Abstract
The global spread of avian influenza A viruses in domestic birds is causing increasing socioeconomic devastation. Various mechanistic models have been developed to better understand avian influenza transmission and evaluate the effectiveness of control measures in mitigating the socioeconomic losses caused by these viruses. However, the results of models of avian influenza transmission and control have not yet been subject to a comprehensive review. Such a review could help inform policy makers and guide future modeling work. To help fill this gap, we conducted a systematic review of the mechanistic models that have been applied to field outbreaks. Our three objectives were to: (1) describe the type of models and their epidemiological context, (2) list estimates of commonly used parameters of low pathogenicity and highly pathogenic avian influenza transmission, and (3) review the characteristics of avian influenza transmission and the efficacy of control strategies according to the mechanistic models. We reviewed a total of 46 articles. Of these, 26 articles estimated parameters by fitting the model to data, one evaluated the effectiveness of control strategies, and 19 did both. Values of the between-individual reproduction number ranged widely: from 2.18 to 86 for highly pathogenic avian influenza viruses, and from 4.7 to 45.9 for low pathogenicity avian influenza viruses, depending on epidemiological settings, virus subtypes and host species. Other parameters, such as the durations of the latent and infectious periods, were often taken from the literature, limiting the models’ potential insights. Concerning control strategies, many models evaluated culling (n = 15), while vaccination received less attention (n = 6). According to the articles reviewed, optimal control strategies varied between virus subtypes and local conditions, and depended on the overall objective of the intervention. For instance, vaccination was optimal when the objective was to limit the overall number of culled flocks. In contrast, pre-emptive culling was preferred for reducing the size and duration of an epidemic. Early implementation consistently improved the overall efficacy of interventions, highlighting the need for effective surveillance and epidemic preparedness.
Similar content being viewed by others
1 Introduction
Avian influenza viruses (AIVs) pose a continuous threat to domestic poultry, wildlife, and humans. The diversity of two envelope glycoproteins, hemagglutinin and neuraminidase, are used to divide AIVs into various subtypes (named HxNy) with different characteristics and pathogenicity. Waterfowl, especially Anseriformes (ducks, geese and swans) and Charadriiformes (gulls, terns and sandpipers), are considered to be the natural reservoir of all AIV subtypes [1]. When transmitted to poultry, the H5 and H7 AIV subtypes usually cause mild clinical symptoms, and are designated as low pathogenicity avian influenza (LPAI). However, H5 and H7 AIVs can mutate into highly pathogenic avian influenza (HPAI), causing severe morbidity and mortality in poultry. This transition from LPAI to HPAI usually happens in domestic poultry, either shortly after the first introduction of the virus, or after months or even years of undetected circulation [2]. On some occasions, this phenomenon has caused large poultry outbreaks, such as the HPAI H5N2 epidemic in 1983 in the United States, the HPAI H7N1 epidemic in Italy in 1999–2000, the HPAI H7N7 epidemic in 2003 in the Netherlands and the HPAI H7N3 epidemic in 2004 in Canada [3]. Both LPAI and HPAI viruses can be zoonotic, causing a range of symptoms in humans, including severe respiratory distress and death. One notable example of zoonotic AIV infection was the transmission of LPAI and HPAI H7N9 viruses from poultry to humans in China between 2013 and 2017, causing more than 1,500 human cases and over 600 deaths [4].
The emergence of the A/Goose/Guangdong/1/1996 (Gs/Gd) H5N1 virus lineage in China in 1996 [3] has been associated with a shift in HPAI virus transmission. This Gs/Gd lineage has shown a propensity for transmission among wild migratory birds, leading to an unprecedented global spread. Moreover, numerous genome reassortments with other circulating AIVs has led to the diversification of the Gs/Gd lineage into several clades and subclades [5]. From Southeast Asia, HPAI H5N1 viruses first spread to Europe, the Middle-East and Africa in 2005 and 2006. These viruses became endemic in poultry in many countries in Asia and Africa, further promoting virus evolution and diversification.
Beginning in 2014, H5Nx viruses clade 2.3.4.4 became predominant and spread throughout Asia, Europe, Africa, and, for the first time, America. HPAI H5N8 and H5N6 predominated from 2014 to 2021 [5], and were replaced again by HPAI H5N1 starting in 2021 [6]. These viruses caused many outbreaks in domestic poultry; in Europe alone, 2642 H5N8 and 2740 H5N1 HPAI outbreaks have been reported from October 1, 2014 to September 30, 2023 [7]. These viruses even appear to have persisted in wild birds in Europe throughout the summers of 2021 and 2022, suggesting a fundamental shift toward a potential endemic circulation [8, 9]. In addition to devastating losses in poultry, HPAI H5Nx viruses recently caused mass mortality events in wild birds worldwide, raising serious concerns for conservation [10,11,12].
Control measures against HPAI epidemics in poultry include culling infected birds, pre-emptive culling around infected flocks, movement bans, and screening of at-risk contacts [13]. Poultry vaccination also has been employed in several Asian and African countries [14, 15]. However, this strategy faces several challenges, including difficulties in selecting vaccine strains, monitoring influenza evolution, differentiating vaccinated from infected birds, and maintaining appropriate vaccination coverage [15]. For all of these reasons, poultry vaccination is currently prohibited in the United States (US) [16], and was authorized only recently in the European Union (EU) [17]. Despite significant efforts to limit avian influenza incursions and spread in the poultry sector, the global spread of HPAI viruses, causing devastating epidemics, highlights the need to design and implement adequate prevention and control strategies [18].
Mechanistic models, which describe transmission dynamics by using mathematical expressions, could help in this effort. Models can help evaluate the impact of existing and alternative disease control strategies. They have been used for various animal diseases, including foot-and-mouth disease [19], African swine fever [20, 21], and bluetongue [22]. While numerous mechanistic models have been used to analyze AIV epidemics [23], their results concerning surveillance and control, which could be useful for decision-making, have yet to be subject to a comprehensive review. Reviews conducted so far on avian influenza have focused on virological and clinical aspects [24, 25] or on risk factors [26,27,28], including transmission routes [29, 30]. Two recent studies reviewed transmission parameter values based on experimental studies [31] and on both experimental and field studies [32], but to the best of our knowledge no comprehensive analysis of mechanistic models has been undertaken. To fill this gap, we conducted a systematic review of mechanistic models applied to avian influenza in poultry to (i) describe the mechanistic models used and their epidemiological context, (ii) list AIV transmission parameters, and (iii) provide insights on avian influenza transmission and the impact of control measures. In regard to our second objective, synthesizing AIV transmission parameters is difficult because parameter values often vary depending on the epidemiological context (e.g., virus subtype, host species, production system). However, listing parameter estimates may be useful for simulation models and can provide ranges of possible values that can help parameter estimation. Based on our results, we discuss future avenues and challenges for modeling AIV epidemics and evaluating control strategies.
2 Materials and methods
This systematic review was conducted in compliance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [33].
2.1 Search strategy
Three online databases (PubMed, Web of Science, and CAB Abstracts) were searched for relevant literature on mechanistic models used to study the transmission of avian influenza in domestic poultry populations. Three groups of terms were used for each database and linked with the “AND” Boolean; within each group, we used an association of keywords linked with the “OR” Boolean (Additional file 1). All searches were done in the article title, abstract, and keywords. Articles in languages other than English were excluded. The last search was performed on April 18, 2023.
2.2 Inclusion and exclusion criteria
The final list of articles to include in the review was defined through a two-step screening process (Additional file 2). In the first step, titles and abstracts were independently screened by two reviewers (BB and AM) based on four criteria. Articles were included if they: (1) were primary research articles on avian influenza, (2) focused at least in part on domestic poultry populations, (3) described the propagation of avian influenza epidemics at the population level, and (4) described a mechanistic approach for modeling the spread of avian influenza. Based on these criteria, editorials, commentaries, reviews, and perspective articles were excluded. Articles without any explicit description of transmission processes, or presenting only experimental or molecular data, also were excluded. During this step, a conservative approach was taken, with all articles selected by at least one of the reviewers kept for the next step.
The second screening step was carried out based on the full-text content. Articles were included if they met the inclusion criteria of the first screening, and if the model described was used to achieve at least one of the following two objectives: (1) estimation of model parameters using avian influenza epidemic data, and (2) evaluation of control strategies using a model fitted to field data in the same or in a previous study. Articles that focused solely on simulated epidemics were therefore excluded (i.e., at least one parameter of the model had to be estimated by fitting the model to observed data; models where all parameter values were assumed were excluded). We also looked at the reference lists of the articles included to find further articles that may have been missed in the primary search. Finally, both reviewers discussed their respective final selections until a consensus was reached on each article. In the absence of consensus, the opinion of a third reviewer (SL) was sought.
2.3 Data extraction and analysis
The first three authors worked independently to systematically record the key features of all selected articles. The information extracted included (Additional file 3): contextual information (year of the epidemic(s) studied, poultry population, AIV subtype, virus pathogenicity, geographical location, scale), control strategies (surveillance and control measures evaluated), modeling approach (modeling aim, model paradigm, epidemiological unit, contact structure, transmission routes) and transmission parameters estimated. For within-farm models, when the value of the basic reproduction number R0 was not reported, it was calculated by multiplying the value of the transmission rate β by the value of the average duration of the infectious period, wherever possible. All extracted data were checked for consistency by the first two authors. Descriptive statistics and figures were done using R software [34].
3 Results
3.1 Included articles and epidemiological characteristics
Based on an initial search on April 24, 2019 and two search updates on October 21, 2021 and April 18, 2023, the search query yielded 2920 articles, of which 1140 were duplicates (Figure 1). Of the 1780 remaining articles, 1487 were removed after the first screening and 251 after the second. Four additional articles which met the inclusion criteria were identified by checking the references of the articles that passed both screenings. As a result, a total of 46 articles were included in the review [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80] (Figure 1).
The epidemiological context of the models presented in these articles varied in terms of pathotype (LPAI or HPAI), subtype, and location (Table 1, Figure 2). The vast majority of articles focused on HPAI (n = 41), with three studies on LPAI and two on both HPAI and LPAI simultaneously (Table 1).
The majority of articles (n = 18; Table 1, Figure 2) focused on the HPAI H5N1 subtype. Although HPAI H5N1 has spread globally since its emergence in 1996, it is noteworthy that models were applied to only a limited number of countries. Thirteen articles analyzed HPAI H5N1 spread in Asia (Thailand, Vietnam, Bangladesh, India, Indonesia and South Korea), three in Africa (Nigeria and Egypt), one in Europe (Romania), and two at the global scale.
The second most studied subtype was HPAI H7N7 (n = 7; Table 1, Figure 2). All seven articles, including the earliest modeling article published in 2004 [69], investigated the 2003 HPAI H7N7 epidemic in the Netherlands.
Other past HPAI epidemics analyzed so far included H5N8 in Japan, France and the Netherlands (n = 4), H5N2 in the United States (n = 4), H5N6 in South Korea and the Philippines (n = 3), H7N3 in Canada (n = 1), H7N1 in Italy (n = 1), and multiple H5 subtypes in South Korea and China (n = 3).
Three studies focused on LPAI viruses (Table 1): LPAI H5N2 in the United States (n = 1), LPAI H7N3 in the Netherlands (n = 1) and LPAI H7N9 in China (n = 1). Finally, two studies focused on both LPAI and HPAI H7N9 in China.
3.2 Modeling approaches
In accordance with our inclusion criteria, the most frequent modeling objective was to estimate model parameters (45/46), followed by the evaluation of control strategies (20/46), with 19 articles doing both. Only one article evaluated control strategies without estimating model parameters: Hill et al. [47] re-used the model fitted to epidemic data in Hill et al. [46] to evaluate control strategies in the same epidemiological context.
