Abstract
Patients with spinal cord injury (SCI) have permanent devastating motor and sensory disabilities. Secondary SCI is known for its complex progression and presents with sophisticated aberrant inflammation, vascular changes, and secondary cellular dysfunction, which aggravate the primary damage. Since their initial discovery, the potent neuroprotective effects and powerful delivery abilities of exosomes (Exos) have been reported in different research fields, including SCI. In this study, we summarize therapeutic advances related to the application of Exos in preclinical animal studies. Subsequently, we discuss the mechanisms of action of Exos derived from diverse cell types, including neurogenesis, angiogenesis, blood–spinal cord barrier preservation, anti-apoptosis, and anti-inflammatory potential. We also evaluate the relationship between the Exo delivery cargo and signaling pathways. Finally, we discuss the challenges and advantages of using Exos to offer innovative insights regarding the development of efficient clinical strategies for SCI.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) is one of the most serious neurological disorders, with a global incidence of 1.2–5.8 and 0.2–13.0 cases per 100 000 population in developed and developing countries, respectively [1,2,3,4,5,6]. Approximately 90% of SCIs are caused by traumatic events, such as traffic accidents, falls, or acts of violence [6]. SCI results in enduring impairments, including paralysis, sensory loss, and long-term complications, including muscle atrophy, joint deformities, infections, atelectasis, pneumonia, venous thromboembolism, dysphagia, chronic pain, pressure ulcer, and psychological distress such as depression [7, 8], thereby accounting for a substantial proportion of the worldwide injury burden of lost productivity and high healthcare costs [5]. Currently, no effective treatment is available to mitigate long-term functional impairments attributed to SCI. Available therapies, such as anti-inflammatory medications, have limited efficacy since they are rapidly eliminated by cerebrospinal fluid, and their bioactivity is diminished [9, 10]. This is partly due to a limited understanding of the intricate pathophysiological processes that occur after SCI and a lack of safe and efficient instruments to regulate the already known therapeutic targets [11].
Pathophysiology of SCI
In large, SCI can be classified into primary and secondary phases [12]. Primary SCI results from physical forces exerted during an initial traumatic event. These forces induce dislocation of the vertebral column, as well as destruction of the vasculature, and compromise the integrity of the blood–spinal cord barrier (BSCB). Following the primary injury, a series of secondary injury events occur, leading to an increase in the volume of spinal cord damage and irritating neurological deficits. During this secondary injury cascade, cell dysfunction and death in the spinal cord occur due to cell permeabilization, pro-apoptotic signaling, and ischemia injury resulting from the breakdown of the microvascular supply. These events occur within minutes of the injury. Additionally, inflammatory cells, such as macrophages, microglia, T cells, and neutrophils, migrate to the area of injury due to the disruption of the BSCB [13]. Secondary neurodegenerative alterations, such as the degeneration of axons and changes in the gray matter caused by the injury or compression, extend both toward the head (rostrally) and tail (caudally) from the location of the initial damage. Moreover, the overstimulation of excitatory amino acid receptors leads to excitotoxicity, which causes neuron and glial cell death through both necrotic and apoptotic processes [14,15,16,17]. Given the above, SCI functional recovery depends on growing neuroplasticity to enhance sprouting and regeneration of spared and injured axons, angiogenesis, preserving the integrity of the BSCB, limiting apoptosis, and decreasing inflammation to boost the potency of residual nerve connections and facilitate the development of new connections between neurons [18].
Characterization and role of exosomes (Exos)
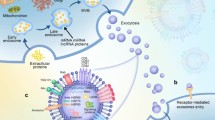
Exos are among the most popular research foci as potential therapeutic agents for overcoming the convoluted pathophysiology process of SCI. Exos are generated by the inward budding of endosomal compartments that later fuse with the plasma membrane, a heterogeneous group of membrane-surrounded particles released by all cell types with a diameter of 30–150 nm [19,20,21]. The isolation methods of Exos progressed as time and technique advanced, from conventional ultracentrifugation, size-based filtration, size-exclusion chromatography, polymer precipitation, and immunoaffinity methods to modern microfluidic-based isolation techniques [22]. Exos has been developed as a new strategy for the non-invasive diagnosis and monitoring of diseases, used as disease markers. Furthermore, Exos can be used as potential non-cell therapeutics. By exchanging functional contents between cells, Exos play fundamental roles in maintaining homeostasis and combatting stress [23]. Additionally, Exos can be engineered to deliver various therapeutic cargos, including short-interfering RNAs (siRNAs), messenger RNAs (mRNAs), microRNAs (miRNAs), antisense oligonucleotides, chemotherapeutic agents, immune modulators, and other bioactive molecules, directly to the desired target [24, 25].
Effects of exos on SCI
In SCI, Exos support many healing mechanisms that have neuroprotective effects by stimulating the regeneration of vessels and nerves and promoting white matter remodeling in the insulted central nerve system (CNS) [26,27,28]. Exos exhibit numerous other advantages, such as good encapsulation and ready penetration of the blood–brain barrier to access the CNS. Furthermore, Exos are important elements of the cellular secretome and are promising options for cell-free therapies because of their potential therapeutic bioactivity, natural compatibility with the body, and ability to target specific cells. This approach helps address concerns regarding the immune response and uncontrolled proliferation or differentiation of cellular transplants. Exos combine the advantages of cell and nanotechnology in drug delivery [29,30,31,32]. Although Exos have advantages in treating SCI, several challenges exist as follows: the potency of Exos large-scale production remains challenging; the off-target effects of Exos remain; systemic clearance limits reach and efficacy of Exos; negatively charged cell membranes and repulsion of Exos induce inefficient uptake; and lysosomal degradation [33, 34].
