Abstract
Natural soil has the ability to suppress the soil-borne pathogen to a certain extent, and the assemblage of soil microbiome plays a crucial role in maintaining such ability. Long-term monoculture accelerates the forms of soil microbiome and leads to either disease conducive or suppressive soils. Here, we explored the impact of soil conditions on bacterial wilt disease (healthy or diseased) under long-term tobacco monoculture on the assemblage of bacterial and fungal communities in bulk and rhizosphere soils during the growth periods. With Illumina sequencing, we compared the bacterial and fungal composition of soil samples from tobacco bacterial wilt diseased fields and healthy fields in three growth periods. We found that Proteobacteria and Ascomycota were the most abundant phylum for bacteria and fungi, respectively. Factors of soil conditions and tobacco growth periods can significantly influence the microbial composition in bulk soil samples, while the factor of soil conditions mainly determined the microbial composition in rhizosphere soil samples. Next, rhizosphere samples were further analyzed with LEfSe to determine the discriminative taxa affected by the factor of soil conditions. For bacteria, the genus Ralstonia was found in the diseased soils, whereas the genus Flavobacterium was the only shared taxon in healthy soils; for fungi, the genus Chaetomium was the most significant taxon in healthy soils. Besides, network analysis confirmed that the topologies of networks of healthy soils were higher than that of diseased soils. Together, our results suggest that microbial assemblage in the rhizosphere will be largely affected by soil conditions especially after long-term monoculture.
Similar content being viewed by others
Introduction
Bacterial wilt, caused by Ralstonia solanacearum, is one typical soil-borne disease and can bring severe losses to agricultural crops (Genin and Denny 2012; Jiang et al. 2017). In addition, long-term monoculture is more likely to cause rapid accumulation of R. solanacearum in soil (Chen et al. 2020a). Until now, there is currently no effective chemical pesticide to manage this disease (Liu et al. 2013). As an attractive alternative, antagonistic microbes were introduced as potential biocontrol agents (Guo et al. 2014). A number of antagonism studies have dealt with the pairwise interactions between beneficial and pathogenic microbes (reviewed by de Boer 2017). Unfortunately, in real communities, the complexity of the soil environment may largely decrease their inefficiency of pathogen suppression (Mallon et al. 2015; de Boer 2017). However, the phenomenon of ‘disease-suppressive soils’ shows an ideal model by which plant protection can be triggered by soil microbes (reviewed by Wang and Li 2019a). Hence, a better knowledge of understanding the positive functions from indigenous microbial communities in the soil is essential for a sustainable and effective bacterial wilt management strategy.
Indigenous microbial communities in the soil forms complex networks and manipulates plant health (Berendsen et al. 2012). Based on the composition of the resident microbial community, a biological barrier is formed, with microbes interacting with pathogens and defending against invasion by pathogens near the root surface (Raaijmakers et al. 2009; Fu et al. 2017). Therefore, assessing the relationship between the soil community and crop morbidity is a critical step toward understanding potential impacts of these communities on plant health (Rosenzweig et al. 2012; Cha et al. 2016; Xiao et al. 2018). It has been revealed that species-rich biomes are more resistant than species-poor biomes to pathogen invasions (Wei et al. 2015) and high incidence of soil-borne diseases could be due to the deterioration of the soil microecological environment (Gao et al. 2020). Additionally, tobacco farmlands with high biodiversity were more resistant to pathogen infection (Wang et al. 2019).
Soil microbial community changes dramatically during plant growth (Lundberg et al. 2012; Xiong et al. 2015). It is important to understand the composition and interaction of microbial communities during plant development (Chaparro et al. 2014). Evidence suggests that Arabidopsis at different developmental stages can culture specific rhizosphere microbiome members (Yuan et al. 2015). Similarly, the rhizosphere microbiome characteristics of maize change with growth stage (Li et al. 2014). During infection by bacterial wilt, the composition of the microbial communities in the rhizosphere of tomato at different growth stages is significantly different (Wei et al. 2018). Research has demonstrated that plant is a unique determinate of community structure in the rhizosphere at early stages, but that these differences in the microbiome disappear as plant develops (Inceoğlu et al. 2011).
