiac output of the patient, which explains the great variability of the ability to oxygenate the patient. However, with a membrane surface of 1.3 m2, its decarboxylation capacities are satisfactory. The second technique is called the venovenous technique, where the use of a pump is necessary (Fig. 2). The venovenous technique uses low or very low blood flow. Currently, it is the venovenous technique that is conventionally used for ECCO2R. The pumps used are rollers, centrifugal or diagonal, electric or electromagnetic. Figure 3 shows a schematic representation of different ECCO2R systems. The gas exchange membrane is a device with a complex geometry based on hollow fibers. The material used is poly-4-methyl-1-pentene (PMP). The exchange surfaces vary in size from 0.32 to 0.65 m2 for venovenous systems and 1.3 m2 for arteriovenous systems. Circuits such as membranes are coated with heparin for better biocompatibility, better gas exchange and less capillary leakage. The extraction of carbon dioxide is done through the sweeping of the membrane by a fresh gas (O2 or medical air) devoid of CO2. Current systems used to remove CO2 are venovenous and use double-lumen venous catheters/cannulas. The venous approach is classically achieved through the right internal jugular or femoral vein, and puncture of the vessel is performed under ultrasound guidance. The placement of the guidewire and the cannula requires control by transesophageal or subxiphoid transthoracic echocardiography (Fig. 4). Anticoagulation therapy (anti-Xa activity between 0.3 and 0.6 IU/L) is mandatory to avoid thrombosis in the circuit. Thus, any patient with a contraindication to anticoagulation therapy cannot benefit from ECCO2R. There are different types of machines on the market. The devices adapted from the VV-ECMO technique are very effective for CO2 removal but require the insertion of cannulas between 18 and 19 French. The blood flow generated is between 500 and 1500 mL/min. The newest ECCO2R devices are relatively simple to use because they require the insertion of a smaller double-lumen cannula (up to 13–15 Fr) and work with very low blood flow rates (between 0.2 to 0.5 L/min). However, their CO2 removal performance remains limited [11]. The characteristics of the different ECCO2R systems available on the market are summarized in Table 1.
Schematic representation of different ECCO2R systems. ECCO2R: extracorporeal carbon dioxide removal. a Pumpless arteriovenous system. b Venovenous system. Pump and membrane are in series. c Venovenous system. Pump is integrated into the membrane. d Venovenous system. The membrane is integrated into an extrarenal purification system that has its own pump
a Transthoracic echocardiography subcostal view showing the J-tip of the guidewire entering the inferior vena cava. b Transesophageal echocardiography bicaval view showing the guidewire passing through from the superior vena cava into the right atrium and entering into the inferior vena cava. RA right atrium, SVC superior vena cava, IVC inferior vena cava
Use of ECCO2R in severe acute exacerbations of COPD
Noninvasive ventilation remains the gold standard for the treatment of acute hypercapnic respiratory failure [28], but in approximately 20 to 30% of cases, this technique may not be sufficient, and patients need to be intubated and mechanically ventilated. The mortality of patients requiring the use of IMV is higher than those receiving NIV alone. Thus, the combination of ECCO2R therapy with NIV could be a therapeutic option to reduce the failure of NIV and prevent the use of intubation and IMV. In fact, the use of ECCO2R in patients with hypercapnic respiratory failure may improve the efficacy of NIV in terms, that ECCO2R, decreases respiratory rate, and reduces dynamic hyperinflation and intrinsic PEEP. In addition, by avoiding the use of IMV and therefore endotracheal intubation, it is also possible to limit the adverse effects related to analgo-sedation, which include hemodynamic instability, difficult and prolonged respiratory weaning, and a significant number of neurological disorders related to prolonged sedation. The absence of analgo-sedation also allows patients to drink, eat, communicate with relatives, and perform active physiotherapy. In addition, it has recently been demonstrated that ECCO2R, by decreasing the respiratory rate, can reduce the work of breathing and decrease the CO2 production of the respiratory muscles. Therefore, this contributes to the decrease in PaCO2 [29]. As a result, this may facilitate weaning from IMV and promote earlier extubation.