Most articles included a stochastic component (33/46), while the others were deterministic (13/46). Two different modeling approaches (Box 1) were used in the selected articles: population-based models (PBM, 27/46) and individual-based models (IBM, 19/46; Table 2). All PBMs assumed homogeneous mixing between epidemiological units (i.e., where one epidemiological unit could contact any other unit in a population randomly with equal probability). Most of the IBMs (15/19) assumed that effective contacts depended on the distance between two epidemiological units; either contacts occurred with equal probability but only with units within a given radius, or contacts could occur with any other unit but with a probability that decreased with increasing distance. One IBM used a network to represent contact between farms through recorded vehicle movements [61]. Finally, three IBMs considered transmission at two levels, with homogenous mixing of the epidemiological units (individuals, farms) within larger subpopulations (farms, areas), and distance-dependent contacts between these subpopulations [38, 60, 74]. In these three cases, the “individuals” in the IBMs were the subpopulations and not the epidemiological units.
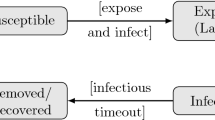
Three types of epidemiological units were considered: individual birds, poultry farms, and geographical areas defined by administrative boundaries (e.g., villages, districts, counties, countries). When considering individuals as epidemiological units (17/46 articles, Table 2), the health statuses of birds were classically defined as susceptible (S), exposed—infected but not yet infectious (E), infectious (I), recovered (R) or dead (D). SID (n = 10) and SEIRD (n = 4) models were used most frequently. When considering farms as epidemiological units, the whole farm was considered as exposed and then infectious after the onset of infection (i.e., neglecting within-farm dynamics). Similarly, for administrative areas, the whole area was considered as exposed when at least one outbreak (e.g., one infected farm) occurred, and then the whole area became infectious at the end of the latent period. At these levels (farms, areas), the recovered state was not considered; after being infectious, the whole epidemiological unit was either completely depopulated, or completely susceptible again. At the farm and administrative area levels (29/46, Table 2), the models used most frequently were SEID (n = 16) and SID (n = 9).
Most of the PBMs presented in the review (16/27; Table 2) defined individual birds as the epidemiological unit. Seven studied the transmission dynamics between individual birds and humans [44, 45, 52, 58, 78,79,80], eight between individual birds in a farm [35, 57, 62, 64, 65, 73, 76, 77], and one between domestic birds and wild birds [66]. The other PBMs (11/27; Table 2) studied transmission between poultry farms [39, 41, 43, 48, 68, 69, 72] or transmission between poultry populations pooled at the administrative unit level [37, 49, 51, 53].
Most IBMs (16/19; Table 2) investigated the transmission process between farms [42, 46, 47, 50, 54,55,56, 59, 61, 63, 67, 70, 71, 75] or between administrative areas [36, 40, 50, 55], with two articles evaluating both in the same study [50, 55]. The remaining three articles considered transmission at two different scales (e.g., within-farm and between-farms) [38, 60, 74].
Data on poultry species explicitly included in the model were specified in only 20 of the 46 articles. The data mainly covered chickens (n = 17), ducks (n = 9) and turkeys (n = 7). Four articles included other species, such as geese, quails, and ostriches [36, 61, 63, 67]. Even when demographic data were available on various poultry species, poultry populations usually were considered to be one homogeneous population. There were a few exceptions that distinguished backyard from commercial farms [36, 68, 72], or that incorporated the heterogeneity in transmission between host species and sometimes estimated their relative contribution to transmission [36, 38, 60, 63, 67].
Similarly, heterogeneity in transmission routes was rarely considered. Most transmission models considered that AIV transmission occurred only via poultry-to-poultry transmission, with only 10 of the 46 considering other transmission routes (e.g., contacts with wild birds or contaminated environment). For example, some authors modeled explicitly the environment [45, 66, 79] or the disease dynamics in wild birds [36, 45, 49, 66], while others considered a constant, distance-independent parameter to implicitly capture other undefined transmission sources (e.g., infectious backyard farms or wild birds, long-distance movements of infected birds or contaminated equipment) [38, 46, 47, 56, 63].
3.3 Parameter estimations
The between-individual transmission rate \(\beta\) was systematically estimated in all nine articles considering within-farm transmission (eight PBMs and one IBM; Table 3). Although all nine models assumed homogeneous mixing and frequency-dependent contact rates, the values of the between-individual transmission rate ranged from 0.6 to 34.4 per day for HPAI viruses, and from 0.5 to 3.9 per day for LPAI viruses (Table 3). Accordingly, values for the between-individual basic reproduction number R0 ranged widely, from 2.18 to 86 for HPAI viruses, and from 4.7 to 45.942 for LPAI viruses (Table 3). The most common parameters other than β and R0, i.e., the average durations of the latent and infectious periods and the case fatality risk, were most often fixed using published values from infection experiments (Additional file 4). The average duration of the latent period was always less than two days, irrespective of subtype and pathogenicity, and the average duration of the infectious period ranged from 1 to 15 days (Additional file 4). The case fatality risk ranged from 20 to 100% for HPAI viruses, while it was very low (0–1%) for LPAI viruses. Five of the nine articles also estimated the time of virus introduction in addition to the above-mentioned parameters [62, 64, 65, 76, 77]. All parameter values in within-farm transmission models of HPAI viruses were estimated using daily recorded mortality data, while egg production data [77] or diagnostic testing data [76] were used for LPAI viruses (Table 3).
Similarly, the between-farm transmission rate β and the between-farm reproduction number Rh were the most commonly estimated parameters in the 24 articles studying between-farm transmission of HPAI (Table 3). The average duration of the infectious period at the farm level was the third most frequently estimated parameter, and ranged quite widely depending on epidemiological settings and virus subtypes. However, the smallest estimated value was 6.4 days, indicating rather long infectious periods at the farm level (Additional file 5).
In addition, 14 of the 24 articles modeling between-farm transmission used spatial transmission kernels to describe how the relative risk of transmission between farms changed with the distance between a susceptible and an infectious farm. The resulting spatial transmission kernels are illustrated in Figure 3, except for that of Seymour et al. [75] who used a nonparametric transmission kernel that could not be reproduced. Two studies used a step function where the relative risk was one below a certain distance threshold, and zero otherwise [42, 63]. Le Menach et al. [70] estimated three transmission rates for pre-determined ranges of between-farms distances (less than 3 km, between 3 and 10 km, and more than 10 km), which we converted into a step-function kernel (Figure 3). The other studies used parametric kernels, with three different functions (Table 4):
-
a Pareto distribution [46, 47]: \(\left\{\begin{array}{c}1, 0\le {d}_{ij}<{x}_{min}\\ {\left(\frac{{x}_{min}}{{d}_{ij}}\right)}^{\alpha +1}, {d}_{ij}\ge {x}_{min}\end{array}\right.\)
-
a power-law function [50, 54, 55]: \(1-{e}^{-{\left(\frac{\delta }{{d}_{ij}}\right)}^{\rho }}\)
-
or a logistic expression [56, 59, 67, 71, 74]: \(\frac{1}{1+{\left(\frac{{d}_{ij}}{{r}_{0}}\right)}^{\alpha }}\)
Comparison of spatial transmission kernels used in between-farm transmission model. Hill et al. [47] and Backer et al. [74] used the same kernel and same parameter values as Hill et al. [46] and Boender et al. [71], respectively. Note that for Rorres et al. [54, 55] and Pelletier et al. [50], estimated parameter values were close and therefore the three curves are indistinguishable. For these three models, although the relative risk of transmission between farms decreased to very low values at small distances, the absolute probability of transmission remained substantial even at long distances because the sizes of both susceptible and infectious farms were included in the force of infection.
The parameters of these kernels were all estimated (Table 4), except for Backer et al. [74] and Hill et al. [47], who used the same parameter values as Boender et al. [71] and Hill et al. [46], respectively. Rorres et al. [54, 55] and Pelletier et al. [50] estimated large values of the shape parameter ρ and very small values of the distance-scaling parameter δ (Table 4), thus producing a step-like function where the relative risk of transmission between farms decreased sharply to very small values (Figure 3). However, in these articles, the force of infection accounted for the number of birds in the susceptible and infectious farms, meaning that the probability of transmission between farms remained substantial even at high distances. Hill et al. [46] also found a rapidly decreasing probability, but with long right-tails depending on the epidemic used to parameterize the kernel (Figure 3; Table 4). Similar values of the shape parameter α were found in Boender et al. [71], Dorigatti et al. [67] and Bonney et al. [56], thus producing similar shapes. However, they estimated different values of the half-kernel distance r0, with local transmission being more important in Boender et al. [71] and Dorigatti et al. [67], while transmission at moderate distances remaining substantial in Bonney et al. [56]. Conversely, Salvador et al. [59] found a similar half-kernel distance r0 to Dorigatti et al. [67], but a smaller shape parameter α, indicating a larger role of long-distance transmission (Figure 3, Table 4).
Finally, the remaining 14 articles considered transmission within and/or between areas, with individuals or administrative areas as epidemiological units (i.e., not modeling farms). Eleven estimated both the transmission rate and the Rh, three estimated the average duration of the infectious period, and two estimated the average duration of the incubation period and spatial kernel parameters. However, variations between the epidemiological units and spatial scales considered limit comparisons and the possibility to use values for different settings. Therefore, we did not report the estimated values here (but they are presented in Additional file 3).
3.4 Mitigation strategies
Out of the 46 articles selected, 20 evaluated the effectiveness of control measures in containing AIV outbreaks through numerical simulations (Table 5). Most focused only on HPAI outbreaks, with one exception that focused on both LPAI and HPAI H7N9 in China (Table 5). Five studies used PBMs and 15 used IBMs. Most articles (15/20) studied the effect of control measures solely on epidemiological parameters in the poultry population (epidemic size and duration, reproduction number, number of culled flocks). The economic impact of the measures implemented was considered in only three articles [38, 74, 75]. Finally, in two studies [52, 80], the authors estimated the effectiveness of mitigation strategies in poultry for controlling the disease in humans (e.g., reduction of the number of infected humans).
The most commonly evaluated strategy was poultry culling (15 articles, Table 5), either in the form of reactive culling (culling of infected flocks, five articles) or pre-emptive culling (culling of at-risk flocks not necessarily infected, 12 articles), with two articles evaluating both.
Reactive culling was most of the time included in the model baseline mitigation strategy without evaluating its effectiveness on its own [36, 38, 40, 47, 49, 50, 56, 59, 60, 63, 67, 70, 71, 74, 75, 80]. There were two exceptions: (1) Lee et al. [52] showed that reducing the number of infectious poultry led to a substantial reduction in the number of H5N1 infections in humans compared to a scenario without reactive culling; and (2) Chen et al. [66] showed that reactive culling could substantially decrease the number of poultry outbreaks, but could not control the epidemic on its own. In addition, the impact of the timeliness of reactive culling was evaluated in three articles, which all consistently showed a strong positive impact of this parameter on the effectiveness of reactive culling [49, 63, 70]. For example, Andronico et al. [63] showed that reducing the time between detection and culling (from 5 to 2 days) cut in half the expected size of the outbreak in poultry.
Pre-emptive culling consisted of culling flocks within a given radius around detected infected flocks [38, 47, 50, 59, 60, 63, 67, 70, 71, 74, 75]. The radius was under 10 km in most articles, except for Kim et al. [42], who evaluated radii up to 56.25 km. All articles reported the effectiveness of pre-emptive culling on reducing the number of infected flocks and the duration of the epidemic. The effectiveness increased when the culling radii increased. However, the economic costs of the epidemic were also the highest for the highest culling radii [74, 75].
Moreover, the improvement brought by increasing the pre-emptive culling radius saturated rapidly when limited culling capacity was considered [47, 50, 60, 63, 70, 74, 75]. This is because further increasing the culling radius did not change the number of culled flocks once the maximum culling capacity was reached. Limited culling capacity was introduced to represent limited resources and logistical difficulties presented by mass culling over a short period of time. It was modeled either as a number or proportion of preventively culled farms around each infected farm [63, 70], as a maximum number of farms culled at each time step [47, 50, 74, 75], or as a nonlinear culling rate depending on the number of reported farms [60]. Increasing the culling capacity substantially increased the effectiveness of pre-emptive culling on the size and duration of an epidemic [47, 74].