Current Exo research in SCI treatment mostly focuses on those derived from various stem cell sources as the regenerative abilities of the source cells [35,36,37]. To provide new researchers on Exo with a succinct, up-to-date foundation on SCI, we also included Exos that originate from microglia, macrophages, regulatory T cells (Tregs), Schwann cells (SCs), bone marrow mesenchymal stem cells (BMDMs), plasma, and other substances, which have a beneficial effect on nerve reconstruction by facilitating axonal regeneration and eliminating damaged debris, as well as transporting proteins that actively inhibit inflammation [38,39,40,41,42,43,44,45,46]. Overall, Exos originating from various cells, including mesenchymal stem cells (MSCs), have broad application prospects for SCI [31, 40,41,42, 46].
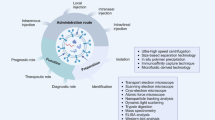
In this review, we discuss how Exos protect neurons, including the underlying processes and other relevant aspects of Exo treatment for SCI. Currently, the promising treatments for SCI are mostly related to Exos derived from MSCs [47, 48]. We focus on the specific mechanisms exerted by different Exos isolated from various progenitor cells rather than stem cells in the complex pathophysiology of SCI, including human epidural adipose cells, neural stem cells (NSCs), Schwann cells, macrophages, and plasma. We also discuss the prospects and challenges of non-stem cell-derived Exos (Fig. 1). Furthermore, the Exos have been classified into five major functions according to their characteristics and cargos, serving as therapeutic strategies for axonal disruption, vascular injury, BSCB disconnection, cell death, and inflammation, as primarily revealed through substantial preclinical animal research. Additionally, we discuss new strategies and information, including origin, effect, cargo, and the current deep molecular mechanisms of Exo-based treatment and the potential for improving treatment efficiency and preventing long-term disease progression to facilitate the transition to clinical trials. Moreover, we compare the Exos and animal model heterogenous in different studies. This review will help facilitate research on using Exos for the efficient management of SCI in the future.
Mechanisms of exo-mediated treatment for SCI
Therapeutic strategies tailored to the pathophysiology of SCI are promising for clinical translation. Nevertheless, SCI is a complex condition that involves multiple aspects. To obtain a better therapeutic outcome, it is necessary to address the simultaneous and subsequent pathogenic processes that occur throughout the evolution of the secondary damage (Fig. 2) [13, 16]. Therefore, there is an urgent need for a multitarget therapeutic approach to counteract secondary injury progression. Exos have shown potential for protection due to their wide-ranging effectiveness and have been extensively studied in various preclinical models of SCI.
Recent research indicates that Exos, together with other payloads, such as RNAs, proteins, and medicine, have a strong protective effect. This effect can modify the functioning of recipient cells in the spinal cord system. Furthermore, the bioactivity and biological composition of Exos are contingent on the phenotype of the parent cell from which they are derived and might exhibit variability in response to stimuli and the local microenvironment. Here, we summarized published studies on SCI treatment with Exos by promoting axonal regeneration and angiogenesis while limiting BSCB disruption, apoptosis, and inflammation in the progression of complicated pathology changes in secondary injury post-SCI.
Mechanism Underlying SCI Changes. Primary and secondary neurodegenerative processes following SCI. The injury leads to the degeneration of the sensory and motor pathways by either anterograde or retrograde axonal degeneration and accompanying demyelination; distant neurons undergo trans-synaptic degeneration. Reproduced with permission [13]. 2019, Lancet Neurology. SCI spinal cord injury; MRI magnetic resonance imaging
Promoting neurogenesis
Adult neurogenesis is essential for maintaining CNS homeostasis and reacting to neurogenic insults. Nevertheless, the adult mammalian spinal cord does not possess the inherent ability for neurogenesis [49]. Nerve regeneration and neurotrophicity are crucial elements in SCI repair and are significant areas of research in SCI treatment [50]. Notably, Exos exhibit considerable potential in SCI neurogenesis therapy [51]. Evidence shows that purified Exos isolated from MSCs and BMDMs, among others, can engage with recipient cells when injected intravenously. This treatment significantly improves bladder dysfunction and motor movement in animal models after SCI by activating NSC differentiation and increasing axonal outgrowth (Fig. 3A) [25, 39, 52, 53].
Growth-associated protein 43 (GAP-43) is a synaptic protein whose expression is significantly elevated during the differentiation of NSCs into cortical neurons. Enhanced production of the neuron-specific proteins microtubule-associated protein 2 (MAP-2) and beta-tubulin III (Tuj1) is vital for the process of NSC differentiation [54,55,56]. Li et al. [57] demonstrated that Exos loaded with overexpressed neuronal growth factor (Exo-oe-NGF) obtained from bone marrow-derived stem cells (BMSCs) enhance the process of NSC differentiation into neuronal cells. Additionally, these Exos promote the neuronal axonal regeneration following SCI that spans a spinal cord cross-section measuring 2 mm in length. This leads to improved locomotor functional recovery, as displayed by a substantial increase in the Basso, Beattie, and Bresnahan (BBB) score compared with SCI mice. This improvement is supported by the upregulated expression of GAP-43, Tuj1, and MAP-2. Jia et al. [58] reported that the levels of Sonic Hedgehog and glioma-associated oncogene homolog 1 increase substantially after injecting BMSC-Exos, along with an increase in GAP-43 expression and the promotion of functional recovery. Moreover, injection with miR-29b Exos and the miR-29b groups significantly promoted SCI (spinal cord contusive injury) characteristics, including increased BBB scores and the numbers of NF200 and GAP-43-positive neurons [59]. Furthermore, Cheng et al. [60] discovered that methacrylated gelatin (GelMA)-Exos stimulated neurogenesis, reduced glial scarring in injury sites, improved the differentiation of Tuj-1-positive neurons, and enhanced axonal outgrowth. Consequently, GelMA-Exos facilitated locomotor functional recovery after SCI (spinal cord contusive injury).