Here we report the results of the bacterial wilt diseased and healthy soil microbial assemblages at different growth stages of tobacco. We included the soils collected from diseased fields, and the ones from healthy fields. Bulk soils were collected in March and rhizosphere soils were collected in July and September. We examined the soil biochemical properties and microbial compositions. We investigated the influences of soil conditions and tobacco growth periods on the bacterial and fungal assemblage.
Materials and methods
Site description and sampling
The sampling sites were located in Chongqing, China. Detailed geographical information was listed in Additional file 1: Table S1. Soils were loamy and had a history of continuous tobacco cropping over 20 years. The variety of tobacco was Yunyan 87, which is a hybrid of Yunyan 2 (origin: China) and K326 (origin: USA) (Chen et al. 2020b). Fertilizers and pesticides were applied under the same standards established by the Chongqing Tobacco Corporation. Bacterial wilt diseases caused by R. solanacearum severely affected the production in sites of Wuling area in recent years, whereas there was no soil-borne disease in sites of Qinba area. In this study, soils from Wuling area were treated as bacterial-wilt diseased soils while soils from Qinba area were healthy soils.
Soil samples were collected in March, July and September 2017, which represented the period before transplanting tobacco seedling into the soil, the start of budding stage and during mature stage of tobacco, respectively. As there was no tobacco planting in March, bulk soil samples were collected at a depth of 10–20 cm; besides, rhizosphere soil samples were collected in July and September following the methods mentioned in our previous study (Liu et al. 2016). Soil samples were sieved (2 mm) and stored at − 80 °C for DNA extraction within one week. Additionally, in each March sample, one subsample was previously prepared for analysis of soil physical and chemical properties.
Soil physical and chemical properties analyses
All soil physical–chemical properties were determined according to Bao (2010). Briefly, soil pH was measured with a glass electrode in deionized water suspensions (soil: water = 1:2.5, w/v). The soil organic matter (SOM) was assayed by the potassium dichromate method. Total nitrogen (TN) was estimated by the semi-micro Kjeldahl method. Available nitrogen (AvN), phosphorus (AvP) and potassium (AvK) were determined using the NaOH hydrolyzation diffusion method, the molybdenum blue colorimetric method and the flame photometer method, respectively. Exchangeable calcium (ExCa) and magnesium (ExMg) were measured by the inductively coupled plasma atomic emission spectrometer (ICP-AES) method.
Tobacco bacterial wilt disease investigation
Bacterial wilt symptoms of tobacco were apparently observed in the diseased fields (Wuling area) from June to October. In September of both 2016 and 2017, the end stage of bacterial wilt outbreak, around 40 plants in each sampling site were evaluated to calculate the disease incidence according to (Qi et al. 2020). Meanwhile, due to the healthy state of tobacco in the healthy fields (Qinba area), we only described the disease incidence of diseased fields.
DNA extraction and sequencing processing
Soil microbial DNA was extracted from 0.5 g of soil using a FastDNA Spin kit (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer’s protocol. DNA quality and concentration were evaluated by Nanodrop (NanoDrop ND-1000, Thermo Fisher, Wilmington, DE, USA). Amplification of the V4-region of the bacterial 16S rRNA gene was performed using primers 515F and 806R (Bergmann et al. 2011). Fungal ITS1 region was amplified using primers ITS1F and ITS2R (Bellemain et al. 2010). The PCR reaction was carried out in triplicate Each sample was amplified under the following conditions: 94 °C for 5 min, followed by 35 cycles including 94 °C for 45 s, 50 °C for 60 s and 72 °C for 90 s, then 72 °C for 10 min for bacteria; and 94 °C for 3 min, followed by 30 cycles including 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, then 72 °C for 10 min for fungi. The PCR products were mixed and purified by Agarose Gel DNA purification kit (TaKaRa, Dalian, China). The amplicons were equally combined to produce two separate PCR pools (keeping bacterial and fungal amplicons separate) that were sent to Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China) for 300-bp paired-end sequencing on an Illumina MiSeq platform in two separate runs. The raw reads were deposited into the NCBI short-reads archive database under accession number SRP270966.
Bioinformatics analysis
The Raw Illumina FASTQ files were demultiplexed, quality filtered, and analyzed using QIIME v1.7.0 (Quantitative Insights Into Microbial Ecology) (Caporaso et al. 2010). Operational taxonomic units (OTUs) were grouped with a threshold of 97% pairwise identity by QIIME. Any sample that had fewer than 20 useable reads was discarded, resulting the unnormalized usable OTU table. Based on this table, a rarefied table was made by rarefying it to the minimum reads. Next, a frequency table was created normalized by transforming the data into relative abundance (Total Sum Scaling normalization (Lundberg et al. 2012)).
