Abstract
Purpose
High-flow oxygen therapy delivered through nasal cannulae improves oxygenation and decreases work of breathing in critically ill patients. Little is known of the physiological effects of high-flow oxygen therapy applied to the tracheostomy cannula (T-HF). In this study, we compared the effects of T-HF or conventional low-flow oxygen therapy (conventional O2) on neuro-ventilatory drive, work of breathing, respiratory rate (RR) and gas exchange, in a mixed population of tracheostomized patients at high risk of weaning failure.
Methods
This was a single-center, unblinded, cross-over study on fourteen patients. After disconnection from the ventilator, each patient received two 1-h periods of T-HF (T-HF1 and T-HF2) alternated with 1 h of conventional O2. The inspiratory oxygen fraction was titrated to achieve an arterial O2 saturation target of 94–98% (88–92% in COPD patients). We recorded neuro-ventilatory drive (electrical diaphragmatic activity, EAdi), work of breathing (inspiratory muscular pressure–time product per breath and per minute, PTPmusc/b and PTPmusc/min, respectively) respiratory rate and arterial blood gases.
Results
The EAdipeak remained unchanged (mean ± SD) in the T-HF1, conventional O2 and T-HF2 study periods (8.8 ± 4.3 μV vs 8.9 ± 4.8 μV vs 9.0 ± 4.1 μV, respectively, p = 0.99). Similarly, PTPmusc/b and PTPmusc/min, RR and gas exchange remained unchanged.
Conclusions
In tracheostomized patients at high risk of weaning failure from mechanical ventilation, T-HF did not improve neuro-ventilatory drive, work of breathing, respiratory rate and gas exchange compared with conventional O2 after disconnection from the ventilator. The present findings might suggest that physiological effects of high-flow therapy through tracheostomy substantially differ from nasal high flow.
Similar content being viewed by others
Introduction
High-flow nasal cannula oxygen therapy is the administration of a warmed and humidified air/oxygen mixture at a flow rate between 20 and 60 L/min [1] through nasal cannulae (HFNC). HFNC is increasingly used in several clinical contexts, particularly in de novo hypoxemic respiratory failure [2,3,4,5]; post-extubation [6, 7] and in post-cardiothoracic surgery [8]. Compared with conventional oxygen therapy (conventional O2), HFNC produces several physiological effects [9], which include: a better matching between the delivered gas flow mixture and patient’s spontaneous inspiratory flow; a positive end-expiratory pressure (PEEP) effect (generally between 2 and 8 cm H2O [10, 11]) and a “CO2 wash out” effect from the upper airways [12, 13]. Further, the humidified and warmed gas mixture favors the mucociliary function and reduces upper airway resistance [2, 14]. The synergistic combination of these mechanisms leads to improved oxygenation [2], decrease in neuro-ventilatory drive and work of breathing [15,16,17].
High-flow oxygen therapy can also be applied to the tracheostomy opening (T-HF) in tracheostomized patients. Since HFNC decreases the risk of re-intubation in patients at risk of extubation failure [6, 7], it is reasonable to hypothesize that T-HF could aid the separation from mechanical ventilation in mechanically ventilated, tracheostomized patients at high risk of weaning failure. However, T-HF is a novel therapy and it is not known whether it replicates the physiological advantages of HFNC.
Our study hypothesis was that T-HF, compared to conventional O2, would decrease the neuro-ventilatory drive, work of breathing, respiratory rate and improve gas exchange, in a mixed population of tracheostomized patients at high risk of weaning failure [18].
Methods
Study design
This was a single-center, unblinded, cross-over study of ventilated patients via tracheostomy with prolonged weaning from mechanical ventilation. After disconnection from the ventilator, each patient received two 1-h periods of T-HF (T-HF1 and T-HF2) alternated with 1 h of conventional O2.
