Abstract
Background
Low levels of ascorbic acid (AA) have been detected in critically ill patients in which AA supplementation leads to promising outcomes. However, the ability of AA to reduce mortality in critically ill patients remains controversial. In this study, we have performed a meta-analysis to evaluate the effects of AA dose on the mortality of critically ill adults.
Methods
Electronic databases were searched for trials in which AA had been intravenously administered to critically ill patients regardless of the dose or the co-administration of antioxidant agents. The predefined primary outcome included all-cause mortality at final follow-up.
Results
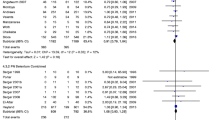
The included trials enrolled a total of 1210 patients. Intravenous (IV) AA doses of 3–10 g/d reduced the mortality of critically ill patients (OR 0.25; 95% CI (0.14–0.46); p < 0.001; I2 = 0.0%), while low (< 3 g/d) and high AA doses (≥ 10 g/d) had no effect (OR 1.44; 95% CI (0.79–2.61); p = 0.234; I2 = 0.0% versus OR 1.12; 95% CI (0.62–2.03); p = 0.700; I2 = 0.0%). AA was associated with a decreased duration of vasopressor support and mechanical ventilation, but did not influence fluid requirement or urine output during the first 24 h of admission. The number of patients suffering from acute kidney injury and the length of intensive care unit or hospital stays were also unaffected by the AA.
Conclusion
Intravenous AA reduces the duration of vasopressor support and mechanical ventilation; 3–10 g AA results in lower overall mortality rates. Given the limitations of the primary literature, further studies are required to fully clarify the effectiveness of AA during the management of critically ill patients.
Similar content being viewed by others
Background
Ascorbic acid (AA) is a water-soluble vitamin and an essential endogenous trace element that scavenges reactive oxygen species (ROS) [1, 2] and reduces immunosuppression [3]. Previous studies have shown that patients with critical illness, particularly sepsis, have low levels of AA in the plasma [4,5,6,7] which holds prognostic value due to its inverse correlation with multiple organ failure [7]. Given the low levels of AA in critically ill patients, supplemental AA has been administered to animal models of sepsis [8,9,10,11,12,13] and intensive care unit (ICU) patients [4, 14,15,16,17,18,19,20,21,22,23,24, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Results from these studies suggest that AA improves the condition of critically ill patients. Its beneficial effects include the attenuation lipid peroxidation, reduced vascular permeability, low levels of microvascular dysfunction, the preservation of endothelial function and microcirculatory flow, improved endogenous vasopressor synthesis, increased vasopressor sensitivity, and hemodynamic stability. This ultimately leads to reduced organ injury and dysfunction in critically ill patients.
Despite promising preliminary results, the benefits of AA remain controversial. Marik and coworkers [20] stated that AA as part of a “cocktail” therapy can reduce mortality in critically ill patients (8.5% vs. 40.4%, p < 0.01), while Lin et al. [19] reported no significant effects of AA infusion (26% vs. 23%, p = 0.8). Notably, the dose of AA varied between these studies, which may account for the discrepancies. Furthermore, the small sample size and single-center nature of the studies questions their reproducibility.
In this review, we provide a comprehensive meta-analysis (MA) of all studies in which AA has been intravenously administered to critically ill patients. We aimed to identify whether the dose of AA impacts mortality and other clinical parameters in this setting, including resuscitation fluid requirement, urine output, acute kidney injury (AKI), vasopressor requirement, the duration of mechanical ventilation, and the length of ICU and/or hospital stay.
Materials and methods
This study was performed and prepared according to the guidelines proposed by Cochrane Collaboration in the Cochrane Handbook for Systematic Reviews of Interventions (http://www.cochrane handbook.org) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PROSMA) statement [25, 26].
Search strategy
We searched articles of all languages published from inception to November 2018 in PubMed, Embase, Ovid and the Cochrane Central Register of Controlled Trials using the following keywords along with MeSH terms: “ascorbic acid” and “sepsis” or “critical illness” or “Intensive Care Unit” or “burn”. We collected all studies in which AA was intravenously administered to adult patients with critical illness.
Study selection criteria
The following trails were included
-
1.
Performed on adults with critical illness.
-
2.
Intravenous AA regardless of the dose vs. placebo or no-intervention.
