Abstract
Glycosaminoglycans, such as hyaluronic acid and chondroitin sulphate, are not only more and more required as main ingredients in cosmeceutical and nutraceutical preparations, but also as active principles in medical devices and pharmaceutical products. However, while biotechnological production of hyaluronic acid is industrially established through fermentation of Streptococcus spp. and recently Bacillus subtilis, biotechnological chondroitin is not yet on the market. A non-hemolytic and hyaluronidase negative S. equi subsp. zooepidemicus mutant strain was engineered in this work by the addition of two E. coli K4 genes, namely kfoA and kfoC, involved in the biosynthesis of chondroitin-like polysaccharide. Chondroitin is the precursor of chondroitin sulphate, a nutraceutical present on the market as anti-arthritic drug, that is lately being studied for its intrinsic bioactivity. In small scale bioreactor batch experiments the production of about 1.46 ± 0.38 g/L hyaluronic acid and 300 ± 28 mg/L of chondroitin with an average molecular weight of 1750 and 25 kDa, respectively, was demonstrated, providing an approach to the concurrent production of both biopolymers in a single fermentation.
Similar content being viewed by others
Introduction
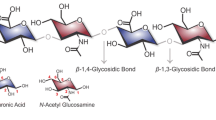
Hyaluronic acid (HA) and chondroitin sulphate (CS) are important glycosaminoglycans (GAG) found in the extracellular matrix of vertebrates, and, although their applications in the biomedical field are quite diverse, growing interest towards both these compounds has indeed been observed in the last decade (Liu et al. 2011; De Angelis 2012). Due to its multiple properties including a high biocompatibility and lack of immunogenicity, HA is a heavily researched molecule with applications in cosmetics and viscosurgery, healing and regeneration of wounds and lately investigated as a drug delivery agent (Maytin 2016; Tripodo et al. 2015; Moscovici 2015). CS on the other hand, is a nutraceutical marketed as anti-arthritic drug, and also an active pharmaceutical ingredient (API) in oral formulations for osteoarthritis (Henrotin et al. 2010); studies towards other interesting applications such as the production of biomaterials for tissue engineering are also under development (Christiani et al. 2016; Bishnoi et al. 2016). Moreover the combination of both HA and CS was shown to be effective for the treatment of urinary infections (Lazzeri et al. 2016; Ciani et al. 2016; Cervigni et al. 2016), cartilage repair (Henson et al. 2012), and early symptomatic knee osteoarthritis (Galluccio et al. 2015).
HA was the first GAG produced by fermentation, and more than 90% of HA present on the market is obtained from group C Streptococcus zooepidemicus, as well as more recently from Bacillus subtilis (de Olivera et al. 2016). K4 and K5 polysaccharides are consolidated precursors of chondroitin and heparin; however, CS is still obtained from limited and potentially harmful, animal sources such as chicken keel, shark fins, and swine or bovine trachea. Several efforts have been made to use E. coli K4, or its biosynthetic machinery in a different background, to generate cell factories for the production of chondroitin, and encouraging results were obtained (Cimini et al. 2013, 2015; He et al. 2015; Jin et al. 2016). The reason lies in the fact that the capsular polysaccharide of this uropathogenic strain, composed of alternating residues of N-acetylgalactosamine (GalNAc) and glucuronic acid (GlcA), has a structure that closely resembles the backbone of CS. Differences regard the presence of a fructose side branch on the GlcA residues and the absence of sulphate groups. Biotechnological chondroitin (BC), produced from recombinant E. coli K4 (Cimini et al. 2013), was evaluated in comparison with CS showing a higher reduction of the inflammatory response in IL-1b treated chondrocytes and the enhancement of their proliferation and phenotype preservation (Stellavato et al. 2016). Furthermore, the chemical conversion of biotechnological chondroitin into CS by fructose branches cleavage and selective sulphation was recently accomplished (Bedini et al. 2011).
However, the gram− E. coli K4 releases in the medium also lipopolysaccharides that are the main product contaminants and need to be removed during the purification strategy. For this reason we focused on the use of a gram+ strain belonging to a genus that is widely used for the production of hyaluronan (Liu et al. 2011).
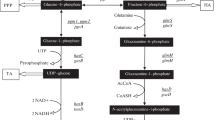
The gene cluster responsible for the biosynthesis of the K4 polysaccharide is composed of three regions, two of which (1 and 3) are conserved among group II E. coli strains and dedicated to the transport and secretion of the CPS. The central region, region 2, includes genes involved in the synthesis of the serotype specific polysaccharide, and in polymer assembly and fructosylation. Among the known gene functions kfoC codes for chondroitin polymerase (Ninomiya et al. 2002), a bifunctional enzyme that transfers nucleotide sugars to the growing end of the polysaccharide, and kfoA codes for an epimerase that converts N-acetylglucosamine (GlcNAc) into GalNAc. In the present work the E. coli K4 genes, kfoC and kfoA, were expressed in a previously obtained non hemolytic, hyaluronidase negative S. equi subsp. zooepidemicus BA06 host (Schiraldi et al. 2010) to implement its UDP-biosynthesis pathway and polymer assembly machinery for the production of chondroitin, thereby generating a cell factory for the obtainment of both biopolymers in a single fermentation event.
Materials and methods
Bacterial strains
Lactococcus lactis NZ900 was used as an intermediate host for plasmid construction. The mutant strain (hyaluronidase-free and haemolytic negative) S. equi subsp. zooepidemicus BA06 strain was obtained as previously described (Schiraldi et al. 2010). Plasmid pNZ8148 was obtained from Nizo (Netherlands).