Patient selection
Patients admitted to the intensive care unit (ICU) of the Bari University Hospital (Italy) between June 2017 and May 2018, requiring mechanical ventilation via tracheostomy, were screened for inclusion in the study. Inclusion criteria were age ≥ 18 and a diagnosis of prolonged weaning according to WIND study (i.e., patients not weaned after more than 7 days from the first separation attempt) [18]. Exclusion criteria were: contraindications to the insertion of an electrical activity of the diaphragm (EAdi) catheter (e.g., upper gastrointestinal surgery, esophageal varices, esophageal trauma) cardiopulmonary instability, concomitant neuro-muscular pathologies and/or known phrenic nerve dysfunction. Furthermore, we excluded patients showing signs of paradoxical abdominal movement or use of accessory inspiratory muscles. The local ethics committee approved the study protocol (Azienda Ospedaliero-Universitaria Policlinico di Bari Ethics Committee, Protocol No.: 55276/C.E. Lug 2016). Patients or next of kin gave their written consent to participate in the study.
Based on our clinical protocol [19], patients were disconnected from the ventilator and allowed to breath unassisted through the tracheostomy cannula if they met the following criteria: (a) resolution or improvement in the condition leading to acute respiratory failure; (b) positive end-expiratory pressure (PEEP) lower than 5 cm H2O and an inspiratory oxygen fraction (FiO2) lower than 0.5 with a PaO2/FiO2 ratio greater than 150 mm Hg; (c) arterial pH > 7.35; Richmond Agitation-Sedation Scale (RASS) between 0 and − 1 [20], on no sedation or on a continuous infusion of dexmedetomidine (0.1–1.4 μg/kg/h); (d) ability to trigger the ventilator as demonstrated by a decrease in pressure airway opening (PAO) > 3–4 cm H2O during a brief (5–10 s) end-expiratory occlusion test. Other criteria were hemodynamic stability without vasoactive drugs (excluding a dobutamine and/or dopamine infusion lower than 5 μg/kg/min and a 3 μg/kg/min, respectively) and normothermia.
Patients were ventilated with a Servo-i ventilator (Maquet, Getinge group Critical Care, Solna, Sweden) equipped with the EAdi software (Maquet, Getinge group Critical Care, Solna, Sweden). The EAdi catheter was positioned based on the corrected nose–earlobe–xyphoid distance formula, and its position was subsequently adjusted using the ventilator EAdi catheter position tool (Servo-i ventilator NAVA software) in accordance with the manufacturer’s instructions [21].
Measurements
Patients were studied in the semi-recumbent position. The EAdi signal was collected from the RS232 port of the ventilator at a sampling rate of 100 Hz (NAVA tracker software, Getinge Critical Care, Solna, Sweden) and stored in a personal computer. The NAVA tracker files were subsequently converted and analyzed using the ICU Lab software package (Kleistek Engineering; Bari, Italy).
The inspiratory EAdi peak (EAdiPEAK), the integral of the inspiratory EAdi deflection over time (EAdiPTP), the slope of the EAdi from the beginning of inspiration to the peak (EAdiSLOPE), the respiratory rate (RR) and the neural inspiratory time (TiNEUR) were measured from the EAdi trace [22].
The pressure generated by the inspiratory muscles (Pmusc) was estimated from the EAdi signal and the neuro-muscular efficiency index (NME), through to a method previously validated by Bellani and coworkers [23]. The NME is the ratio between the negative pressure generated by the inspiratory muscles and the corresponding EAdi [17, 24,25,26] and is therefore expression of the efficiency of the diaphragmatic depolarization (as expressed by the EAdi) to generate a muscular contraction.