Use of ECCO2R decreases the use of IMV in patients with COPD exacerbation
Kluge et al. [30] investigated the feasibility of a pumpless extracorporeal assist (PECLA) system in 21 patients with COPD who did not respond to NIV. The use of PECLA system was associated with decreased PaCO2 levels and improved pH after 24 h and avoided the use of intubation and IMV in 90% of treated patients. Retrospective analysis with a control group showed no significant difference in mortality at 28 days (19% with ECCO2R and 24% without ECCO2R) or at 6 months (both groups at 33%) or in the median duration of ICU or hospital length of stay (15 vs 30 days and 23 vs 42 days, respectively). In the study conducted by Burki et al. [4], 20 hypercapnic patients with COPD were treated with ECCO2R using a 15.5-Fr dual-lumen cannula, allowing an average blood flow of 430 mL/min. The authors reported improvement in both hypercapnia and respiratory acidosis, and IMV was avoided in the nine patients treated with NIV. More recently, Del Sorbo et al. [31] reported that ECCO2R with a 14-Fr dual-lumen catheter and blood flow rates of 177 to 333 mL/min not only improved respiratory acidosis, but also reduced the need for intubation in 25 patients with COPD who have a high risk of NIV failure. Compared with the control group, intubation risk and hospital mortality were significantly lower in the ECCO2R group. These results were challenged in a recent study by Braune et al. [32], which showed that IMV was avoided in 56% of patients treated with ECCO2R but was associated with a higher incidence of complications. However, several differences were found between these two studies, including the inclusion of patients with contraindications for NIV and the unexpectedly high incidence of hypoxemic patients [33]. In another study, Morelli et al. [34] confirmed the efficacy of ECCO2R (with a flow rate of 250 to 450 mL/min via a 13-Fr dual-lumen catheter) to reduce PaCO2 in a case series of 30 patients with acute hypercapnic respiratory failure due to COPD exacerbation who refused endotracheal intubation after NIV failure. The duration of ECCO2R treatment was 2 to 16 days, and it was possible to avoid endotracheal intubation in 27 patients. Finally, in a round table, 14 European experts' views were collated to better understand how ECCO2R therapy is used, how patients are selected and managed. In COPD patients with acute exacerbation, a consensus was found that, in patients at risk of NIV failure, no decrease in PaCO2 and no decrease in respiratory rate were principal criteria for starting with ECCO2R therapy. Main treatment targets in COPD patients were patient well-being, pH (> 7.30–7.35), respiratory rate (< 20–25 breaths/min), decrease of PaCO2 (by 10–20%), weaning from NIV, decrease in HCO3− and maintaining hemodynamic stability [35].
Use of ECCO2R to help weaning from IMV
In the case series of Elliot et al. of two patients with severe acute asthma [36], the addition of pumpless ECCO2R to IMV corrected hypercapnia and related acidosis and reduced other support measures, including hemodynamics, and allowed weaning from IMV. In the study by Burki et al. [4], in the subgroup of 11 mechanically ventilated patients, ECCO2R allowed weaning from IMV in only 3 patients. Nevertheless, even if they were not completely weaned, in three other patients, ventilatory assistance could be reduced. Using a double-lumen cannula (20–23 Fr) with a blood flow of 1 to 1.7 L/min, Abrams et al. [37] successfully weaned and extubated five COPD patients with acute respiratory acidosis after only 24 h of IMV. All of these patients survived until discharge from the hospital. Similarly, using a pediatric VV-ECMO system (with blood flow rates of 0.9 L/min through a 19-Fr double-lumen cannula placed in the right internal jugular vein) in two adult patients with COPD exacerbation, Roncon-Albuquerque Jr [38] reported early extubation after 72 h and patient mobilization on day 6. A retrospective analysis of data from 12 patients with hypercapnic respiratory failure confirms the efficacy of ECCO2R with median blood flow rates of 1.2 to 1.4 L/min in the correction of hypercapnia and in the reduction of ventilation pressures and minute ventilation. Of the patients studied, six patients with mainly hypercapnic pulmonary insufficiency due to COPD or fibrosis were promptly weaned off of IMV and survived until discharge from the hospital. It should be noted that five patients were awake and spontaneously breathing during ECCO2R therapy [39].
Taken together, these results support the notion that ECCO2R may be useful for the avoidance of intubation during NIV and for the facilitation of weaning from IMV. Nevertheless, the observational nature of the available data makes it difficult to understand the efficacy and safety of such strategies in these patients.
The relevant clinical studies on ECCO2R in COPD are summarized in Table 2.