Materials
Genomic DNA and plasmid DNA were isolated using Qiagen DNeasy kit, Qiagen miniprep kit, (Qiagen, Valencia, CA) respectively according to the manufacturer’s instructions. Restriction endonuclease digestions, DNA ligations, agarose gel electrophoresis were performed using standard techniques (Sambrook and Russel 2001).
Construction of the kfoA and kfoC overexpressing strains
The kfoA and kfoC genes were amplified from E. coli K4 genomic DNA. kfoA was amplified with primers kfoA_Up: 5′- GGACGGTGCCATGGATGAATATATTAGTTACAGGTGGAG-3′containing the NcoI recognition site, and KfoA_Dw 5′-TCTCTAGACGCGGATCCTTAAATATAACCATTTGGGTTTTTCA-3′ containing the BamHI and XbaI restriction sites. kfoC was amplified with primers kfoC_ Up 5′-CGGGATCCAGTGAGGAGTTACTGATGAGTATTCTTAATCAAG-3′ and kfoC_Dw 5′-GAGCTCTTATAAATCATTCTCTATTTTTTCC-3′, containing the BamHI and SacI restriction sites respectively. Polymerase chain reaction (PCR) was carried out with Expand High fidelity PCR System (Roche, Monza. Italy) according to the manufacturer’s protocol. DNA fragments were recovered from agarose gels using the Qiaquick gel extraction kit (Qiagen, Valencia, CA). The kfoA and kfoC genes were sequentially cloned into plasmid pNZ8148 using L. lactis as intermediate host to improve transformation efficiency (Additional file 1).
Efficiency of sub-cloning was verified by performing double digestions with BamHI and SacI on the recombinant plasmid extracted from colonies of L. lactis growing on selective medium. Restriction endonucleases were purchased from New England Biolabs and ligases were purchased from Invitrogen (Carlsband, CA). Nucleotide sequencing of all PCR fragments cloned was carried out at BMR Genomics (Padova, Italy) to check whether any mutation was introduced.
pNZ8148kfoAkfoC was isolated from L. lactis-pNZ8148kfoAkfoC and electroporated into S. equi subsp. zooepidemicus BA06 slightly changing the protocol described by Marcellin et al. (2010). Briefly, cells were grown on THY supplemented with 2 M glycine. About 250 mL of fresh medium were inoculated with an o/n pre-culture and grown for 3 h at 37 °C until OD530 reached a value of about 0.6. Prior to harvesting, 0.2 mg/mL of hyaluronidase (H3506, Sigma-Aldrich) were added to the broth and incubated for 30 min at the same temperature. After centrifugation the pellet was resuspended in 0.5 M sucrose and washed twice; finally it was resuspended in about 600 μL of the same solution. The recombinant plasmid pNZ8148kfoAkfoC was added to the ice cold cell aliquot and electroporation was carried out on a Biorad Bio-Rad Gene Pulser (2 mm cuvettes, 2.0 kV, 200 Ω, 25 μF) according to the protocol suggested by the manufacturer. Selection was performed on THY supplemented with 5 μg/mL of chloramphenicol. Plates were incubated o/n at 37 °C.
Shake flask experiments
All cultivations were conducted in 3 L flasks filled with 0.6 L of medium in order to keep a 5:1 air–liquid ratio. Growth temperature was set at 37 °C and the culture was agitated at 140 rpm, in a rotary shaker incubator (model Minitron, Infors, Bottmingen, Switzerland). Experiments were performed on the medium containing per L: sucrose 17 ± 1 g, yeast extract 10 g, KH2PO4 2 g, K2HPO4 9.7 g/L, MnSO4·4H2O 0.1 g, and 1 mL of microelement solution (CaCl2 2 g/L, MnSO4·4H2O 0.05 g/L, CuSO4·5H2O 0.019 g/L, ZnCl2 0.046 g/L). pH was adjusted to 7.2 before strain inoculation. The medium also contained 5 μg/mL of chloramphenicol to avoid plasmid loss. Twenty ng/mL of nisin were added about 2–3 h after inoculating the culture to induce expression of recombinant proteins.
Experiments with the addition of 0.5 mM GalNAc, or 2 mM phosphatidylcholine after induction were also performed.
Determination of sucrose and acids produced during growth was performed by HPLC (UHPLC Dionex Ultimate 3000; Thermofisher) on a Alltech IOA-2000 column (250 mm × 6.5 mm ID). Three mL of broth collected at T0 and at the end of the process were centrifuged and supernatants were ultrafiltered on 3 kDa centricon devices (Millipore, Bedford, MA, USA) at 5000×g and the flow through was used for analyses. Runs were performed at 40 °C with 0.1% v/v sulphuric acid in water as mobile phase at a flow rate of 0.6 mL/min. Detection was performed via UV absorbance at 200 nm and refraction index (Shodex RI-101 detector, Max auto step 5.1 s, temperature 32 °C, rise time 1 s, polarity plus, record range 512 µRIU, integrator range 500 µRIU/UV).