Accordingly, Pmusc may be estimated through the formula:
In our study, we obtained the NME by the ratio between the negative pressure in the airway opening (PAO) generated by a single inspiratory effort against the occluded airways and the corresponding positive peak in EAdi (EAdiPEAK). Briefly, we performed a short (5–10 s) end-expiratory occlusion and recorded the PAO and EAdi traces that were subsequently digitally recorded and analyzed offline. We analyzed a single inspiratory effort, the first that occurred during the end-expiratory airway occlusion. Of note, this method allows calculating the NME without an esophageal catheter, since the fall in PAO during a spontaneous inspiratory effort against the occluded airways is, by definition, equal to the corresponding fall in esophageal pressure (PES) [27, 28]. However, the PAO-based NME overestimates by a factor of 1.25 the PES-based NME, and therefore we corrected our Pmusc calculation as follows [23]:
The inspiratory Pmusc pressure–time product per breath (PTPmusc/b) was calculated as the area under the Pmusc signal. The inspiratory Pmusc pressure–time product per minute (PTPmusc/min) was calculated as:
The tidal volume (VT) was not measured to avoid any modification in breathing pattern caused by the measurement apparatus.
Study protocol
Before disconnection from the ventilator, each patient underwent a 30-min spontaneous breathing trial (SBT) in pressure support ventilation (PSV), with a pressure support of 5 cm H2O and a PEEP of 5 cm H2O [29]. The SBT was successful if no signs of distress occurred, defined as: (a) paradoxical abdominal movements or other signs of accessory respiratory muscle fatigue; (b) cardiovascular instability (systolic blood pressure, SBP > 160 or < 90 mmHg—or a 20% change from the pre-SBT values; heart rate, HR > 120 or < 60 beats/min or 20% change from the pre-SBT values; (c) arterial desaturation with SaO2 < 88%; (d) hypercapnia defined as respiratory acidosis with pH < 7.35 [19]. Patients succeeding the SBT were separated by the ventilator and included in the study.
The NME was calculated during the SBT, 5–10 min before the disconnection from mechanical ventilation, after careful tracheal suctioning in order to improve the quality of the measurement.
Weaning from the ventilator was defined as the ability to breathe spontaneously through the tracheostomy cannula without signs of respiratory distress (see above) and without the need for reconnection to the mechanical ventilator for at least 48 h.
Immediately after disconnection from the ventilator, patients underwent a cross-over protocol with an ON–OFF–ON design, alternating T-HF with conventional O2 delivered through a T-piece (i.e., T-HF1—conventional O2 therapy—T-HF2), with each phase lasting for 1 h (Fig. 1).
Study protocol timeline. ABG, arterial blood gas analysis; EAdi, diaphragm electrical activity; T-HF 1, first period of high-flow tracheostomy cannula oxygen therapy (1 h); conventional O2, period of conventional low-flow oxygen therapy (1 h); TP, T-piece; T-HF 2, second period of high-flow tracheostomy cannula oxygen therapy (1 h)
The T-HF was administered through the AIRVO™ 2 system (Fisher and Paykel Healthcare, Auckland, New Zealand) with a specific interface for the tracheostomy tube (OPT870, Fisher and Paykel Healthcare, Auckland, New Zealand). The system allows administration of humidified and warmed gas flow at 10–60 L/min in the adult configuration. The interface (Fig. 2) is composed of a connector (length = 38 mm) equipped with a side stream gas delivery tube (diameter 12 mm). The angle between the axis of the connector and the delivery tube is 60°. The gas flow was set at 50 L/min in all the patients. At the beginning of each T-HF period, the FiO2 was titrated to achieve an arterial hemoglobin oxygen saturation (SaO2) of 94–98% (or 88–92% in patients with chronic obstructive pulmonary disease). The temperature of the heater–humidifier was set at 37 °C.
Gas flows through the specific interface for the tracheostomy tube (OPT870, Fisher and Paykel, Healthcare, Auckland, New Zealand) tested in the present study. The interface (a) is composed of a connector (length = 38 mm) equipped with a side stream gas delivery tube (diameter 12 mm). The angle between the axes of the connector and the delivery tube is 60° (b)
Conventional O2 therapy was administered through a T-piece weaning kit connected to a standard O2/air mixer (0–15 L/min). The T-piece gas flow was set to 10 L/min in all the patients. At the beginning of the conventional O2 period, the FiO2 was titrated to match the same oxygenation targets of the T-HF periods (see above).
