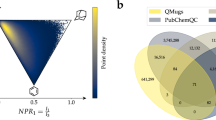
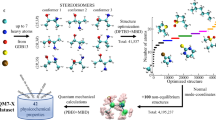
Abstract
Previous studies have shown that the three-dimensional (3D) geometric and electronic structure of molecules play a crucial role in determining their key properties and intermolecular interactions. Therefore, it is necessary to establish a quantum chemical (QC) property database containing the most stable 3D geometric conformations and electronic structures of molecules. In this study, a high-quality QC property database, called QuanDB, was developed, which included structurally diverse molecular entities and featured a user-friendly interface. Currently, QuanDB contains 154,610 compounds sourced from public databases and scientific literature, with 10,125 scaffolds. The elemental composition comprises nine elements: H, C, O, N, P, S, F, Cl, and Br. For each molecule, QuanDB provides 53 global and 5 local QC properties and the most stable 3D conformation. These properties are divided into three categories: geometric structure, electronic structure, and thermodynamics. Geometric structure optimization and single point energy calculation at the theoretical level of B3LYP-D3(BJ)/6-311G(d)/SMD/water and B3LYP-D3(BJ)/def2-TZVP/SMD/water, respectively, were applied to ensure highly accurate calculations of QC properties, with the computational cost exceeding 107 core-hours. QuanDB provides high-value geometric and electronic structure information for use in molecular representation models, which are critical for machine-learning-based molecular design, thereby contributing to a comprehensive description of the chemical compound space. As a new high-quality dataset for QC properties, QuanDB is expected to become a benchmark tool for the training and optimization of machine learning models, thus further advancing the development of novel drugs and materials. QuanDB is freely available, without registration, at https://quandb.cmdrg.com/.
Scientific contribution
-
The QuanDB database contains comprehensive quantum chemical properties of diverse organic molecular entities, and all data have been rigorously pretreated and manually cleaned to ensure high accuracy.
-
By utilizing the quantum chemical properties provided by QuanDB, relevant three-dimensional (3D) electronic structural information can be included in comprehensive molecular representation models to facilitate drug and material design.
-
Compared to other similar databases, QuanDB covers a broader space of chemical compounds, adopts a higher level of theoretical calculations, and offers a user-friendly interface.
Graphical Abstract

Similar content being viewed by others
Introduction
Currently, the fundamental assumption of an AI-assisted molecular (drug or material) design is that “structurally similar molecules have similar properties.” A comprehensive molecular representation is crucial for facilitating the discovery of novel molecules [1]. Generally, molecular representations with stronger discriminative ability tend to demonstrate superior performance in downstream molecular design tasks [2,3,4,5]. Traditional molecular descriptors require manual feature engineering, making it difficult to comprehensively represent molecules without expert knowledge [6]. Consequently, data-driven representation models are increasingly used to extract unbiased features from molecules [7,8,9,10]. Additionally, as the relationships between the molecular structure and physicochemical (PC) properties, reactivity, and bioactivity are becoming better understood, researchers are gradually incorporating features that can include the three-dimensional (3D) conformation of molecules in representation models [11,12,13,14]. The electronic and structural parameters of stable 3D conformations are of particular interest because they critically affect several crucial properties of molecules in 3D space, such as their reactivity, strong electrostatic interactions, and chemical adsorption. Density functional theory (DFT) remains the most reliable and accurate method for obtaining the electronic structure information of the most stable 3D molecular conformations, which can be reflected by quantum chemical (QC) properties [15,16,17,18]. By incorporating QC properties into the training phase of the molecular representation models, their ability to represent the electronic structural space can be effectively enhanced, thereby improving the performance of downstream tasks, such as predicting molecular properties [12, 19]. Therefore, the construction of a DFT-based QC property database for small organic molecules is of great significance for the virtual evaluation, screening, and reverse design of novel molecules.