Extraction of intracellular polysaccharides
In order to extract intracellular chondroitin and HA, about 10 g of wet cells were resuspended in 20 mL of distilled water and autoclaved at 120 °C for 15 min. After centrifugation the supernatant was recovered and precipitated on ice with 4 volumes of cold ethanol 96% v/v, and stored at 4 °C o/n. The pellet, recovered after centrifugation was treated with 1 mg/mL DNase I (Applichem) in a buffer containing 100 mM Tris pH 7.5, 50 mM MgCl2 and 10 mM CaCl2 for 1 h at 37 °C and successively digested with 2.5 mg/mL of Protease K from Aspergillus (Sigma-Aldrich) for 2 h at 56 °C. A second precipitation was repeated on the sample o/n at 4 °C and the resulting pellet was dried and used for: (a) hydrodynamic characterization (b) quantification of uronic acids through carbazole assay (Bitter and Muir 1962) (c) determination of relative ratios of HA and chondroitin through high performance anion exchange chromatography with pulsed amperometric detection (HPAE-PAD) monosaccharide determinations after hydrolysis.
The powder obtained from the control sample was further purified to verify the production of chondroitin in S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC, through structure determination by NMR analysis.
Purification of intracellular chondroitin from S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC by fast protein liquid chromatography (FPLC)
About 100 mg of powder were dissolved in 5 mL of buffer A (20 mM sodium acetate, 0.5 M sodium chloride, pH 7.4) and loaded on an anion exchange column (HiPrep Q Sepharose 16/10 HP, 1.6 × 10.0 cm, GE Healthcare, Milan, Italy), previously equilibrated with the same buffer, using an ÄKTA Explorer 100 purifier system (GE Healthcare, Milan, Italy), connected to the software UNICORN (GE Healthcare, Milan, Italy). The samples were eluted by applying a three steps gradient at 5, 10.5 and 100% of buffer B (20 mM sodium acetate, 3 M sodium chloride, pH 7.4) in 3 column volumes, at a flow rate of 3 mL/min and collected in 2 mL fractions. The chromatographic profiles were registered detecting the signal at 215 nm. The fractions containing a single peak were pooled together and each pool was loaded on a desalting column (HiPrep 26/10 Desalting, 2.6 × 10.0 cm, GE Healthcare, Milan, Italy) previously equilibrated with pure water. Elution was performed by using the same ÄKTA purifier system, at flow rate of 1 mL/min, in 1.5 column volumes; the chromatographic profiles were detected at 215 nm, the eluted peaks were collected and then freeze-dried (Christ Epsilon 2-6D, Martin Christ, Germany). After lyophilisation the peak fractions were analyzed by Size exclusion chromatography-triple detector array (SEC-TDA), NMR and hydrolysed for HPAE-PAD sugar nucleotide determinations.
Precipitation of extracellular HA and chondroitin
After growth in shakeflask the broth was centrifuged at 6000×g, 4 °C for 30 min. The supernatant was precipitated with 1.8 volumes of cold ethanol (99.9% v/v) o/n at 4 °C to remove HA. The precipitate was recovered by centrifugation and vacuum dried at 40 °C for 18 h. The supernatant underwent a second precipitation, to recover chondroitin, with ethanol up to 4 volumes at 4 °C o/n and the recovered precipitate was dried at 40 °C under vacuum.
Fermentation experiments
Fermentation experiments were carried out on a Biostat C plus reactor (Sartorius Stedim; Melsungen, Germany), with 1.6 L working volume. Agitation was provided by 1 rushton impeller and 2 paddle impellers, suitable for viscous liquid mixing. Fermentation medium contained per L: sucrose 70 g, MgSO4·7H2O 2 g, yeast extract 20 g, Na2HPO4·12H2O 2.23 g, K2SO4 1.3 g, arginin 0.05 g and 2.5 mL of microelement solution. The preculture was performed in 1 L shakeflasks filled with 100 mL of medium, reported in the previous section, and grown on a rotary shaker at 200 rpm and 37 °C for 12–14 h. When pH dropped to 5 the flask culture was aseptically used to inoculate the fermenter: inoculum size was 5% of the volume of fermentation medium. A constant pH was maintained at 7.2 by automated addition of 6 M NaOH and 30% v/v sulfuric acid. Stirring and airflow rate were set at 200 rpm and 1.2/1.4 vvm for the duration of the experiment. The medium also contained 5 μg/mL of chloramphenicol and addition of 20 ng/mL of nisin was performed about 3 h after fermentation start. A pulse of sucrose re-establishing a concentration of 20 g/L in the broth was performed after 10 h of growth. At least four samples were withdrawn during the initial batch phase and at the end of the experiments to measure substrate consumption and production of lactic acid by HPLC as described in “Shakeflask experiments” section. The overall process lasted 18 h.
Fermentation broth downstream processing to obtain heteropolysaccharides (HA and chondroitin)
The recovered fermentation broth was added with 5% v/v of trichloroacetic acid (from a 50% w/v solution) before centrifugation at 6000×g for 60 min. The pH of the clarified supernatant was adjusted to 6.5 ± 0.2 with 6 M NaOH. A first ultrafiltration (UF) step was performed on a tangential flow filtration system (Sartoflow Alpha crossflow system, Sartorius) by using a polyethersulfone membrane (0.1 m2) with a cut-off of 100 kDa. The supernatant was concentrated about eightfold, diafiltered with 2 volumes of bidistilled water and the retentate was collected. The cassette was washed with 100 mL of water and the recovered solution was added to the concentrated supernatant and precipitated with 1.8 volumes of cold ethanol (4 °C) o/n. The precipitate was vacuum dried at 40 °C for 18 h.