-
3.
Primary outcome was mortality at the final follow-up.
The following trails were excluded
-
1.
Performed on children.
-
2.
AA administrated orally or enterally.
-
3.
Lack of mortality data.
Data extraction
Data were independently extracted by the first and third authors. Extracted data consisted of the first author name, year of publication, type of the study, study population, number of patients, AA dose, antioxidant agent, treatment initiation, treatment duration, mortality at follow-up, and other clinical parameters. We resolved disagreements through discussions until a consensus was reached.
Outcome measurements and definitions
The primary outcome was all-cause mortality at final follow-up. Secondary outcomes included resuscitation fluid requirement, urine output, patients suffering from AKI, vasopressor requirement, duration of mechanical ventilation, and length of ICU and/or hospital stay.
Assessment of risk of bias
We used the Cochrane Collaboration tool to assess the risk of bias in randomized controlled trials (RCTs) [26, 27]. Domains containing random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of the outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias were assessed. The remaining observational trials were assessed using the ROBINS-I tool [28]. Domains include bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviation from intend intervention, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported results. We rated each domain of the trials as low risk, unclear, or high risk. Trials were considered low risk when each independent domain was rated as low risk. Any domain rated as unclear or high risk increased the overall risk score.
Statistical analysis
Data were analyzed using Statistics/Data Analysis 15.1. The results of dichotomous data were presented as forest plots through the odds ratios (ORs) with 95% confidence intervals (CIs). Forest plots using SMD with 95% CI were performed for the assessment of continuous data. We quantified heterogeneity via the I2 statistic. Data were pooled through random (M-H heterogeneity) models if the value of I2 was greater than 50%, regarded as heterogeneity [29]. A p value ≤ 0.05 was considered statistically significant, except when otherwise specified.
Results
Literature search
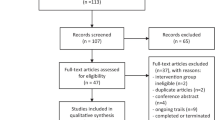
Through database searches (Fig. 1), 2296 records were identified. We screened both titles and abstracts according to the inclusion and exclusion criteria, leaving 63 studies that were deemed suitable for inclusion. After reviewing the full texts, 12 studies [14,15,16,17,18,19,20,21,22,23,24, 36] were finally included (Table 1). Studies were excluded for the following reasons: duplication (n = 32), performed on children (n = 1) [30], enteral administration of AA (n = 11) [31,32,33,34,35, 37,38,39,40,41,42], lack of mortality data (n = 5) [4, 43,44,45,46], both groups administrated AA (n = 2) [47, 48].
Study characteristics
Of the 12 included trials, eight were RCTs and four were retrospective studies. All studies were published from 1997 to 2018. The characteristics of each trial are shown in Table 1. The studies recruited patients with severe sepsis or septic shock [15, 16, 20, 24], burn shock [17,18,19], critical injury [21, 22], post-operation [14, 16, 36], trauma [36] and those in need of contrast-enhanced CT in the ICU [23]. The sample sizes ranged from 20 to 595. The included studies enrolled a total of 1210 patients of which 624 were administrated IV AA, and 586 were control subjects. The dose of AA ranged from 450 mg/d to 66 mg/kg/h.
Risk of bias and quality of evidence
The risk of bias is summarized in Fig. 2. No trials were considered low risk, and no specific details were used for assessment of the blinding outcomes. The trial performed by Tanaka and colleagues [18] despite being classed as a randomized study was deemed a high risk of bias as participants were allocated to groups according to months. Studies by Ramzkon et al. [21] and Galley et al. [24] lacked specific allocations and were classed as an unclear risk. In total, 11 trials were defined as an unclear risk and a single trial was deemed high risk.
