Abstract
Background
The aim was to investigate whether the use of N-acetylcysteine and ascorbic acid reduce contrast-induced nephropathy incidence in critical care patients.
Methods
This was a one-center, two-arm, prospective, randomized, open-label, controlled trial in the Intensive Care Unit of the University Hospital of Larissa, Greece. Patients with stable renal function, who underwent non urgent contrast-enhanced computed tomography for diagnostic purposes, were included in the study. Patients in the treatment group (NacA, n = 60) received intravenously N-acetylcysteine (1200 mg) and ascorbic acid (2 g) dissolved separately in 100 ml of normal saline 2 hours before, and at 10 hours and 18 hours following the infusion of contrast agent, while control group patients (CG, n = 64) received only normal saline. All patients received additional hydration. Contrast-induced nephropathy was defined as relative increase by 25% of the baseline values of serum creatinine.
Results
Contrast-induced nephropathy in NacA and CG were 18.33% and 15.6%, respectively (p = 0.81). The percentage change median (interquartile range (IR)) of serum cystatin-C (mg/L) from baseline in patients who underwent contrast-induced tomography, were 37.23% (28.53) and 93.20% (46.90) in NacA and in CG, respectively (p = 0.03). The 8-isoprostane serum levels in NacA were significantly lower compared to CG at 2 hours (p = 0.012) and 24 hours (p = 0.006) following radiocontrast infusion. Multivariate analysis revealed that contrast-induced nephropathy was independently associated with a higher baseline ratio of serum urea/creatinine (odds ratio, 1.02; 95 CI%, 1.00–1.05) and with the use of nephrotoxic medications (odds ratio, 0.24; 95 CI%, 0.06–0.94).
Conclusion
Intravenous administration of N-acetylcysteine and ascorbic acid failed to reduce contrast-induced nephropathy in critically ill patients who underwent contrast-enhanced computed tomography, despite a significant reduction of 8-isoprostane levels in treated patients.
Trial registration
ClinicalTrials.gov, NCT01017796. Registered on 20 November 2009.
Similar content being viewed by others
Background
Critically ill patients represent a group vulnerable to renal function deterioration due to the severity of the illness and because they are under several potential nephrotoxic hazards. Moreover, critically ill patients often have to undergo computed tomography (CT) or angiography for diagnostic/therapeutic purposes, with the use of contrast agents that may induce nephropathy - commonly known as contrast-induced nephropathy (CIN) [1]. CIN increases the need for renal replacement therapy (RRT), prolongs hospitalization and increases morbidity and mortality [2,3,4,5,6,7,8].
However, those data on the impact of radiocontrast material on renal function come mainly from patients with cardiac conditions who underwent coronary percutaneous procedures [2, 7,8,9,10,11,12]. Only a few studies have addressed this issue in the critical care setting. Hence, the incidence of CIN varies considerably from 1.4% to 50% depending on the characteristics of the studied population, the material and dose used and the criteria that have been used for renal impairment detection [13,14,15]. In a previous study we showed that in relation to their younger counterparts, older critically ill patients are more prone to developing renal dysfunction after the intravenous infusion of the contrast agent [16].
CIN has been reported to be associated with increased oxidative stress [17] and several clinical factors have been identified that increase its risk in patients with diabetes mellitus or patients with cardiac conditions [9, 10, 18]. Yet, data on the use of various protective measures in the critical care setting are limited [16, 19]. In this study we therefore aimed to investigate the impact of the combination of two antioxidant agents, N-acetylcysteine (Nac) and ascorbic acid (Aa), in critically ill patients who undergo contrast-enhanced CT. We hypothesized that the use of antioxidant agents could balance the increased oxidative burden that is associated with the use of the radiocontrast material [17].
Methods
Design and population
The present study is a one-center, two-arm, randomized, open-label, controlled trial. The study took place in the Intensive Care Unit (ICU) of the University Hospital of Larissa (12 beds) between 2010 and 2013. Inclusion criteria were age ≥14 years and diagnostic need for contrast-enhanced CT. Exclusion criteria were history of intravascular administration of contrast agent during the 6-day period prior to randomization, the use of antioxidant agents during the last week before the examination, unstable renal function, use of RRT in the 3-day period before randomization and pregnancy. We defined the unstable renal function as a change in serum creatinine values greater than 20% between 2 consecutive days during the 3 days prior to randomization, independently from crude baseline renal function.