Microbial α-diversity and β-diversity analysis were performed using the free online platform of Majorbio Cloud Platform (www.majorbio.com). Indies of Sobs, Shannon, Ace and Chao1 were calculated as microbial α-diversity. The dissimilarity of the microbial communities was determined using principal coordinate analysis (PCoA) on Bray–Curtis distance. Permutational multivariate analyses of variance (PERMANOVA) using Bray–Curtis distances with 999 permutations was performed within each sample type to explore the percentage of variance explained by the factors of soil conditions and tobacco growth periods.
The LEfSe (Linear Discriminate Analysis (LDA) Effect Size) (Segata et al. 2011) algorithm was performed on the website (http://huttenhower.sph.harvard.edu/galaxy). A factorial Kruskal–Wallis sum-rank test (α = 0.05) was used to identify taxa with significant differential abundances between categories (using all-against-all comparisons) for the sequencing data collected in both July and September. The value of LDA was used to estimate the effect size of each differentially abundant feature. Taxa with logarithmic LDA value over 4.00 were selected to perform histogram figures. The number and relative abundance of shared taxa in healthy soils were further shown in Venn diagram and histogram.
Network analyses were performed using the molecular ecological network analyses (MENA) pipeline (http://ieg4.rccc.ou.edu/mena) with default settings and recommended similarity thresholds (Deng et al. 2012). The co-occurrence networks were visualized in Gephi (Ver. 0.9.2) (Bastian et al. 2009). OTUs with relative abundance over 0.1% in rhizosphere samples of July and September were used for network construction.
Statistical analysis
All statistical analyses were performed in SPSS Statistics 17.0 software program (SPSS Inc., USA). Significant differences in physical and chemical properties of soil samples and alpha diversity indices of soil microbial community across sampling region were determined by independent-sample t test.
Results
Bulk soil properties and tobacco bacterial wilt disease incidence
Differences of physical and chemical properties of bulk soil collected in March between diseased and healthy soils were shown in Table 1. Interestingly, although there were many differences in the chemical properties (AvN, AvK etc.), none of them reached the significance (p > 0.05).
Disease incidences of tobacco bacterial wilt in diseased soils were recorded in July of both 2016 and 2017. There were over 50% of tobacco plants showing typical bacterial-wilt symptoms in all the sampling sites (Table 2).
Soil microbial community α-diversity
For bacteria, a total of 27,609 raw sequence reads was obtained through 16S rRNA gene sequencing. There was no significant difference in the richness or evenness indices of the bacterial community in both March and September samples (Additional file 1: Table S2). The Shannon index was significantly higher in the healthy soils than diseased soils in July (p < 0.05).
For fungi, a total of 30,581 raw sequence reads was obtained through ITS sequencing. All the diversity indices of the fungal community in the diseased soils gradually decreased throughout the plant growth periods, and was lowest by September (Additional file 1: Table S2). In contrast, the richness and diversity indices of the healthy samples gradually increased, reaching the highest value in July.
Soil microbial community β-diversity
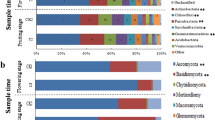
Proteobacteria (36.94 ± 0.64%; mean ± SE) was the most dominated bacterial phylum in soil samples, followed by Actinobacteria (19.42 ± 0.92%). Relative abundance of bacterial classes changed during different tobacco growth stages in both healthy and diseased soils (Fig. 1a). For healthy soils, a clear shift was seen from dominance of Alphaproteobacteria (18.89%) in the bulk soil samples collected in March to that of Betaproteobacteria (13.65%) in the rhizosphere samples collected in both July and September. For diseased soils, the decrease in relative abundance of Alphaproteobacteria (17.94%) were also seen in rhizosphere samples; however, this compensation of Betaproteobacteria (13.21%) was only in July samples.