Meta-analysis results
Intravenous AA administration and ICU/hospital mortality
The MA included 12 trials comprising 1210 participants, of whom 624 received AA and 586 received placebo treatment. The analysis indicated that IV AA did not reduce the mortality of critically ill patients (OR 0.71; 95% CI (0.41–1.23); p = 0.219; I2 = 53.1%, Additional file 1: Fig. S1). A random (M-H heterogeneity) model was applied due to an I2 = 53.1%. Subgroup analysis suggested that the administration of AA alone or in combination with other antioxidant agents did not lower the mortality rates (OR 0.64; 95% CI (0.30–1.37); p = 0.253; I2 = 58.9% vs. OR 0.84; 95% CI (0.37–1.91); p = 0.678; I2 = 48.5%, Additional file 1: Fig. S1). As the dose of AA varied between the trials, subgroup analysis was performed. Doses < 3 g/d were defined as low, ≥ 10 g/d as high, and 3–10 g/d as medium. The medium AA dose was found to reduce the mortality of critically ill patients (OR 0.25; 95% CI (0.14–0.46); p < 0.001; I2 = 0.0%, Fig. 3), while neither low-dose AA (< 3 g/d) nor high-dose AA (≥ 10 g/d) influenced the mortality (OR 1.44; 95% CI (0.79–2.61); p = 0.234; I2 = 0.0% vs. OR 1.12; 95% CI (0.62–2.03); p = 0.700; I2 = 0.0%, Fig. 3). Due to homogeneous, a fixed Mantel–Haenszel model was applied. Subgroup analysis was adopted according to patient characteristics. The results revealed that in all patient conditions (including sepsis, burns, and others), AA did not decrease the mortality rates (OR 0.30; 95% CI (0.08–1.08); p = 0.066; I2 = 63.9% vs. OR 1.26; 95% CI (0.61–2.59); p = 0.538; I2 = 0.0% vs. OR 0.88; 95% CI (0.46–1.69); p = 0.706; I2 = 35.6%, Fig. 4). Sensitivity analysis was performed through the removal of each single trial and the reanalysis of the remaining trials according to sepsis subgroups. Upon excluding the Galley et al. study [24], the analysis was homogeneous and AA decreased the mortality of patients with sepsis (OR 0.16; 95% CI (0.07–0.37); p < 0.001; I2 = 0.0%, Additional file 2: Fig. S2).
Length of ICU and hospital stay
Three trials [16, 22, 23] compared the length of ICU stays between AA and control groups. AA was found to have no influence on the length of ICU residence (SMD = 0.34; 95% CI (− 0.50–1.19); p = 0.424; I2 = 87.7%, Fig. 5). As the I2 value = 87.7%, a random (M-H heterogeneity) model was applied. Sensitivity analysis was performed through the removal of each individual trial and through reanalysis of the remaining trials. When excluding the trial performed by Palli and colleagues [23], the analysis became homogeneous and the application of AA did not reduce the length of the ICU stay (SMD = − 0.08; 95% CI (− 0.49–0.32); p = 0.680; I2 = 0.0%).
Two trials [18, 22] were included to assess the impact of the length of hospital stay on which AA had no influence (SMD = − 0.35; 95% CI (− 0.73–0.04); p = 0.080; I2 = 0.0%, Fig. 6). As the data from these trials were homogeneous, a fixed Mantel–Haenszel model was employed.
Fluid requirement, urine output and patients suffering from AKI
Our analysis among trials [16, 17, 19] suggested that AA intervention did not reduce the fluid requirement during the first 24 h of admission (SMD = − 0.52; 95% CI (− 1.63–0.58); p = 0.351; I2 = 88.2%, Fig. 5). A random (M-H heterogeneity) model was applied due to the I2 value = 88.2%. Sensitivity analysis was performed through the removal of each individual trial and reanalysis of the remaining trials. The results remained unaffected except for the removal of the Kahn et al. study [17] from which the analysis became homogeneous (SMD = 0.09; 95% CI (− 0.29–0.47); p = 0.650; I2 = 0.0%).
Upon AA administration, no increase in urine output was observed during the first 24 h of admission (SMD = 0.50; 95% CI (− 0.11–1.12); p = 0.110; I2 = 55.9%, Fig. 5). For heterogeneity analysis, a random (M-H heterogeneity) model was applied. For sensitivity analysis, removal of the Zabet et al. [16] study led to an increased urine output in response to AA (SMD = 0.72; 95% CI (0.23–1.21); p = 0.004; I2 = 47.1, Additional file 3: Fig. S3).
No differences were observed in the number of patients suffering from AKI (OR 1.40; 95% CI (0.74–2.63); p = 0.298; I2 = 38.5%, Additional file 4: Fig. S4). We applied a fixed Mantel–Haenszel model for this analysis [19, 20, 36].