The effectiveness of pre-emptive culling came at the cost of culling more flocks. In most cases, the total number of culled flocks (by reactive and pre-emptive culling) increased as the pre-emptive culling radius increased. This means that reactive culling without pre-emptive culling would be preferred if the objective was to reduce the number of culled flocks instead of reducing the epidemic size [38, 47, 63, 67, 74, 75]. However, in some cases the total number of culled flocks was non-monotonic as the pre-emptive culling radius increased [42, 59, 60]. The total number of culled flocks first decreased, as the number of infected flocks decreased faster than the number of pre-emptively culled flocks increased. The total number of culled flocks then increased again as there were more and more pre-emptively culled flocks. Therefore, there was a minimum number of culled flocks at an optimal radius, which ranged from 1 to 18.75 km depending on the epidemiological setting [42, 59, 60]. In Lee et al. [60], this non-monotonic behavior was only observed in areas with R0 above one, but not in areas with R0 below one.
Vaccination strategies were evaluated less frequently than culling strategies (six articles; Table 5). Vaccination was applied either within a radius of 1 to 10 km around infected flocks [47, 50, 74] or to a proportion of the total poultry population [38, 40, 50, 58]. One article evaluated the impact of a vaccination strategy that was applied in real life and not as a theoretical scenario [40]. The results showed that the countrywide vaccination strategy implemented in Vietnam starting in 2005 with ~60% coverage allowed the transmissibility of HPAI infection to be substantially reduced. However, this was coupled with a substantial increase of the time from infection to detection, possibly because lower levels of mortality and symptomatic infections made outbreaks harder to detect [40]. When considering hypothetical vaccination strategies, the results showed the effectiveness of vaccination from an epidemiological point of view in most articles, although ring vaccination was found ineffective in some cases [47, 50]. Pelletier et al. [50] showed that ring vaccination was less effective than countrywide vaccination with 92–97% coverage. The effectiveness of countrywide vaccination was further improved when the order in which premises were vaccinated was determined by known risk factors, such as flock size or proximity to an infected flock [50]. Increasing the vaccination radius brought limited improvements when considering limited capacity [47].
Vaccination was compared to pre-emptive culling in four articles [38, 47, 50, 74]. Their respective effectiveness depended on the objective behind their implementation: vaccination was more effective in reducing the number of culled flocks, while pre-emptive culling was more effective in reducing epidemic size and duration [38, 47, 50, 74]. In Pelletier et al. [50], countrywide vaccination was the most effective in reducing the number of culled flocks, while at the same time having similar effectiveness as pre-emptive culling in reducing the epidemic size. However, the effort required by these two control strategies was substantially different: 92 to 97% of flocks were vaccinated while only 17 to 27% were culled [50]. Increasing the management capacity was less effective for ring vaccination than for ring culling [47, 74].
Only two studies compared the relative costs of pre-emptive culling and vaccination strategies [38, 74]. Backer et al. [74] found that the duration and size of the epidemics were lower for pre-emptive culling than for ring vaccination for a same radius (3 km). However, the total costs (direct costs—such as compensation of culled poultry, costs of culling, cleaning and disinfecting, costs of vaccine doses and vaccination, and surveillance costs—as well as indirect costs—such as lower prices for eggs and slaughtered poultry, and loss of revenue) of the two strategies were only marginally different. Retkute et al. [38] showed that the choice of a strategy depended on the relative costs of pre-emptive culling and vaccinating compared to the economic impact of an infected flock (e.g., costs of reactive culling, compensation paid for destroyed animals, or costs associated with potential zoonotic transmission to poultry professionals). If the cost of culling was low compared to the cost of an infected flock, pre-emptive culling was preferred regardless of the cost of vaccination. If the costs of culling and vaccinating were both high compared to the cost of an infected flock, then neither strategy were preferred and reactive culling alone minimized the total costs of the epidemic. However, if the cost of culling was high compared to the cost of an infected flock but the cost of vaccination was low, then vaccination was preferred [38].
Seven articles evaluated the effectiveness of surveillance strategies (Table 5). Andronico et al. [63] demonstrated that the surveillance zone implemented as part of the mandatory EU strategy during the 2016–2017 H5N8 epidemic in France was effective in reducing transmission. Walker et al. [36] found that intensive surveillance campaigns reduced the period between infection and reporting during the 2004–2005 H5N1 epidemic in Thailand. The other five articles all showed that improving surveillance to reduce the time between infection and detection had a large effect on the epidemic [40, 42, 47, 59, 70]. For instance, reducing the average duration between infection and detection by 35% reduced the epidemic size by 66.8% in Walker et al. [40]. Hill et al. [47] even concluded that improving surveillance was in most cases a more effective strategy than pre-emptive culling or ring vaccination.
Various other strategies (e.g., movement bans, biosecurity measures, closure of live poultry markets) were evaluated in eight articles (Table 5). Most articles assumed that farms emptied of poultry during an epidemic, either because they had completed a production cycle or because their flocks had been culled, were banned from restocking. However, only Dorigatti et al. [67] demonstrated the effectiveness of this mitigation measure. They showed that if the ban on restocking had not been implemented, the epidemic size would have been 155% times higher on average [67]. During the HPAI H5N2 epidemic in Minnesota (US) in 2015, a strategy called “early marketing” was used [56]. This strategy consisted in sending flocks to slaughter earlier than the normally scheduled date to reduce the density of poultry farms. By sufficiently decreasing densities, it was possible to decrease the reproduction number to below one in a high-density area, thereby reducing virus spread [56]. The authors therefore considered this strategy to be an interesting alternative or complement to conventional pre-emptive culling [56].
Finally, the early implementation of mitigation strategies was always more effective than implementing them later in an epidemic [67, 70, 80]. When considering the impact of mitigation strategies on zoonotic transmission to humans, it was demonstrated that combining measures in poultry with measures in humans was better than implementing measures in poultry alone [52]. Given the potential difficulties of implementing a single strategy at highly effective levels, the joint implementation of two strategies at less effective levels may be both easier and as (or even more) effective, thus representing an interesting alternative [52].
4 Discussion
The results presented in this study summarize the state of the art in mechanistic modeling applied to field outbreaks of low pathogenicity and highly pathogenic avian influenza in poultry. Our objectives were to describe the models and their epidemiological context, to list current estimates of AIV transmission parameters, to provide insights on avian influenza transmission and the impact of control strategies, and finally to discuss future avenues for modeling AIV transmission. We limited our analysis to articles describing a model fitted to epidemic data to ascertain the articles’ relevance for this review. The search was restricted to three online databases for articles in English based on a selected set of keywords. Therefore, it is possible that we missed a few relevant publications in the gray literature which did not have the selected keywords or were in a language other than English. However, we are confident that this analysis provides an accurate literature synthesis.
4.1 Epidemiological insights
4.1.1 Parameter estimates
Two recent studies reviewed values of the transmission rate, the basic reproduction number, and the average durations of the latent and infectious periods estimated from experimental studies [31, 32]. In addition, Kirkeby and Ward [32] included estimates from field data, but only if no disease control strategies had been implemented when the data used to perform the estimation was collected. Our review of parameter values therefore differed from these previous studies, as we excluded studies based on experimental infections and included all estimates from field data, including those from periods with active disease control strategies. This was in line with our objectives, which were to review both parameter values and effectiveness of disease control strategies estimated using mechanistic models fitted to field outbreaks.
In our study, only two articles estimated the average durations of the latent and infectious periods in individual birds (Additional file 4) [64, 77], preventing comparisons with the previous reviews. In Kirkeby and Ward [32], the average latent period ranged from 0.2 to 2 days for HPAI, and from 0.02 to 8.6 days for LPAI. The average infectious period for HPAI ranged from 1 to 6.8 days in [32], and from 1.47 days to 13.4 days in [31]. For LPAI, the average infectious period ranged from 2.3 to 13.32 days in [32], and from 4.25 to 10.03 days in [31].
As in our study, both of these reviews found a large variation in the estimated between-individual transmission rates and basic reproduction number R0 [31, 32]. The values of the between-individual reproduction number ranged from 2.18 to 86 for HPAI viruses in our study (Table 3), from 0.17 to 208 in [32] and from 1.6 to 34 in [31]. For LPAI, R0 ranged from 4.7 to 45.9 in our study (Table 3), from 0.3 to 15.6 in [32] and from 0.22 to 15.3 in [31].
In our review, estimates of the average duration of the infectious period at the farm level ranged between 6.4 and 17.22 days for HPAI (Additional file 5). In comparison, estimated values of this parameter ranged between 7.9 and 13.8 days in Kirkeby and Ward [32]. The between-farm reproduction number Rh for HPAI ranged from 0.03 to 15.7 in our review (Additional file 5), and from 0.85 to 22.4 in [32].
The estimates presented in the studies selected for the current review were from a wide range of geographical regions and virus subtypes. This will undoubtedly improve risk analysis and the creation of future models by constructing more realistic simulation studies. While some insights may be gained into the virus spread dynamics in these areas, caution should be taken to extrapolate parameters from one region to the next as disease dynamics depend on numerous factors, including local conditions, farm density, and production systems [81].
4.1.2 Transmission mechanisms
As illustrated in Figure 3, different transmission models used distance-based kernels to study HPAI spread between poultry farms. The estimated kernel parameters (Table 5) suggest that most HPAI transmissions happened at a short to moderate distance range, irrespective of the subtype or geographical location. This is in line with results from observational studies [27, 82]. However, it does not mean that long-distance transmission is impossible. For example, the spatial kernels used in Bangladesh [46] and the Philippines [59] were long-tailed, indicating that some transmission events were often sourced over long distances. Differences in spatial kernels were observed between epidemics in different countries. Differences also could appear between different epidemics of the same subtype in a given country, as highlighted in Hill et al. [46], or even between different waves in a given epidemic, as suggested in Bonney et al. [56]. Such differences could arise from differences in poultry production, distinct characteristics of different HPAI subtypes, or specific control strategies.
Poultry farming systems vary widely regarding the species raised, farm sizes, and management systems [83]. This heterogeneity likely plays a role in epidemic dynamics, but it was rarely accounted for explicitly. However, when species heterogeneity was explicitly modeled, it was possible to determine the respective contribution of each species to transmission, which appeared to depend on the virus subtype. For example, Andronico et al. [63] explicitly modeled galliformes (e.g., chickens) and palmipeds (e.g., ducks and geese) to account for their contribution to the 2016–2017 HPAI H5N8 propagation dynamics in France. They were able to show that palmiped farms were 2.6 (95% CI: 1.2–10) more susceptible and 5.0 (95% CI: 3.7–6.7) times more infectious than galliform farms. Similar results were found in the Republic of Korea for the 2016–2017 HPAI H5N8 spread, with transmissibility between duck farms being as much as 1.6 times higher than either between chicken farms or between different farm types [60]. In contrast, chicken farms were up to 10.7 times more infectious than palmiped farms during the 2004 HPAI H5N1 epidemic in Thailand [36].
Similarly, heterogeneity between management systems was explicitly accounted for in three articles by considering separate compartments for commercial and backyard poultry farms [36, 68, 72]. Both Bavinck et al. [72] and Smith and Dunipace [68] found that backyard farms played a limited role in HPAI transmission, with reproduction numbers below one for transmission between backyard farms and between commercial and backyard farms (note that Walker et al. [36] used a common compartment for backyard poultry and wild birds, preventing comparison).