Use of ECCO2R in acute respiratory distress syndrome (ARDS)
The latest feasibility and safety pilot study of 20 patients with moderate and/or severe ARDS, in whom ECCO2R was performed with a new standalone platform (without concomitant extrarenal treatment), Prismalung® (Gambro-Baxter), integrated on the Prismaflex® platform (Gambro-Baxter), showed a reduction in the tidal volume from 6 to 4 mL/kg of the predicted body weight and in Pplat below 25 cmH2O, thus achieving ultraprotective ventilation. However, the results show that despite maximal ECCO2R treatment (sweep gas flow at 10 ± 0.3 L/min and blood flow at 421 ± 40 mL/min, corresponding to the maximum that this platform can generate), patients ventilated at 4 mL/kg of their predicted body weight become acidotic (pH decreased from 7.39 ± 0.1 to 7.32 ± 0.10 and PaCO2 increased from 43 ± 8 mmHg to 53 ± 9 mmHg) [5].
A larger prospective multicenter international phase II study aimed to assess the feasibility and safety of extracorporeal carbon dioxide removal (ECCO2R) to facilitate ultraprotective ventilation (VT 4 mL/kg and Pplat ≤ 25 cmH2O) in patients with moderate ARDS. The primary endpoint was the proportion of patients achieving ultraprotective ventilation with PaCO2 not increasing more than 20% from baseline and arterial pH > 7.30. Both lower CO2 extraction and higher CO2 extraction devices (membrane lung cross-sectional area 0.59 vs. 1.30 m2; flow 300–500 mL/min vs. 800–1000 mL/min, respectively) were used in this study. 59 patients were included. The proportion of patients who achieved ultraprotective settings by 8 h and 24 h was 78% (74 out of 95 patients; 95% confidence interval 68–89%) and 82% (78 out of 95 patients; 95% confidence interval 76–88%), respectively. ECCO2R was maintained for 5 [3–8] days. A total of 69 patients (73%) were alive at day 28. Fifty-nine patients (62%) were alive at hospital discharge. The authors concluded that the use of ECCO2R to facilitate ultraprotective ventilation was feasible [40]. In the recent round table of European experts on ECCO2R, an agreement was reached that the main treatment goal of ECCO2R therapy in patients with ARDS was to carry out ultraprotective lung ventilation through handling CO2 levels. Driving pressure with plateau pressure optimization was estimated as the principal criteria for ECCO2R introduction. Main targets for patients with ARDS starting with ECCO2R included pH (> 7.30), respiratory rate (< 25 or < 20 cycles/min), Pplat (< 25 cmH2O) and driving pressure (< 14 cmH2O) [35]. Finally, using data from the SUPERNOVA trial (95 patients with early moderate ARDS), Goligher et al. assessed the independent effects of alveolar dead space fraction (ADF), respiratory system compliance (Crs), hypoxemia (PaO2/FiO2), and device performance (higher vs lower CO2 extraction) on the magnitude of reduction in Vt, driving pressure and mechanical power permitted by ECCO2R were assessed. The authors demonstrated that patients with higher ADF or lower Crs and patients treated with higher CO2 extraction are most likely to benefit from ECCO2R [41].
The combination of continuous renal replacement therapy (CRRT) and ECCO2R with very low blood flow is a promising concept. The hypothesis in a study by Moerer et al. is that this combined system can remove enough CO2 to facilitate protective ventilation in mechanically ventilated patients. In 11 ventilated patients with acute renal failure placed under CRRT, a very-low-flow ECCO2R (300 mL/min) was added to the circuit. During 6 h of combined therapy, the elimination of CO2 and its effect on the possibility of achieving protective ventilation were evaluated. The ventilation settings were maintained in assisted mode or in controlled pressure mode, allowing spontaneous breathing. With very-low-flow ECCO2R, a significant decrease in minute ventilation, tidal volume and paCO2 was possible after 1–3 h but not after 6 h of treatment. On the other hand, no significant reduction in the driving pressure was observed during the combined treatment. The CO2 removal was 20.73 mL CO2/min. Therefore, the very low blood flow in ECCO2R associated with CRRT treatment is not enough to significantly reduce respiratory work. The absolute cause could be the absolute amount of CO2 removed by approximately 10% of CO2 production in the resting adult. Therefore, the effectiveness of ECCO2R with very low blood flow in allowing protective ventilation is very limited [42]. Moreover, in another recent study including 20 hypercapnic ARDS patients requiring CRRT who were treated with a system combining very-low-flow ECCO2R (membrane lung 0.32 m2) and renal replacement therapy, the pH increased from 7.18 ± 0.09 to 7.22 ± 0.08 (p < 0.05). There was a significant reduction in ventilation requirements with a decrease in tidal volume from 6.2 ± 0.9 to 5.4 ± 1.1 mL/kg PBW (p < 0.05), associated to a reduced pulmonary stress and strain [43]. Even if these results were statistically significant, we can question their clinical relevance. The relevant clinical studies on ECCO2R in ARDS are summarized in Table 3.