Duration of vasopressor requirement
The analysis consisted of 4 trials [15,16,17, 20] over which the duration of vasopressor requirement declined (SMD = − 1.04; 95% CI (− 1.69 to − 0.38); p = 0.002; I2 = 72.1%, Fig. 5). Upon consideration of the I2 value, we employed a random (M-H heterogeneity) model for the analysis. Upon sensitivity analysis, no significant changes were observed.
Duration of mechanical ventilation
From the analysis among trials [14, 16, 18], a lower duration of mechanical ventilation (SMD = − 0.59; 95% CI (− 1.02 to − 0.16); p = 0.008; I2 = 0.0%, Fig. 6) was proposed. A fixed Mantel–Haenszel model was employed for this analysis.
Publication bias
A Begg test was performed to assess the publication bias of the 11 included studies (p = 0.640). The analysis suggested that minimal publication bias occurred.
Discussion
Main Findings
IV AA administration and ICU/hospital mortality
Compared to other MAs [49, 50, 52], the major finding of this study was that IV medium doses (3–10 g/d) of AA were associated with decreased mortality, with neither low doses (< 3 g/d) nor high doses (≥ 10 g/d) having a significant impact. The MA conducted by Langlois and coworkers [52] included enteral and IV supplementation and suggested no association of AA with reduced mortality; subgroup analysis revealed that oral-enteral or parenteral, low or high administration of doses of AA (1.5 g/d as a boundary) did not significantly influence mortality.
Both dosing and bio-distribution data in humans suggest that pharmacological concentrations of AA are only attainable through IV administration due to the saturation of intestinal transporters (sodium-vitamin C transporter-1) [51]. Previous studies have shown that critically ill patients often have low AA plasma levels [4,5,6,7]. Furthermore, during the post-injury period, 2 days of 3000 mg/day AA significantly increase plasma AA concentrations [4]. Other studies [48] have shown that 10 g/d AA is associated with supranormal plasma concentrations. Considerable adverse effects have not been reported at high AA doses (66 mg/kg/h) [17,18,19]. In this study, we defined a dose lower than 3 g/d (not inclusive of 3 g/d) as low, higher than 10 g/d (inclusive of 10 g/d) as high, and 3–10 g/d as medium. Our analysis revealed that low-dose AA had little effect on mortality partly due to patients receiving IV AA ≤ 2 g/d (Table 1). Studies have shown that IV AA 2 g/d leads to only normal plasma concentrations [48]. In addition, AA is depleted by free iron, free radical scavengers in the plasma, and the destruction of oxidized AA and dehydroascorbic acid [43]. Thus, low AA doses do not influence mortality. For medium doses, improved patient prognosis was observed. Low levels of plasma AA in septic patients inversely correlated with the incidence of multiple organ failure [7], while medium doses restored the AA concentrations to normal plasma levels [48]. Additionally, studies performed by Straaten and coworkers [68] suggested that high-dose AA (3–6 g/d) decreases the formation of superoxide and peroxynitrite, bidirectly scavenges superoxides, augments antibacterial defenses, and protects against oxidative stress in critically ill patients. AA can counteract lipid peroxidative damage through the scavenging of oxygen-derived free radicals and the restoration of vascular function [53]. Counteracting oxidative stress represents a likely mechanism by which moderate AA reduces mortality. For high doses of AA, no loss of mortality was observed. Among the three trials [15,16,17,18,19, 21] participants suffering from burn injuries were recruited and the majority of deaths did not occur during resuscitation, but from subsequent infections [54]. In addition, patients in group B of the study by Razmkon and coworkers [21] received 10 g on the first day of admission which was repeated on day 4, followed by 4 g/d for the remaining 3 days. The final follow-up of mortality in these trials differed, partly accounting for the outcomes.
Through subgroup analysis of the patient characteristics, AA had no effect on sepsis or burns. For sepsis, heterogeneity was observed, mainly due to the Galley et al. [24] study. The Galley trial was performed in 1997, and the study protocols drastically differed from those of the more recent studies. When the Galley et al. trial was excluded, AA had a positive effect on mortality. For burn patients, AA had little influence. Further studies are warranted to dissect this relationship given that burn deaths mainly occur after resuscitation [54].