QC property databases aim to comprehensively represent the electronic structural information of the most stable 3D molecular conformations using a broad set of QC properties [20,21,22]. The QM9 database is currently the most extensively used and authoritative source of QC properties [22]. It comprises data for 134,000 molecules taken from the GDB-17 database [23]. Presently, QM9 plays a crucial role as a benchmark dataset for evaluating molecular representation models [2, 3] and producing 3D molecular representations [24,25,26,27,28,29]. However, the QM9 dataset has several limitations. First, the geometric structural optimization in QM9 is performed at the B3LYP/6-31G(2df,p) theoretical level, which allows for potential improvements in the calculation accuracy [30]. Second, to reduce computational complexity, QM9 restricts the number of heavy atoms to a maximum of 9 and contains only 5 elements: H, C, O, N, and F. This limitation severely restricts the representation of diverse molecular structures and chemical compound spaces. Despite these limitations, the simple molecular structures in the QM9 dataset have yielded excellent predictive results when used as input data for current deep-learning models [3, 12], some of which achieved prediction errors close to zero [4]. Additionally, the molecules in the QM series datasets are computed and thus deviate to some extent from real materials. Finally, QM9 lacks a user-friendly visualization interface, which makes it difficult for researchers outside the field to take full advantage of its utility. In conclusion, it is imperative to develop a new database of high-quality QC properties that contains real compounds, has broad coverage of the chemical space, and provides a user-friendly interface.
To address the need for a new database, this study aimed to develop a new high-quality QC property database, which comprises diverse labeled compounds with more comprehensive QC properties than previous databases and a user-friendly interface. It can not only further enrich and supplement high-value molecular structure representation information, but also provide a benchmark for the training and optimization of machine learning models, thereby facilitating the design and development of novel materials and drugs.
Construction and content
Data collection and curation
First, to identify the target molecular entity in the database based on the research requirements, 23 endpoints were defined, covering three categories: bioactivity, toxicity, and PC properties (Table 1). We used a semi-automatic text-mining method to collect experimental data from databases such as OCHEM [31], PubChem [32], and DrugBank [33], which include the one of above 23 endpoints for compounds and annotated the literature sources. A good database should cover the largest possible chemical space. However, since the computational time is exponentially related to the number of atoms, the maximum learning space was limited by the computational resources available to our research group. Therefore, we restricted the range of elements to C, H, O, N, P, S, F, Cl, and Br, with a maximum of 40. Based on these constraints, we removed small molecules from the original data that exceeded these limits, along with their corresponding experimental values. Considering that different experimental data can be obtained for the same molecule and endpoint (e.g., due to variations in the experimental conditions), we performed data deduplication and cleaning steps. The cleaning strategy was as follows: if the maximum ratio of logarithmic values for duplicate molecule entries exceeded 1.17 (log10 15), all data were deleted; otherwise, the mean value was used as the final experimental value. Because the geometric structure of a molecule significantly affects its quantifiable properties at the microscopic level, molecules with different conformations were treated as different entities. Finally, we calculated the basic properties such as relative molecular mass, Canonical SMILES, InChI, and InChIKey for each molecule and annotated them. Finally, we obtained 154,610 molecule entities with 334,781 property data entries for 23 endpoints. The overall data-cleaning process is shown in Fig. 1.
Data collection and cleaning process in QuanDB. The 23 proposed endpoint properties are listed in Table 1. Each molecule in QuanDB contains 53 global and 5 local QC properties, as well as the lowest energy conformation
Calculation and extraction of QC properties
Based on the tradeoff between the calculation accuracy and computational time, the basis set with the highest accuracy obtainable using our limited computing resources was chosen. For geometric structural optimization, which has low conformational sensitivity to the basis set and is highly time-consuming, we chose a common 3-zeta basis set, viz., 6-311G(d) [34]. For the single-point energy calculation, which is sensitive to the basis set, we chose a higher-level 3-zeta basis set (def2-TZVP) [35].