Wild birds were sometimes included to account for sources of infection other than domestic poultry farms. Different approaches were used, either by implementing a constant parameter fitted to the epidemic data and implicitly considering various sources of transmission (including wild birds) [38, 46, 56, 63], by including seasonal introductions into poultry farms via migratory birds [45], or by considering a specific compartment for the number of wild birds in the vicinity of domestic farms [36, 49]. When the relative role of wild birds and domestic poultry was assessed, commercial poultry farms were found to be the main sources of infection [45, 49, 63]. For instance, Andronico et al. [63] quantified that wild birds and backyard poultry only accounted for 11% of transmission events. However, wild birds may be playing an important role in introducing and re-introducing the virus to the local environment [45, 49].
4.1.3 Control strategies
About half of the included articles evaluated control strategies (20/46). Overall, both reactive and pre-emptive culling remained the most studied control measures, reflecting the widespread use of these measures in real life. In contrast, vaccination was evaluated more rarely. The use of vaccination as a routine component of the control strategy was until 2023 prohibited in the EU [84], and currently remains prohibited in the US [16] because of the trade implications. However, it was authorized in the EU in 2023 [17] due to the recent devastating epidemics.
The effectiveness of control measures likely depends on the virus subtype and on local conditions, such as farm density and the distribution of farm types (backyard/commercial) and poultry species. For instance, the optimal radius minimizing the number of preventively culled flocks varied between locations and epidemic years [42, 59, 60]. Similarly, the optimal strategies minimizing the costs of HPAI epidemics depend on the relative costs of various control measures [38], which may vary over time and between countries. Therefore, whenever possible, modeling approaches should consider the local context and time period when assessing control strategies.
Nonetheless, we identified two general findings regarding the evaluation of control strategies. First, the optimal control measure depended on the objective. The best strategy often differed depending on whether the objective was to reduce the epidemic duration and size or whether it was to reduce the number of culled flocks [38, 47, 50, 74]. In particular, vaccination was often the strategy associated with the lowest number of culled flocks, thus possibly reducing the costs of an epidemic (e.g., median of 278 culled farms for the baseline scenario vs only 163 culled farms in the same scenario but with ring vaccination [74]). However, vaccination strategies may incur other costs by increasing epidemic durations (e.g., median of 67 days for ring vaccination compared to a minimum of 26 days for ring culling [74]) or by disrupting international trade. It should be noted that losses due to restrictions on exports were not included in the three models that compared control strategies based on economic costs [38, 74, 75]. Therefore, a careful definition of the objective and costs associated with each measure is necessary to have a more comprehensive evaluation of the cost-effectiveness of each strategy.
Second, the early implementation of control measures supported by effective surveillance was always beneficial. For instance, reactive culling of infected flocks soon after their detection had a substantial impact on the course of HPAI epidemics [49, 63, 70]. Similarly, improving surveillance to reduce the time between infection and detection/reporting, thus allowing earlier implementation of various strategies (e.g., reactive culling of the infected flock, pre-emptive culling around it), substantially reduced the impact of HPAI epidemics [36, 40, 42, 47, 59, 70]. Moreover, the effectiveness of control strategies increased as the period between the first detected outbreak and the first implementation of control measures decreased [67, 70, 80]. For the 1999–2000 HPAI H7N1 epidemic in Italy, the number of outbreaks would have been reduced from 385 to 222 on average if preventive culling had been implemented 20 rather than 54 days after the epidemic started [67]. All of these results highlight the importance of epidemic preparedness, with effective surveillance systems and quick responses of policy makers and veterinary services.
4.1.4 Different insights from different modeling approaches?
Different modeling approaches have been used to evaluate AIV transmission during the same epidemics in the same countries (Table 1). This was the case for the 2003 HPAI H7N7 epidemic in the Netherlands [69,70,71,72, 74, 75] and for the 2004 HPAI H5N1 epidemic in Thailand [36,37,38]. In these epidemics, different modeling approaches were applied to the same datasets with varying levels of details, providing varying parameter estimates. The 2003 HPAI H7N7 epidemic in the Netherlands was by far the most studied (Table 1), and provided a good case study of how various modeling approaches influence parameter estimations and recommendations to policy makers. Two population-based models neglected the spatial aspect of between-farm transmission [69, 72]. Contrary to Stegeman et al. [69], Bavinck et al. [72] accounted for backyard flocks and neglected temporal changes in transmission rates and infectious periods during the study period (22 February-3 April 2003). Nonetheless, they estimated a between-farm reproduction number (Rh) of 1.33 (Table 3), quite similar to the 1.2 (95% CI: 0.6–1.9) estimates for the same area between 1 March 2003 and 3 April 2003 in Stegeman et al. [69]. Bavinck et al. [72] had the additional advantage of estimating the contribution of backyard and commercial flocks to transmission. They showed that culling backyard flocks may not be necessary as these flocks only played a minor role [72].
Compared to PBMs, spatial IBMs had the additional ability to produce at-risk maps [71] and to evaluate spatially-explicit control strategies such as preventive culling around infected flocks [70, 74, 75]. Various ways of accounting for spatial heterogeneity in transmission during the 2003 HPAI H7N7 epidemic have been used. Le Menach et al. [70] estimated three transmission rates depending on the distance between farms, and found substantially lower transmission rates for long-distance ranges (above 10 km) than for short and medium-ranges (under 3 km and between 3 and 10 km, respectively – Figure 3). Boender et al. [71] used a parametric kernel with a logistic functional form to represent how the relative risk of transmission decreased with increasing between-farm distance (Figure 3). The same kernel was re-used by Backer et al. [74]. The estimates of the spatial kernel parameters indicated a rapid decrease of the relative risk of transmission with increasing distance, where the risk was already halved at 1.9 km and reached very low values beyond 10 km (Figure 3; [71]). Finally, Seymour et al. [75] developed a nonparametric kernel to represent the same phenomenon but in a more flexible way. The estimated nonparametric kernels were of similar shape and scale as the parametric kernel used in Boender et al. [71], but with a higher degree of uncertainty, especially at lower distances [75]. The authors therefore argued that the assumptions imposed by parametric kernels may underestimate the uncertainty, and that nonparametric kernels may better reflect the actual uncertainty in the data [75].
The last point of comparison is the evaluation of control strategies, and more specifically of pre-emptive culling. Boender et al. [71] concluded that culling farms within a 1 km radius was not very effective in reducing the Rh, but that culling farms within a 5 km radius reduced the Rh of all farms below one, and thus was fully effective. In contrast, Backer et al. [74] and Seymour et al. [75] both found a strong effect of pre-emptive culling within a 1 km radius on the number of infected farms compared to no pre-emptive culling. This difference may be explained by the way the control strategies were evaluated. In Boender et al. [71], the effectiveness of pre-emptive culling was evaluated on the Rh of all farms. The other two studies simulated the epidemics under different scenarios and compared the model outputs, such as the number of infected and culled farms. Therefore, the criteria used to evaluate the effectiveness of control strategies need to be chosen carefully. Interestingly, Seymour et al. [75] found that increasing the culling radius beyond 1 km did not substantially reduce the number of infected farms despite a larger number of culled farms. In contrast, Backer et al. [74] found that the effectiveness increased when expanding the radius from 1 to 3 km, but not from 3 to 10 km. This difference in the optimal pre-emptive culling radius (1 vs 3 km) could be explained by the different culling capacity used, up to 20 farms per day in Backer et al. [74] vs only up to six farms per day in Seymour et al. [75]. This difference highlights the importance of this parameter on the evaluation of control strategies. Overall, these results also emphasize the importance of comparing several models with different structures and assumptions to inform policy recommendations [85, 86].
4.2 Model limitations and future avenues for modeling AIV transmission
4.2.1 Research gaps on avian influenza transmission and control
It is worth noting that until the winter of 2021–2022, the HPAI H5N8 subtype was responsible for the largest epidemics ever recorded in the EU in terms of the number of poultry outbreaks, geographical extent, and the number of dead wild birds, spreading across more than 29 European countries in the winters of 2016–2017 and of 2020–2021 [87]. However, only three studies in our review focused on parameter estimation and evaluation of control strategies of HPAI H5N8 in the EU [62,63,64]. Given the animal welfare and economic impact that these epidemics had, further research is needed on mechanistic modeling of HPAI H5N8 to better understand how the virus spread and how best to control it [32]. Since 2021, the HPAI H5N1 subtype has become predominant and caused thousands of outbreaks in domestic poultry [87,88,89]. Modeling studies will therefore also be required for this new devastating and widespread epidemic. In particular, the evaluation of vaccination in some European countries should be a priority given that it may become part of their strategy to mitigate the impact of HPAI [16].
Only five studies in our review included data on LPAI viruses, including three studies on the LPAI H7N9 viruses that were first discovered in China in 2013. Given their zoonotic potential and the ability of H5 and H7 LPAI viruses to mutate into HPAI, further research is needed on this topic [32]. This is especially true as each virus subtype and context is unique, and therefore a better understanding of the transmission of LPAI viruses in different settings is needed.
4.2.2 Fine-tuning models
Models are created for a specific situation with three key (and often opposing) characteristics to be balanced: accuracy (the ability to reproduce the observed data), tractability (the capacity to solve and understand the model analytically or numerically), and flexibility (the ease with which the model can be adapted to new situations) [90]. The trade-off between these characteristics depends on the question being addressed and the availability of data. Even when data are available, simplification is often necessary, for example to get a more flexible and tractable model or save computational time. In doing so, some processes that could impact transmission dynamics may be overlooked.
In the articles reviewed, this was the case for the impact of host species and production systems. Many models (24/46) considered a homogeneous “poultry” population. However, as was demonstrated in some of the articles, galliformes and palmipeds contributed differently to different epidemics [36, 60, 63], and transmission parameters may vary between species depending on the virus subtype [32, 36, 60, 63] and between commercial and backyard farms [68, 72]. Thus, caution should be taken in pooling all poultry populations into one. Whenever possible and according to the available data, models should account for heterogeneity between host species and production systems to gain a better understanding of the contribution of each poultry population to AIV transmission and design control strategies accordingly. Even if host heterogeneity is not included in the model, future studies should at least report information on which poultry populations are included, e.g., to clarify which parameter values are applicable to which species [32].
Another modeling assumption that should be refined is the homogeneous mixing of individual birds, especially at the national [52, 53, 66] and global scales [44, 45]. This assumption means that every host interacts uniformly and randomly with every other host with the same probability, thus neglecting any heterogeneity that may arise from differences in age, behavior, and most importantly, spatial location [90]. This assumption helps simplify models in the absence of detailed data, and may be appropriate at small scales, such as for within-farm epidemic dynamics [35, 57, 62, 64, 65, 73, 74, 76, 77]. However, it may not adequately reflect transmission dynamics at larger scales.
Similarly, the homogeneous mixing of farms [39, 41, 43, 48, 68, 69, 72] may not be adequate, especially for large geographical areas. To refine this assumption and introduce spatial heterogeneity, spatial kernels were most of the time included at the between-farm transmission scale (Figure 3). This may represent a simple but adequate alternative that is more representative of the spatial spread of AIV than homogeneous mixing.
Finally, the scale of the epidemiological unit was also important. Eight articles used administrative areas as the epidemiological unit [36, 37, 40, 49,50,51, 53, 55]. These administrative areas ranged from villages or communes up to US counties or Nigerian states. In all these articles, the administrative area was considered as a single unit, i.e., the whole area became exposed or infectious at the same time, neglecting within-area dynamics. Rorres et al. [55] compared models using three different epidemiological units in Pennsylvania (US): farms, ZIP-codes and counties. Interestingly, the epidemic dynamics were consistent with those at the farm level when aggregating at the ZIP-code level, but not at the county level. These results suggest that US counties are too large to neglect the within-area transmission dynamics [55]. Similar results were found when producing risk maps in the Netherlands: similar risk maps were obtained at the farm or municipality level, but not at the regional level [31]. Therefore, we recommend, depending on the available data and the modeling objective, to use the smallest spatial resolution possible.
4.2.3 Estimating parameters from field data
In most studies, only the transmission parameter was estimated using field data (Table 3). Other parameters, such as the frequently used average durations of the latent and infectious periods, were more often assumed (Additional files 4 and Additional file 5).