Study protocol
Patients were randomized to intravenously receive Nac (1200 mg) and Aa (2 g) dissolved separately in 100 ml of normal saline (N/S) 0.9%, 2 hours before and at 10 hours and 18 hours following the infusion of contrast agent (treatment group - NacA) or 200 ml of intravenous N/S 0.9% (control group - CG) at the same time points as NacA. The choice of Nac and Aa and their doses were based on previous studies that aimed to manage oxidative damage [9], whereas Nac presents vasodilatory properties [20] that could overcome vasoconstriction caused by the contrast agent [21].
All participants received the same amount of additional hydration with 1000 ml N/S 0.9% given intravenously before CT as protection against the intravenous constant medium, unless this was contraindicated based on concurrent hemodynamic assessment; the latter was assessed either by ultrasonography or thermo-dilution [22]. Randomization was performed using tables of random numbers.
Serum urea, creatinine concentrations were assessed before the infusion of the contrast agent and once daily until the 5th day following radiocontrast infusion; serum cystatin-C assessed before and at 24 and 48 hours following radiocontrast infusion and 8-isoprostane was assessed before and at 2, 24 and 48 hours following radiocontrast infusion. Timing of renal function assessment was based on creatinine and cystatin-C expected peak serum levels following the infusion of radiocontrast agent; serum creatinine peaks at 3–5 days [23] while serum cystatin-C peaks at 1–2 days after the infusion of contrast agent [24].
CIN was defined as relative increase by 25% of serum creatinine from the baseline value within 5 days [24]. CT scans were performed according to the institute’s standard protocol with the use of the same agent, iopamidol, a low osmolarity, non ionic, iodinated contrast medium (Iopamiro 370, Bracco). The quantity of infused contrast agent was determined by the radiologists depending on the type of CT imaging, and patients related characteristics and was recorded in a dedicated chart. The dose of contrast medium for contrast-enhanced CT was generally 0.5–2 ml/Kg of body weight. The minimum used dose was 100 ml and 150 ml was the maximum dose that was given. Measurement of serum cystatin-C and 8-isoprostane were performed with commercial enzyme-linked immunoassay kits (Cayman CC, USA).
Outcomes
The main outcome was the incidence of CIN. In addition, we assessed serial changes in serum creatinine within 5 days, serum cystatin-C within 48 hours and 8-isoprostane serum levels within 48 hours. Secondary indices of outcome were the need for RRT for a 10-day period following the infusion of the contrast agent, ICU stay and mortality.
Assuming that the incidence of CIN is 50% [25] we estimated that a sample size of 58 patients per treatment group would be required to detect 50% relative reduction in the incidence of CIN in the NacA with 80% power and 95% confidence level.
Statistical analysis
All analysis was performed on an intention-to-treat basis. The results are expressed as means ± standard error (SE) unless otherwise stated. Data were compared between groups using Fisher’s exact test for categorical variables and the t test or Mann–Whitney test as appropriate for continuous variables. Differences in changes in serum creatinine, cystatin-C and 8-isoprostane concentrations (dependent variables) during time were analyzed by linear mixed model analysis. The kinetic of serum creatinine, cystatin-C and 8-isoprostane are indicated by mean regression lines. The slope of the regression line is the rate at which the examined parameter’s value changes day after day. The intercept of the regression line represents the value of the y (dependant variable) axis where the mean regression line crosses the y axis at theoretical day 0. Receiver operating characteristic (ROC) analysis was performed to illustrate the performance of clinical or laboratory variables in identifying patients with CIN. Only variables that were associated with CIN in univarate analysis were used in ROC analysis. P values <0.05 were considered to be statistically significant. Statistical analysis and preparation of graphs were performed using the statistical package SPSS 21.0 (SPSS Inc., Chicago, IL, USA), and GraphPad Prism (version 5.01).