Role of ECCO2R while awaiting lung transplantation
It is well known that patients who develop acute gas exchange impairment requiring IMV while awaiting lung transplantation are more likely to die than patients who do not require IMV [44]. The reason for using ECCO2R in such patients is the possibility of the avoidance of endotracheal intubation and IMV, thus limiting their adverse effects (i.e., ventilator-associated pneumonia) that may preclude transplantation. In addition, by using ECCO2R, it is possible to avoid analgo-sedation, which allows the patient to maintain the tone of the respiratory muscles and to continue to perform active physiotherapy. Despite this pathophysiological rationale, studies regarding the use of ECCO2R in this subgroup of hypercapnic patients are still rare. Schellongowski et al. [45] performed a retrospective study of 20 patients with bronchiolitis obliterans, cystic fibrosis and idiopathic pulmonary fibrosis with indication for lung transplantation (n = 13) or retransplantation (n = 7). The use of venovenous ECCO2R and pumpless arteriovenous ECCO2R was associated with an improvement in hypercapnia and acidosis during the first 12 h of treatment. After a transition period of 4 to 11 days, 19 patients (95%) were successfully transplanted. Survival at the hospital was 75%. A very recent retrospective study confirmed that patients treated with ECCO2R before lung re-transplantation tended to have better survival [46]. In light of these findings, ECCO2R may even be useful in thoracic surgical procedures other than lung transplantation [47]. Nevertheless, given the complexity and the difficult clinical conditions of these patients awaiting lung transplantation, the use of ECCO2R in these patients should be performed only in experienced centers.
ECCO2R-related complications and technical limitations
The use of ECCO2R may have pulmonary and hemodynamic consequences and may be associated with complications. Adverse events include events related to the patient, the circuit and mechanical events (Table 4). In four studies of ARDS patients, the use of ECCO2R was associated with hypoxemia and the need for an increase in FiO2. The present fact could be explained by lung derecruitment related to decrease in ventilation (favoring atelectasis). Moreover, PaO2/FiO2 worsening in ECCO2R may at least in part, reflect a modification of the alveolar gas content due to ECCO2R (modification of the respiratory quotient) [48]. To correct this phenomenon, IMV was implemented in spontaneously breathing patients [49] with both the use of high levels of PEEP and prone position to maintain functional residual capacity [24, 49,50,51]. In case of refractory hypoxemia a switch to VV-ECMO [52] was performed.
The major adverse effects may be caused by venous and/or arterial cannulation, with increased risk depending on the choice of vascular access and the type and size of cannulas. Transient ischemia of the lower limb, "false" aneurysm of the femoral artery [50] and fatal perforation following retroperitoneal bleeding have been described [4, 33].
Anticoagulation protocols with heparin are necessary to maintain the efficacy and performance of ECCO2R [53]. Thus, hemorrhagic events may be considered the most common complication and are associated with a higher number of blood transfusions during ECCO2R therapy [4, 30, 33, 49, 50, 52].
Transient thrombocytopenia, probably related to the use of heparin, has also been noted [4, 33, 51]. However, thrombocytopenia and decreased coagulation factors, certainly due to an activation of coagulation and fibrinolysis as well as an inflammatory response mediated by the complement system [54] may also be the result of interactions between blood components and the circuit. Future research should focus on improvements in anticoagulation protocols and the development of practical guidelines [55].
Despite anticoagulation protocols, clot formation in the circuits often occurs reducing the clearance of CO2 in the membrane and resulting in a rapid increase in PaCO2. The occurrence of membrane thrombosis should be considered a life-threatening event and necessitates rapid circuit changes, changes in ventilator parameters, and endotracheal intubation in the case of NIV [33, 51, 52]. Moreover, it seems that the reduction in blood flow through the membrane may be linked to an increase in the risk of thrombosis of the system. In the study of Schmidt et al. including 20 patients with mild or moderate ARDS, VT was gradually lowered from 6 to 5, 4.5, and 4 ml/kg. When arterial PaCO2 increased by > 20% from its initial value, a very-low-flow standalone ECCO2R was initiated to reduce respiratory acidosis. The authors showed that despite a heparin-infusion protocol that also included a bolus at catheter insertion, 50% of the treated patients experienced membrane clotting before the end of the experimental protocol [5]. In a retrospective study carried out by our group on 3 patients with severe COPD also assisted by a very-low-flow ECCO2R, thrombosis of the circuit occurred in 2 patients. In contrast, in our study, the 6 patients assisted by a higher blow flow ECCO2R did not experience circuit thrombosis [56]. It therefore appears that the blood flow passing throughout the circuit has a role in the occurrence of circuit thrombosis.