Fluid requirement, urine output, and patients suffering from AKI
The results demonstrate that AA has little effect on fluid requirements or urine output during the initial 24 h, or the number of patients suffering from AKI. Upon consideration of the trial reported by Zabet et al. [16], although a significant increase in urine output during the first 24 h did not occur, a tendency toward increasing urine output over time was noted, which may have influenced the overall significance of the analysis. AA is hyperosmolar and is a risk factor for osmotic diuresis [17]. Despite the trial of Tanaka et al. [18], AA did not cause osmotic diuresis and no differences in the patients’ urine and serum osmolality were observed. In the trial of Kahn and colleagues [17], several patients showed signs of hypovolemia in the absence of decreased urine output, which was noted as a possible sign of osmotic diuresis. Other trials lacked data on urine output and osmotic diuresis and future studies should consider these parameters.
Previous studies suggested that following a severe burn injury, potent oxygen-free radicals (OFRs) are produced from the ischemia and reperfusion of burnt skin [60]. Animal studies reveal that antioxidant therapy through the administration of high-dose AA, an ORF scavenger [61], reduces post-burn lipid peroxidation [9], decreases vascular permeability [57], decreases burn and no-burn tissue edema [58], and reduces the requirement for resuscitation fluid [10, 18, 59]. However, this was not observed in this study. Possible reasons for these discrepancies include the varied initiation of treatment between the studies. In the study by Kahn et al. [17], treatment was initiated at 52 ± 26 min post-admission, while in study by Lin et al. [19] treatment was initiated at 4.01 ± 15 h. Zabet and coworkers [16] did not provide a specific treatment time. All the included studies were single-center, and the fluid levels received by the patients were variable. In the study by Lin and colleagues [19], although a reduction in fluid requirement was noted, both groups had higher weights and had a higher % of total body surface area (TBSA) of burn injuries than would be estimated based on the Parkland formula. The trials lacked data on fluid requirement for longer periods and future studies should consider these parameters.
It has been reported that AA promotes acute renal failure [62, 63]. In patients with renal impairment who received high AA doses, increased levels of oxalate (typically excreted by the kidney) were observed in conjunction with crystallization, leading to impaired kidney function [64, 65]. In this study, effects on patients suffering from AKI were not observed partly due to the AA dose. In studies by Marik and Nathens [20, 36], AA was administrated at doses of 6 g/d and 3 g/d, respectively, which was considered medium doses, while Lin and colleagues [19] assessed the effects of 66 mg/kg/h AA. These trials included lacked data on time span during which AKI was measured. This may account for the overall outcome observed.
Duration of vasopressor requirement
From our studies, AA significantly reduced the duration of vasopressor requirement. AA is an essential cofactor for the copper-containing enzyme dopamine β-hydroxylase during catecholamine (dopamine, norepinephrine, and epinephrine) synthesis [55]. It was found that IV AA improved cardiovascular function and decreased the requirement for catecholamine in a patient with septic shock [56]. Daniel and colleagues [69] stated that AA was a cofactor during collagen synthesis that was required to support cardiovascular functions. Additionally, Carr et al. [13] found that AA increases vasopressor sensitivity.
Duration of mechanical ventilation
A decreased duration of mechanical ventilation upon AA administration was observed, but whether this reflected improved pulmonary function was uncertain. Tanaka et al. [18] commented that fewer days of mechanical ventilation and improved early respiratory function were associated with fluid reduction. The opposite scenario of fluid requirement was observed in this study. Animal models of sepsis suggest that IV AA attenuates proinflammatory and procoagulant states, reducing lung vascular injury [11] and oxidative stress, induced histopathological alterations, thus improving pulmonary function [66]. Only the Tanaka et al. [18] study provided pulmonary function measurements. Future studies should consider these parameters.
Comparison with other studies
The major strength of this MA was the investigation of how different AA doses contribute to the clinical outcomes of patients with critical illness. We compared the effects of AA alone or in combination with other agents for its effects on mortality and compared the effects of AA on different patient characteristics (sepsis, burn, or others). Such studies have not been previously performed. Furthermore, a varied mix of medical, surgical, and burn injury patients was included, and each trial equally contributed to the final study outcomes.