The 100 kDa permeate was further concentrated on UF polyethersulfone membrane (0.1 m2) with a cut-off of 10 kDa, concentrated tenfold and diafiltered with 300 mL of bidistilled water. The cassette was washed with 100 mL of purified water. Conductivity of the 10 kDa retentate and wash fraction was adjusted to about 15 mS/cm with NaCl before precipitation with 4 volumes of cold ethanol (4 °C) o/n. The recovered precipitate was vacuum dried at 40 °C for 18 h. The dried powder obtained from the 100 and 10 kDa retentate samples were analysed by SEC-TDA, HPAE-PAD and carbazole assay.
Intracellular polysaccharides were extracted as described in the previous paragraph (“Extraction of intracellular polysaccharides” section), and quantified as described above.
Molecular weight determination
The molecular mass determinations of HA and chondroitin were carried out using the SEC-TDA 305 equipment by Viscotek (Malvern Instruments, Italy). Dried samples obtained after fermentation downstream treatments were dissolved in water and analysed whereas samples obtained from shakeflask experiments were dissolved in water and diafiltered with 2 volumes of bidistilled water on 3 kDa centrifugal filter devices (YM-10 Centricon, Millipore, Bedford, MA, USA) at 5000×g to remove salts and low molecular weight contaminants. Analyses were performed at concentrations ranging from 0.1 to 0.4 g/L for HA, and from 0.5 to 4 g/L for chondroitin, to have a column load for each sample (injection volume × sample concentration × intrinsic viscosity) of approximately 0.2 dL, and runs were performed at 40 °C with a running time of 50 min. The fragment molecular weight distribution, molecular size distribution, polydispersity, hydrodynamic radius, and intrinsic viscosity were determined as described by La Gatta et al. (2010) and Restaino et al. (2017).
HPAE-PAD
The aminosugars were identified in extracellular and intracellular polysaccharide samples by using the high-performance chromatographic system equipped with a pulsed amperometric detector (PAD, Thermo Fisher Scientific, Italy) on an anion exchange column (Carbopac PA1, Thermo Fisher Scientific, Italy) as previously demonstrated by Marcellin et al. (2009). About 7 mg of powder (obtained after lyophilisation) were hydrolyzed in 1 M HCl for 18 h at 100 °C and analysed as described by Restaino et al. (2017).
NMR analysis
NMR spectra were recorded with a Bruker DRX-600 (1H: 600 MHz, 13C: 150 MHz) instrument equipped with a cryo probe, in D2O (acetone as internal standard, 1H: (CH3)2CO at δ = 2.22 ppm; 13C: (CH3)2CO at δ = 31.5 ppm). The distorsionless enhancement by polarization transfer-heteronuclear single-quantum correlation (DEPT-HSQC) experiments were measured in the 1H-detected mode by single quantum coherence with proton decoupling in the 13C domain, by using data sets of 2048 × 256 points and typically 32 increments.
Results
Construction of recombinant S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC
The pathways leading to HA production in Streptococcus spp. and the implemented changes performed in this work to obtain concurrent biosynthesis of chondroitin are shown in Fig. 1. PCR products were obtained utilizing E. coli K4 chromosomal DNA as a template, and specific oligonucleotide primers as indicated in the “Materials and methods” section. Sub-cloning for the construction of the recombinant plasmid pNZ8148kfoAkfoC, was performed in L. lactis. After electroporation recombinant cells were selected on medium containing chloramphenicol. Double digestions that yielded bands of the expected molecular weight, and gene sequence analyses confirmed the absence of mutations. S. equi subsp. zooepidemicus BA06 was transformed by electroporation with pNZ8148kfoAkfoC and positive clones were selected on chloramphenicol.
Production of chondroitin and HA in shake flask experiments
Growth of S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC was performed on medium containing sucrose and yeast extract as main C and N sources, and expression of recombinant genes was induced by the addition of nisin. The supernatant and the biomass were recovered and treated as reported in the “Materials and methods” section to establish the ratio of polysaccharides produced based on HPAE-PAD analyses of monomeric components (amino sugars and uronic acid); total polysaccharides produced were evaluated by carbazole assay based on uronic acid determination and results were consistent with those obtained by SEC-TDA. The recombinant strain demonstrated to consume about 9 g/L of sucrose within 18 h of growth, and to produce about 11 g/L of lactic acid. Strain performance was also evaluated in the presence of 0.5 mM GalNAc and 2 mM phosphatidylcholine. GalNAc administration did not alter biomass and lactic acid production, however sucrose consumption was 30% lower compared to control conditions, resulting in higher yields (Table 1, Additional file 2). Upon addition of phosphatidylcholine medium opalescence was observed; this did not allow absorbance determinations during strain cultivations. Lactic acid final concentration and sucrose consumption were not altered by the addition of this phospholipid.
As reported in Table 1 about 90 mg/L of chondroitin in addition to 415 mg/L of HA were obtained from the recombinant strain after induction of kfoC and kfoA expression with nisin (control), demonstrating the proof of principle of the engineering strategy. Chondroitin concentration remained unchanged following medium supplementation with 0.5 mM GalNAc, whereas it was slightly lower in the presence of phosphatidylcholine. Also the concentration of HA was not significantly altered in all conditions. However, due to the lower concentration of sugar consumed with 0.5 mM GalNAc in the medium, the yield of chondroitin and HA with respect to starting sucrose (YBC/Suc and YHA/Suc, respectively) were 80 and 54% higher compared to those obtained in control conditions (Table 1). The addition of a higher concentration of GalNAc (5 mM) improved HA production and decreased the amount of produced chondroitin (data not shown).