At the end of each study period, arterial blood for gas analysis was obtained. The EAdi trace was digitally recorded and analyzed for the entire duration of the study period.
Statistical analysis
The power analysis indicated a sample size of 14 patients taking the EAdiPEAK as the main study endpoint. We have hypothesized that the variability explained by the study condition had to be at least 5% of total variability that approximatively corresponds to an expected effect size of 0.25, with a power of 0.8 and a significance level of 0.05. The sample size was determined using the software GPower version 3.1.9.2.
Continuous quantitative variables were summarized as mean ± standard deviation if normally distributed or as median and interquartile range if non-normally distributed. Comparisons were performed with ANOVA for repeated measures or Friedman test as appropriate. A p value < 0.05 was considered as statistically significant, except in multiple comparison procedure, when the p-value was adjusted. The analyses were carried out with SAS 9.4 for Windows PC.
Results
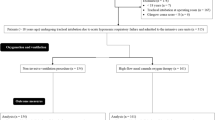
The CONSORT study diagram (Fig. 3) shows that in the study period, 22 out of 38 tracheostomized patients with prolonged weaning from mechanical ventilation at high risk of weaning failure were eligible for the study and 14 were finally enrolled and completed the study protocol. Patients’ demographics and clinical characteristics are shown in Table 1.
Breathing pattern and gas exchange
Table 2 shows the breathing pattern and gas exchange parameters. The oxygenation target was achieved with similar FiO2 levels in the three study steps (0.51 ± 0.1; 0.51 ± 0.12; and 0.51 ± 0.11 in the T-HF1, conventional O2 and T-HF2 periods, respectively; p = NS). The arterial pO2 was 109 ± 27 mm Hg in the T-HF1 period, 92 ± 17 mm Hg in the conventional O2 period and 111 ± 28 mm Hg in the T-HF2 period, p = 0.09. Arterial PCO2, RR and TiNEUR, remained similar throughout the study (Table 2).
Neuro-ventilatory drive and work of breathing
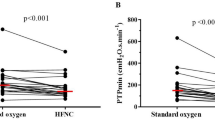
Figure 4 is an experimental record showing the EAdi traces obtained at the end of each of the three experimental conditions, in four representative patients. The neuro-ventilatory drive, as expressed by the EAdiPEAK, EAdiPTP and EAdiSLOPE, remained unchanged in the three experimental periods. The same occurred for work of breathing, as expressed by PTPmusc/b and PTPmusc/min (Table 3). Figure 5 shows the individual changes in EAdiPEAK and work of breathing parameters, in the three experimental conditions.
Experimental record showing the diaphragm electrical activity (EAdi) in the three experimental conditions in four representative patients. T-HF 1, first period of high-flow tracheostomy cannula oxygen therapy; conventional O2, period of conventional low-flow oxygen therapy with T-piece; T-HF 2, second period of high-flow tracheostomy cannula oxygen therapy
Trend of the neuro-ventilatory drive, as expressed by the diaphragm electrical activity peak EAdiPEAK, and of work of breathing, as expressed by the inspiratory muscular pressure–time product per breath (PTPmusc/b) and per minute (PTPmusc/min). Other abbreviations: T-HF 1, first period of high-flow tracheostomy cannula oxygen therapy; conventional O2, period of conventional low-flow oxygen therapy with T-piece; T-HF 2, second period of high-flow tracheostomy cannula oxygen therapy
Discussion
In this study, we tested the effects of high-flow oxygen therapy through a dedicated tracheostomy interface on gas exchange, neuro-ventilatory drive, respiratory rate and work of breathing in tracheostomized patients at high risk of weaning failure. Our data suggest that T-HF does not produce the same physiological effects as HFNC on these outcome parameters. Although our study was not designed for a detailed examination of the physiological mechanisms generated by high flow at the tracheal level, we discuss the possible physiological mechanisms that may explain our results.