The gene regulatory networks that trigger NSC differentiation at an early stage have been elucidated in part through research on the mechanisms underlying neuronal differentiation and axon regeneration (Fig. 3B) [61]. Based on this, Exos originated from miR-26a-modified MSCs were used to enhance neurogenesis and restrict glial scar formation by activating the phosphatase and tensin homolog deleted on chromosome ten (PTEN)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling cascade (Fig. 3C) [62]. As the extracellular signal-related kinase (ERK)/cAMP response element-binding (CREB) and wingless/integrated (Wnt)/β-catenin pathway also participates in the regulation of neurogenesis (Fig. 4A) [63,64,65], HpMSC-derived Exos could enhance the proliferation of NSCs by activating the MEK/ERK/CREB signaling pathway and increasing the levels of phosphorylation in MAPK/ERK kinase (MEK), ERK, and CREB [63]. Moreover, neuronal differentiation triggered by the novel paclitaxel-delivered MExos–collagen scaffold via the Wnt/β-catenin signaling pathway could effectively instruct NSCs to differentiate into neurons, thereby promoting neuronal regeneration and minimizing scar formation (Fig. 4B) [65, 66].
Additionally, exosomal miRNAs perform an essential function in neuron protection in the initial stages of SCI and promote functional recovery. MiR-133b-modified Exos inhibit ras homolog family member A expression and activate ERK1/2, signal transducer and activator of transcription 3 (STAT3), and CREB, thereby reducing the lesion area, preserving neuronal tissues, and stimulating nerve fiber regeneration after a SCI caused by an aneurysm clip [67]. MiR-151-3p, which targets phospho-protein 53 (p53), is abundant in microglia-derived Exos; this miRNA suppresses the p53/cyclin-dependent kinase inhibitor 1 A (p21)/cyclin-dependent kinase 1 (CDK1) signaling pathway, reduces neuronal apoptosis, and promotes axonal regrowth [38]. The expression levels of miR-199a-3p/145-5p are relatively high in Exos. MiR-mediated knockdown of Cblb, which is specifically targeted by miR-199a-3p, and Cbl, which is specifically targeted by miR-145-5p, subsequently activates the NGF/tropomyosin receptor kinase A (TrkA) downstream pathways AKT and ERK (Fig. 4C) [68]. MiR-431-3p delivered by Exos from a subtype of BMSCs (CD271+CD56+ BMSC) significantly caused an exacerbation in the length of axon extension and an increase in the number of branches in the axons of the dorsal root ganglion by targeting Repulsive Guidance Molecule Family Member A [69]. EGFR+NSC, a subpopulation of endogenous NSC-enriched exosomal miR-34a-5p, can facilitate axonal regeneration at the injured site by directly binding to HDAC6 and inhibiting expression [37]. Moreover, Exos secreted by oxygen- and glucose-deprived astrocytes increased Exo-associated miR-92b-3p to exert a neuroprotective effect [70].
Exos promote neurogenesis through PTEN/phosphatidylinositide 3-kinases (PI3K)/AKT/mTOR, Wnt/β-catenin, MEK/ERK/STAT3/CREB, and NGF/TrkA signaling cascades [71,72,73]. This creates a favorable environment for neurite outgrowth, accelerates NSC differentiation, promotes neuronal survival and axon regeneration, and attenuates glial scar formation (Table 1).
Characteristics of Exos and the Underlying Signaling Mechanisms for Neurogenesis. A) The specific characteristics of Exos that can deliver various types of DNA/RNA/siRNA, protein, and drugs. Reproduced with permission [25]. Copyright 2015, Journal of Controlled Release. B) After axotomy, injured adult CNS neurons present with low regenerative capacity; diverse molecular mechanisms promote axons to become the high regenerative type. Modified from 2018, Annual Review of Cell and Developmental Biology [61]. (By Figdraw.) C) MSC-derived Exos promote axonal regeneration via the phosphatase and tensin homolog (PTEN)/AKT/mammalian target of rapamycin (mTOR) pathway following SCI. Reproduced with permission [62]. Copyright 2021, Stem Cell Research & Therapy. SCI spinal cord injury; CNS central nervous system; MSC mesenchymal stem cell
Neurogenesis Following SCI. A) Exos extracted from human placental MSCs enhance the proliferation of endogenous neural progenitor cells (NPCs) and promote their differentiation into mature neurons through the activation of MEK/ERK/CREB phosphorylation. Reproduced with permission [63]. Copyright 2021, Stem Cell Research & Therapy. B) Exos extracted from human umbilical cord-derived MSCs (hUC-MSCs) loaded on a multifunctional collagen scaffold (LBMP) promote endogenous NSC migration and differentiation. Reproduced with permission [66]. Copyright 2021, Advanced Healthcare Materials. C) Exos extracted from hUC-MSCs promote neurite outgrowth. Exos secrete high levels of miRNA-199a-3p/145-5p, which specifically target Cblb and Cbl mRNAs to prevent TrkA degradation. Sustained activation of phosphorylated ERK (p-ERK) and p-AKT results in the sustainable expression of NEU-N, neurofilament H (NF-H), and β-tubulin-III. Reproduced with permission [68]. Copyright 2021, Stem Cell Research & Therapy. SCI spinal cord injury; MSC mesenchymal stem cell; NSC neural stem cell
Promoting angiogenesis
To accelerate regeneration following SCI, neurogenesis must be coupled with angiogenesis. In addition to the regeneration of nerve cells, the assistance of the surrounding microenvironment, including blood vessels and the extracellular matrix, is necessary for restoring neural function. Specifically, rejuvenated blood vessel formation plays a significant role in tissue repair [83]. The vasculature can serve as a supportive structure and guide axonal sprouting after injury, thereby promoting axonal guidance [84]. Moreover, vascularization following SCI facilitates nourishment for restoring and sustaining neuronal network stability, which in turn is favorable for functional recovery after SCI [85]. Notably, the administration of MSC-Exos significantly promotes angiogenesis [86].