The displacement or twisting of the catheter/cannula may cause pump malfunction and promote thrombosis of the membrane. Finally, episodes of intravascular hemolysis have been reported in two case series, including one requiring a transfusion [51, 52].
Finally, CO2 extraction capacity differed between the devices available on the market. While re-analyzing the results of the SUPERNOVA trial according to the ECCO2R devices used (lower blood flow (area of membrane length 0.59 m2; blood flow 300–500 mL/min) vs higher blood flow (membrane area 1.30 m2; blood flow between 800 and 1000 mL/min), Combes et al. showed that reduction of VT to 4 mL/kg was achieved in 55% and 64% of patients with the lower extraction versus 90% and 92% of patients with higher extraction devices at 8 and 24 h from baseline, respectively (p < 0.001) [57]. Moreover, ECCO2R-related hemolysis and bleeding were higher with lower than with higher extraction devices. In our retrospective study on COPD patients, we showed that when compared with a higher blood flow ECCO2R system, very low-flow device was not able to remove sufficient CO2, normalize pH or decrease respiratory rate [56].
New technologies and ongoing research on ECCO2R
ECCO2R devices remove CO2 directly from the blood, facilitating ultraprotective ventilation or even offering an alternative to IMV. However, ECCO2R is not widely available, while dialysis is available in most intensive care units. Recent technological advances are focused on the development of minimally invasive devices that provide adequate CO2 removal with increased safety and simple use. Previous attempts to perform ECCO2R with dialysis by removing CO2 as bicarbonate have been affected by metabolic acidosis. Bicarbonate dialysis is possible, provided that the difference between the strong ions in the plasma is maintained. Using a mathematical model to study the effects of bicarbonate removal on pH and CO2 in plasma, in vitro experiments were performed to test CO2 removal using three dialysates with different bicarbonate concentrations (0, 16 and 32 mmol/L). This model predicts a reduction in partial CO2 pressure (PaCO2) and an increase in pH with a progressive reduction in plasma bicarbonate, provided that the strong ion difference and the maintenance of plasma proteins are preserved. In these in vitro experiments, CO2 removal with an adult-size filter was maximal with a dialysate not containing bicarbonate, equivalent to 94 mL/min (± 3.0) of CO2 eliminated. Under the same conditions, the dialysate containing a conventional concentration of bicarbonates (32 mmol/L) eliminated only 5 mL/min (± 4, p < 0.001). As expected, the pH increased after the removal of the bicarbonate. These data show that dialysis with low-bicarbonate dialysates is feasible and results in a reduction in plasma PaCO2. When scaled to estimate equivalent CO2 removal with an adult dialysis circuit, the amount eliminated competes with that of existing low-flow ECCO2R devices [58]. However, these methods may be impractical for clinical use due to acid–base disturbances, hemolysis, cardiac arrhythmias and micronutrient depletion despite several attempts to replace bicarbonate [59, 60]. Finally, other techniques were evaluated, including the combination of ECCO2R and continuous renal replacement therapy, the acidification of blood with lactic acid, the addition of carbonic anhydrase to the membrane and electrodialysis [60,61,62]. ECCO2R technique based on infusion of metabolizable acids exploits bicarbonate for gas exchange. An innovative lung support technique, called respiratory electrodialysis has been developed, consisting in a combination of a hemofilter, a membrane lung, and an electrodialysis unit. By applying electrodialysis to hemodiafiltrate, the pH and the electrolyte concentration are selectively modulated in specific sections of the extracorporeal circuitry. Blood is regionally acidified, bicarbonate is exchanged with chloride, and the PaCO2 is increased, leading to facilitated membrane lung CO2 removal [61]. These strategies can enhance the physiological benefits of ECCO2R while reducing its risks. However, studies demonstrating safety and efficacy are necessary before putting these technological innovations into clinical practice.
Several studies of ECCO2R are currently underway in patients with hypercapnic respiratory failure (ClinicalTrials.gov). Details of these studies are available in Additional file 2. These various ongoing clinical studies on the use of ECCO2R in COPD and ARDS are summarized in Additional file 2: Tables S1, S2, respectively.