Recent studies have shown that during HFNC, patients decrease their minute inspiratory volume maintaining stable the PaCO2 [15,16,17]. These findings indirectly support the hypothesis that HFNC decreases the neuro-ventilatory drive and work of breathing mainly through its “CO2 wash out” effect [11, 13, 15,16,17]. Since we found that T-HF does not impact on the neuro-ventilatory drive and work of breathing, we speculate that these negative results are explained by the fact that T-HF has negligible “CO2 wash out effect” compared with HFNC. This can be explained by the fact that HFNC washes out the CO2 mainly from the nasopharyngeal anatomical dead space [12, 13], whereas the tracheostomy tube bypasses the upper airways. The effect of tracheostomy on dead space has been confirmed by Chadda et al. [30] who show that physiological dead space increases from 156 ± 67 mL before decannulation to 230 ± 82 ml after decannulation.
We were not able to show any impact of T-HF on the PaO2/FiO2 ratio, compared with conventional O2 (Table 2). We have no data on tracheal pressures and lung volumes, but—given that conventional O2 and T-HF achieved similar results on a multitude of parameters—it is unlikely that a “PEEP effect” occurred in our patients. Our results are in contrast with those reported in a research letter by Corley et al. [31], showing a slight, but statistically significant improvement in oxygenation with T-HF compared to conventional O2 in prolonged wean patients. Corley et al. applied T-HF for 15 min, whereas our study periods lasted 1 h and the gas delivery flow rate during conventional O2 was 15 L/min versus 10 L/min in our study. However, it seems unlikely that these methodological differences could explain the discrepancy between Corley’s results and ours.
The EAdi is a “processed” diaphragmatic electromyography signal recorded through an array of electrode pairs mounted on the wall of a nasogastric feeding tube [32] and is proportional to the intensity of the electrical stimuli directed to the diaphragm [24, 33, 34]. In other words, the EAdi is a measure of the discharge rate of phrenic nerves that have been shown to reliably reflect the neuro-ventilatory drive and work of breathing [24, 32,33,34,35,36]. Recently, Bellani and coworkers [23] demonstrated that EAdi can be used to estimate the instantaneous work of breathing, as we did in our study. Liu and coworkers found that an EAdiPEAK lower than 15–20 μV during a spontaneous breathing trial was associated with extubation success [25]. Dres et al. [37] and Barwing et al. [38] obtained similar results. In our study, we found that the EAdiPEAK was below 20 μV (Fig. 5) both during the T-HF and the conventional O2 periods. We speculate that these findings could be explained by the fact that our patients were tracheostomized. Previous studies have shown that the neuro-ventilatory drive is up to 30% lower in tracheostomized patients [30], probably because the tracheostomy decreases the overall airway resistance [39] and the physiological dead space [30]. Furthermore, to our knowledge, data on the normal ranges of neuro-ventilatory drive in tracheostomized patients are scanty. In analogy with EAdi, the PTPmusc/min, a well-known index of work of breathing, was within its “acceptable” range (i.e., between 50 and 150 cm H2O/s/min [40, 41]), in 72%, 65% and 72% of our patients in the T-HF1, conventional O2 and T-HF2 periods, respectively (Fig. 5).