Ascomycota (60.35 ± 2.81%) was the most abundant fungal phylum in soil samples, including Sordariomycetes (35.42 ± 4.47%), Eurotiomycetes (9.85 ± 3.17%), Dothideomycetes (7.09 ± 2.50%), Leotiomycetes (2.66 ± 1.22%) and Pezizomycetes (2.16 ± 1.31%) listed as top-10 classes (Fig. 1b). Sordariomycetes were higher in healthy soils (47.08 ± 0.50%) than in diseased ones (36.12 ± 1.06%). For the rhizosphere samples, there was a consistent enrichment of Eurotiomycetes in diseased soils (10.37 ± 3.92%), while Pezizomycetes was largely seen in healthy soils (6.27 ± 0.58%).
Besides, effects of soil conditions on both bacterial and fungal composition were clearly seen from the PCoA analysis (Fig. 2 and Additional file 1: Table S1). PERMANOVA analyses were further confirmed with significance of the factor of soil conditions in samples with and without bulk soil samples (p < 0.001; Additional file 1: Tables S3 and S4). Specifically, PCoA explained 22% and 12% of total variation in compositions of bacterial and fungal bacterial communities in rhizosphere soils. Additionally, there were few effects of tobacco growth periods on both bacterial and fungal community compositions in rhizosphere samples (Additional file 1: Table S4).
Rhizosphere microbial taxa discriminated by soil conditions
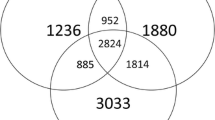
Rhizosphere soil samples collected in July and September were further compared with LEfSe to determine the discriminative taxa affected by the factor of soil conditions. In July samples, there were 10 taxa found in the heathy soils and 9 in the diseased soils (Fig. 3). The genus Ralstonia, to which the pathogen R. solanacearum belongs, was found in the diseased soils (logarithmic LDA = 4.25, p = 0.02). The most significant taxon in the healthy soils was the phylum Bacteroidetes (logarithmic LDA = 4.45, p < 0.001). In September samples, a total of 35 taxa were found: 13 taxa in the healthy soils and 14 taxa in the diseased soils (Fig. 3). The class Alphaproteobacteria (logarithmic LDA = 4.78, p = 0.02) was the most significant taxon, followed by the genus Sphingomonas (logarithmic LDA = 4.62, p < 0.001) in diseased soils, while the order Flavobacteriales (logarithmic LDA = 4.78, p < 0.001) had the largest effects in healthy soils. Among those discriminative taxa in healthy soils, there were 6 taxa shown in both July and September samples (Fig. 4a), and Flavobacterium (in July samples, 1.64 ± 0.11%; in September samples, 3.29 ± 0.42%) was the only shared genera (Fig. 4b).
With respect to fungi, the LEfSe analysis revealed that there were more discriminative taxa in healthy soils than diseased soils. In July samples, 13 taxa were identified for the healthy soils, and 4 taxa for the diseased soils (Fig. 3). The genus Fusarium (logarithmic LDA = 4.72, p = 0.02) was found as the most significant taxon in diseased soils, while the order Sordariales (logarithmic LDA = 4.86, p < 0.001) had the largest effects in healthy soils. In September samples, there were 15 taxa found in the heathy soils and 2 in the diseased soils (Fig. 3). Among those discriminative taxa in healthy soils, there were 9 taxa shown in both July and September samples (Fig. 4c). The genus Chaetomium (in July samples, 10.32 ± 3.22%; in September samples, 10.52 ± 6.23%) was the most significant genus in healthy soils collected in both July and September (Fig. 4d).
Rhizosphere microbial networks explained by soil conditions
For bacterial networks, both the network size and the degree of connectivity showed large differences between healthy and diseased soils (Fig. 5a). The topologies of the healthy networks, namely, the number of nodes and the number of links, were higher than that of the diseased networks (Additional file 1: Table S5). In total, there were 231 nodes and 433 links in healthy soils, whereas 156 nodes and 216 links in diseased soils (Fig. 5a). Moreover, using betweenness centrality, Flavobacterium (OTU3316, OTU4570) and Pseudomonas (OTU1200, OTU3398, OTU9417) were found as key genera in the networks of healthy soils.