After SCI, the injured spinal column becomes hypoxic [87]. The preservation of endothelial cells ensures limited secondary injury to the blood vessels following SCI, enabling the provision of vital oxygen and nutrients required for the repair of microenvironment and nerve circuits. Vascular endothelial cells, which are an essential part of the blood vessel wall, increase the absorption of Exos produced by hypoxia-treated MSCs. Mu et al. [31] found that hypo-Exo-treated rats had improved locomotor functional recovery. The hypoxia-inducible factor 1-alpha (HIF-1a) content was significantly increased in hypoxia-stimulated Exos (hypo-Exos), leading to the upregulation of vascular endothelial growth factor (VEGF) in the Exo treatment system, indicating the immense potential of prominent angiogenesis in SCI (a 4.0 ± 0.5-mm spinal cord cross-section and fragments were removed) and repair (Fig. 5A). Regarding VEGF, NSC-Exos are highly expressed VEGF-A and can facilitate the angiogenic capabilities of spinal cord microvascular endothelial cells (SCMECs); promote SCMEC migration, tube formation, and proliferation; mediate pro-angiogenic effects; and promote tissue healing [88]. Exos from M2 macrophages enhance angiogenesis and functional recovery following SCI; this is partially attributed to the activation of the HIF-1/VEGF signaling pathway [89]. Exos that originate from miR-126-modified MSCs enhance the process of microvascular regeneration and human umbilical vein endothelial cell (HUVEC) migration by suppressing Sprouty-related EVH1 domain-containing protein 1 and phosphoinositide-3-kinase regulatory subunit 2 (PIK3R2) expression [80]. These proteins function as inhibitors of the VEGF pathway [90].
Moreover, several studies have shown that Exos promote blood vessel formation independent of VEGF. For example, macrophage membrane-fused Exo-mimetic nanovesicles (MF-NVs) target the ischemic endothelium and promote angiogenesis (Fig. 5B) [91]. SCs-Exos promote angiogenesis by delivering integrin-β1 through the effect of control VE-cadherin localization and blood vessel stability [92]. The administration of human placenta-derived MSC-Exos (hpMSC-Exos) enhances the process of tube formation and HUVEC migration; moreover, BMS scores improved significantly in the hPMSCs-Exos group [93]. Treatment with Exo-educated macrophages (EEMs), defined as M2-like macrophages generated using Exos isolated from BMSCs, significantly improved the angiogenic activity of HUVECs and facilitated the development of axonal growth in cortical neurons (Fig. 5C) [94].
The JNK/c-Jun, Wnt/β-catenin, and PTEN/mTOR signaling pathways participate in the regulation of angiogenesis. Specifically, unlike those prepared from untreated hMSCs, iron oxide nanoparticle-incorporated Exo-mimetic nanovesicles (NV-IONPs) actuate the JNK and c-Jun signaling pathway, and accumulated NV-IONPs enhance blood vessel formation [95]. In turn, the M2-Exo-derived ubiquitin isopeptidase OTULIN can activate the Wnt/β-catenin signaling pathway by upregulating the expression of β-catenin, which in turn triggers the upregulation of angiogenesis-related genes in SCMECs. These genes are known to be modulated by the Wnt/β-catenin signaling pathway (Fig. 5D) [85]. Subsequently, the use of MSC-Exos containing siRNA (ExoPTEN) reduces PTEN expression and promotes axonal growth and neovascularization [81]. Ultimately, cerebrospinal fluid-derived extracellular vesicles from pigs with SCI promote angiogenesis by activating the PI3K/AKT signaling pathway [96].
In angiogenesis, Exos interact with the PTEN/PI3K/AKT/mTOR, Wnt/β-catenin, and JNK/c-Jun pathways to promote SCMEC migration, tube formation, and proliferation. Particularly, JNK is tightly linked to the release of growth factors; similarly, c-Jun participates in the VEGF receptor 2 signaling axis (Table 2), [97, 98].