Extremely low fractions of HA (0.6–1%) on the total polysaccharide produced, were found inside S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC recombinant cells. Inversely, intracellular chondroitin amounts ranged from 15 to 27% of the total produced polysaccharide.
Hydrodynamic characterization
The molecular weight (Mw) of the produced polysaccharides (HA and chondroitin) was assessed by SEC-TDA chromatography and results are reported in Table 2. The supernatants recovered from S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC grown in shakeflasks, were initially precipitated with 1.8 volumes of ethanol in order to recover HA. As reported in Table 2A in all growth conditions this precipitate contained three peak distributions, the most abundant of which was that with higher molecular weight with values ranging from 51 to 59% (1st Peak, representativeness). Medium supplementation with 2 mM phosphatidylcholine increased the average Mw of this population by 18% compared to that obtained in control conditions; moreover, the analysis of Mw distributions of the subpopulations within this peak indicated a 10% larger subpopulation of molecules with a Mw above 2000 kDa. This result is also confirmed by the higher intrinsic viscosity (IV) of the sample recovered from recombinant cells growing in the presence of the above mentioned phosphatidylcholine. The presence of the latter also changed Mw distributions within the 3rd peak, in fact the amount of polymers with Mw > 50 kDa and with 10 kDa < Mw < 50 kDa were 34% lower and 36% higher in this condition, respectively (Table 2A).
The supernatant was further precipitated with 4 volumes of ethanol to recover chondroitin and eventually residual low Mw HA. In all conditions two peaks with an average molecular weight of 27 and 19 kDa were found (Table 2B).
Chondroitin purification and determination of chemical structure
Purification of chondroitin from the biomass was performed, after cell lysis and precipitation, by anion exchange chromatography. The three step gradient allowed the identification of four major fractions that subsequently underwent salt removal on a desalting column, hydrolysis, and HPAE-PAD for determination of monosaccharide components. The peak eluted at 5% v/v of buffer B showed the highest concentration of GalN and was further analysed by SEC-TDA and NMR.
SEC-TDA analysis of the above indicated fraction showed a single peak (96% of representativeness) with a Mw of 28.4 kDa, a polydispersity index of 1.24 and an intrinsic viscosity equal to 1.09 as shown in the figure (Fig. 2). The chemical structure of the purified fraction was confirmed to be that of chondroitin by comparison of 1H and 13C NMR chemical shift data obtained by a DEPT-HSQC experiment (Fig. 3) with those reported in literature (Mucci et al. 2000).
SEC-TDA chromatogram of intracellular chondroitin purified by ethanol precipitation, anion exchange and desalting chromatographies from S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC. RI signal (red), viscometer signal (blue), right angle light scattering (green) and low angle light scattering (black)
1H and DEPT-HSQC NMR (600 MHz, 298 K, D2O, acetone as internal standard) spectra of chondroitin polysaccharide from S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC [chemical structure and numbering of chondroitin is shown in the inset; in the parenthesis below signal attributions are indicated the 1H and 13C (in italic) chemical shift values]
This confirmed the production of chondroitin polysaccharide upon expression of the two E. coli K4 genes namely kfoA, that converted UDP-GlcNAc into UDP-GalNAc, and kfoC, coding for chondroitin polymerase, that is essential for polymer assembly, in S. equi subsp. zooepidemicus BA06.
Batch experiments on 3 L-bioreactors
S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC was grown in a 3 L reactor on semidefined medium and expression of the foreign genes, kfoC and kfoA, was induced during exponential phase by the addition of nisin. The process lasted 18 h with a final concentration of biomass of about 8.34 ± 0.92 OD600 and a production of 50 ± 8 g/L of lactic acid (Table 3). The C source was not completely consumed, in fact, a residue of about 21 ± 2 g/L of sucrose was found in the medium at the end of the process (Additional file 3). The fermentation broth was treated with TCA to lower viscosity and to precipitate proteins in the medium and after centrifugation it was ultrafiltered/diafiltered on 100 kDa membranes to recover the high molecular weight fraction of exopolysaccharides; the permeate was concentrated and diafiltered on 10 kDa membranes. Both retentates were precipitated with ethanol in different proportion to recover HA and chondroitin enriched fractions, respectively (Additional file 4). An average production of 1.44 ± 0.36 and 0.22 ± 0.01 g/L of secreted HA and chondroitin, respectively, were found in the broth at the end of the process (Table 3; Additional file 3). Mw and polydispersity of the two produced polymers is reported in Fig. 4. The polysaccharide fraction extracted from the biomass after 18 h of growth consisted of about 0.021 and 0.082 g/L of HA and BC; data were normalized on the volume of recovered broth and on the concentration of wet biomass obtained at harvesting (Additional file 3).
Discussion
In the present work we reported the construction of a S. equi subsp. zooepidemicus recombinant strain that expresses two E. coli K4 genes, namely kfoC and kfoA, involved in the biosynthesis of chondroitin-like capsular polysaccharide. The aim of the study was to use a non-hemolytic and hyaluronidase negative microorganism, that already represents a consolidated industrial host for the production of HA, and endow it with the ability to additionally produce chondroitin, generating a cell factory for both biopolymers.