The calculation of the QC properties involves the following three steps. (1) The GMMX3.0 module in GaussView6 [36] is used to search for molecular conformations. The lowest-energy conformation is then subjected to geometric structure optimization and frequency analysis using Gaussian16 [37] at the B3LYP-D3(BJ)/6-311G(d)/SMD/water [38] theoretical level. After obtaining the lowest-energy conformation without imaginary frequencies, the single-point energy calculation is performed at the B3LYP-D3(BJ)/def2-TZVP/SMD/water [38] theoretical level. (2) The Gaussian16 wavefunction file (.chk) is analyzed using Multiwfn software to obtain a.txt file containing the molecular electrostatic surface properties. (3) QC properties are extracted automatically in batches using internal scripts. In total, we obtained 53 global and 5 local QC properties, as well as the lowest-energy conformation for each molecule (Fig. 1). Therefore, the QC properties in QuanDB are derived from three sources: (i) properties obtained from the geometric structural optimization and frequency analysis; (ii) properties calculated from the single-point energy of the lowest-energy conformation obtained in (i); and (iii) properties obtained from quantitative surface analysis of the wavefunction file using Multiwfn software [39].
Online database implementation
The backend service of the QuanDB database was built using the Python web framework FastAPI, whereas the frontend pages were developed using Vue 3.0 [40]. The entire database follows a backend/frontend separation, essentially implementing the MVVM pattern. All data are stored and managed using MySQL software. For molecular visualization, the RDKit toolkit [41] is used to generate two-dimensional (2D) graphs and 3D structures are displayed using 3Dmol.js [42]. All visualizations in QuanDB are implemented using ECharts [43]. QuanDB has undergone comprehensive testing to ensure functionality across multiple operating systems and web browsers.
Current database content and statistics
QuanDB is a comprehensive and user-oriented QC property database that is proposed as a high-quality benchmark for QC properties. QuanDB can be used to represent the most stable 3D conformations and electronic structures of small organic molecules, such as drugs and other materials. In turn, it could play an important role in a range of downstream tasks like property prediction, molecule generation, and inverse molecule design.
The primary objective of most chemical databases is to explore a wide chemical compound space. To cover the chemical space of practically relevant compounds as much as possible while minimizing computational complexity, QuanDB restricts the elemental composition to H, C, O, N, P, S, F, Cl, and Br and loosens the upper limit for the total number of atoms to 40. In total, QuanDB includes 154,610 molecular entities and 10,125 scaffolds. On average, each scaffold covers 14 molecules, and more than 85% of scaffolds contain fewer than 10 molecules. Approximately 46% of the scaffolds are distinct. A cloud diagram of the top 200 scaffolds is shown in Fig. 2, as implemented using the Scopy toolkit [44].
In terms of molecular composition, the distribution of total atoms and heavy atoms in QuanDB can is shown be seen in Fig. 3. On an average, the molecules in QuanDB contain 19 heavy atoms, whereas the most widely used QC property database (QM9the most widely used QC property database) has a limit, QM9, contains a maximum of 9 heavy atoms. In terms of heteroatoms, the three most frequently occurring elements are N, O, and F. Overall, compared with other popular QC property benchmarks, QuanDB provides a better means to test and evaluate the representation and generalization ability of models, and presents a greater challenge to their goodness of fit.
Another advantage of QuanDB is that the molecular entities are accompanied by experimental data. Currently, QuanDB contains 334,781 rigorously validated experimental data points for 23 endpoint properties (Fig. 4). Among these properties, bioactivity, toxicity, and PC properties account for 79%, 2%, and 19%, respectively. Among them, IC50 has the highest number of entries (136,746), accounting for 47% of the entire dataset. For this subset of data, we annotated the target of compound action and the PubMed ID. Overall, QuanDB provides a high-quality, standardized dataset for drug and material design.
QuanDB provides 53 global QC properties (e.g., zero-point energy) and 5 local QC properties (four atomic charges and one chemical bond order) for each molecule. These QC properties were categorized into three types based on their representative level: geometric, electronic, and thermodynamic properties. Additionally, QuanDB provides the lowest-energy conformation for each molecule, thus serving as a standard dataset for conformer-generation research. The high-quality experimental data and accurate QC properties provided by QuanDB highlight its potential as a benchmark dataset for evaluating model performance. In addition, the electronic structural information is expected to be useful for molecular representation learning, thus enhancing model representation capabilities. A complete list of the 58 QC properties in QuanDB is presented in Table 2.