Angiogenesis of Exos. A) Hypoxia-stimulated MSC-derived Exos encapsulated in hydrogel promote microvascular and nerve regeneration at the spinal lesion through the upregulation of VEGF. Modified from 2022, Biomaterials Science [31]. (By Figdraw). B) MF-NVs (macrophage membrane-fused Exo-mimetic nanovesicles) target the SCI lesion by binding to ischemic endothelium to exert multiple protective effects. Reproduced with permission [91]. Copyright 2020, International Journal of Molecular Sciences. C) BMSC-derived Exos cultured with macrophages to obtain EEMs. EEMs loaded on hydrogel promote axonal regeneration and angiogenesis in the injured spinal cord. Thus, EEMs accelerate microvasculature regeneration of the spinal cord. Reproduced with permission [94]. Copyright 2021, Frontiers in Cellular Neuroscience. D) Hydrogel-loaded M2-Exos facilitate microvascular regeneration and diminish the lesion area by activating the Wnt/β-catenin pathway following SCI. Reproduced with permission [85]. Copyright 2021, Acta Biomaterialia. SCI spinal cord injury; MSC mesenchymal stem cell; BMSC bone marrow mesenchymal stem cells; EEM Exo-educated macrophage; VEGF vascular endothelial growth factor
Preserving the integrity of the BSCB
The BSCB is crucial in preserving the stability of the microenvironment in the spinal cord; accordingly, BSCB disruption is detrimental to locomotor function recovery. Consistent with this, preserving the integrity of the BSCB can enhance spinal cord tissue repair and lead to movement improvement following SCI [99, 100].
BMSC-Exos help preserve the integrity of BSCB and enhance the process of motor recovery after SCI, partly by regulating tissue inhibitors of the matrix metalloproteinase 2 (TIMP2)/matrix metalloproteinase (MMP) signaling pathway. TIMP2 in BMSC-Exos alleviates BSCB damage by suppressing the MMP pathway, thereby protecting the expression of cell junction proteins (e.g., claudin-5, occludin, zonula occludens-1 (ZO-1), and β-catenin) [100, 101]. Moreover, extracellular vesicles that originate from MSCs can boost the levels of transforming growth factor-beta (TGF-β), TGF-β receptors, and tight junction proteins and decrease the permeability of the BSCB. These vesicles achieved an average BBB score of 7.96 ± 0.83, which was greater than that of the Vehicle group (4.74 ± 0.67) [102]. NSC-Exos containing FTY720, an immunomodulatory agent, can preserve the integrity of the endothelial barrier of SCMECs within a hypoxic environment via the PTEN/PI3K/AKT pathway [103]. Additionally, pericytes are crucial constituents of the neurovascular structure and present with various regulatory effects in preserving the BSCB integrity [104]. BMSC-Exos strengthen the BSCB integrity by preventing aberrant pericyte migration and improving pericyte coverage at the barrier. This is achieved by downregulating the nuclear factor-kappa B (NF-κB) pathway [78]. Moreover, BMSC-Exos can protect BSCB and alleviate edema by suppressing pericyte pyroptosis through the inhibition of the Nod1 inflammasome. This improves the coverage of the pericyte and results in better functional recovery following injury [74]. High expression of MiR-210-5p in pericyte-derived Exos can inhibit the Janus kinase (JAK1)/STAT3 signaling pathway. This inhibition helps to regulate lipid peroxidation levels, improve mitochondrial function, and regulate endothelial barrier function [105].
To preserve the BSCB, Exos regulate the PTEN/PI3K/AKT/mTOR, JAK/STAT3, and TIMP2/MMP signaling pathways to limit the reduction of cell junction proteins, upregulate TGF-β and its receptor, inhibit pericyte migration, improve the rate of pericyte coverage, and inhibit pericyte pyroptosis (Table 3) [74, 100].
Inhibiting apoptosis
SCI is characterized by axonal disruption and neuronal apoptosis and results in profound motor and sensory impairments [106]. Increasing evidence indicates that axonal growth and neuronal apoptosis are important areas of focus during SCI treatment. MiRNAs, which play critical roles in regulating cellular activities, are the predominant nucleic acids found in Exos and have been shown to positively influence the outcome of SCI.
Following SCI, treatment with MSC-miR-338-5p increases cyclic AMP (cAMP) accumulation and cAMP-mediated repressor activator protein 1 (Rap1) activation. The eventual PI3K/AKT pathway activation reduces cell apoptosis and enhances the survival of neurons [107]. BMSC-miR-181c inhibits PTEN and NF-κB signaling, ultimately decreasing the inflammation process and cell apoptosis in the spinal cord tissue and improving SCI (spinal cord contusive injury) [108]. MSC-miR-21/miR-19b depletes PTEN mRNA/protein, significantly promotes axon growth, prevents neuronal apoptosis following nerve injury, and promotes functional recovery; it has also been shown to raise BBB scores in rats with SCI [109]. MSC-miR-21 enhances the locomotor recovery of rats with contusive SCI by inhibiting cell death through the miR-21/PTEN/programmed cell death 4 signaling pathway and activates the JAK/STAT signaling pathways [110, 111]. Exos that originate from hypoxia-conditioned adipose tissue-derived stromal cells (ADSCs) (Hypo-Exos) that are enriched in miR-499a-5p significantly reduce neuronal apoptosis by regulating the c-jun N-terminal kinase 3 (JNK3)/c-jun apoptotic signaling pathway by targeting JNK3 [112]. Conversely, low miR-429 expression in SCI (spinal cord contusive injury) plasma Exos promotes neuronal apoptosis by facilitating PTEN expression and affecting PI3K/AKT signaling [46], whereas human neuroepithelial stem cell-miR-29b downregulates PTEN/caspase-3 expression and subsequently suppresses neuronal apoptosis [113]. BMSC-miR-455-5p directly targets neurite outgrowth inhibitor A (Nogo-A), a myelin-associated axonal growth inhibitory protein, to promote autophagy and inhibit neuronal apoptosis [114]. Exo-miR-494 suppresses inflammation factors and cell apoptosis in the insulted region [52], while MiR-126 Exos enhance neurogenesis and inhibit apoptosis following SCI (spinal cord contusive injury) [80].