For instance, most within-farm model parameters were assumed based on experimental studies for the same or different virus subtype. However, the course of the infection could be different in field situations compared to experimental studies, and could also differ between virus subtypes. Therefore, further insights could be gained on within-farm transmission dynamics and on the course of infection in individual birds by estimating parameters such as the durations of the latent and infectious periods from field outbreak data, when possible. Of course, results from experimental studies could still be used when these parameters cannot be estimated, or as a priori knowledge if the estimation is performed in a Bayesian framework [64]. The introduction time of the virus is another parameter that was estimated in only a few studies, despite its importance for contact tracing and identifying risk factors for the introduction of AIVs in a farm [62]. Using modeling approaches and epidemic data to estimate this parameter thus represents a potential avenue for future research. Moreover, this parameter could be used to inform the time between the onset of infection and its detection (incubation period) in between-farm transmission models.
The duration of the incubation period at the farm level was estimated in only four of the 24 articles that considered between-farm transmission (Additional file 5). Results from within-farm modeling could be used to better inform this parameter and how it varies between farms according to their characteristics (species, size…). Alternatively, this parameter could be estimated, as was done in Kim et al. [42], Hill et al. [46] and Andronico et al. [63]. This would provide useful information on the effectiveness of surveillance.
4.2.4 Improving the evaluation of control strategies
Given the frequency of devastating epidemics and the potential switch toward endemic circulation of HPAI viruses worldwide, preparedness and prevention strategies are critical for the sustainability of poultry production. Mechanistic models of AIV transmission are highly valuable since they provide the possibility to compare several strategies in a given epidemiological setting, which is rarely feasible in the field. The evaluation of different combinations of AIV control strategies deserves further attention. Yet except for combining various strategies with the conventional practice of reactive culling, most articles included in this review compared strategies in isolation. However, the repeated occurrence of devastating HPAI H5Nx epidemics around the world raises serious concerns about the capacity of existing strategies to control HPAI viruses, and highlights that no strategy alone is sufficient [18]. For instance, investigating the use of vaccination in combination with other control strategies could provide useful insights. The barriers to vaccine usage in the EU are progressively being removed, especially with the catastrophic impact of the recent epidemics of HPAI H5N1. The Netherlands and France, among others, already have performed clinical trials of vaccines [91, 92]. Mechanistic models can help assess the effectiveness of control strategies, including vaccination. The implementation of additional mitigation approaches, such as reinforcing biosecurity measures and reducing the density of poultry farms, is a prerequisite to successful vaccination campaigns [18]. In this context, mechanistic models offer a promising route to decipher the effects of associated mitigation and control strategies.
Furthermore, greater attention needs to be paid to the economic costs of control strategies. Effective control strategies from an epidemiological point of view do not necessarily translate into efficient strategies from an economic point of view. In this review, only three articles evaluated the economic costs of an epidemic under various control strategy scenarios [38, 74, 75]. Further work combining mechanistic epidemiological models with economic models is therefore needed.
While no strategy can be used in every context, future investigations could be done with optimization algorithms that incorporate the parameters defining the mitigation strategies as input to determine the set of strategies required to attain the desired outcomes (e.g., minimize the overall epidemic or economic impact) [93]. This approach could complement the traditional method of comparing a set of predefined control strategies. Examples of parameters could be pre-emptive culling and vaccination efforts, and this method would find the optimal strategy (i.e., how much culling and how much vaccination is required) to reach the objective (e.g., minimizing the epidemic impact). Moreover, this could also help in designing time-varying control strategies [94]. The optimal control measure may indeed change over time according to epidemic dynamics and the effects of the interventions themselves. Such optimization approaches were used, for example, to determine the cost-effective surveillance strategies for invasive species management in Australia [95] and to choose the optimal allocation of resources between quarantine and surveillance for protecting islands from pest invasion [93].
Real-time modeling could also provide useful insights to decision makers by comparing scenarios in the early stages of an ongoing epidemic [96]. However, limited data and the resulting uncertainty in parameter values may limit the ability of models to provide useful insights. In this review, only one article retrospectively assessed the ability of its model to be used in real-time by evaluating interventions with models fitted to available data at different time points during the epidemic [38]. Interestingly, despite high uncertainty in model projections, the ranking of control measures was consistent early on during the course of the epidemic (even when using only the first 10 days of the epidemic) when the objective was to reduce the epidemic size or the number of culled flocks. Similar results were found for two epidemics of foot-and-mouth disease [96], indicating the ability of models to provide accurate comparisons of interventions relatively early during an epidemic. However, when the objective was to reduce the epidemic duration, using more data (25 days) was needed to obtain consistent rankings. This means that recommendations made to policy makers could be wrong if they were made before a sufficient amount of data was available [38]. Further research is therefore needed to assess the models’ ability to be used in real-time for the evaluation of control strategies.
Finally, we identified in our review the importance of accounting for limited management capacity in mechanistic models of HPAI. Not accounting for limited resources may overestimate the efficacy of interventions [47, 50, 60, 63, 70, 74, 75], which may lead to suboptimal or even wrong recommendations to policy makers. However, it may be difficult to quantify culling or vaccination capacities and how these change over the course of an epidemic. Different assumptions regarding this parameter may lead to different conclusions, as seen for the 2003 HPAI H7N7 epidemic in the Netherlands [74, 75]. How to accurately model limited management capacity, and how this capacity changes over the course of an epidemic, is therefore an avenue for future research. Collaboration with veterinary services and policy makers will be crucial to make realistic assumptions about this parameter, among others.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files.
References
Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, Fouchier RAM (2006) Global patterns of influenza A virus in wild birds. Science 312:384–388. https://doi.org/10.1126/science.1122438
Abdelwhab E-SM, Veits J, Mettenleiter TC (2013) Genetic changes that accompanied shifts of low pathogenic avian influenza viruses toward higher pathogenicity in poultry. Virulence 4:441–452. https://doi.org/10.4161/viru.25710
Lycett SJ, Duchatel F, Digard P (2019) A brief history of bird flu. Philos Trans R Soc Lond B Biol Sci 374:20180257. https://doi.org/10.1098/rstb.2018.0257
Wang X, Jiang H, Wu P, Uyeki TM, Feng L, Lai S, Wang L, Huo X, Xu K, Chen E, Wang X, He J, Kang M, Zhang R, Zhang J, Wu J, Hu S, Zhang H, Liu X, Fu W, Ou J, Wu S, Qin Y, Zhang Z, Shi Y, Zhang J, Artois J, Fang VJ, Zhu H, Guan Y et al (2017) Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013–17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis 17:822–832. https://doi.org/10.1016/S1473-3099(17)30323-7
Shi W, Gao GF (2021) Emerging H5N8 avian influenza viruses. Science 372:784–786. https://doi.org/10.1126/science.abg6302
Wille M, Barr IG (2022) Resurgence of avian influenza virus. Science 376:459–460. https://doi.org/10.1126/science.abo1232
FAO (Food and Agriculture Organization) (2022) Empres-i. https://empres-i.apps.fao.org/epidemiology. Accessed 8 Nov 2022.
Pohlmann A, King J, Fusaro A, Zecchin B, Banyard AC, Brown IH, Byrne AMP, Beerens N, Liang Y, Heutink R, Harders F, James J, Reid SM, Hansen RDE, Lewis NS, Hjulsager C, Larsen LE, Zohari S, Anderson K, Bröjer C, Nagy A, Savič V, van Borm S, Steensels M, Briand F-X, Swieton E, Smietanka K, Grund C, Beer M, Harder T (2022) Has epizootic become enzootic? Evidence for a fundamental change in the infection dynamics of highly pathogenic avian influenza in Europe, 2021. mMBio 13:e0060922. https://doi.org/10.1128/mbio.00609-22
EFSA (European Food Safety Authority) ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory for Avian Influenza), Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Marangon S, Niqueux E, Staubach C, Terregino C, Guajardo IM, Chuzhakina K, Baldinelli F (2022) Avian influenza overview June–September 2022. EFSA J 20:e07597. https://doi.org/10.2903/j.efsa.2022.7597
Miller BJ (2022) Why unprecedented bird flu outbreaks sweeping the world are concerning scientists. Nature 606:18–19. https://doi.org/10.1038/d41586-022-01338-2
Ramey AM, Hill NJ, DeLiberto TJ, Gibbs SEJ, Camille Hopkins M, Lang AS, Poulson RL, Prosser DJ, Sleeman JM, Stallknecht DE, Wan X-F (2022) Highly pathogenic avian influenza is an emerging disease threat to wild birds in North America. J Wildl Manag 86:e22171. https://doi.org/10.1002/jwmg.22171
Gamarra-Toledo V, Plaza PI, Gutiérrez R, Luyo P, Hernani L, Angulo F, Lambertucci SA (2023) Avian flu threatens Neotropical birds. Science 379:246–246. https://doi.org/10.1126/science.adg2271
European Commission (2019) Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for surveillance, eradication programmes, and disease-free status for certain listed and emerging diseases.
Swayne DE, Spackman E, Pantin-Jackwood M (2014) Success factors for avian influenza vaccine use in poultry and potential impact at the wild bird–agricultural interface. EcoHealth 11:94–108. https://doi.org/10.1007/s10393-013-0861-3
Yoo SJ, Kwon T, Lyoo YS (2018) Challenges of influenza A viruses in humans and animals and current animal vaccines as an effective control measure. Clin Exp Vaccine Res 7:1–15. https://doi.org/10.7774/cevr.2018.7.1.1
Stokstad E (2022) Wrestling with bird flu, Europe considers once-taboo vaccines. Science 376:682–683. https://doi.org/10.1126/science.adc9450
European Commission (2023) Commission Delegated Regulation (EU) 2023/361 of 28 November 2022 supplementing Regulation (EU) 2016/429 of the European Parliament and the Council as regards rules for the use of certain veterinary medicinal products for the purpose of prevention and control of certain listed diseases.