Utility and discussion
Web design and interface
QuanDB offers researchers a user-friendly interface to facilitate the access and use of its extensive data. The QuanDB database is available at https://quandb.cmdrg.com. The search box at the top enables users to input the SMILES of a molecule or draw its structure. The ‘Browse’ option in the navigation bar allows users to explore the entire dataset, whereas the ‘Download’ feature provides multiple methods for data retrieval. Further assistance can be found in the ‘Help’ section.
Data browsing
By default, the browsing interface of QuanDB displays all molecular structures in the QuanDB identifier order. On the left-hand side of the browsing page, the filters are designed based on the endpoints of the experimental properties, and users can filter compounds that contain the desired endpoints based on the requirements of their specific application. To select multiple endpoints, the database uses the “OR” logical operator for processing. In addition, the number of molecules displayed per page can be controlled using a selector at the top of the page. Finally, clicking on a compound card in the browsing interface opens the corresponding page with a detailed description, including the basic chemical properties and quantitative descriptors of the molecule.
Data searching
The search box on the right-hand side banner on any QuanDB page provides a quick search function. Users can enter the SMILES code or draw the structure of the query molecule using tools provided by the database in the search box, and then click the search icon to initiate the search. After a few seconds, the user is redirected to the search results page. The search interface page is similar to the browsing interface, but the molecules are sorted in the descending order of similarity to the query molecule (based on the Tanimoto Index and 1024-bit ECFP4 Fingerprints), and the query structure is fixed on the right-hand side of the page. Similarly, users can filter the experimental data and perform other operations. Clicking on a compound card redirects the user to the corresponding detailed information page.
Data retrieval
The information page for a selected molecule consists of three sections: basic information, experimental and QC property data, and corresponding charts of the properties. As shown in Fig. 5A, the Basic Information section provides the QuanDB ID and other characteristics, including the molecular formula, molecular weight, SMILES, InChI, and InChIKey. To provide a high-quality dataset for 3D molecular representation learning, QuanDB provides two-dimensional structures and the lowest-energy conformations obtained by energy optimization using Gaussian 16 (based on B3LYP-D3(BJ)/6-311G(d)/SMD/water). Additionally, upon clicking “Search 2D Similar Compounds” on the structure card, a search is performed using the current molecule as the query structure and the user is redirected to the search results page. Next, in the Experimental Data section, the elemental composition and distribution are presented in the form of bar and pie charts, respectively, to provide an approximate representation of the chemical space of the molecule. The green line in the bar chart represents the cumulative number of atoms (Fig. 5B). Below the elemental composition diagram, experimental data related to bioactivity, PC properties, and toxicity are displayed separately (Fig. 5C). Each record is processed using the method mentioned in the “Data collection and curation” section. If available, links to relevant literature are provided for user reference. For bioactivity data, QuanDB not only indicates the endpoint (e.g., IC50), but also provides the corresponding UniProt ID for the target. In the case of toxicity data, information on the organism and administration route are provided to clarify the nature of the endpoint as much as possible.
Molecular information page in QuanDB: A Basic information of the molecule, including common molecular identifiers and 2D and 3D structures (QuanDB 69403); B Composition of the molecule, with a bar chart on the left showing the frequency distribution of each element, a line chart showing the cumulative distribution of heavy atoms, and a pie chart on the right showing the proportion of each heavy atom; C Three major categories of experimental data for the molecule; D Display of global QC properties of the molecule, with a radar chart on the right showing the distribution of properties relative to the overall database mean; E Interactive table for displaying local QC properties design; F Download area
Finally, the QC properties in the QuanDB database are divided into three sections based on the method of acquisition: “Geometric Structure Optimization and Frequency Analysis,” “Single-point Energy Calculation,” and “Quantitative Molecular Surface Analysis.” The calculated values of the corresponding properties are displayed in the tables on the left side of each section. The radar chart on the right shows the distribution of molecular properties in the overall database. The blue line in the chart represents the properties of the molecule, whereas the red area represents the geometric mean value of all data in the database for that endpoint (Fig. 5D). Additionally, in the “Single-point Energy Calculation” section, five types of local QC properties are provided, i.e., four types of atomic charges and one chemical bond order. To present the results intuitively, QuanDB offers an interactive table to enable the user to hover over specific atoms or chemical bonds, which are then highlighted in the structure above (Fig. 5E). Furthermore, the download section on the right side of the page allows users to download desired information based on their requirements (Fig. 5F).