Co-occurrence patterns of a bacteria and b fungi in healthy and diseased rhizosphere samples. Nodes indicate taxonomic affiliation at phylum level. Red lines indicate positive correlations, and green lines indicate negative correlations. The size of each node is proportional to the betweenness centrality. Detailed properties of network indices were listed in Additional file 1: Table S5
For fungal networks, the number of nodes and links was lower in diseased soils than healthy soils (Fig. 5b and Additional file 1: Table S5). In July samples, there were 125 links and 70 nodes in healthy soils, and 115 links and 63 nodes in diseased soils (Fig. 5b). In September sample, the network of healthy soils contained 163 links and 81 nodes, while the network of diseased soils contained 128 links and 65 nodes (Fig. 5b). High betweenness centrality values were found for the genera Fusarium (OTU672, OTU4590) and Mortierella (OTU1037, OTU1987) in the networks of healthy soils.
Discussion
Here, we investigated bacterial and fungal communities from the early growth stage to the last two growth periods and the microbial community in the later stages of tobacco growth plays an integral role in plant-pathogen interactions. Increasing evidence has shown that the rhizosphere microbial community plays an indispensable role in relieving nutrient stress and responding to pathogenic micro-invasion by using root exudates from plant roots (Okubo et al. 2016). Plants are able to recruit specific bacteria and fungi for defense against bacterial wilt in the rhizosphere (Lareen et al. 2016). Additionally, the specific selection of microbiome by plants in the rhizosphere mainly differs at different developmental stages (Yang and Crowley 2000; Bulgarelli et al. 2012). Infection by pathogenic bacteria is the main cause of plant recruitment of beneficial microorganisms in the rhizosphere (Bakker et al. 2013), and the antagonistic effect on pathogens is enhanced during plant development (Hu et al. 2020). Specific resident plant rhizosphere bacterial communities that adapt to plants play important roles in both optimize growth and protecting against infection by pathogens. The recruitment of beneficial microorganisms can also change the physiological function of plants to allow them to resist aerial pathogens (Kumar et al. 2012).
Although the rhizosphere effects on microbial assemblage is proved to be crucial in plant health, it also have been reported that the initial variation in soil bacterial composition and functioning can determine the outbreak of bacterial wilt disease (Wei et al. 2019). And thus, understanding the difference of microbial community in healthy and diseased soils are important regarding to plant-pathogen interactions. In this study, we confirmed significant shifts in the diversity and abundance of bacterial and fungal communities associated with healthy and diseased soils. Indeed, there are increasing studies focusing on the microbial indicators associated with the suppression of tobacco bacterial wilt (Liu et al. 2016; She et al. 2017). Here, we used LEfSe and co-occurrence network to investigate the keystone species as well.
Network analysis have been widely used to determine the association and co-occurrence complexity of microorganisms (Su et al. 2020). Our study shows that the rhizosphere soil co-occurance network of healthy tobacco plants is more complicated than that of diseased tobacco plants. This is consistent with previous research results (Yang et al. 2017). Indeed, microbial communities with relatively high diversity have better resistance to invasion by pathogenic bacteria (Hu et al. 2020). Interactions between microbial species can affect disease dynamics by changing the relative and absolute density of pathogens in the host-associated microbiome (Mendes et al. 2011; Mueller and Sachs 2015). In the healthy and diseased rhizosphere soil networks, we observed positive interactions between nodes, indicating niche overlap, as well as negative interactions, which suggest competition or exclusion (Faust and Raes 2012). Competitive interactions and the production of antimicrobial compounds play an important role in controlling pathogen density and disease dynamics (Wei et al. 2015). Further experimentation is needed to decipher the impact of competitive microbes on soil microbial ecological networks and plant health.
The greater variety of potential key taxa observed in the rhizosphere samples might be beneficial to maintain plant health. Analyses of LEfSe and network analysis showed that Flavobacterium and Pseudomonas may be the most active microbial species in healthy soil. Flavobacterium can play a role in biological control by producing antibacterial effect factors, antibacterial substances, extracellular macromolecular degrading enzymes, etc. (Bernardet and Nakagawa 2006; Kwak et al. 2018; Carrion et al. 2019). Pseudomonas which can produce antifungal/inhibitory compounds and siderophores that can control against bacterial wilt disease (Ramesh and Phadke 2012; Chandrasekaran et al. 2016). High Pseudomonas diversity can reduce R. solanacearum density in the rhizosphere and decrease the disease incidence due to both intensified resource competition and interference with the pathogen (Hu et al. 2016). Notably, the network analysis revealed the Mortierella and Fusarium were key species in healthy soils. It was also reported from previous studies that Mortierella was an indicator species in disease suppressive soils (Expósito et al. 2017; Xiong et al. 2017). Mortierella can produce antibiotics, and has potential antagonist activity against various plant pathogens (Tagawa et al. 2010). F. oxysporum confers biocontrol against root diseases in various plants (Lamo and Takken 2020). Thus, a potentially beneficial microbiome may form cooperative associations with other taxa to maintain plant health.