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory for Avian Influenza), Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Marangon S, Niqueux E, Staubach C, Terregino C, Aznar I, Muñoz Guajardo I, Baldinelli F (2021) Avian influenza overview September–December 2021. EFSA J 19:94. https://doi.org/10.2903/j.efsa.2021.7108
Keeling MJ, Woolhouse MEJ, Shaw DJ, Matthews L, Chase-Topping M, Haydon DT, Cornell SJ, Kappey J, Wilesmith J, Grenfell BT (2001) Dynamics of the 2001 UK foot and mouth epidemic: stochastic dispersal in a heterogeneous landscape. Science 294:813–817
Halasa T, Bøtner A, Mortensen S, Christensen H, Toft N, Boklund A (2016) Control of African swine fever epidemics in industrialized swine populations. Vet Microbiol 197:142–150. https://doi.org/10.1016/j.vetmic.2016.11.023
Andraud M, Halasa T, Boklund A, Rose N (2019) Threat to the French swine industry of African swine fever: surveillance, spread, and control perspectives. Front Vet Sci 6:248. https://doi.org/10.3389/fvets.2019.00248
Courtejoie N, Zanella G, Durand B (2018) Bluetongue transmission and control in Europe: a systematic review of compartmental mathematical models. Prev Vet Med 156:113–125. https://doi.org/10.1016/j.prevetmed.2018.05.012
Stegeman A, Bouma A, de Jong MCM (2010) Use of epidemiologic models in the control of highly pathogenic avian influenza. Avian Dis 54:707–712. https://doi.org/10.1637/8821-040209-Review.1
El Zowalaty ME, Bustin SA, Husseiny MI, Ashour HM (2013) Avian influenza: virology, diagnosis and surveillance. Future Microbiol 8:1209–1227. https://doi.org/10.2217/fmb.13.81
Germeraad EA, Sanders P, Hagenaars TJ, de Jong MCM, Beerens N, Gonzales JL (2019) Virus shedding of avian influenza in poultry: a systematic review and meta-analysis. Viruses 11:812. https://doi.org/10.3390/v11090812
Harris KA, Freidl GS, Munoz OS, von Dobschuetz S, De Nardi M, Wieland B, Koopmans MPG, Stärk KDC, van Reeth K, Dauphin G, Meijer A, de Bruin E, Capua I, Hill AA, Kosmider R, Banks J, Stevens K, van der Werf S, Enouf V, van der Meulen K, Brown IH, Alexander DJ, Breed AC, the FLURISK Consortium (2017) Epidemiological risk factors for animal influenza A viruses overcoming species barriers. EcoHealth 14:342–360. https://doi.org/10.1007/s10393-017-1244-y
Gilbert M, Pfeiffer DU (2012) Risk factor modelling of the spatio-temporal patterns of highly pathogenic avian influenza (HPAIV) H5N1: a review. Spat Spatio-temporal Epidemiol 3:173–183. https://doi.org/10.1016/j.sste.2012.01.002
Wang Y, Li P, Wu Y, Sun X, Yu K, Yu C, Qin A (2014) The risk factors for avian influenza on poultry farms: a meta-analysis. Prev Vet Med 117:1–6. https://doi.org/10.1016/j.prevetmed.2014.06.008
Van Kerkhove MD, Mumford E, Mounts AW, Bresee J, Ly S, Bridges CB, Otte J (2011) Highly pathogenic avian influenza (H5N1): pathways of exposure at the animal-human interface, a systematic review. PLoS One 6:e14582. https://doi.org/10.1371/journal.pone.0014582
Hautefeuille C, Dauphin G, Peyre M (2020) Knowledge and remaining gaps on the role of animal and human movements in the poultry production and trade networks in the global spread of avian influenza viruses—a scoping review. PLoS One 15:e0230567. https://doi.org/10.1371/journal.pone.0230567
Central Veterinary Institute, Animal and Plant Health Agency, de Koeijer A, Arnold M, Gonzales J, Boender GJ (2017) Data analysis and predictive modelling of HPAI H5 and H7 outbreaks in the EU 2005–2015. EFSA Supp Publ 14:1285E. https://doi.org/10.2903/sp.efsa.2017.EN-1285
Kirkeby C, Ward MP (2022) A review of estimated transmission parameters for the spread of avian influenza viruses. Transbound Emerg Dis 69:3238–3246. https://doi.org/10.1111/tbed.14675
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/.
Tiensin T, Nielen M, Vernooij H, Songserm T, Kalpravidh W, Chotiprasatintara S, Chaisingh A, Wongkasemjit S, Chanachai K, Thanapongtham W, Srisuvan T, Stegeman A (2007) Transmission of the highly pathogenic avian influenza virus H5N1 within flocks during the 2004 epidemic in Thailand. J Infect Dis 196:1679–1684. https://doi.org/10.1086/522007
Walker PGT, Cauchemez S, Hartemink N, Tiensin T, Ghani AC (2012) Outbreaks of H5N1 in poultry in Thailand: the relative role of poultry production types in sustaining transmission and the impact of active surveillance in control. J R Soc Interface 9:1836–1845. https://doi.org/10.1098/rsif.2012.0022
Marquetoux N, Paul M, Wongnarkpet S, Poolkhet C, Thanapongtharm W, Roger F, Ducrot C, Chalvet-Monfray K (2012) Estimating spatial and temporal variations of the reproduction number for highly pathogenic avian influenza H5N1 epidemic in Thailand. Prev Vet Med 106:143–151. https://doi.org/10.1016/j.prevetmed.2012.01.021
Retkute R, Jewell CP, Van Boeckel TP, Zhang G, Xiao X, Thanapongtharm W, Keeling M, Gilbert M, Tildesley MJ (2018) Dynamics of the 2004 avian influenza H5N1 outbreak in Thailand: the role of duck farming, sequential model fitting and control. Prev Vet Med 159:171–181. https://doi.org/10.1016/j.prevetmed.2018.09.014
van den Broek J (2021) Modelling the reproductive power function. J Appl Stat 48:176–190. https://doi.org/10.1080/02664763.2020.1716696
Walker PGT, Cauchemez S, Métras R, Dung DH, Pfeiffer D, Ghani AC (2010) A Bayesian approach to quantifying the effects of mass poultry vaccination upon the spatial and temporal dynamics of H5N1 in northern Vietnam. PLoS Comput Biol 6:e1000683. https://doi.org/10.1371/journal.pcbi.1000683
Delabouglise A, Choisy M, Phan TD, Antoine-Moussiaux N, Peyre M, Vu TD, Pfeiffer DU, Fournié G (2017) Economic factors influencing zoonotic disease dynamics: demand for poultry meat and seasonal transmission of avian influenza in Vietnam. Sci Rep 7:5905. https://doi.org/10.1038/s41598-017-06244-6
Kim T, Hwang W, Zhang A, Sen S, Ramanathan M (2010) Multi-agent modeling of the South Korean avian influenza epidemic. BMC Infect Dis 10:236. https://doi.org/10.1186/1471-2334-10-236
Kim W-H, Cho S (2021) Estimation of the basic reproduction numbers of the subtypes H5N1, H5N8, and H5N6 during the highly pathogenic avian influenza epidemic spread between farms. Front Vet Sci 8:597630. https://doi.org/10.3389/fvets.2021.597630
Smirnova A, Tuncer N (2014) Estimating time-dependent transmission rate of avian influenza via stable numerical algorithm. J Inverse Ill-Posed Probl 22:31–62. https://doi.org/10.1515/jip-2012-0097
Tuncer N, Martcheva M (2013) Modeling seasonality in avian influenza H5N1. J Biol Syst 21:1340004. https://doi.org/10.1142/S0218339013400044
Hill EM, House T, Dhingra MS, Kalpravidh W, Morzaria S, Osmani MG, Yamage M, Xiao X, Gilbert M, Tildesley MJ (2017) Modelling H5N1 in Bangladesh across spatial scales: model complexity and zoonotic transmission risk. Epidemics 20:37–55. https://doi.org/10.1016/j.epidem.2017.02.007
Hill EM, House T, Dhingra MS, Kalpravidh W, Morzaria S, Osmani MG, Brum E, Yamage M, Kalam MdA, Prosser DJ, Takekawa JY, Xiao X, Gilbert M, Tildesley MJ (2018) The impact of surveillance and control on highly pathogenic avian influenza outbreaks in poultry in Dhaka division, Bangladesh. PLoS Comput Biol 14:e1006439. https://doi.org/10.1371/journal.pcbi.1006439
Ssematimba A, Okike I, Ahmed GM, Yamage M, Boender GJ, Hagenaars TJ, Bett B (2018) Estimating the between-farm transmission rates for highly pathogenic avian influenza subtype H5N1 epidemics in Bangladesh between 2007 and 2013. Transbound Emerg Dis 65:e127–e134. https://doi.org/10.1111/tbed.12692
Pandit PS, Bunn DA, Pande SA, Aly SS (2013) Modeling highly pathogenic avian influenza transmission in wild birds and poultry in West Bengal. India Sci Rep 3:2175. https://doi.org/10.1038/srep02175
Pelletier STK, Rorres C, Macko PC, Peters S, Smith G (2012) Models of highly pathogenic avian influenza epidemics in commercial poultry flocks in Nigeria and Ghana. Trop Anim Health Prod 44:1681–1687. https://doi.org/10.1007/s11250-012-0124-2
Bett B, Henning J, Abdu P, Okike I, Poole J, Young J, Randolph TF, Perry BD (2012) Transmission rate and reproductive number of the H5N1 highly pathogenic avian influenza virus during the December 2005–July 2008 epidemic in Nigeria. Transbound Emerg Dis 61:60–68. https://doi.org/10.1111/tbed.12003
Lee EK, Liu Y, Pietz FH (2017) A computational framework for a digital surveillance and response tool: application to avian influenza. AMIA Annu Symp Proc 2017:1090–1099
Ward MP, Maftei D, Apostu C, Suru A (2009) Estimation of the basic reproductive number (R0) for epidemic, highly pathogenic avian influenza subtype H5N1 spread. Epidemiol Infect 137:219–226. https://doi.org/10.1017/S0950268808000885
Rorres C, Pelletier STK, Bruhn MC, Smith G (2011) Ongoing estimation of the epidemic parameters of a stochastic, spatial, discrete-time model for a 1983–84 avian influenza epidemic. Avian Dis 55:35–42. https://doi.org/10.1637/9429-061710-Reg.1
Rorres C, Pelletier STK, Smith G (2011) Stochastic modeling of animal epidemics using data collected over three different spatial scales. Epidemics 3:61–70. https://doi.org/10.1016/j.epidem.2011.02.003
Bonney PJ, Malladi S, Boender GJ, Weaver JT, Ssematimba A, Halvorson DA, Cardona CJ (2018) Spatial transmission of H5N2 highly pathogenic avian influenza between Minnesota poultry premises during the 2015 outbreak. PLoS One 13:e0204262. https://doi.org/10.1371/journal.pone.0204262
Ssematimba A, Malladi S, Hagenaars TJ, Bonney PJ, Weaver JT, Patyk KA, Spackman E, Halvorson DA, Cardona CJ (2019) Estimating within-flock transmission rate parameter for H5N2 highly pathogenic avian influenza virus in Minnesota turkey flocks during the 2015 epizootic. Epidemiol Infect 147:e179. https://doi.org/10.1017/S0950268819000633
Lee H, Lao A (2018) Transmission dynamics and control strategies assessment of avian influenza A (H5N6) in the Philippines. Infect Dis Model 3:35–59. https://doi.org/10.1016/j.idm.2018.03.004
Salvador R, Tanquilut N, Macmac R, Lampang KN, Chaisowwong W, Pfeiffer D, Punyapornwithaya V (2020) Evaluation of strategies using simulation model to control a potential outbreak of highly pathogenic avian influenza among poultry farms in Central Luzon, Philippines. PLoS One 15:e0238815. https://doi.org/10.1371/journal.pone.0238815
Lee J, Ko Y, Jung E (2019) Effective control measures considering spatial heterogeneity to mitigate the 2016–2017 avian influenza epidemic in the Republic of Korea. PLoS One 14:e0218202. https://doi.org/10.1371/journal.pone.0218202
Yoo D-S, Chun BC, Kim Y, Lee K-N, Moon O-K (2021) Dynamics of inter-farm transmission of highly pathogenic avian influenza H5N6 integrating vehicle movements and phylogenetic information. Sci Rep 11:24163. https://doi.org/10.1038/s41598-021-03284-x
Hobbelen PHF, Elbers ARW, Werkman M, Koch G, Velkers FC, Stegeman A, Hagenaars TJ (2020) Estimating the introduction time of highly pathogenic avian influenza into poultry flocks. Sci Rep 10:12388. https://doi.org/10.1038/s41598-020-68623-w