The difference between disaccharide units composing HA and chondroitin lies in the presence of either GlcNAc or GalNAc, respectively, bound to GlcA residues. Expression of kfoA encoding an epimerase that converts UDP-GlcNAc into UDP-GalNAc was not sufficient to redirect the pathways dedicated to HA biosynthesis in S. equi subsp. zooepidemicus to the production of chondroitin as well, probably due to the absence of a specific polymerase needed to bind nucleotide sugars and allow polymer assembly (data not shown). Concurrent expression of the kfoC encoded chondroitin polymerase, was necessary to produce chondroitin as confirmed by HPAE-PAD and NMR analyses. The recombinant strain S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC co-produced about 400 and 90 mg/L of HA and chondroitin, respectively, in shakeflasks on semidefined medium containing sucrose and yeast extract as main C and N sources. A productivity of 23 mg/Lh of HA was obtained considering the entire process duration. The final titer and productivity of chondroitin was lower with respect to HA, but comparable to that obtained by growing a natural producer such as E. coli K4 in shakeflasks on semidefined medium (Cimini et al. 2010). While HA was completely secreted from recombinant cells with a residual intracellular concentration of about 1% of the total polysaccharide produced, a higher concentration of chondroitin, ranging between 15 and 27%, was found upon biomass extraction (Table 1), probably indicating a lower efficiency of the secretion system with the new polymer. Two aspects were further investigated in shakeflasks, namely (i) whether GalNAc supplementation could partially redirect fluxes towards chondroitin production (ii) whether addition of phosphatidylcholine would increase HA molecular weight.
The crystal structure of chondroitin polymerase suggested a higher binding affinity for UDP-GalNAc (Zanfardino et al. 2010) and, a simulated implementation of the UDP-GalNAc productive branch by glutamine addition boosted capsular polysaccharide production in recombinant E. coli K4 (Cimini et al. 2015). However, in the present work addition of GalNAc only resulted in a slight reduction of HA titers, leaving the production of chondroitin unchanged and a higher (80%) concentration of the latter inside the cells compared to that found in control conditions. Addition of growing concentrations of GalNAc up to 5 mM did not improve results leading, on the contrary, to higher HA final titers thereby also decreasing the amount of chondroitin produced (data not shown).
One of the most pursued research goals about HA production is to obtain HA with the highest possible molecular weight and several critical cultivation parameters such as pH, temperature, aeration conditions etc. were investigated so far (Liu et al. 2011). The ratio of UDP-GlcA and UDP-GlcNAc influences the molecular weight of HA produced by Streptococcus strains (Badle et al. 2014), however genetic changes made in this work did not exert such effect.
Sun et al. (2012) interestingly found that the presence of phosphatidylcholine in the growth medium directed more carbon to HA synthesis (production raised by 17%) by enhancing ATP production. It also increased the polymer molecular weight by 67% in controlled bioreactor experiments. Addition of the same phospholipid here did not enhance HA production. Nevertheless, an 18% higher average molecular weight was observed, thereby obtaining HA with average Mw of 1.98 kDa. In fact, interestingly, SEC-TDA showed that within the highest molecular weight peak (1st peak) obtained after the first precipitation step with ethanol, a 10% larger subpopulation of molecules with a Mw above 2000 kDa was found, compared to that observed in the absence of phosphatidylcholine (Table 2). Similar polydispersity (Mw/Mn) and higher IV compared to the control (27.0 vs 24.5 dL/g) further confirm data robustness. Moreover, the same sample showed, within the 3rd peak, a smaller amount of polymers with Mw > 50 kDa indicating a broader effect of phosphatidylcholine on the distribution of high and low molecular weight HA polymers.
SEC-TDA analyses of supernatants further precipitated with 4 volumes of ethanol indicated the presence of two peaks with an average molecular weight of 27 and 19 kDa. By comparing the relative abundance of the two peaks identified by SEC-TDA to results obtained by hydrolysis and HPAE-PAD, the two populations principally correspond to chondroitin together with residual polysaccharides from the medium and a lower HA contamination.
Constant air sparging and pH control led to the production of about 1.46 and 0.3 g/L of HA and BC respectively in 3 L bioreactors, in batch mode. Twenty-seven percent of chondroitin was retained inside the cells, whereas most HA was found in the extracellular environment (1.5% inside on the total produced), confirming shakeflask results. The polymers produced after 18 h of growth were characterized after 1/2 ultrafiltration steps and precipitation with differential ethanol percentages showing an average Mw of 1.75 ± 0.14 MDa and 25 ± 3 kDa for HA and BC, respectively.
Engineering of S. equi subsp. zooepidemicus BA06, a hyaluronidase and haemolysin-free natural producer of HA, was accomplished in this work. The strain was enriched by recombinant inducible expression of two E. coli K4 genes, kfoA and kfoC, that are involved in the biosynthesis of chondroitin-like polysaccharide. As proved by HPAE-PAD and NMR the recombinant strain produced both polymers with an HA:BC ratio equal to 5:1, for extracellular species, and 1:4/5 for intracellular species. To our knowledge this is the first study that provides an approach to the concurrent production of HA and chondroitin in a highly exploited industrial workhorse, that differently from other potentially harmful Streptococcus strains is non-hemolytic and hyaluronidase negative. This gives the opportunity to obtain both polymers from a single bioprocess; since the HA secretion apparatus is not as efficient for chondroitin release, biomass could be the source of a chondroitin rich polysaccharide fraction of low Mw (<40 kDa), whereas the supernatant the source of almost pure and high Mw HA.