Results
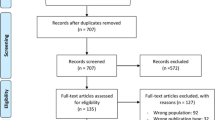
There were 124 patients who participated in the study, with 64 patients in the CG and 60 patients in the NacA (Fig. 1). Table 1 and Additional file 1: Tables S1 and S2 represent characteristics of participants at the entry of the study. There was a trend towards a larger percentage of patients with diabetes mellitus to have been included in the NacA, and for patients in the NacA to have received more contrast agent, whereas patients in the CG tended to have received more fluids (Table 2). Length of ICU stay and mortality were not significantly different between the two groups. Additional file 1: Table S3 indicates significant characteristics in univariate analysis of survival. Mortality was independently associated only with age (odds ratio, 1.06; 95% CI, 1.0–1.13) in multivariate analysis.
CIN
The incidence of CIN in the CG and NacA was 15.6% and 18.33%, respectively (p = 0.81). There was no significant difference between the two groups in serum creatinine during the examination period. Table 3 shows characteristics of participants according to the presence of CIN or not. Additional file 1: Table S4 shows a classification of patients with CIN based on serum creatinine or cystatin-C changes or the Risk, Injury, Failure, Loss, End-Stage Renal Failure (RIFLE) score. Compared to patients without CIN, patients with CIN had significantly increased baseline values of urea/creatinine ratio (p = 0.01) and had more often received colimycin or at least one of the following drugs that have a known adverse impact in renal function: aminoglycosides, amphotericin, colimycin, vancomycin, teicoplanin or non-steroid anti-inflammatory drugs (Additional file 1: Table S5). Additional file 1: Table S6 shows fluid balance in patients with or without CIN. Multivariate analysis revealed that CIN was associated with a higher baseline ratio of serum urea/creatinine (odds ratio, 1.02; 95 CI%, 1.00–1.05) and with the concomitant use of nephrotoxic medications (odds ratio, 0.24; 95 CI%, 0.06–0.94), or with a higher baseline ratio of serum urea/creatinine (odds ratio, 1.03; 95 CI%, 1.00–1.05) and with the concomitant use of colimycin (odds ratio, 0.25; 95 CI%, 0.08–0.78) as independent risk factors. CIN did not have significant impact on ICU mortality (28.57% versus 19.42%) or on ICU stay (29 versus 25 days) (Table 3).
Serum cystatin-C
Serum cystatin-C (sCysC) concentration did not present significant differences between groups or during the examination period (p = 0.658). Patients in the CG who had CIN had significantly increased cystatin-C levels compared to patients with NacA who had CIN (p = 0.03) (Fig. 2). The percentage change (median ± interquartile range (IR)) in sCysC (mg/L) from baseline in patients who had CIN was 37.23% ± 12.54 and 93.20% ± 33.54 in NacA and in CG, respectively (p = 0.03). Data were also analyzed according to 25% increase of cystatin-C levels from the baseline value (25% DeltaCystC) - similarly to creatinine-based definition of CIN, but there was no significant difference between the NacA and CG (p = 0.26).
Serum cystatine C levels changes (%) between baseline and time of radio contrast induced nephropathy (CIN) diagnosis in controls and in patients who received antioxidants (NacA group). The horizontal lines in the low-high bar graphs represent median values. The statistical significance is indicated with the capped line
Serum 8-isoprostane
Serum levels of 8-isoprostane are presented in Fig. 3. Although the mean regression lines of serum levels of 8-isoprostane did not differ significantly between the two groups over time, significant differences between the NacA and CG were detected at 2 and 24 hours after the infusion of the contrast agent and in the CG between baseline and 48 hours.
Serum 8-isoprostane levels changes during 48 hours following administration of radio contrast material in controls and in patients who received antioxidants (NacA group). The significant differences are marked with brackets. Forty-eight hours kinetics is indicated by the corresponding mean regression lines
Diagnostics
ROC curve analysis showed that the best cutoff baseline value of the urea/creatinine ratio to predict CIN was 63.79 (area under the curve (AUC) (95% CI) 0.712 ± 0.076 (0.56–0.86), p = 0.002) (Fig. 4). ROC curve analysis also showed that the best cutoff baseline value of the serum urea/creatinine ratio to predict CIN in a patient who receives nephrotoxic medication was 65.70 (sensitivity 68.75%, specificity 69.81%, AUC (95%CI) 0.74 ± 0.078 (0.58–0.89), p = 0.003) (Fig. 5).