We must acknowledge some study limitations. First, we conducted a non-randomized ON–OFF–ON cross-over study that, for practical reasons, could not be blinded. Furthermore, we were not able to measure several respiratory parameters (VT, EELV, airways pressure) that could have provided us with a more complete interpretation of the treatment effect. However, our study was conducted in spontaneously breathing patients and we sought to avoid any modification in breathing pattern caused by the measurement apparatus. Second, we estimated Pmusc based on a method recently validated by Bellani and coworkers [22] and applied in different studies, but we must point out that this method assumes a diaphragm contribution to the overall Pmusc of 75%, as occurs in normal conditions [42]. On the other hand, if the contraction of the accessory inspiratory muscles is dominant (as occurs in case of paradoxical abdominal movements) the estimation of Pmusc from EAdi could be biased. However, we excluded from our study patients showing signs of paradoxical abdominal movement and use of accessory inspiratory muscles (see “Methods” section). In addition, the method described by Bellani et al. [23] assumes a linear relationship between EAdi and Pmusc at different lung volumes but other authors have shown a nonlinearity between EAdi and lung volumes [24]. However, this occurs only in case of intense diaphragmatic contractions [24]. Fourth, our physiological study was not designed to test the impact of T-HF on patients’ comfort. Over a prolonged period, however, T-HF could better preserve the mucociliary function compared with conventional O2 [14], improving the secretion clearance and patient’s comfort. Further studies are needed to test this hypothesis. Fifth, we used a fixed high flow rate (50 L/min) but it is possible that, in analogy with HFNC [43], our results would have been different at different flow rates. Further studies are needed to construct a dose–response between T-HF flow rate and physiological effects. On the other hand, a different interface design, for example, with a different flow delivery angle (Fig. 2), could improve the CO2 clearing from the tracheal space, compared with the interface tested in the present study.
Conclusions
In conclusion, in our cohort of tracheostomized patients at high risk of weaning failure, after disconnection from the ventilator T-HF did not affect neuro-ventilatory drive, work of breathing, respiratory rate and gas exchange, compared to conventional O2. The present findings might suggest that physiological effects of high-flow therapy through tracheostomy substantially differ from nasal high flow.
Abbreviations
- COPD:
-
chronic obstructive pulmonary disease
- Conventional O2 :
-
conventional low-flow oxygen therapy
- EAdi:
-
electric activity of the diaphragm
- FiO2 :
-
inspiratory oxygen fraction
- HFNC:
-
high-flow nasal cannula oxygen therapy
- ICU:
-
intensive care unit
- NIV:
-
noninvasive ventilation
- NME:
-
neuro-muscular efficiency
- P AO :
-
pressure airway opening
- PEEP:
-
positive end-expiratory pressure
- PEEPi:
-
intrinsic positive end-expiratory pressure
- PTPmusc/b :
-
pressure–time product per breath
- PTPDmuscmin :
-
pressure–time product per minute
- RR:
-
respiratory rate
- SaO2 :
-
arterial hemoglobin oxygen saturation
- SBP:
-
systolic blood pressure
- SBT:
-
Spontaneous Breathing Trial
- T-HF:
-
high-flow tracheostomy oxygen therapy
- TiNEUR :
-
neural inspiratory time
- VT:
-
tidal volume
References
Papazian L, Corley A, Hess D, Fraser JF, Frat JP, Guitton C, Jaber S, Maggiore SM, Nava S, Rello J, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42(9):1336–49.
Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, Ricard JD. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37(11):1780–6.
Messika J, Ben Ahmed K, Gaudry S, Miguel-Montanes R, Rafat C, Sztrymf B, Dreyfuss D, Ricard JD. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: a 1-year observational study. Respir Care. 2015;60(2):162–9.
Nagata K, Morimoto T, Fujimoto D, Otoshi T, Nakagawa A, Otsuka K, Seo R, Atsumi T, Tomii K. Efficacy of high-flow nasal cannula therapy in acute hypoxemic respiratory failure: decreased use of mechanical ventilation. Respir Care. 2015;60(10):1390–6.
Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–96.
Hernandez G, Vaquero C, Colinas L, Cuena R, Gonzalez P, Canabal A, Sanchez S, Rodriguez ML, Villasclaras A, Fernandez R. Effect of Postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–74.