Abbreviations
- CS:
-
chondroitin sulphate
- BC:
-
biotechnological chondroitin
- HA:
-
hyaluronic acid
- GlcA:
-
glucuronic acid
- GalNAc:
-
N-acetylgalactosamine
- GlcNAc:
-
N-acetylglucosamine
References
Badle SS, Jayaraman G, Ramachandran KB (2014) Ratio of intracellular precursors concentration and their flux influences hyaluronic acid molecular weight in Streptococcus zooepidemicus and recombinant Lactococcus lactis. Bioresour Technol 163:222–227. doi:10.1016/j.biortech.2014.04.027
Bedini E, De Castro C, De Rosa M, Di Nola A, Iadonisi A, Restaino OF, Schiraldi C, Parrilli M (2011) A microbiological-chemical strategy to produce chondroitin sulphate A, C. Angew Chem Int Ed Engl 50:6160–6163
Bishnoi M, Jain A, Hurkat P, Jain SK (2016) Chondroitin sulphate: a focus on osteoarthritis. Glycoconj J 33(5):693–705. doi:10.1007/s10719-016-9665-3
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334
Cervigni M, Sommariva M, Tenaglia R, Porru D, Ostardo E, Giammò A, Trevisan S, Frangione V, Ciani O, Tarricone R, Pappagallo GL (2016) A randomized, open-label, multicenter study of the efficacy and safety of intravesical hyaluronic acid and chondroitin sulphate versus dimethyl sulfoxide in women with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. doi:10.1002/nau.23091
Christiani TR, Toomer K, Sheehan J, Nitzl A, Branda A, England E, Graney P, Iftode C, Vernengo AJ (2016) Synthesis of thermogelling poly(N-isopropylacrylamide)-graft-chondroitin sulfate composites with alginate microparticles for tissue engineering. J Vis Exp. doi:10.3791/53704
Ciani O, Arendsen E, Romancik M, Lunik R, Costantini E, Di Biase M, Morgia G, Fragalà E, Roman T, Bernat M, Guazzoni G, Tarricone R, Lazzeri M (2016) Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulphate (CS) for the treatment of female recurrent urinary tract infections: a European multicentre nested case–control study. BMJ Open 6(3):e009669. doi:10.1136/bmjopen-2015-009669
Cimini D, Restaino OF, Catapano A, De Rosa M, Schiraldi C (2010) Production of capsular polysaccharide from Escherichia coli K4 for biotechnological applications. Appl Microbiol Biotechnol 85(6):1779–1787
Cimini D, De Rosa M, Carlino E, Ruggiero A, De Rosa M, Schiraldi C (2013) Homologous overexpression of RfaH in E. coli K4 improves the production of chondroitin-like capsular polysaccharide. Microb Cell Fact 12:46–54
Cimini D, Carlino E, Giovane A, Argenzio O, Dello Iacono I, De Rosa M, Schiraldi C (2015) Engineering a branch of the UDP-precursor biosynthesis pathway enhances the production of capsular polysaccharide in Escherichia coli O5:K4:H4. Biotechnol J 10(8):1307–1315. doi:10.1002/biot.201400602
De Angelis PL (2012) Glycosaminoglycan polysaccharide biosynthesis and production: today and tomorrow. Appl Microbiol Biotechnol 94(2):295–305. doi:10.1007/s00253-011-3801-6
de Olivera JD, Carvalho LS, Vieira Gomes AM, Rezende Queiroz L, Magalhaes BM, Skorupa Parachin N (2016) Genetic basis for hyperproduction of hyaluronic acid in natural and engineered microorganisms. Microb Cell Fact 15:119–138
Galluccio F, Barskova T, Matucci Cerinic M (2015) Short-term effect of the combination of hyaluronic acid, chondroitin sulfate, and keratin matrix on early symptomatic knee osteoarthritis. Eur J Rheumatol 3:106–108
He W, Fu L, Li G, Andrew Jones J, Linhardt RJ, Koffas M (2015) Production of chondroitin in metabolically engineered E. coli. Metab Eng 27:92–100. doi:10.1016/j.ymben.2014.11.003
Henrotin Y, Mathy M, Sanchez C, Lambert C (2010) Chondroitin sulphate in the treatment of osteoarthritis: from in vitro studies to clinical recommendations. Ther Adv Musculoskelet Dis 2(6):335–348
Henson FM, Getgood AM, Caborn DM, McIlwraith CW, Rushton N (2012) Effect of a solution of hyaluronic acid-chondroitin sulfate-N-acetyl glucosamine on the repair response of cartilage to single-impact load damage. Am J Vet Res 73(2):306–312. doi:10.2460/ajvr.73.2.306
Jin P, Zhang L, Yuan P, Kang Z, Du G, Chen J (2016) Efficient biosynthesis of polysaccharides chondroitin and heparosan by metabolically engineered Bacillus subtilis. Carbohydr Polym 140:424–432. doi:10.1016/j.carbpol.2015.12.065
La Gatta A, De Rosa M, Marzaioli I, Busico T, Schiraldi C (2010) A complete hyaluronan hydrodynamic characterization using a size exclusion chromatography-triple detector array system during in vitro enzymatic degradation. Anal Biochem 404(1):21–29
Lazzeri M, Hurle R, Casale P, Buffi N, Lughezzani G, Fiorini G, Peschechera R, Pasini L, Zandegiacomo S, Benetti A, Taverna G, Guazzoni G, Barbagli G (2016) Managing chronic bladder diseases with the administration of exogenous glycosaminoglycans: an update on the evidence. Ther Adv Urol 8(2):91–99. doi:10.1177/1756287215621234