Additionally, Exos inhibit apoptosis via multiple signaling pathways. G protein-coupled receptor kinase 2 interacting protein 1 (GIT1)-BMSC-Exos alleviate neuronal apoptosis by promoting PI3K/AKT signaling pathway activation [77]. Li et al. [115] demonstrated that BMSCs-Exos efficiently triggered the upregulation of the Wnt/β-catenin signaling cascade, resulting in a significant decrease of Bax, cleaved caspase-3, and cleaved caspase-9. hUC-MSC-derived Exos reduced apoptosis via the Bcl-2/Bax pathway, facilitated the low-density lipoprotein receptor-related protein 6/Wnt/β-Catenin signaling cascade, and enhanced the level of c-myc and Cyclin D1 in damaged lesions after SCI [116]. Moreover, cellular damage can be mitigated by enhancing autophagy by administering treatments targeting the constituents of the lesion [117]. BMSC-Exos can inhibit cell apoptosis by activating autophagy by enhancing the autophagy-related proteins microtubule-associated protein 1 A/1B-light chain 3 IIB and beclin-1 expression, enabling autophagosome formation and promoting the potential efficacy of locomotor recovery in rats with SCI (spinal cord contusive injury) [118].
Overall, Exos exert antiapoptotic effects in various pathological conditions mainly through the PTEN/PI3K/AKT/mTOR, Wnt/β-catenin, JNK/c-Jun, JAK/STAT, Bcl-2/Bax, and caspase signaling pathways to inhibit endoplasmic reticulum (ER) stress and promote autophagy (Table 4) [119, 120].
Regulating inflammation
Excessive neuroinflammation impedes neuronal regeneration, thereby contributing to the poor prognosis of patients with SCI [41, 127]. Severe neuroinflammation hinders the ability of axonal regeneration in the lesion site to rebuild connections with neighboring neurons, which leads to long-lasting neurological deficits [61, 128]. Notably, the inhibitory effect of inflammatory and scarring activities observed following the application of progenitor cells in SCI are mediated by their secreted Exos [129].
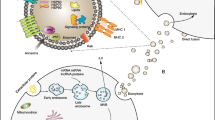
Exos are paracrine factors released by progenitor cells, which inhibit dysregulated neuroinflammatory cascades. Exos transport and discharge anti-inflammatory molecules such as berberine, miR-181c, LncGm37494, and insulin growth factor-1 (IGF-1) [44, 76, 108, 130]. These molecules can reduce the level of reactive oxygen species (ROS) and inflammatory cytokines at the lesion site of the spinal cord tissue during the initial stages of secondary damage [79, 129, 131]. Additionally, exosomal miRNAs can be exchanged between different immune cells and repress gene expression, with such Exo-mediated intercellular communication potentially influencing immune cell maturation [24, 131]. Exosomal miRNAs regulate target cells and may be crucial for modulating biological processes [41, 132]. MiR-544-modified BMSC-Exos markedly suppress the generation of the inflammatory cytokines interleukin 1α (IL-1α), tumor necrosis factor-alpha (TNF-α), IL-17β, and IL-36β at lesion site of spinal cord tissues following SCI (spinal cord contusive injury) [133]. BMSC-Exo-miR-494 effectively suppresses inflammation and apoptosis in the affected region [52]. Furthermore, BMSC-derived exosomal miR-9-5p has been reported to enhance BBB scores at days 1, 3, 7, 14, and 28 post-treatment compared with the sham group by promoting fibroblast growth factor 2 expression by downregulating histone deacetylase 5-mediated deacetylation, thereby ameliorating inflammation and ER stress (Fig. 6A) [125]. Exos can also act as safe and efficient siRNA delivery vectors that can traverse the BSCB to convey biological genetic information. Exo-siRNA, which can specifically silence the connective tissue growth factor gene, significantly quenches inflammatory response and hinders neural cell apoptosis along with A1 astrocyte activation and glial scar deposition [124].