Discussion
The main findings of the present study are: (a) intravenous infusion of the combination of two antioxidant agents, N-acetylcysteine and ascorbic acid, failed to reduce the incidence of CIN; (b) CIN was associated with increased baseline values of the serum urea/creatinine serum ratio and the concomitant use of nephrotoxic medications; (c) antioxidants may have reduced oxidative stress as indicated by 8-isoprostane serum levels, which were significantly lower in the NacA compared to the CG at 2 hours and 48 hours; (d) in patients with CIN in the NacA, serum cystatin-C values increased less than in patients with CIN in the CG.
The pathogenesis of CIN is considered multifactorial, with renal vasoconstriction and direct cell toxicity, which both lead to medullary hypoxia and the production of reactive oxygen species [21, 26]. The co-administration of the antioxidant agents could help in managing the potential oxidative burden added by the radiocontrast material. Yet, there was no significant difference in the incidence of CIN between the NacA and controls. Several explanations could be given for the absence of a difference between groups. Critically ill patients present with many predisposing factors for deterioration of renal function. In this respect it might be difficult to assess the net impact of the contrast agent in renal function [27]. Individual cases of nephropathy occurring in critically ill patients after use of intravenous radiocontrast material cannot be attributed with certainty to the contrast exposure. In this respect, the definition of CIN is challenging and the incidence of CIN presents varies greatly between studies, i.e. from 1.5 to 33% [13, 14]. In the present study, we included renally stable patients and the definition of CIN was based on change in serum creatinine, an index that is widely accepted and available [28]. The incidence of CIN was 15.6%.
Another point that could be noted to help explain the absence of a difference between NacA and CG was that there were indications that CG included fewer patients with diabetes mellitus or that patients in the NacA received higher volumes of radiocontrast material (Table 1) or that patients in the CG received more fluids at 1 day before and on the day of radiocontrast material infusion (Table 2). These factors might have obscured any significant prophylactic impact of the antioxidant agents on renal function.
In order to provide further insight into the relationship between CIN and potential risk factors, we analyzed the impact of several clinical factors associated with renal deterioration. We found that CIN was associated with the concomitant use of nephrotoxic medications (p = 0.03). This has been underlined by previous studies on the etiology of CIN in critically ill patients [29]. Notably in this study, we found that colimycin was related to CIN, which underlines the adverse impact of this antibiotic, which is unfortunately often necessary for management of gram-negative infections nowadays.
Other well-known etiologic factors for CIN such as co-morbidities (i.e. diabetes mellitus or preexisting renal failure) were not associated with CIN. This is in accordance with other studies [29, 30] addressing CIN risk factors in ICU patients. A possible explanation for this fact may be related to the great heterogeneity of critically ill patients or to the population size in this study, which could not depict such differences. We should also underline here that the present study did not include a special strategy for fluids administration/balance and all relevant decisions were based on treating physicians’ decisions, which were according to current clinical practice recommendations [22]. Yet, we found no difference in fluid balance between groups.
In this study the main outcome was CIN based on serum creatinine values. One might argue that creatinine may not be a sensitive index of glomerular filtration rate (GFR) alterations [31]. Previous studies suggested that creatinine metabolism can be affected by Nac, so that the observed changes in serum creatinine concentration after administration of Nac may not be indicative of GFR improvement [32]. In order to overcome these obstacles we assessed sCysC concentration, which can serve as an endogenous marker of renal function and is believed to be superior to plasma creatinine concentration as it does not depend on age, sex and muscle mass, and so it has been considered as a simple, reliable and accurate marker of renal function [33, 34]. Thus, we identified patients with 25% DeltaCysC - similarly to creatinine-based definition of CIN. Although no significant difference was depicted, more patients presented with severe CIN (increase of more than 50% from baseline values) in the CG compared to the NacA. We acknowledge that other sensitive markers such as urine neutrophil gelatinase-associated lipocalin (NGAL) [35] could be more accurate to define the impact of CIN; however, this was not studied in this investigation and might be the aim of a future study in ICU patients.