Limitations
This MA had several weaknesses that should be noted. Firstly, 12 trials were included of which 8 were RCTs and 4 were retrospective trials. The study sizes were relatively small, and all trials were single-center. The included participants varied in terms of medical, surgical, and burn status characteristics, which may have led to study heterogeneity. Secondly, the initiation of treatment, the duration of therapy, and follow-up varied between the trials, which may have influenced the outcomes. The adequate dosing of antioxidants, administration routes, timings, the initiation of treatment, the duration of therapy, and the role of single versus combination therapy still requires clarification in future studies [67].
Conclusion
Based on the current available evidence, the IV administration of AA can narrow the duration of vasopressor requirement and mechanical ventilation, but plays little role in fluid requirement or urine output during the first 24 h of admission, or the number of patients suffering from AKI, as well as the length of ICU or hospital stay. Furthermore, medium dose (3–10 g/d) AA has a positive role in mortality, which is not achieved by low (< 3 g/d) or high doses (≥ 10 g/d). However, given the limitations of the study combined with the heterogeneity, further studies are required to clarify the role of AA during the management of critically ill patients.
Availability of data and materials
Not applicable.
Abbreviations
- AA:
-
ascorbic acid
- MA:
-
meta-analysis
- IV:
-
intravenous
- AKI:
-
acute kidney injury
- ICU:
-
intensive care unit
- ROS:
-
reactive oxygen species
- RCTs:
-
randomized controlled trials
- Ors:
-
odds ratios
- CIs:
-
confidence intervals
- OFRs:
-
oxygen-free radical
- TBSA:
-
total body surface area of burn injury
References
Goode HF, Webster NR. Free radicals and antioxidants in sepsis. Crit Care Med. 1993;21(11):1770.
Wu F, Schuster DP, Tyml K, et al. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic Biol Med. 2007;42(1):124–31.
Yu-Lei G, Bin L, Jian-Hua Z, et al. The parenteral vitamin c improves sepsis and sepsis-induced multiple organ dysfunction syndrome via preventing cellular immunosuppression. Mediators Inflamm. 2017;2017:1–12.
Long CL, Maull KI, Krishnan RS, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res. 2003;109(2):144–8.
Schorah CJ, Downing C, Piripitsi A, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr. 1996;63(5):760–5.
Hunt C, Chakravorty NK, Annan G, et al. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vitam Nutr Res. 1994;64(3):212–9.
Borrelli E, Rouxlombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med. 1996;24(3):392–7.
John A, Karel T, Darcy L, et al. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat [J]. J Appl Physiol. 2001;90(3):795–803.
Matsuda T, Tanaka H, Yuasa H, et al. The effects of high-dose vitamin C therapy on post burn lipid peroxidation. J Burn Care Rehabil. 1993;14(6):624–9.
Matsuda T, Tanaka H, Reyes HM, et al. Antioxidant therapy using high dose vitamin C: reduction of post burn resuscitation fluid volume requirements. World J Surg. 1995;19(2):287–91.
Fisher BJ, Seropian IM, Kraskauskas D, et al. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury*. Crit Care Med. 2011;39(6):1454–60.
May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium [J]. Antioxid Redox Signal. 2013;19(17):2068–83.
Carr AC, Shaw GM, Fowler AA, et al. Ascorbate-dependent vasopressor synthesis: a rationale for Vitamin C administration in severe sepsis and septic shock? Crit Care. 2015;19(1):418.
Ferrón-Celma I, Mansilla A, Hassan L, et al. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J Surg Res. 2009;153(2):224–30.
Fowler AA, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12(1):32.
Zabet MH, Mohammadi M, Ramezani M, et al. Effect of high-dose ascorbic acid on vasopressor’s requirement in septic shock. J Res Pharm Pract. 2016;5(2):94–100.
Kahn SA, Beers RJ, Lentz CW. Resuscitation after severe burn injury using high-dose ascorbic acid: a retrospective review. J Burn Care Res. 2011;32(1):110–7.
Tanaka H, Matsuda T, Miyagantani Y, et al. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135(3):326–31.
Lin J, Falwell S, Greenhalgh D, et al. High-dose ascorbic acid for burn shock resuscitation may not improve outcomes. J Burn Care Res. 2017;39(5):708–12.
Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2016;151(6):1229–38.
Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133–7.