Conclusion
ECCO2R may be a promising adjunctive therapeutic strategy for the management of patients with severe COPD exacerbation and for the establishment of protective or ultraprotective ventilation in patients with ARDS without prognosis-threatening hypoxemia. To date, only the feasibility and the relative safety of this therapy have been studied and demonstrated and large randomized controlled studies are definitively warranted. In the meantime, a careful clinical evaluation of patients should be performed to select the most appropriate ECCO2R device in terms of extracorporeal blood flow and the potential complications of ECCO2R need to be considered.
Take home messages
-
Chronic obstructive pulmonary disease (COPD) exacerbation and protective mechanical ventilation of acute respiratory distress syndrome (ARDS) patients may induce hypercapnic respiratory acidosis.
-
Extracorporeal carbon dioxide removal (ECCO2R) is an efficient technique which by eliminating blood CO2 fights against the adverse effects of hypercapnia and related acidosis.
-
ECCO2R may be a promising adjunctive therapeutic strategy for the management of patients with severe COPD exacerbation and for the establishment of protective or ultraprotective ventilation in patients with ARDS.
-
A careful clinical evaluation of patients should be performed to both select the most appropriate ECCO2R device in terms of extracorporeal blood flow and consider the potential complications of ECCO2R.
Availability of data and materials
Not applicable.
References
Giraud R, Bendjelid K, Banfi C. Obesity-related respiratory failure: a new area for extracorporeal lung support? Swiss Med Wkly. 2018;148:w14651.
d’Andrea A, Banfi C, Bendjelid K, Giraud R. The use of extracorporeal carbon dioxide removal in acute chronic obstructive pulmonary disease exacerbation: a narrative review. Can J Anaesth. 2019;67:462–74.
Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256(7):881–6.
Burki NK, Mani RK, Herth FJ, Schmidt W, Teschler H, Bonin F, et al. A novel extracorporeal CO2 removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest. 2013;143(3):678–86.
Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care. 2018;22(1):122.
Diehl JL, Boisrame-Helms J, Chardon-Couteau A, Commereuc M, Augy JL, Sokoloff A, et al. The role of extracorporeal removal of CO2 (ECCO2R) in the management of respiratory diseases. Rev Mal Respir. 2017;34(6):598–606.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–36.
Macchia A, Rodriguez Moncalvo JJ, Kleinert M, Comignani PD, Gimeno G, Arakaki D, et al. Unrecognised ventricular dysfunction in COPD. Eur Respir J. 2012;39(1):51–8.
Ismaiel NM, Henzler D. Effects of hypercapnia and hypercapnic acidosis on attenuation of ventilator-associated lung injury. Minerva Anestesiol. 2011;77(7):723–33.
Lund LW, Federspiel WJ. Removing extra CO2 in COPD patients. Curr Respir Care Rep. 2013;2:131–8.
Morelli A, Del Sorbo L, Pesenti A, Ranieri VM, Fan E. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med. 2017;43(4):519–30.
Sklar MC, Beloncle F, Katsios CM, Brochard L, Friedrich JO. Extracorporeal carbon dioxide removal in patients with chronic obstructive pulmonary disease: a systematic review. Intensive Care Med. 2015;41(10):1752–62.
Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294–323.
Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest. 2006;130(6):1906–14.
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Acute Respiratory Distress Syndrome N, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175(2):160–6.
Hager DN, Krishnan JA, Hayden DL, Brower RG, Network ACT. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172(10):1241–5.
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55.
Curley G, Contreras MM, Nichol AD, Higgins BD, Laffey JG. Hypercapnia and acidosis in sepsis: a double-edged sword? Anesthesiology. 2010;112(2):462–72.
Vadasz I, Hubmayr RD, Nin N, Sporn PH, Sznajder JI. Hypercapnia: a nonpermissive environment for the lung. Am J Respir Cell Mol Biol. 2012;46(4):417–21.
Richard JC, Marque S, Gros A, Muller M, Prat G, Beduneau G, et al. Feasibility and safety of ultra-low tidal volume ventilation without extracorporeal circulation in moderately severe and severe ARDS patients. Intensive Care Med. 2019;45(11):1590–8.
Feihl F, Eckert P, Brimioulle S, Jacobs O, Schaller MD, Melot C, et al. Permissive hypercapnia impairs pulmonary gas exchange in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;162(1):209–15.