Liu L, Liu Y, Li J, Du G, Chen J (2011) Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microb Cell Fact 10:99–107. doi:10.1186/1475-2859-10-99
Marcellin E, Nielsen LK, Abeydeera P, Krömer JO (2009) Quantitative analysis of intracellular sugar phosphates and sugar nucleotides in encapsulated streptococci using HPAEC-PAD. Biotechnol J 4(1):58–63. doi:10.1002/biot.200800197
Marcellin E, Chen WY, Nielsen LK (2010) Understanding plasmid effect on hyaluronic acid molecular weight produced by Streptococcus equi subsp. zooepidemicus. Metab Eng 12(1):62–69. doi:10.1016/j.ymben.2009.09.001
Maytin EV (2016) Hyaluronan: more than just a wrinkle filler. Glycobiology 26(6):553–559. doi:10.1093/glycob/cww033
Moscovici M (2015) Present and future medical applications of microbial exopolysaccharides. Front Microbiol 6:1012. doi:10.3389/fmicb.2015.01012 (eCollection)
Mucci A, Schenetti L, Volpi N (2000) H-1 and C-13 nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydr Polym 41(1):37–45
Ninomiya T, Sugiura N, Tawada N, Sugimoto K, Watanabe H, Kimata K (2002) Molecular cloning and characterization of chondroitin polymerase from Escherichia coli strain K4. J Biol Chem 277:21567–21575
Restaino OF, Finamore R, Diana P, Marseglia M, Vitiello M, Casillo A, Bedini E, Parrilli M, Corsaro MM, Trifuoggi M, De Rosa M, Schiraldi C (2017) A multi-analytical approach to better assess the keratan sulphate contamination in animal origin chondroitin sulfate. Anal Chim Acta 958:59–70
Sambrook J, Russel DW (2001) Molecular cloning. A laboratory manual. Cold Spring Harbour Laboratory Press, Cold Spring Harbour, New York
Schiraldi C, Andreozzi L, Marzaioli I, Vinciguerra S, D’Avino A, Volpe F, Panariello A, De Rosa M (2010) Hyaluronic acid degradation during initial steps of downstream processing. Biocatal Biotransform 28(1):83–89
Stellavato A, Tirino V, de Novellis F, Della Vecchia A, Cinquegrani F, De Rosa M, Papaccio G, Schiraldi C (2016) Biotechnological chondroitin a novel glycosamminoglycan with remarkable biological function on human primary chondrocytes. J Cell Biochem 117(9):2158–2169. doi:10.1002/jcb.25556
Sun J, Wang M, Chen Y, Shang F, Ye H, Tan T (2012) Understanding the influence of phosphatidylcholine on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus. Appl Biochem Biotechnol 168(1):47–57
Tripodo G, Trapani A, Torre ML, Giammona G, Trapani G, Mandracchia D (2015) Hyaluronic acid and its derivatives in drug delivery and imaging: recent advances and challenges. Eur J Pharm Biopharm 97:400–416. doi:10.1016/j.ejpb.2015.03.032
Zanfardino A, Restaino OF, Notomista E, Cimini D, Schiraldi C, De Rosa M, DeFelice M, Varcamonti M (2010) Isolation of an Escherichia coli K4 kfoC mutant over-producing capsular chondroitin. Microb Cell Fact 17:9–34
Authors’ contributions
DC and CS conceived the study, DC wrote the manuscript, DC and EC constructed the recombinant strains, EC and ID performed growth and purification experiments, RF performed FPLC runs, OFR performed hydrolysis and HAE-PAD quantifications, PD performed SEC-TDA runs, EB performed NMR structure determinations. All authors read and approved the final manuscript.
Acknowledgements
We kindly thank Sabrina Reale and Ottavia Argenzio for helpful discussions on SEC-TDA analyses and shakeflask cultivations.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included in the main manuscript file and the additional file.
Funding
The study was supported by the Ministero dell’Università e della Ricerca Scientifica project PON03PE_00060_7 “Sviluppo preclinico di nuove terapie e di strategie innovative per la produzione di molecole ad azione farmacologica”.
Author information
Authors and Affiliations
Corresponding authors
Additional files
13568_2017_364_MOESM1_ESM.docx
Additional file 1. Sequence of kfoA and kfoC genes amplified from E. coli K4 and cloned in pNZ8148; Map of pNZ8148kfoAkfoC.
13568_2017_364_MOESM2_ESM.xlsx
Additional file 2. Complete data of shake flask experiments of S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC grown in shakeflasks on semidefined medium in the presence of inducer (nisin) alone or in combination with either GalNAc or phosphatidylcholine. Three experiments were performed in each condition. Mean and sd resulting from these experiments are reported in Table 1.
13568_2017_364_MOESM4_ESM.docx
Additional file 4. Downstream processing of broths recovered from growth of S. equi subsp. zooepidemicus-pNZ8148kfoAkfoC on 3 L bioreactors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cimini, D., Iacono, I.D., Carlino, E. et al. Engineering S. equi subsp. zooepidemicus towards concurrent production of hyaluronic acid and chondroitin biopolymers of biomedical interest. AMB Expr 7, 61 (2017). https://doi.org/10.1186/s13568-017-0364-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-017-0364-7