Downloads and updates
Users can download the experimental properties, QC properties, and the lowest-energy conformations of molecules from the database without the need to log in or register. The dataset is divided into multiple subsets based on the 23 experimental endpoints as most applications of the database are expected to be focused on specific molecular endpoints. We hope that these datasets will assist researchers in exploring QC properties and establishing more comprehensive molecular representation models. In the future, we will continue to maintain and update the database; the QC properties and experimental data will be updated every 6 months as new molecules are computed and processed. In addition, we plan to perform calculations for more complex molecules.
Conclusions
Many key PC properties and biological activities of molecules are closely related to their 3D geometric and electronic structures. Therefore, the construction of a high-quality property database is very important for facilitating the further development of molecular representation models. Considering the limitations of existing available public QC property databases, we developed QuanDB as a more targeted and higher-quality QC database. Currently, the QuanDB contains 154,610 molecular entities, 10,125 scaffolds and 334,781 experimental labels. For each molecule, 53 global and 5 local QC properties, as well as the lowest-energy conformation are provided, with a total computational cost of more than 107 core-hours. The advantages of this database compared to existing ones are as follows: (i) All the molecular entities are labeled compounds, and the experimental data cover 23 experimental property endpoints. (ii) The molecular structure types are more diverse, covering a wider chemical compound space, including nine elements (C, H, O, N, P, S, F, Cl, and Br), and allowing up to 40 atoms in each molecule. (iii) More comprehensive QC properties are provided, and the automated batch calculations and extraction of QC properties are realized by combining Gaussian16, Multiwfn, and in-house scripts. (iv) The database has a user-friendly interface, with intuitive and interactive features. The 23 endpoints are categorized into three major classes: bioactivity, toxicity, and PC properties. Users can download the entire dataset or specific subsets according to their needs.
In general, QuanDB is a high-value QC database supported by current computing power. We expect that QuanDB will become a valuable tool for enhancing the representation capability of molecular representation models, while providing a new benchmark for researchers to develop QC property prediction models. This will ultimately contribute to advancement in molecular design research.
Availability of data and materials
The whole data in QuanDB can be retrieved via https://quandb.cmdrg.com/#/download without any requests, and the calculation scripts used in this work can be obtained through https://github.com/kotori-y/scripts_4_quandb. Besides, for developers we also provide a REST API for accessing: https://quandb.cmdrg.com/api/docs.