Bein T, Weber F, Philipp A, Prasser C, Pfeifer M, Schmid FX, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med. 2006;34(5):1372–7.
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111(4):826–35.
Fitzgerald M, Millar J, Blackwood B, Davies A, Brett SJ, McAuley DF, et al. Extracorporeal carbon dioxide removal for patients with acute respiratory failure secondary to the acute respiratory distress syndrome: a systematic review. Crit Care. 2014;18(3):222.
Combes A, Pesenti A, Ranieri VM. Fifty years of research in ARDS. Is extracorporeal circulation the future of acute respiratory distress syndrome management? Am J Respir Crit Care Med. 2017;195(9):1161–70.
Schmidt M, Hodgson C, Combes A. Extracorporeal gas exchange for acute respiratory failure in adult patients: a systematic review. Crit Care. 2015;19:99.
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–22.
Pisani L, Fasano L, Corcione N, Comellini V, Guerrieri A, Ranieri MV, et al. Effects of extracorporeal CO2 removal on inspiratory effort and respiratory pattern in patients who fail weaning from mechanical ventilation. Am J Respir Crit Care Med. 2015;192(11):1392–4.
Kluge S, Braune SA, Engel M, Nierhaus A, Frings D, Ebelt H, et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med. 2012;38(10):1632–9.
Del Sorbo L, Pisani L, Filippini C, Fanelli V, Fasano L, Terragni P, et al. Extracorporeal Co2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med. 2015;43(1):120–7.
Braune S, Burchardi H, Engel M, Nierhaus A, Ebelt H, Metschke M, et al. The use of extracorporeal carbon dioxide removal to avoid intubation in patients failing non-invasive ventilation—a cost analysis. BMC Anesthesiol. 2015;15:160.
Del Sorbo L, Fan E, Nava S, Ranieri VM. ECCO2R in COPD exacerbation only for the right patients and with the right strategy. Intensive Care Med. 2016;42(11):1830–1.
Morelli A, D’Egidio A, Orecchioni A, Alessandri F, Mascia L, Ranieri VM. Extracorporeal co2 removal in hypercapnic patients who fail noninvasive ventilation and refuse endotracheal intubation: a case series. Intensive Care Med Exp. 2015;3(1):A824.
Combes A, Auzinger G, Capellier G, du Cheyron D, Clement I, Consales G, et al. ECCO2R therapy in the ICU: consensus of a European round table meeting. Crit Care. 2020;24(1):490.
Elliot SC, Paramasivam K, Oram J, Bodenham AR, Howell SJ, Mallick A. Pumpless extracorporeal carbon dioxide removal for life-threatening asthma. Crit Care Med. 2007;35(3):945–8.
Abrams DC, Brenner K, Burkart KM, Agerstrand CL, Thomashow BM, Bacchetta M, et al. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(4):307–14.
Roncon-Albuquerque R Jr, Carona G, Neves A, Miranda F, Castelo-Branco S, Oliveira T, et al. Venovenous extracorporeal CO2 removal for early extubation in COPD exacerbations requiring invasive mechanical ventilation. Intensive Care Med. 2014;40(12):1969–70.
Hermann A, Staudinger T, Bojic A, Riss K, Wohlfarth P, Robak O, et al. First experience with a new miniaturized pump-driven venovenous extracorporeal CO2 removal system (iLA Activve): a retrospective data analysis. ASAIO J. 2014;60(3):342–7.
Combes A, Fanelli V, Pham T, Ranieri VM, European Society of Intensive Care Medicine Trials G, the "Strategy of Ultra-Protective lung ventilation with Extracorporeal CORfN-OmtsAi. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. 2019;45(5):592–600.
Goligher EC, Combes A, Brodie D, Ferguson ND, Pesenti AM, Ranieri VM, et al. Determinants of the effect of extracorporeal carbon dioxide removal in the SUPERNOVA trial: implications for trial design. Intensive Care Med. 2019;45(9):1219–30.
Moerer O, Harnisch LO, Barwing J, Heise D, Heuer JF, Quintel M. Minimal-flow ECCO2R in patients needing CRRT does not facilitate lung-protective ventilation. J Artif Organs. 2018;22:68–76.
Nentwich J, Wichmann D, Kluge S, Lindau S, Mutlak H, John S. Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: a pilot study. Ann Intensive Care. 2019;9(1):3.
Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185(7):763–8.
Schellongowski P, Riss K, Staudinger T, Ullrich R, Krenn CG, Sitzwohl C, et al. Extracorporeal CO2 removal as bridge to lung transplantation in life-threatening hypercapnia. Transpl Int. 2015;28(3):297–304.