Despite the fact that this strategy failed to reduce the incidence of CIN, or other indices of outcome we found evidence that it may at least partially balance the oxidative stress burden, as patients in the NacA had lower 8-isoprostane levels following radiocontrast material infusion and patients with CIN had attenuation of the increase in serum cystatin-C levels. Serum levels of 8-isoprostane represent a sensitive index of oxidative stress in vivo [36]. In this study, although the overall kinetics of serum levels of 8-isoprostane did not differ between the two groups, as indicated by the mean regression lines, significant differences between the two groups were detected at 2 hours and 24 hours after the infusion of antioxidant agents, and in the CG between baseline and 48 hours (Fig. 3).
Conclusion
In the present study the use of the combination of antioxidant agents, Nac and Aa, failed to reduce the incidence of CIN in critically ill patients undergoing CT with radiocontrast material. Furthermore, our findings indicate that an increased urea/creatinine ratio and the use of nephrotoxic medications, especially of colimycin, may have an adverse impact on renal function in this setting. Despite the absence of a significant impact of Nac-Aa in the incidence of CIN, the use of antioxidants partially balanced the oxidative stress burden following contrast infusion and decreased renal injury, as it was assessed using serum cystatin-C in patients who presented with CIN. In this respect, our results should be validated in a large number of patients in a multi-center randomized clinical trial in the future.
Abbreviations
- Aa:
-
Ascorbic acid
- ACEi:
-
Angiotensin converting enzyme inhibitor
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation
- ARBs:
-
Angiotensin II receptor blockers
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CG:
-
Control group
- CI:
-
Confidence interval
- CIN:
-
Contrast-induced nephropathy
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- ESRD:
-
End-stage renal disease
- GFR:
-
Glomerular filtration rate
- ICU:
-
Intensive care unit
- IR:
-
Interquartile range
- N/S:
-
Normal saline
- Nac:
-
N-acetylcysteine
- NacA:
-
Treatment group
- RIFLE:
-
Risk Injury Failure Loss End-Stage Renal Failure
- ROC:
-
Receiver operating characteristic
- RRT:
-
Renal replacement therapy
- sCysC:
-
Serum cystatin-C
- SE:
-
Standard error
- SOFA:
-
Sequential Organ Failure Assessment
References
Barrett BJ, Parfrey PS. Prevention of nephrotoxicity induced by radiocontrast agents. N Engl J Med. 1994;331(21):1449–50.
McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–75.
Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–8.
Subramanian S, Tumlin J, Bapat B, et al. Economic burden of contrast-induced nephropathy: implications for prevention strategies. J Med Econ. 2007;10:119–34.
Solomon RJ, Mehran R, Natarajan MK, et al. Contrast-induced nephropathy and long-term adverse events: cause and effect? Clin J Am Soc Nephrol. 2009;4:1162–9.
Goldenberg I, Chonchol M, Guetta V. Reversible acute kidney injury following contrast exposure and the risk of longterm mortality. Am J Nephrol. 2009;29:136–44.
Bartholomew BA, Harjai KJ, Dukkipati S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–9.
Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–8.
Manske CL, Sprafka JM, Strony JT, et al. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–20.
Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150:1237–42.
Albabtain MA, Almasood A, Alshurafah H, et al. Efficacy of ascorbic acid, N-acetylcysteine, or combination of both on top of saline hydration versus saline hydration alone on prevention of contrast-induced nephropathy: a prospective randomized study. J Interv Cardiol. 2013;26(1):90–6.
Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110:2837–42.
Haveman JW, Gansevoort RT, Bongaerts AH, et al. Low incidence of nephropathy in surgical ICU patients receiving intravenous contrast: a retrospective analysis. Intensive Care Med. 2006;32(8):1199–205.
Chousterman BG, Bouadma L, Moutereau S, et al. Prevention of contrast-induced nephropathy by N-acetylcysteine in critically ill patients: different definitions, different results. J Crit Care. 2013;28(5):701–9.
Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–9.
Palli E, Makris D, Papanikolaou J, et al. Contrast-induced nephropathy in aged critically ill patients. Oxid Med Cell Longev. 2014;2014:756469.
Katholi RE, Woods Jr WT, Taylor GJ, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32(1):64–71.
Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int. 1994;45:259–65.
Joannidis M, Wiedermann C. Radiocontrast-induced acute kidney injury in the ICU: worse than presumed? Intensive Care Med. 2011;37:1904–6.
Kay J, Chow WH, Chan TM. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention. A randomized controlled clinical trial. ACC Curr J Rev. 2003;12:337–8.
Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium induced nephropathy. Kidney Int. 2005;68:14–22.
Wetterslev M, Møller-Sørensen H, Johansen RR, et al. Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med. 2016;42:1223–33.
Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, et al. NGAL (neutrophil gelatinase-associated lipocalin) and cystatin C: are they good predictors of contrast nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine? Int J Cardiol. 2008;127(2):290–1.
Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin. C Kidney Int. 2004;66(3):1115–22.
Narula A, Mehran R, Weisz G, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35(23):1533–40.
Tumlin J, Stacul F, Adam A, et al. Pathophysiology of contrast induced nephropathy. Am J Cardiol. 2006;18:98(6A):14K–20K. Epub 2006 Feb 17.
McCullough PA, Zhang J, Ronco C. Volume expansion and contrast-induced acute kidney injury. Lancet. 2017;389(10076):1277–8. https://doi.org/10.1007/s80109000008610.1016/S0140-6736(17)30540-8 Epub 2017 Feb 21.
Ribichini F, Gambaro G, Graziani MS, et al. Comparison of serum creatinine and cystatin C for early diagnosis of contrast-induced nephropathy after coronary angiography and interventions. Clin Chem. 2012;58(2):458–64.
Ehrmann S, Badin J, Savath L, et al. Acute kidney injury in the critically ill: is iodinated contrast medium really harmful? Crit Care Med. 2013;41(4):1017–26.
Rashid AH, Brieva JL, Strokes B. Incidence of contrast induced nephropathy in intensive care patients undergoing computerised tomography and prevalence of risk factors. Anaesth Intensive Care. 2009;37(6):968–75.
Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–53.
Alioglou E, Saygi S, Turk U, et al. N-acetylcysteine in preventing contrast-induced nephropathy assessed by cystatin C. Cardiovasc Ther. 2013;31(3):168–73.
Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function—a review. Clin Chem Lab Med. 1999;37:389–95.
Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–8.
Kafkas N, Liakos C, Zoubouloglou F, et al. Neutrophil gelatinase-associated lipocalin as an early marker of contrast-induced nephropathy after elective invasive cardiac procedures. Clin Cardiol. 2016;39(8):464–70.
Tiryaki BS, Tasliyurt T, Yelken BM, et al. Evaluation of oxidative stress using exhaled breath 8-isoprostane levels on chronic kidney disease. Niger J Clin Pract. 2014;17(3):356–60.
Acknowledgements
The authors wish to thank Mrs S. Donis-Tzioumakis for her assistance in editing the manuscript.
Funding
The study was funded by the department of Critical Care of the University Hospital of Larissa and the University of Thessaly.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
EP designed the study, enrolled patients, collected the data, contributed to statistical analysis, performed the literature review and wrote the manuscript. DM contributed to designation of the study and statistical analysis and supervised the preparation of the manuscript. JP contributed to statistical analysis and reviewed the manuscript. GG and PZ contributed to the collection of data and reviewed the manuscript. IT performed the measurement of serum cystatin-C and 8-isoprostane. EZ conceived and designed the study and reviewed the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
Not applicable.
Ethics approval and consent to participate
The ethics committee of the University Hospital of Larissa approved the protocol. Written informed consent was obtained from each patient or from appropriate surrogates for patients unable to consent. Ref number:122,6/05/2009.
Consent for publication
All patients provided necessary consent for publication.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1.
Classification of participants renal function according to RIFLE criteria at the entry study. Table S2. Medications with potential impact on renal function received by participants according to treatment group. Table S3. Univariate analysis of characteristics of survivors and non survivors. Table S4. CIN based on serum creatinine or cystatin-C changes or RIFLE score (between the day of radiocontrast material infusion and the day of CIN diagnosis). Table S5. Medications with potential impact on renal function received by participants according to the presence of CIN or not. Table S6. Fluid balance of patients included in the study according to the presence of CIN or not. (DOC 110 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Palli, E., Makris, D., Papanikolaou, J. et al. The impact of N-acetylcysteine and ascorbic acid in contrast-induced nephropathy in critical care patients: an open-label randomized controlled study. Crit Care 21, 269 (2017). https://doi.org/10.1186/s13054-017-1862-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-017-1862-3