Sandesc M, Rogobete AF, Bedreag OH, et al. Analysis of oxidative stress-related markers in critically ill polytrauma patients: An observational prospective single-center study. Bosn J Basic Med Sci. 2018;10:15–20. https://doi.org/10.17305/bjbms.2018.2306.
Palli E, Makris D, Papanikolaou J, et al. The impact of N-acetylcysteine and ascorbic acid in contrast-induced nephropathy in critical care patients: an open-label randomized controlled study[J]. Crit Care. 2017;21(1):269.
Galley HF, Howdle PD, Walker BE, et al. The effects of intravenous antioxidants in patients with septic shock. Free Radic Biol Med. 1997;23(5):768–74.
PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses (PRISMA).http://prisma-statement.org. Accessed 8 Sept 2018.
Higgins J, Green S (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0. March 2011.https://handbook-5-1.cochrane.org. Accessed 8 Sept 2018.
Higgins JPT, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomized trials. Br Med J. 2011;343:d5928.
Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016;355:i4919.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Barbosa E, Faintuch J, Moreira EAM, et al. Supplementation of vitamin E, vitamin C, and zinc attenuates oxidative stress in burned children: a randomized, double-blind, placebo-controlled pilot study. J Burn Care Res Off Publ Am Burn Assoc. 2009;30(5):859.
Carr AC, Rosengrave PC, Bayer S, et al. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21(1):300.
Nogueira CR, Borges F, Lameu E, et al. Effects of supplementation of antioxidant vitamins and lipid peroxidation in critically ill patients. Nutr Hosp. 2013;28(28):1666–72.
Preiser JC, Van Gossum A, Berré J, et al. Enteral feeding with a solution enriched with antioxidant vitamins A, C, and E enhances the resistance to oxidative stress. Crit Care Med. 2000;28(12):3828–32.
Heyland D, Heyland D. Early enteral supplementation with key pharmaconutrients improves sequential organ failure assessment score in critically ill patients with sepsis: outcome of a randomized, controlled, double-blind trial. Crit Care Med. 2008;36(1):131–44.
Howe KP, Clochesy JM, Goldstein LS, et al. Mechanical ventilation antioxidant trial. Am J Crit Care. 2015;10:15–20. https://doi.org/10.4037/ajcc2015335.
Surgery AO. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236(6):814–22.
Giladi AM, Dossett LA, Fleming SB, et al. High-dose antioxidant administration is associated with a reduction in post-injury complications in critically ill trauma patients. Injury. 2011;42(1):78–82.
Theilla M, Singer P, Cohen J, et al. A diet enriched in eicosapentanoic acid, gamma-linolenic acid and antioxidants in the prevention of new pressure ulcer formation in critically ill patients with acute lung injury: a randomized, prospective, controlled study. Clin Nutr. 2007;26(6):752–7.
Crimi E, Liguori A, Condorelli M, Cioffi M, Astuto M, Bontempo P, Pignalosa O, Vietri MT, Molinari AM, Sica V, Della Corte F. NapoliC: the beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Anesth Analg. 2004;99:857–63.
Mirhoseini MF, Hamblin SE, Moore WP, Pouliot J, Jenkins JM, Wang W, Chandrasekhar R, Collier BR, Patel MB. Antioxidant supplementation and atrial arrhythmias in critically ill trauma patients. J Surg Res. 2018;222:10–6.
Raposio E, Grieco MP, Caleffi E. Evaluation of plasma oxidative stress, with or without antioxidant supplementation, in superficial partial thickness burn patients: a pilot study. J Plast Surg Hand Surg. 2017;51(6):393–8.
Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, et al. Reducing deaths due to oxidative stress (the redoxs study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc. 2006;65(3):250–63.
Galley HF, Davies MJ, Webster NR. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic Biol Med. 1996;20(1):139–43.
Angdin M, Settergren G, Starkopf J, et al. Protective effect of antioxidants on pulmonary endothelial function after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2003;17(3):314–20.
Rümelin A, Jaehde U, Kerz T, et al. Early postoperative substitution procedure of the antioxidant ascorbic acid. J Nutr Biochem. 2005;16(2):104–8.
Bradley JA, King RF, Schorah CJ, et al. Vitamins in intravenous feeding: a study of water-soluble vitamins and folate in critically ill patients receiving intravenous nutrition. Br J Surg. 2010;65(7):492–4.