Exos Extracted From MSCs Mitigate Inflammation Following SCI. A) Exos derived from BMSCs release miR-9-5p and mitigate neuronal inflammation following SCI. Reproduced with permission [125]. Copyright 2022, Molecular Immunology. B) HMSC-derived Exos immobilized in hydrogel can eliminate ROS and inflammatory factors, promoting nerve repair in SCI. Reproduced with permission [134]. Copyright 2020, Nano Letters. C) EXO-C@P accelerates locomotor function recovery through M2 macrophage polarization and injury volume restriction of SCI. Reproduced with permission [135]. Copyright 2021, Materials Science & Engineering C, Materials for Biological Applications. SCI spinal cord injury; MSC mesenchymal stem cell; BMSC bone marrow mesenchymal stem cells; HMSC human mesenchymal cell
Recent studies have further demonstrated that exosomal miRNAs inhibit both canonical and noncanonical inflammatory signaling pathways. BMSC-Exos can suppress the apoptosis and inflammatory response after injury and promote locomotor recovery by downregulating the Toll-like receptor 4 (TLR4)/myeloid differentiation primary response gene 88 (MyD88)/NF-κB signaling pathway [136]. MiR-181c in BMSC-Exos inhibits PTEN and the NF-κB expression on the decrease of I kappa B kinase alpha/beta (IKKα/β) phosphorylation and p65 expression in microglia nuclei, thereby mediating inflammation [108]. BMSC-Exos exert a protective effect in SCI (right semicircular spinal cord severed model) by suppressing the production and release of complement mRNA, as well as SCI-activated NF-κB (as indicated by significantly downregulated levels of p-p65 and p-IκBα) by binding to microglia [137]. MSC-Exos promote hind limb function recovery, and miR-145-5p expression is increased at the lesion site of SCI, leading to the suppression of the TLR4/NF-κB pathway and thereby suppressing the inflammatory reaction [138]. Liu et al. [79] and Wang et al. [122] clarified that MSCs-Exos treatment significantly improved the BBB score and that MSC-Exos reduced SCI (spinal cord contusive injury)-induced neurotoxic reactive A1 astrocyte activation following traumatic SCI. Wang et al. [122] also found that A1 astrocyte diminish was likely induced by suppressing the nuclear translocation of NFκB p65; the BBB score in the MSC-Exo treatment group was 14.450 ± 0.411. Hypo-Exos from MSCs mediate microglial polarization via enriched miR-216a-5p to modulate TLR4/NF-κB/PI3K/AKT signaling cascades. Hypoxia Exos from MSCs (HExos) enrich miR-216a-5p, which can modulate the TLR4/NF-κB/PI3K/AKT signaling pathway and mediate microglial polarization [139]. The overexpression of miR-544 in BMSC-Exos reduces inflammation following SCI (spinal cord contusive injury) [133].
NSC-derived Exos formed in the presence of IGF-1 upregulate miR-219a-2-3 expression to inhibit the yin yang 1/NF-κB pathway, thereby inhibiting inflammation and promoting neuroprotective effects following SCI (spinal cord contusive injury) [76]. Human epidural adipose tissue-derived MSCs can reverse thrombospondin 4 (THBS4) and B-cell lymphoma 3 (Bcl3) levels in SCI (clip compression injury); specifically, THBS4 supports local vascular inflammation, while BCL3 is aggregated at the nucleus and controls the activity of NF-κB in gene transcription [140]. Exos derived from long non-coding RNA (lncRNA) tectonic family member 2-modified MSCs attenuate inflammation via the miR-329-3p/IGF1R axis [123]. MSC-Exo lncGm36569, which acts as both an inhibitor and mimic of miR-5627-5p, inhibits neural cell ferroptosis via the miR-5627-5p/ferroptosis suppressor protein 1 axis [53].
Exos secreted by immune cells other than MSCs can also inhibit inflammation. Schwann cell-derived Exos (SCDEs) can enhance normal function restoration in mice following SCI by reducing the accumulation of chondroitin sulfate proteoglycan (CSPG). This is achieved by boosting TLR2 levels of astrocytes via the NF-κB/PI3K signaling cascade [43]. Peripheral macrophage (PM)-Exos activate microglial autophagy by downregulating the PI3K/AKT/mTOR signaling cascade [40]. MiR-126-3p originated from hypoxia-preconditioned VSC 4.1 neuron-derived Exos alleviate the hypersensitivity to pain caused by infrared radiation by restoring miR-126-3p expression in the affected site after SCI. This, in turn, regulates the activity of the PIK3R2-mediated PI3K and NF-κB pathways [141, 142].
Additionally, studies have explored different aspects of traditional mechanical research, including Exo application methods, modifications, and cell phagocytosis. Romanelli et al. [143] reported that intralesional application of Exos secreted by human umbilical cord mesenchymal stromal cells was more potent than intravenous administration regarding the restriction of the inflammatory process and glial scarring following SCI (spinal cord contusive injury). HMSC-derived Exos loaded on peptide-modified adhesive hydrogel (Exo-pGel) effectively mitigate inflammation and oxidation (Fig. 6B) [134]. EXO-C@Ps, incorporating a CAQK peptide to convey CRISPR/Cas9 components with the ability to edit the genome to upregulate soluble TNF receptor-1 (sTNFR1) at the lesion site, neutralize TNF-α and quench the inflammatory process activated by TNF-α (Fig. 6C) [135]. MF-NVs efficiently target ischemic and inflammatory organs and attenuate apoptosis and inflammation [91]. Finally, BMSC-Exos increase the expression of collagenous structure receptors (MARCOs) on macrophages, resulting in improved phagocytosis of engulfed myelin debris (Fig. 7) [75].
BMSC-Exo Hydrogel Promotes the Macrophage Phagocytosis Effect after SCI. A) BMSC-Exo administration mixed with hydrogel upregulates MARCO, which in turn enhances the ability of macrophage to clear myelin debris that promotes the regeneration of axons following SCI. B) Hematoxylin & eosin (H&E) staining shows that BMSC-Exo-Hydrogel diminishes the SCI area following SCI in vivo. C) Immunofluorescence images show that BMSC-Exo-Hydrogel improves axon growth following SCI in vivo. Reproduced with permission [75]. Copyright 2021, Frontiers in Cell and Developmental Biology. SCI spinal cord injury; BMSC bone marrow mesenchymal stem cells
Additionally, pyroptosis, an innate immune response, is regulated by Exos in patients with SCI. M2 microglial Exos (M2-Exos) rich in miR-672-5p can downregulate the absent in melanoma 2 (AIM2)/apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC)/caspase-1 signaling pathway by blocking AIM2 activity, thereby preventing neuronal pyroptosis [39]. Tregs target NF-κB-activating protein via exosomal miR-709 to reduce microglia pyroptosis [41].