In conclusion, our results showed that there are significant differences in microbial composition between healthy and diseased soils. Both factors of soil conditions and tobacco growth periods can have an influence on the bulk and rhizosphere microbial composition. Yet, the impact of soil conditions is larger than that of tobacco growth periods in the rhizosphere soils. Discriminative taxa determined by LEfSe and network analysis in healthy soils showed beneficial potentials. This implies that steering soil microbiome in a beneficial way could have great opportunities to maintaining soil-borne disease. However, these findings need to be further confirmed in greenhouse experiments.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional files. Sequence datasets for all the samples were deposited into the NCBI short-reads archive database under accession number SRP270966.
Abbreviations
- HM/DM:
-
Soils collected from healthy (H) and diseased (D) fields in March
- HJ/DJ:
-
Soils collected from healthy (H) and diseased (D) fields in July
- HS/DS:
-
Soils collected from healthy (H) and diseased (D) fields in September
- OTU:
-
Operational taxonomic units
- PCoA:
-
Principal coordinate analysis
- PERMANOVA:
-
Permutational multivariate analyses of variance
- LEfSe:
-
Linear discriminate analysis effect size
- LDA:
-
Linear discriminant analysis
References
Bakker PAHM, Berendsen RL, Doornbos RF, Wintermans PCA, Pieterse CMJ (2013) The rhizosphere revisited: root microbiomics. Front Plant Sci 30:165
Bao S (2010) Analytical methods of soil agro-chemistry. Beijing, China.
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. In: Proceedings of the third international conference on weblogs and social media, San Jose, California, 17–20 May 2009
Bellemain E, Carlsen T, Brochmann C, Coissac E, Taberlet P, Kauserud H (2010) ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol 10:189
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bergmann GT, Bates ST, Eilers KG, Lauber CL, Caporaso JG, Walters WA, Knight R, Fierer N (2011) The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol Biochem 43:1450–1455
Bernardet JF, Nakagawa Y (2006) An introduction to the family flavobacteriaceae. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes. Springer, New York
Bulgarelli D, Rott M, Schlaeppi K, Themaat EVLV, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner F, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley REC, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows integration and analysis of high-throughput community sequencing data. Nat Methods 7:335–356
Carrión VJ, Perez-Jaramillo JE, Cordovez V, Tracanna V, de Hollander M, Buck RD, Mendes LW, van Ijcken WFJ, Expósito RG, Elsayed SS, Mohanraju P, Arifah A, van der Oost J, Paulson JN, Mendes R, van Wezel G, Medema MH, Raaijmakers JM (2019) Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 366:606–612
Cha JY, Han S, Hong HJ, Cho H, Kim D, Kwon Y, Kwon SK, Crusemann M, Lee YB, Kim JF, Giaever G, Nislow C, Moore BS, Thomashow LS, Weller DM, Kwak YS (2016) Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J 10:119–129
Chandrasekaran M, Subramanian D, Yoon E, Kwon T, Chun SC (2016) Meta-analysis reveals that the genus pseudomonas can be a better choice of biological control agent against bacterial wilt disease caused by Ralstonia solanacearum. Plant Pathol J 32:216–227
Chaparro JM, Badri DV, Vivanco JM (2014) Rhizosphere microbiome assemblage is affected by plant development. ISME J 8:790–803
Chen S, Qi G, Ma G, Zhao X (2020a) Biochar amendment controlled bacterial wilt through changing soil chemical properties and microbial community. Microbiol Res 231:1–9
Chen X, Krug L, Yang M, Berg G, Cernava T (2020b) Conventional seed coating reduces prevalence of proteobacterial endophytes in Nicotiana tabacum. Ind Crops Prod 155:112784
de Boer W (2017) Upscaling of fungal-bacterial interactions: from the lab to the field. Curr Opin Microbiol 37:35–41
Deng Y, Jiang Y, Yang Y, He Z, Luo F, Zhou J (2012) Molecular ecological network analyses. BMC Bioinf 13:113