Pontesarruda A, Aragão AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crtit Care Med. 2006;34(9):2325–33.
De Grooth HJ, Manubulu-Choo W-P, Zandvliet AS, et al. Vitamin-C pharmacokinetics in critically ill patients: a randomized trial of four intravenous regimens. Chest. 2018;153(6):1368–77.
Langlois PL, Szwec C, D’Aragon F, et al. Vitamin D supplementation in the critically ill: a systematic review and meta-analysis. Clin Nutr. 2018;37(4):1238–46.
Li J. Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis. Critical Care (BioMed Central). 2018;22(1):258.
Padayatty SJ. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533.
Langlois PL, Manzanares W, et al. Vitamin C supplementation in the critically ill: a systematic review and meta-analysis. J Parenter Enter Nutr. 2018;10:15–20. https://doi.org/10.1177/2050312118807615.
Bielli A, Scioli MG, Mazzaglia D, et al. Antioxidants and vascular health. Life Sci. 2015;143:209–16.
Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13(6):R183.
May JM, Qu ZC, Nazarewicz R, et al. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res Bull. 2013;90(Complete):35–42.
Kieffer P, Thannberger P, Wilhelm JM, et al. Multiple organ dysfunction dramatically improving with the infusion of vitamin C: more support for the persistence of scurvy in our “welfare” society. Intensive Care Med. 2001;27(2):448.
Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560–6.
Tanaka H, Hanumadass M, Matsuda H, et al. Hemodynamic effects of delayed initiation of antioxidant therapy (beginning two hours after burn) in extensive third-degree burns. J Burn Care Rehabil. 1995;16(6):610.
Tanaka H, Broaderick P, Shimazaki S, et al. How long do we need to give antioxidant therapy during resuscitation when its administration is delayed for two hours? J Burn Care Rehabil. 1992;13(5):567.
Till GO, Guilds LS, Mahrougui M, et al. Role of xanthine oxidase in thermal injury of skin. Am J Pathol. 1989;135(1):195.
Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine–xanthine oxidase system. Biochem Biophys Res Commun. 1975;63(2):463–8.
Mashour S, Turner JMR. Acute renal failure, oxalosis, and vitamin C supplementation: a case report and review of the literature. Chest. 2000;118(2):561–3.
Rutkowski M, Grzegorczyk K. Adverse effects of antioxidative vitamins. Int J Occup Med Environ Health. 2012;25(2):105–21.
Massey LK. Ascorbate increases human oxaluria and kidney stone risk. J Nutr. 2005;135(7):1673–7.
Wandzilak TR, Dandre SD, Davis PA, et al. Effect of high dose vitamin C on urinary oxalate levels. J Urol. 1994;151(4):834–7.
Galvao AM, Wanderley MSO, Silva RA, et al. Intratracheal co-administration of antioxidants and ceftriaxone reduces pulmonary injury and mortality rate in an experimental model of sepsis [J]. Respirology. 2014;19(7):1080–7.
Reddell L, Cotton BA. Antioxidants and micronutrient supplementation in trauma patients [J]. Curr Opin Clin Nutr Metab Care. 2012;15(2):181–7.
Oudemans-van Straaten HM, Man SD, Waard MCD. Vitamin C revisited. Crit Care. 2014;18(4):1–13.
Daniel G. Increasing vitamin C Content in plant foods to improve their nutritional value—successes and challenges. Nutrients. 2013;5(9):3424–46.
Funding
The authors received no financial support for the study, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing of interest nor any financial interest in any product mentioned in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1.
Forest plot of the effect of IV AA on mortality at the final follow-up when compared by administration of AA alone or in combination with other antioxidant agents.
Additional file 2.
Forest plot of the effect of IV AA on mortality at the final follow-up in the subgroup of sepsis by removing the trial of Galley.
Additional file 3.
Forest plot of the effect of IV AA on the urine output in the first 24 h of admission when removing the trials of Zabet [16].
Additional file 4.
Forest plot of the effect of intravenous ascorbic acid administration on the number of patients suffered from AKI.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, Y., Lin, H., Lin, Bw. et al. Effects of different ascorbic acid doses on the mortality of critically ill patients: a meta-analysis. Ann. Intensive Care 9, 58 (2019). https://doi.org/10.1186/s13613-019-0532-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-019-0532-9