Andronico A, Courcoul A, Bronner A, Scoizec A, Lebouquin-Leneveu S, Guinat C, Paul MC, Durand B, Cauchemez S (2019) Highly pathogenic avian influenza H5N8 in south-west France 2016–2017: a modeling study of control strategies. Epidemics 28:100340. https://doi.org/10.1016/j.epidem.2019.03.006
Vergne T, Gubbins S, Guinat C, Bauzile B, Delpont M, Chakraborty D, Gruson H, Roche B, Andraud M, Paul M, Guérin J-L (2021) Inferring within-flock transmission dynamics of highly pathogenic avian influenza H5N8 virus in France, 2020. Transbound Emerg Dis 68:3151–3155. https://doi.org/10.1111/tbed.14202
Hayama Y, Sawai K, Yoshinori M, Yamaguchi E, Yamamoto T (2022) Estimation of introduction time window of highly pathogenic avian influenza virus into broiler chicken farms during the 2020–2021 winter season outbreak in Japan. Prev Vet Med 208:105768. https://doi.org/10.1016/j.prevetmed.2022.105768
Chen Y, Jin Z, Zhang J, Wang Y, Zhang J (2022) Global dynamical analysis of H5 subtype avian influenza model. Int J Biomath 15:2250058. https://doi.org/10.1142/S1793524522500589
Dorigatti I, Mulatti P, Rosà R, Pugliese A, Busani L (2010) Modelling the spatial spread of H7N1 avian influenza virus among poultry farms in Italy. Epidemics 2:29–35. https://doi.org/10.1016/j.epidem.2010.01.002
Smith G, Dunipace S (2011) How backyard poultry flocks influence the effort required to curtail avian influenza epidemics in commercial poultry flocks. Epidemics 3:71–75. https://doi.org/10.1016/j.epidem.2011.01.003
Stegeman A, Bouma A, Elbers ARW, de Jong MCM, Nodelijk G, de Klerk F, Koch G, van Boven M (2004) Avian influenza A virus (H7N7) epidemic in the Netherlands in 2003: course of the epidemic and effectiveness of control measures. J Infect Dis 190:2088–2095. https://doi.org/10.1086/425583
Le Menach A, Vergu E, Grais RF, Smith DL, Flahault A (2006) Key strategies for reducing spread of avian influenza among commercial poultry holdings: lessons for transmission to humans. Proc Biol Sci 273:2467–2475. https://doi.org/10.1098/rspb.2006.3609
Boender GJ, Hagenaars TJ, Bouma A, Nodelijk G, Elbers ARW, de Jong MCM, van Boven M (2007) Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comp Biol 3:e71. https://doi.org/10.1371/journal.pcbi.0030071
Bavinck V, Bouma A, van Boven M, Bos MEH, Stassen E, Stegeman JA (2009) The role of backyard poultry flocks in the epidemic of highly pathogenic avian influenza virus (H7N7) in the Netherlands in 2003. Prev Vet Med 88:247–254. https://doi.org/10.1016/j.prevetmed.2008.10.007
Bos MEH, Nielen M, Koch G, Bouma A, de Jong MCM, Stegeman A (2009) Back-calculation method shows that within-flock transmission of highly pathogenic avian influenza (H7N7) virus in the Netherlands is not influenced by housing risk factors. Prev Vet Med 88:278–285. https://doi.org/10.1016/j.prevetmed.2008.12.003
Backer JA, van Roermund HJW, Fischer EAJ, van Asseldonk MAPM, Bergevoet RHM (2015) Controlling highly pathogenic avian influenza outbreaks: an epidemiological and economic model analysis. Prev Vet Med 121:142–150. https://doi.org/10.1016/j.prevetmed.2015.06.006
Seymour RG, Kypraios T, O’Neill PD, Hagenaars TJ (2021) A Bayesian nonparametric analysis of the 2003 outbreak of highly pathogenic avian influenza in the Netherlands. J R Stat Soc Ser C Appl Stat 70:1323–1343. https://doi.org/10.1111/rssc.12515
Bonney PJ, Malladi S, Ssematimba A, Spackman E, Torchetti MK, Culhane M, Cardona CJ (2021) Estimating epidemiological parameters using diagnostic testing data from low pathogenicity avian influenza infected turkey houses. Sci Rep 11:1602. https://doi.org/10.1038/s41598-021-81254-z
Gonzales JL, Elbers ARW, van der Goot JA, Bontje D, Koch G, de Wit JJ, Stegeman JA (2012) Using egg production data to quantify within-flock transmission of low pathogenic avian influenza virus in commercial layer chickens. Prev Vet Med 107:253–259. https://doi.org/10.1016/j.prevetmed.2012.06.010
Li R, Bai Y, Heaney A, Kandula S, Cai J, Zhao X, Xu B, Shaman J (2017) Inference and forecast of H7N9 influenza in China, 2013 to 2015. Euro Surveill 22:30462. https://doi.org/10.2807/1560-7917.ES.2017.22.7.30462
Bai N, Zhang J, Li L, Jin Z (2019) Evaluating the effect of virus mutation on the transmission of avian influenza H7N9 virus in China based on dynamical model. Math Biosci Eng 16:3393–3410. https://doi.org/10.3934/mbe.2019170
Zhu G, Kang M, Wei X, Tang T, Liu T, Xiao J, Song T, Ma W (2021) Different intervention strategies toward live poultry markets against avian influenza A (H7N9) virus: model-based assessment. Environ Res 198:110465. https://doi.org/10.1016/j.envres.2020.110465
Ypma RJF, Jonges M, Bataille A, Stegeman A, Koch G, van Boven M, Koopmans M, van Ballegooijen WM, Wallinga J (2013) Genetic data provide evidence for wind-mediated transmission of highly pathogenic avian influenza. J Infect Dis 207:730–735. https://doi.org/10.1093/infdis/jis757
Guinat C, Nicolas G, Vergne T, Bronner A, Durand B, Courcoul A, Gilbert M, Guérin J-L, Paul MC (2018) Spatio-temporal patterns of highly pathogenic avian influenza virus subtype H5N8 spread, France, 2016 to 2017. Euro Surveill 23:1700791. https://doi.org/10.2807/1560-7917.ES.2018.23.26.1700791
FAO (Food and Agriculture Organization) (2022) Gateway to poultry production and products. https://www.fao.org/poultry-production-products/en/. Accessed 15 Nov 2022.
European Commission (2006) Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC.
den Boon S, Jit M, Brisson M, Medley G, Beutels P, White R, Flasche S, Hollingsworth TD, Garske T, Pitzer VE, Hoogendoorn M, Geffen O, Clark A, Kim J, Hutubessy R (2019) Guidelines for multi-model comparisons of the impact of infectious disease interventions. BMC Med 17:163. https://doi.org/10.1186/s12916-019-1403-9
Hollingsworth TD, Medley GF (2017) Learning from multi-model comparisons: collaboration leads to insights, but limitations remain. Epidemics 18:1–3. https://doi.org/10.1016/j.epidem.2017.02.014
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory for Avian Influenza), Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Marangon S, Niqueux E, Staubach C, Terregino C, Aznar I, Muñoz Guajardo I, Baldinelli F (2022) Avian influenza overview December 2021–March 2022. EFSA J 20:64. https://doi.org/10.2903/j.efsa.2022.7289
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory for Avian Influenza), Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Marangon S, Niqueux E, Staubach C, Terregino C, Aznar I, Muñoz Guajardo I, Baldinelli F (2022) Avian influenza overview March 2022–June 2022. EFSA J 20:67. https://doi.org/10.2903/j.efsa.2022.7415
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory for Avian Influenza), Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Mirinaviciute G, Niqueux E, Stahl K, Staubach C, Terregino C, Broglia A, Kohnle L, Baldinelli F (2023) Avian influenza overview March–April 2023. EFSA J 21:e08039. https://doi.org/10.2903/j.efsa.2023.8039
Keeling MJ, Rohani P (2008) Modeling infectious diseases in humans and animals. Princeton University Press, Princeton
Ministère de l’Agriculture et de la Souveraineté alimentaire (2023) All you need to know on the Highly Pathogenic Avian Influenza vaccination action plan in France. https://agriculture.gouv.fr/tout-ce-quil-faut-savoir-sur-le-plan-daction-vaccination-iahp-en-france. Accessed 6 Jul 2023.
Beerens N (2023) Two vaccines effective against bird flu. https://www.wur.nl/en/research-results/research-institutes/bioveterinary-research/show-bvr/two-vaccines-effective-against-bird-flu.htm. Accessed 6 Jul 2023.
Moore JL, Rout TM, Hauser CE, Moro D, Jones M, Wilcox C, Possingham HP (2010) Protecting islands from pest invasion: optimal allocation of biosecurity resources between quarantine and surveillance. Biol Conserv 143:1068–1078. https://doi.org/10.1016/j.biocon.2010.01.019
Fenichel EP, Horan RD (2007) Gender-based harvesting in wildlife disease management. Am J Agric Econ 89:904–920. https://doi.org/10.1111/j.1467-8276.2007.01025.x
Hauser CE, McCarthy MA (2009) Streamlining ‘search and destroy’: cost-effective surveillance for invasive species management. Ecol Lett 12:683–692. https://doi.org/10.1111/j.1461-0248.2009.01323.x
Probert WJM, Jewell CR, Werkman M, Fonnesbeck CJ, Goto Y, Runge MC, Sekiguchi S, Shea K, Keeling MJ, Ferrari MJ, Tildesley MJ (2018) Real-time decision-making during emergency disease outbreaks. PLoS Comput Biol 14:e1006202. https://doi.org/10.1371/journal.pcbi.1006202
Kirkeby C, Brookes VJ, Ward MP, Dürr S, Halasa T (2021) A practical introduction to mechanistic modeling of disease transmission in veterinary science. Front Vet Sci 7:546651. https://doi.org/10.3389/fvets.2020.546651
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled? Trends Ecol Evol 16:295–300. https://doi.org/10.1016/S0169-5347(01)02144-9
Vynnycky E, White RG (2010) An introduction to infectious disease modelling. Oxford University Press, New York
Webster RG, Webby RJ, Hoffmann E, Rodenberg J, Kumar M, Chu H-J, Seiler P, Krauss S, Songserm T (2006) The immunogenicity and efficacy against H5N1 challenge of reverse genetics-derived H5N3 influenza vaccine in ducks and chickens. Virology 351:303–311. https://doi.org/10.1016/j.virol.2006.01.044
Tian G, Zhang S, Li Y, Bu Z, Liu P, Zhou J, Li C, Shi J, Yu K, Chen H (2005) Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology 341:153–162. https://doi.org/10.1016/j.virol.2005.07.011
Lee C-W, Suarez DL, Tumpey TM, Sung H-W, Kwon Y-K, Lee Y-J, Choi J-G, Joh S-J, Kim M-C, Lee E-K, Park J-M, Lu X, Katz JM, Spackman E, Swayne DE, Kim J-H (2005) Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol 79:3692–3702. https://doi.org/10.1128/JVI.79.6.3692-3702.2005
Swayne DE, Lee C-W, Spackman E (2006) Inactivated North American and European H5N2 avian influenza virus vaccines protect chickens from Asian H5N1 high pathogenicity avian influenza virus. Avian Pathol 35:141–146. https://doi.org/10.1080/03079450600597956
Li KS, Guan Y, Wang J, Smith GJD, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie ATS, Chaisingh A, Auewarakul P, Long HT, Hanh NTH, Webby RJ, Poon LLM, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JSM (2004) Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209–213. https://doi.org/10.1038/nature02746
Capua I, Alexander DJ (2004) Avian influenza: recent developments. Avian Pathol 33:393–404. https://doi.org/10.1080/03079450410001724085
Spackman E, Pantin-Jackwood MJ, Kapczynski DR, Swayne DE, Suarez DL (2016) H5N2 Highly Pathogenic Avian Influenza Viruses from the US 2014–2015 outbreak have an unusually long pre-clinical period in turkeys. BMC Vet Res 12:260. https://doi.org/10.1186/s12917-016-0890-6
Cardona C, Alexander C, Bergeron J, Bonney P, Culhane M, Goldsmith T, Halvorson D, Linskens E, Malladi S, Ssematimba A, Walz E, Weaver T, Umber J (2018) An assessment of the risk associated with the movement of turkeys to market into, within, and out of a control area during a highly pathogenic avian influenza outbreak in the United States. Collaborative agreement between USDA:APHIS:VS and University of Minnesota Center for Secure Food Systems. Fort Collins, CO, USA