Change history
11 June 2024
A Correction to this paper has been published: https://doi.org/10.1186/s13321-024-00864-7
Abbreviations
- 3D:
-
Three-dimensional
- DFT:
-
Density functional theory
- PC:
-
Physicochemical
- QC:
-
Quantum chemical
References
Cherkasov A, Muratov EN, Fourches D, Varnek A, Baskin II, Cronin M, Dearden J, Gramatica P, Martin YC, Todeschini R et al (2014) QSAR modeling: where have you been? Where are you going to? J Med Chem 57(12):4977–5010
Fang X, Liu L, Lei J, He D, Zhang S, Zhou J, Wang F, Wu H, Wang H (2022) Geometry-enhanced molecular representation learning for property prediction. Nat Mach Intell 4(2):127–134
Wang Y, Wang J, Cao Z, Barati Farimani A (2022) Molecular contrastive learning of representations via graph neural networks. Nat Mach Intell 4(3):279–287
Zhou G, Gao Z, Ding Q, Zheng H, Xu H, Wei Z, Zhang L, Ke G (2022) Uni-Mol: a universal 3D molecular representation learning framework. https://openreview.net/forum?id=6K2RM6wVqKu. Accessed 20 Mar 2024
Atz K, Grisoni F, Schneider G (2021) Geometric deep learning on molecular representations. Nat Mach Intell 3(12):1023–1032
Walters WP, Barzilay R (2021) Applications of deep learning in molecule generation and molecular property prediction. Acc Chem Res 54(2):263–270
Wu Z, Wang J, Du H, Jiang D, Kang Y, Li D, Pan P, Deng Y, Cao D, Hsieh C-Y et al (2023) Chemistry-intuitive explanation of graph neural networks for molecular property prediction with substructure masking. Nat Commun 14(1):2585
Fang Y, Zhang Q, Zhang N, Chen Z, Zhuang X, Shao X, Fan X, Chen H (2023) Knowledge graph-enhanced molecular contrastive learning with functional prompt. Nat Mach Intell 5(5):542–553
Born J, Manica M (2023) Regression Transformer enables concurrent sequence regression and generation for molecular language modelling. Nat Mach Intell 5(4):432–444
Zhang X, Wang S, Zhu F, Xu Z, Wang Y, Huang J (2018) Seq3seq fingerprint: towards end-to-end semi-supervised deep drug discovery. Proceedings of the 2018 ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics:404–413.
Stärk H, Beaini D, Corso G, Tossou P, Dallago C, Günnemann S, Liò P (2022) 3D Infomax improves GNNs for molecular property prediction. https://arxiv.org/abs/2110.04126. Accessed 20 Mar 2024
Wang X, Zhao H, Tu W, Yao Q (2023) Automated 3D pre-training for molecular property prediction. https://arxiv.org/abs/2306.07812. Accessed 20 Mar 2024
Liu S, Wang H, Liu W, Lasenby J, Guo H, Tang J (2022) Pre-training molecular graph representation with 3D geometry. https://arxiv.org/abs/2306.07812. Accessed 20 Mar 2024
Fuchs F, Worrall D, Fischer V, Welling M (2020) Se (3)-transformers: 3d roto-translation equivariant attention networks. Adv Neural Inf Process Syst 33(1):1970–1981
Parr RG, Yang W (1995) Density-functional theory of the electronic structure of molecules. Annu Rev Phys Chem 46(1):701–728
Cartier A, Rivail JL (1987) Electronic descriptors in quantitative structure—activity relationships. Chemom Intell Lab Syst 1(4):335–347
Wang L, Ding J, Pan L, Cao D, Jiang H, Ding X (2021) Quantum chemical descriptors in quantitative structure–activity relationship models and their applications. Chemom Intell Lab Syst 217:104384
Karelson M, Lobanov VS, Katritzky AR (1996) Quantum-chemical descriptors in QSAR/QSPR studies. Chem Rev 96(3):1027–1044
Kao PY, Yang YC, Chiang WY, Hsiao JY, Cao Y, Aliper A, Ren F, Aspuru-Guzik A, Zhavoronkov A, Hsieh MH et al (2023) Exploring the advantages of quantum generative adversarial networks in generative chemistry. J Chem Inf Model 63(11):3307–3318
Chen G, Chen P, Hsieh C-Y, Lee C-K, Liao B, Liao R, Liu W, Qiu J, Sun Q, Tang J et al (2019) Alchemy: a quantum chemistry dataset for benchmarking AI models. https://arxiv.org/abs/1906.09427. Accessed 20 Mar 2024
Ghahremanpour MM, van Maaren PJ, van der Spoel D (2018) The Alexandria library, a quantum-chemical database of molecular properties for force field development. Sci Data 5(1):180062
Ramakrishnan R, Dral PO, Rupp M, von Lilienfeld OA (2014) Quantum chemistry structures and properties of 134 kilo molecules. Sci Data 1(1):140022