Hernandez G, Vaquero C, Gonzalez P, Subira C, Frutos-Vivar F, Rialp G, Laborda C, Colinas L, Cuena R, Fernandez R. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354–61.
Stephan F. High-flow nasal oxygen therapy for postextubation acute hypoxemic respiratory failure-reply. JAMA. 2015;314(15):1644–5.
Goligher EC, Slutsky AS. Not just oxygen? Mechanisms of benefit from high-flow nasal cannula in hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1128–31.
Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58(10):1621–4.
Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107(6):998–1004.
Moller W, Celik G, Feng S, Bartenstein P, Meyer G, Oliver E, Schmid O, Tatkov S. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol. 2015;118(12):1525–32.
Moller W, Feng S, Domanski U, Franke KJ, Celik G, Bartenstein P, Becker S, Meyer G, Schmid O, Eickelberg O, et al. Nasal high flow reduces dead space. J Appl Physiol. 2017;122(1):191–7.
Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1):253–61.
Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, Pesenti A. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–15.
Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, Montini L, De Gaetano A, Navalesi P, Antonelli M. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190(3):282–8.
Di Mussi R, Spadaro S, Stripoli T, Volta CA, Trerotoli P, Pierucci P, Staffieri F, Bruno F, Camporota L, Grasso S. High-flow nasal cannula oxygen therapy decreases postextubation neuroventilatory drive and work of breathing in patients with chronic obstructive pulmonary disease. Crit Care. 2018;22(1):180.
Beduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, Grelon F, Runge I, Nicolas T, Grange S, et al. Epidemiology of weaning outcome according to a new definition. The WIND study. Am J Respir Crit Care Med. 2017;195(6):772–83.
Schmidt GA, Girard TD, Kress JP, Morris PE, Ouellette DR, Alhazzani W, Burns SM, Epstein SK, Esteban A, Fan E, et al. Official executive summary of an American Thoracic Society/American College of Chest Physicians clinical practice guideline: liberation from mechanical ventilation in critically ill adults. Am J Respir Crit Care Med. 2017;195(1):115–9.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–44.
Barwing J, Ambold M, Linden N, Quintel M, Moerer O. Evaluation of the catheter positioning for neurally adjusted ventilatory assist. Intensive Care Med. 2009;35(10):1809–14.
Di Mussi R, Spadaro S, Mirabella L, Volta CA, Serio G, Staffieri F, Dambrosio M, Cinnella G, Bruno F, Grasso S. Impact of prolonged assisted ventilation on diaphragmatic efficiency: NAVA versus PSV. Crit Care. 2016;20(1):1.
Bellani G, Mauri T, Coppadoro A, Grasselli G, Patroniti N, Spadaro S, Sala V, Foti G, Pesenti A. Estimation of patient’s inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41(6):1483–91.
Beck J, Sinderby C, Lindstrom L, Grassino A. Effects of lung volume on diaphragm EMG signal strength during voluntary contractions. J Appl Physiol. 1998;85(3):1123–34.
Liu L, Liu H, Yang Y, Huang Y, Liu S, Beck J, Slutsky AS, Sinderby C, Qiu H. Neuroventilatory efficiency and extubation readiness in critically ill patients. Crit Care. 2012;16(4):R143.
Schmidt M, Kindler F, Cecchini J, Poitou T, Morawiec E, Persichini R, Similowski T, Demoule A. Neurally adjusted ventilatory assist and proportional assist ventilation both improve patient-ventilator interaction. Crit Care. 2015;19:56.
Hedenstierna G. Esophageal pressure: benefit and limitations. Minerva Anestesiol. 2012;78(8):959–66.
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126(5):788–91.
Ouellette DR, Patel S, Girard TD, Morris PE, Schmidt GA, Truwit JD, Alhazzani W, Burns SM, Epstein SK, Esteban A, et al. Liberation from mechanical ventilation in critically ill adults: an official American College of Chest Physicians/American Thoracic Society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2017;151(1):166–80.