Collaud S, Benden C, Ganter C, Hillinger S, Opitz I, Schneiter D, et al. Extracorporeal life support as bridge to lung retransplantation: a multicenter pooled data analysis. Ann Thorac Surg. 2016;102(5):1680–6.
Redwan B, Ziegeler S, Semik M, Fichter J, Dickgreber N, Vieth V, et al. Single-site cannulation venovenous extracorporeal CO2 removal as bridge to lung volume reduction surgery in end-stage lung emphysema. ASAIO J. 2016;62(6):743–6.
Diehl JL, Mercat A, Pesenti A. Understanding hypoxemia on ECCO2R: back to the alveolar gas equation. Intensive Care Med. 2019;45(2):255–6.
Braune S, Sieweke A, Brettner F, Staudinger T, Joannidis M, Verbrugge S, et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med. 2016;42(9):1437–44.
Bein T, Weber-Carstens S, Goldmann A, Muller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy ( approximately 3 ml/kg) combined with extracorporeal CO2 removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39(5):847–56.
Moss CE, Galtrey EJ, Camporota L, Meadows C, Gillon S, Ioannou N, et al. A retrospective observational case series of low-flow venovenous extracorporeal carbon dioxide removal use in patients with respiratory failure. ASAIO J. 2016;62(4):458–62.
Fanelli V, Ranieri MV, Mancebo J, Moerer O, Quintel M, Morley S, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress syndrome. Crit Care. 2016;20:36.
Beloncle F, Brochard L. Extracorporeal Co2 removal for chronic obstructive pulmonary disease: too risky or ready for a trial? Crit Care Med. 2015;43(1):245–6.
Cardenas VJ Jr, Miller L, Lynch JE, Anderson MJ, Zwischenberger JB. Percutaneous venovenous CO2 removal with regional anticoagulation in an ovine model. ASAIO J. 2006;52(4):467–70.
Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev. 2015;29(2):90–101.
Giraud R, Banfi C, Assouline B, De Charriere A, Bendjelid K. Very low blood flow carbon dioxide removal system is not effective in a chronic obstructive pulmonary disease exacerbation setting. Artif Organs. 2020. https://doi.org/10.1111/aor.13867.
Combes A, Tonetti T, Fanelli V, Pham T, Pesenti A, Mancebo J, et al. Efficacy and safety of lower versus higher CO2 extraction devices to allow ultraprotective ventilation: secondary analysis of the SUPERNOVA study. Thorax. 2019;74(12):1179–81.
Cove ME, Vu LH, Ring T, May AG, Federspiel WJ, Kellum JA. A proof of concept study, demonstrating extracorporeal carbon dioxide removal using hemodialysis with a low bicarbonate dialysate. ASAIO J. 2018;65:605–13.
Cove ME, MacLaren G, Federspiel WJ, Kellum JA. Bench to bedside review: Extracorporeal carbon dioxide removal, past present and future. Crit Care. 2012;16(5):232.
Taccone FS, Malfertheiner MV, Ferrari F, Di Nardo M, Swol J, Broman LM, et al. Extracorporeal CO2 removal in critically ill patients: a systematic review. Minerva Anestesiol. 2017;83(7):762–72.
Zanella A, Castagna L, Salerno D, Scaravilli V, Abd El Aziz El Sayed Deab S, Magni F, et al. Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med. 2015;192(6):719–26.
Zanella A, Castagna L, Abd El Aziz El Sayed Deab S, Scaravilli V, Ferlicca D, Magni F, et al. Extracorporeal CO2 removal by respiratory electrodialysis: an in vitro study. ASAIO J. 2016;62(2):143–9.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and design: RG; administrative support: CB; provision of study materials or patients: all authors; collection and assembly of data: RG and CB; data analysis and interpretation: all authors; manuscript writing: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Pathophysiology of respiratory acidosis and Pathophysiology of COPD and Figure S1: Pathophysiology of COPD exacerbation.
Additional file 2.
Ongoing research on ECCO2R Table S1: Ongoing clinical studies on the use of ECCO2R in COPD and Table S2: Ongoing clinical studies on the use of ECCO2R in ARDS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giraud, R., Banfi, C., Assouline, B. et al. The use of extracorporeal CO2 removal in acute respiratory failure. Ann. Intensive Care 11, 43 (2021). https://doi.org/10.1186/s13613-021-00824-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-021-00824-6