Expósito RG, de Bruijn I, Postma J, Raaijmakers JM (2017) Current insights into the role of rhizosphere bacteria in disease suppressive soils. Front Microbiol 8:2529
Faust K, Raes J (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550
Fu L, Penton CR, Ruan Y, Shen Z, Xue C, Li R, Shen Q (2017) Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol Biochem 104:39–48
Gao L, Wang R, Gao J, Li F, Huang G, Huo G, Guang H, Liu Z, Tang W, Shen G (2020) Analysis of the structure of bacterial and fungal communities in disease suppressive and disease conducive tobacco-planting soils in China. Soil Res 58:35–40
Genin S, Denny TP (2012) Pathogenomics of the Ralstonia solanacearum species complex. Annu Rev Phytopathol 50:67–89
Guo Q, Dong W, Li S, Lu X, Wang P, Zhang X, Wang Y, Ma P (2014) Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol Res 169:533–540
Hu J, Wei Z, Friman VP, Gu S, Wang X, Eisenhauer N, Yang T, Ma J, Shen Q, Xu Y, Jousset A (2016) Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. Mbio 7:e01790-e1816
Hu J, Wei Z, Kowalchuk GA, Xu Y, Shen Q, Jousset A (2020) Rhizosphere microbiome functional diversity and pathogen invasion resistance build up during plant development. Environ Microbiol 22:5005–5018
Inceoglu OL, Al-Soud WA, Salles JF, Alexander VS, Elsas JDV (2011) Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS ONE 6:e23321
Jiang G, Zhong W, Jin X, Chen H, Yong Z, She X, Macho AP, Wei D, Liao B (2017) Bacterial wilt in China: history, current status, and future perspectives. Front Plant Sci 8:1549
Kumar AS, Lakshmanan V, Caplan JL, Powell D, Czymmek KJ, Levia DF, Bais HP (2012) Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J 72:694–706
Kwak MJ, Kong HG, Choi K, Kwon SK, Song JY, Lee J, Lee PA, Choi SY, Seo M, Lee HJ, Jung EJ, Park H, Roy N, Kim H, Lee MM, Rubin EM, Lee SW, Kim JF (2018) Rhizoshere microbiome structure alters to enable wilt resistance in tomato. Nat Biotechnol 36:1100–1109
Lamo FJD, Takken FLW (2020) Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front Plant Sci 10:187
Lareen A, Burton F, Schäfer P (2016) Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol 90:575–587
Li X, Rui J, Mao YJ, Yannarell A, Mackie R (2014) Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar. Soil Biol Biochem 68:392–401
Liu Y, Shi J, Feng Y, Yang X, Li X, Shen Q (2013) Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol Fert Soils 49:447–464
Liu X, Zhang S, Jiang Q, Bai Y, Shen G, Li S, Ding W (2016) Using community analysis to explore bacterial indicators for disease suppression of tobacco bacterial wilt. Sci Rep 6:36773
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Trembkay J, Engelbrektson A, Kunin V, del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90
Mallon CA, Poly F, Le Roux X, Marring I, van Elsas JD, Salles JF (2015) Resource pulses can alleviate the biodiversity-invasion relationship in soil microbial communities. Ecology 96:915–926
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JHM, Piceno YM, DeSantis TZ, Andersen GL, Bakker PAHM, Raaijmakers JM (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100
Mueller UG, Sachs JL (2015) Engineering microbiomes to improve plant and animal health. Trends Microbiol 23:606–617
Okubo A, Matsusaka M, Sugiyama S (2016) Impacts of root symbiotic associations on interspecific variation in sugar exudation rates and rhizosphere microbial communities: a comparison among four plant families. Plant Soil 399:345–356
Qi G, Chen S, Ke L, Ma G, Zhao X (2020) Cover crops restore declining soil properties and suppress bacterial wilt by regulating rhizosphere bacterial communities and improving soil nutrient contents. Microbiol Res 238:126505
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Ramesh R, Phadke GS (2012) Rhizosphere and endophytic bacteria for the suppression of eggplant wilt caused by Ralstonia solanacearum. Crop Prot 37:35–41
Rosenzweig N, Tiedje JM, Quensen IJF, Meng Q, Hao JJJPD (2012) Microbial communities associated with potato common scab-suppressive soil determined by pyrosequencing analyses. Plant Dis 96:718–725