Ruddigkeit L, van Deursen R, Blum LC, Reymond J-L (2012) Enumeration of 166 billion organic small molecules in the chemical universe database GDB-17. J Chem Inf Model 52(11):2864–2875
Zhou G, Gao Z, Wei Z, Zheng H, Ke G (2023) Do deep learning methods really perform better in molecular conformation generation? https://arxiv.org/abs/2302.07061. Accessed 20 Mar 2024
Ganea O, Pattanaik L, Coley C, Barzilay R, Jensen K, Green W, Jaakkola T (2021) Geomol: Torsional geometric generation of molecular 3d conformer ensembles. https://arxiv.org/abs/2106.07802. Accessed 20 Mar 2024
Shi C, Luo S, Xu M, Tang J (2021) Learning gradient fields for molecular conformation generation. https://arxiv.org/abs/2105.03902. Accessed 20 Mar 2024
Axelrod S, Gómez-Bombarelli R (2022) GEOM, energy-annotated molecular conformations for property prediction and molecular generation. Sci Data 9(1):185
Zhu J, Xia Y, Liu C, Wu L, Xie S, Wang T, Wang Y, Zhou W, Qin T, Li H (2022) Direct molecular conformation generation. https://arxiv.org/abs/2202.01356. Accessed 20 Mar 2024
Zhang H, Li S, Zhang J, Wang Z, Wang J, Jiang D, Bian Z, Zhang Y, Deng Y, Song J et al (2023) SDEGen: learning to evolve molecular conformations from thermodynamic noise for conformation generation. Chem Sci 14(6):1557–1568
Narayanan B, Redfern PC, Assary RS, Curtiss LA (2019) Accurate quantum chemical energies for 133 000 organic molecules. Chem Sci 10(31):7449–7455
Sushko I, Novotarskyi S, Körner R, Pandey AK, Rupp M, Teetz W, Brandmaier S, Abdelaziz A, Prokopenko VV, Tanchuk VY et al (2011) Online chemical modeling environment (OCHEM): web platform for data storage, model development and publishing of chemical information. J Comput Aided Mol Des 25(6):533–554
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B et al (2022) PubChem 2023 update. Nucleic Acids Res 51(D1):D1373–D1380
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z et al (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46(D1):D1074-d1082
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72(1):650–654
Zheng J, Xu X, Truhlar DG (2011) Minimally augmented Karlsruhe basis sets. Theor Chem Acc 128(3):295–305
Roy D, Keith Todd A, Millam, John M, Semichem Inc., Shawnee Mission KS (2016) GaussView, Version 6
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H et al (2016) Gaussian 16 Rev
Schröder H, Hühnert J, Schwabe T (2017) Evaluation of DFT-D3 dispersion corrections for various structural benchmark sets. JCP. 146(4).
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Vue.js developers (2014) Vue.js—the progressive JavaScript framework v3.0. https://vuejs.org/guide/introduction.html. Accessed 20 Mar 2024
RDKit developers (2021) RDKit: open-source cheminformatics. https://www.rdkit.org/. Accessed 20 Mar 2024
Rego N, Koes D (2014) 3Dmol.js: molecular visualization with WebGL. Bioinformatics 31(8):1322–1324
Li D, Mei H, Shen Y, Su S, Zhang W, Wang J, Zu M, Chen W (2018) ECharts: a declarative framework for rapid construction of web-based visualization. Vis Inform 2(2):136–146
Yang ZY, Yang ZJ, Lu AP, Hou TJ, Cao DS (2021) Scopy: an integrated negative design python library for desirable HTS/VS database design. Brief Bioinform. 22(3):bbaa194
Acknowledgements
We would like to express our gratitude to the State Key Laboratory of NBC Protection for Civilians for providing support and technical assistance to the computer cluster and selfless open-source developers.
Author information
Authors and Affiliations
Contributions
Z. Yang contributed to the main code, data collection, and manuscript writing. T. Huang and L. Pan tested the functionality of the database. J. Wang contributed to the visualization. L. Wang designed the web UI, computed QC properties, and revised the manuscript. J. Ding and J. Xiao directed the study and involved in experimental deign, data analysis and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Z., Huang, T., Pan, L. et al. QuanDB: a quantum chemical property database towards enhancing 3D molecular representation learning. J Cheminform 16, 48 (2024). https://doi.org/10.1186/s13321-024-00843-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13321-024-00843-y