Chadda K, Louis B, Benaissa L, Annane D, Gajdos P, Raphael JC, Lofaso F. Physiological effects of decannulation in tracheostomized patients. Intensive Care Med. 2002;28(12):1761–7.
Corley A, Edwards M, Spooner AJ, Dunster KR, Anstey C, Fraser JF. High-flow oxygen via tracheostomy improves oxygenation in patients weaning from mechanical ventilation: a randomised crossover study. Intensive Care Med. 2017;43(3):465–7.
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindstrom L. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5(12):1433–6.
Doorduin J, van Hees HW, van der Hoeven JG, Heunks LM. Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med. 2013;187(1):20–7.
Beck J, Sinderby C, Lindstrom L, Grassino A. Influence of bipolar esophageal electrode positioning on measurements of human crural diaphragm electromyogram. J Appl Physiol. 1996;81(3):1434–49.
Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, Rossini M, Sinderby C. Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001;164(3):419–24.
Telias I, Brochard L, Goligher EC. Is my patient’s respiratory drive (too) high? Intensive Care Med. 2018;44(11):1936–9.
Dres M, Schmidt M, Ferre A, Mayaux J, Similowski T, Demoule A. Diaphragm electromyographic activity as a predictor of weaning failure. Intensive Care Med. 2012;38(12):2017–25.
Barwing J, Pedroni C, Olgemoller U, Quintel M, Moerer O. Electrical activity of the diaphragm (EAdi) as a monitoring parameter in difficult weaning from respirator: a pilot study. Crit Care. 2013;17(4):R182.
Diehl JL, El Atrous S, Touchard D, Lemaire F, Brochard L. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am J Respir Crit Care Med. 1999;159(2):383–8.
Sassoon CS, Light RW, Lodia R, Sieck GC, Mahutte CK. Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis. 1991;143(3):469–75.
Mancebo J, Isabey D, Lorino H, Lofaso F, Lemaire F, Brochard L. Comparative effects of pressure support ventilation and intermittent positive pressure breathing (IPPB) in non-intubated healthy subjects. Eur Respir J. 1995;8(11):1901–9.
Tobin MJ. Respiratory monitoring in the intensive care unit. Am Rev Respir Dis. 1988;138(6):1625–42.
Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, Bottino N, Lissoni A, Spadaro S, Volta CA, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med. 2017;43(10):1453–63.
Authors’ contributions
SG, TS and SS contributed to design the conception and design of the study. RDm, FDc, RI, FS and TS were responsible for patient screening and enrollment. PT performed statistical analysis. SG, RDm, FS analyzed the data; SG and TS wrote the manuscript. LC, SS, CAV, FSt and FB critically revised the manuscript for important intellectual content. All authors contributed to interpretation of the data. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
SG has given talks in symposia organized by Getinge Critical Care (Solna Sweden), Estor Critical Care (Mirandola, Italy), Fisher and Paykel (Auckland, New Zealand) and has received compensation for related travel and accommodation fees. He declares no other relationship or activities that could appear to have influenced the submitted work. All the other authors declare: (1) no support from any organization for the submitted work; (2) no financial relationship with any organizations that might have an interest in the submitted work in the previous 3 years; and (3) no other relationship or activities that could appear to have influenced the submitted work.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was obtained by the local ethics committee (Azienda Ospedaliero-Universitaria Policlinico di Bari Ethic Committee, Protocol No.: 55276/C.E. Lug 2016). All patients or their relatives provided informed consent to participate.
Funding
No specific funding was received for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stripoli, T., Spadaro, S., Di mussi, R. et al. High-flow oxygen therapy in tracheostomized patients at high risk of weaning failure. Ann. Intensive Care 9, 4 (2019). https://doi.org/10.1186/s13613-019-0482-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-019-0482-2