Mutinelli F, Capua I, Terregino C, Cattoli G (2003) Clinical, gross, and microscopic findings in different avian species naturally infected during the H7N1 low- and high-pathogenicity avian influenza epidemics in Italy during 1999 and 2000. Avian Dis 47:844–848. https://doi.org/10.1637/0005-2086-47.s3.844
De Benedictis P, Manuel Joannis T, HannatuLombin L, Shittu I, Serena Beato M, Rebonato V, Cattoli G, Capua I (2007) Field and laboratory findings of the first incursion of the Asian H5N1 highly pathogenic avian influenza virus in Africa. Avian Pathol 36:115–117. https://doi.org/10.1080/03079450601161406
Alexander DJ, Parsons G, Manvell RJ (1986) Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol 15:647–662. https://doi.org/10.1080/03079458608436328
Slemons RD, Easterday BC (1972) Host response differences among 5 avian species to an influenza virus—A/turkey/Ontario/7732/66 (Hav5N?). Bull World Health Organ 47:521–525
Kang H-M, Lee E-K, Song B-M, Jeong J, Choi J-G, Jeong J, Moon O-K, Yoon H, Cho Y, Kang Y-M, Lee H-S, Lee Y-J (2015) Novel reassortant influenza A(H5N8) viruses among inoculated domestic and wild ducks, South Korea, 2014. Emerg Infect Dis 21:298–304. https://doi.org/10.3201/eid2102.141268
Grund C, Hoffmann D, Ulrich R, Naguib M, Schinköthe J, Hoffmann B, Harder T, Saenger S, Zscheppang K, Tönnies M, Hippenstiel S, Hocke A, Wolff T, Beer M (2018) A novel European H5N8 influenza A virus has increased virulence in ducks but low zoonotic potential. Emerg Microbes Infect 7:132. https://doi.org/10.1038/s41426-018-0130-1
Song B-M, Kang H-M, Lee E-K, Jeong J, Kang Y, Lee H-S, Lee Y-J (2015) Pathogenicity of H5N8 virus in chickens from Korea in 2014. J Vet Sci 16:237–240. https://doi.org/10.4142/jvs.2015.16.2.237
Tanikawa T, Kanehira K, Tsunekuni R, Uchida Y, Takemae N, Saito T (2016) Pathogenicity of H5N8 highly pathogenic avian influenza viruses isolated from a wild bird fecal specimen and a chicken in Japan in 2014. Microbiol Immunol 60:243–252. https://doi.org/10.1111/1348-0421.12369
Lee D-H, Kwon J-H, Noh J-Y, Park J-K, Yuk S-S, Erdene-Ochir T-O, Lee J-B, Park S-Y, Choi I-S, Lee S-W, Song C-S (2016) Pathogenicity of the Korean H5N8 highly pathogenic avian influenza virus in commercial domestic poultry species. Avian Pathol 45:208–211. https://doi.org/10.1080/03079457.2016.1142502
Bae Y-J, Lee S-B, Min K-C, Mo J-S, Jeon E-O, Koo B-S, Kwon H-I, Choi YK, Kim J-J, Kim J-N, Mo I-P (2014) Pathological evaluation of natural cases of a highly pathogenic avian influenza virus, subtype H5N8, in broiler breeders and commercial layers in South Korea. Avian Dis 59:175–182. https://doi.org/10.1637/10921-081914-Case
Bertran K, Swayne DE, Pantin-Jackwood MJ, Kapczynski DR, Spackman E, Suarez DL (2016) Lack of chicken adaptation of newly emergent Eurasian H5N8 and reassortant H5N2 high pathogenicity avian influenza viruses in the U.S. is consistent with restricted poultry outbreaks in the Pacific flyway during 2014–2015. Virology 494:190–197. https://doi.org/10.1016/j.virol.2016.04.019
Gamoh K, Nakamizo M, Okamatsu M, Sakoda Y, Kida H, Suzuki S (2016) Protective efficacy of stockpiled vaccine against H5N8 highly pathogenic avian influenza virus isolated from a chicken in Kumamoto prefecture, Japan, in 2014. J Vet Med Sci 78:139–142. https://doi.org/10.1292/jvms.15-0324
Kapczynski DR, Tumpey TM, Hidajat R, Zsak A, Chrzastek K, Tretyakova I, Pushko P (2016) Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine 34:1575–1581. https://doi.org/10.1016/j.vaccine.2016.02.011
Kim SM, Kim Y-I, Park S-J, Kim E-H, Kwon H-I, Si Y-J, Lee I-W, Song M-S, Choi YK (2017) Vaccine efficacy of inactivated, chimeric hemagglutinin H9/H5N2 avian influenza virus and its suitability for the marker vaccine strategy. J Virol 91:e01693-e16. https://doi.org/10.1128/JVI.01693-16
Lee E-K, Song B-M, Kang H-M, Woo S-H, Heo G-B, Jung SC, Park YH, Lee Y-J, Kim J-H (2016) Experimental infection of SPF and Korean native chickens with highly pathogenic avian influenza virus (H5N8). Poult Sci 95:1015–1019. https://doi.org/10.3382/ps/pew028
Steensels M, Rauw F, van den Berg T, Marché S, Gardin Y, Palya V, Lambrecht B (2015) Protection afforded by a recombinant turkey herpesvirus-H5 vaccine against the 2014 European highly pathogenic H5N8 avian influenza strain. Avian Dis 60:202–209. https://doi.org/10.1637/11126-050615-Reg.1
Zeng X, Chen P, Liu L, Deng G, Li Y, Shi J, Kong H, Feng H, Bai J, Li X, Shi W, Tian G, Chen H (2016) Protective efficacy of an H5N1 inactivated vaccine against challenge with lethal H5N1, H5N2, H5N6, and H5N8 influenza viruses in chickens. Avian Dis 60:253–255. https://doi.org/10.1637/11179-052015-ResNoteR
Sakuma S, Uchida Y, Kajita M, Tanikawa T, Mine J, Tsunekuni R, Saito T (2021) First outbreak of an H5N8 highly pathogenic avian influenza virus on a chicken farm in Japan in 2020. Viruses 13:489. https://doi.org/10.3390/v13030489
van der Goot JA, Koch G, de Jong MCM, van Boven M (2005) Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proc Natl Acad Sci USA 102:18141–18146. https://doi.org/10.1073/pnas.0505098102
Bouma A, Claassen I, Natih K, Klinkenberg D, Donnelly CA, Koch G, van Boven M (2009) Estimation of transmission parameters of H5N1 avian influenza virus in chickens. PLoS Pathog 5:e1000281. https://doi.org/10.1371/journal.ppat.1000281
Poetri ON, Bouma A, Murtini S, Claassen I, Koch G, Soejoedono RD, Stegeman JA, van Boven M (2009) An inactivated H5N2 vaccine reduces transmission of highly pathogenic H5N1 avian influenza virus among native chickens. Vaccine 27:2864–2869. https://doi.org/10.1016/j.vaccine.2009.02.085
van der Goot JA, de Jong MCM, Koch G, van Boven M (2003) Comparison of the transmission characteristics of low and high pathogenicity avian influenza A virus (H5N2). Epidemiol Infect 131:1003–1013. https://doi.org/10.1017/S0950268803001067
Pillai SPS, Pantin-Jackwood M, Suarez DL, Saif YM, Lee C-W (2010) Pathobiological characterization of low-pathogenicity H5 avian influenza viruses of diverse origins in chickens, ducks and turkeys. Arch Virol 155:1439–1451. https://doi.org/10.1007/s00705-010-0727-8
Iqbal M, Essen SC, Xiao H, Brookes SM, Brown IH, McCauley JW (2012) Selection of variant viruses during replication and transmission of H7N1 viruses in chickens and turkeys. Virology 433:282–295. https://doi.org/10.1016/j.virol.2012.08.001
Pantin-Jackwood MJ, Stephens CB, Bertran K, Swayne DE, Spackman E (2017) The pathogenesis of H7N8 low and highly pathogenic avian influenza viruses from the United States 2016 outbreak in chickens, turkeys and mallards. PLoS One 12:e0177265. https://doi.org/10.1371/journal.pone.0177265
Comin A, Klinkenberg D, Marangon S, Toffan A, Stegeman A (2011) Transmission dynamics of low pathogenicity avian influenza infections in turkey flocks. PLoS One 6:e26935. https://doi.org/10.1371/journal.pone.0026935
Spackman E, Gelb J, Preskenis LA, Ladman BS, Pope CR, Pantin-Jackwood MJ, Mckinley ET (2010) The pathogenesis of low pathogenicity H7 avian influenza viruses in chickens, ducks and turkeys. Virol J 7:331. https://doi.org/10.1186/1743-422X-7-331
Saenz RA, Essen SC, Brookes SM, Iqbal M, Wood JLN, Grenfell BT, McCauley JW, Brown IH, Gog JR (2012) Quantifying transmission of highly pathogenic and low pathogenicity H7N1 avian influenza in turkeys. PLoS One 7:e45059. https://doi.org/10.1371/journal.pone.0045059
Buisch WW, Hall AE, McDaniel HA (1984) 1983–1984 Lethal avian influenza outbreak. Proceedings. Annual meeting - United States Animal Health Association (USA)
Swayne DE, Beck JR (2005) Experimental study to determine if low-pathogenicity and high-pathogenicity avian influenza viruses can be present in chicken breast and thigh meat following intranasal virus inoculation. Avian Dis 49:81–85. https://doi.org/10.1637/7260-081104R
Savill NJ, St Rose SG, Keeling MJ, Woolhouse MEJ (2006) Silent spread of H5N1 in vaccinated poultry. Nature 442:757–757. https://doi.org/10.1038/442757a
van der Goot JA, van Boven M, Koch G, de Jong MCM (2007) Variable effect of vaccination against highly pathogenic avian influenza (H7N7) virus on disease and transmission in pheasants and teals. Vaccine 25:8318–8325. https://doi.org/10.1016/j.vaccine.2007.09.048
Jeong O-M, Kim M-C, Kim M-J, Kang H-M, Kim H-R, Kim Y-J, Joh S-J, Kwon J-H, Lee Y-J (2009) Experimental infection of chickens, ducks and quails with the highly pathogenic H5N1 avian influenza virus. J Vet Sci 10:53–60. https://doi.org/10.4142/jvs.2009.10.1.53
Park S-C, Song B-M, Lee Y-N, Lee E-K, Heo G-B, Kye S-J, Lee K-H, Bae Y-C, Lee Y-J, Kim B (2019) Pathogenicity of clade 2344 H5N6 highly pathogenic avian influenza virus in three chicken breeds from South Korea in 2016/2017. J Vet Sci 20:e27. https://doi.org/10.4142/jvs.2019.20.e27
Busani L, Valsecchi MG, Rossi E, Toson M, Ferrè N, Pozza MD, Marangon S (2009) Risk factors for highly pathogenic H7N1 avian influenza virus infection in poultry during the 1999–2000 epidemic in Italy. Vet J 181:171–177. https://doi.org/10.1016/j.tvjl.2008.02.013
Acknowledgements
The authors wish to thank the two anonymous reviewers for their valuable comments and Grace Delobel for English language editing.
Funding
This work was funded by the FEDER/Région Occitanie Recherche et Sociétés 2018–AI-TRACK. This study was performed in the framework of the “Chair for Avian Health and Biosecurity”, hosted by the National Veterinary School of Toulouse and funded by the Direction Générale de l’Alimentation, Ministère de l’Agriculture et de l’Alimentation, France. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
TV and MP conceived the study design. BB performed the literature search. BB and AM performed the two-step screening, with ad hoc input from SL. SL, BB and AM extracted and analyzed the data, and drafted the first version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Handling editor: Vincent Béringue.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Complete overview of search terms used in PubMed, Web of Science and CAB Abstracts.
Additional file 2:
Summary of the two-step screening.
Additional file 3:
Data extraction from the 46 included articles [35–80].
Additional file 4:
Parameter values used in within-farm transmission models [35, 57, 62, 64, 65, 73, 74, 76, 77, 100–135].
Additional file 5:
Parameter values used in between-farms transmission models. [38, 41–43, 46–48, 50, 54–56, 59–61, 63, 67–72, 75, 101, 102, 116, 122, 126, 129, 136–142].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lambert, S., Bauzile, B., Mugnier, A. et al. A systematic review of mechanistic models used to study avian influenza virus transmission and control. Vet Res 54, 96 (2023). https://doi.org/10.1186/s13567-023-01219-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-023-01219-0