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60
She S, Niu J, Zhang C, Xiao Y, Chen W, Dai L, Liu X, Yin H (2017) Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch Microbiol 199:267–275
Su L, Zhang L, Nie D, Kuramae EE, Shen B, Shen Q (2020) Bacterial tomato pathogen Ralstonia solanacearum invasion modulates rhizosphere compounds and facilitates the cascade effect of fungal pathogen Fusarium solani. Microorganisms 8:806
Tagawa M, Tamaki H, Manom A, KoyamaO KamagataY (2010) Isolation and characterization of antagonistic fungi against potato scab pathogens from potato field soils. FEMS Microbiol Lett 305:136–142
Wang L, Li X (2019) Steering soil microbiome to enhance soil system resilience. Crit Rev Microbiol 45:743–753
Wang W, Luo X, Chen Y, Ye X, Wang H, Cao Z, Ran W, Cui Z (2019) Succession of composition and function of soil bacterial communities during key rice growth stages. Front Microbiol 10:421
Wei Z, Yang T, Friman VP, Xu Y, Shen Q, Jousset A (2015) Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat Commun 6:8413
Wei Z, Hu J, Gu YA, Yin S, Xu Y, Jousset A, Shen Q, Friman VP (2018) Ralstonia solanacearum pathogen disrupts bacterial rhizosphere microbiome during an invasion. Soil Biol Biochem 118:8–17
Wei Z, Gu Y, Friman VP, Kowalchuk GA, Xu Y, Shen Q, Jousset A (2019) Initial soil microbiome composition and functioning predetermine future plant health. Sci Adv 5:eaaw0759
Xiao Y, Liu X, Meng D, Tao J, Gu Y, Yin H, Li J (2018) The role of soil bacterial community during winter fallow period in the incidence of tobacco bacterial wilt disease. Appl Microbiol Biotechnol 102:2399–2412
Xiong W, Zhao Q, Zhao J, Xun W, Li R, Zhang R, Wu H, Shen Q (2015) Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microb Ecol 70:209–218
Xiong W, Rong L, Ren Y, Shen Q (2017) Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol Biochem 107:198–207
Yang CH, Crowley DE (2000) Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl Environ Microbiol 66:345–351
Yang H, Li J, Xiao Y, Gu Y, Liu H, Liang Y, Liu X, Hu J, Meng D, Yin H (2017) An integrated insight into the relationship between soil microbial community and tobacco bacterial wilt disease. Front Microbiol 8:2179
Yuan J, Chaparro JM, Manter DK, Zhang R, Vivanco JM, Shen Q (2015) Roots from distinct plant developmental stages are capable of rapidly selecting their own microbiome without the influence of environmental and soil edaphic factors. Soil Biol Biochem 89:206–209
Acknowledgements
We thank Chongqing Tobacco Corporation for allowing us to take samples from their fields. We also thank anonymous reviewers and editors for their insightful suggestions and careful reading.
Funding
This research was funded by the Chongqing Postdoctoral Science Foundation (cstc2020jcyj-bshX0127), National Natural Science Foundation of China (32102300), China National Tobacco Corporation (110202101047 LS-07) and Chongqing Tobacco Corporation (B20211NY1316).
Author information
Authors and Affiliations
Contributions
XL, LL and WD conceived and designed the experiments; LL, JG, LZ and KH performed the experiments. XL, LL and QJ analyzed the data. XL and WD wrote the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1
. PCoA of the rhizosphere soil a) bacterial and b) fungal community compositions. Abbreviations as in Fig. 2. Table S1. Geographic information of sampling sites. Table S2. Alpha-diversity indices of soil bacterial and fungal community (mean ± SE, N = 3). Table S3. Results of PERMANOVA analysis showing effects of different factors on soil1 microbial composition. Table S4. Results of PERMANOVA analysis showing effects of different factors on rhizosphere2 microbial composition. Table S5. Co-occurrence network properties of rhizosphere microbial communities in different samples. (DOCX 91 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Liu, L., Gong, J. et al. Soil conditions on bacterial wilt disease affect bacterial and fungal assemblage in the rhizosphere. AMB Expr 12, 110 (2022). https://doi.org/10.1186/s13568-022-01455-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-022-01455-1