Macrophage/microglia activity constitutes an important factor in adjusting inflammatory responses in SCI. Modified macrophages release various Exos to regulate the inflammation response (Fig. 8A) [25]. Targeted intervention in SCI involves inhibiting the recruitment and proliferation of macrophages and preventing macrophage polarization. These processes contribute significantly to tissue regeneration and homeostasis maintenance [144, 145]. Exos derived from various cell types can suppress the inflammation process in the spinal tissue microenvironment after SCI and prevent M1 cells and reactive astrocyte activation. Additionally, Exos can facilitate microglia polarization to the M2 type, which has anti-inflammatory properties. Furthermore, Exos can extend the duration of M2 cell stay in the spinal cord [39, 42, 45, 146,147,148]. MSC-derived Exos specifically promote M2 polarization at the injury site after SCI (spinal cord contusive injury) [146] and HUC-MSC-Exos trigger the polarization of BMDMs to the M2 phenotype [149]. Dental pulp stem cell-derived Exos can decrease M1 polarization via the ROS-mitogen-activated protein kinase (MAPK)-NF-κB-P65 signaling pathway in SCI (spinal cord contusive injury) treatment; at 28 days post-SCI, the Exo-treated group presented with greater BMS scores (phosphate-buffered saline vs. Exos: 2.333 ± 1.155 vs. 4.667 ± 0.577) [147]. MiR-222-3P upregulation in endothelial progenitor cell Exos decreases proinflammatory macrophages and increases anti-inflammatory macrophages by activating the suppressor of the cytokine signaling 3 (SOCS3)/JAK2/STAT3 pathway; consequently, the ERC-Exos group showed better BMS scores [150]. BMSC-Exo-enriched miR-125a downregulates interferon regulatory factor 5 expression to promote M2-phenotype polarization [126]. LncRNA-Gm37494 overexpressed in hypoxia-pretreated ADSCs inhibits miR-130b-3p and enhances peroxisome proliferator-activated receptor gamma expression to promote microglial M1/M2 polarization, which suppresses inflammation by inhibiting the STAT/NF-κB pathways [130]. Exos produced from MSCs replicate the effects of a single MSC infusion on many factors, such as the enhanced expression of M2 macrophage markers [102]. HExos enriched in miR-216a-5p promote M2 polarization via the TLR4/NF-κB/PI3K/AKT signaling cascades [139]. BMSC-derived exosomal microRNA-124-3p ameliorates SCI (spinal cord ischemia injury) by inhibiting ER to nucleus signaling 1 and promoting M2 polarization [151]. Berberine-loaded M2 macrophage-derived Exos (Exos-Ber) induce macrophage/microglia phenotype polarization, consequently decreasing the amount of inflammatory cytokines TNF-α, IL-1β, and IL-6 (Fig. 8B) [44]. M2 macrophage-derived Exos inhibit the inflammatory response through macrophage polarization via the miR-23a-3p/PTEN/PI3K/AKT network [45]. MFG-E8, the main component of SCDEs, upregulates M2 polarization via the SOCS3/STAT3 signaling cascade [42]. Nevertheless, PM-Exos promote M2 polarization (Fig. 8C) [40]. Additionally, neural tissue-like electroconductive hydrogels carrying BMSC-Exos promote microglial M2 polarization via the NF-κB pathway and promote axonal regeneration via the PTEN/PI3K/AKT/mTOR pathway (Fig. 9) [82]. Moreover, Exos derived from Schwann cells loaded on hydrogel (NFs@MP-HAh@Exo) suppressed the inflammation process through M2 polarization and promoted neurons survival after SCI via the TLR4/NF-κB, MAPK, and AKT/mTOR pathways (Fig. 10) [121].
To attain anti-inflammatory effects, Exos inhibit complement mRNA synthesis and release, neuronal and microglial pyroptosis, neuronal cell ferroptosis, and excessive accumulation of lipid peroxides and suppress the activation of astrocytes to the A1 type. Exos also promote microglial autophagy, macrophage polarization, and myelin debris phagocytosis. Finally, the expression of proinflammatory cytokines is decreased. These numerous anti-inflammatory effects are achieved mainly by modulating the TLR4/MyD88/NF-kB signaling pathway, which regulates the inflammation process by stimulating ROS secretion and proinflammatory cytokines, including TNF-γ, IL-1, and interferon-γ, thereby eliciting secondary neurotoxicity effects [152]. In summary, Exos and their cargo can modulate diverse important molecular signaling pathways to mitigate the pathological microenvironment following SCI (Table 5).
Exos Inhibit Inflammation and Accelerate Macrophage Polarization to M2. A) The path from Exos derived from macrophage drug formulations to patient application. Reproduced with permission [25]. Copyright 2015, Journal of Controlled Release. B) M2 macrophage-derived Exos loaded with berberine quench inflammatory factors. Reproduced with permission [44]. Copyright 2021, Acta Biomaterialia. C) Macrophage-derived Exos accelerate microglial polarization to the anti-inflammation type by enhancing autophagy. Reproduced with permission [40]. Copyright 2021, International Journal of Biological Sciences
Exo-loaded Electroconductive Hydrogel Exerts Potent Protective Effects Following SCI. A) Exo-loaded electroconductive hydrogel creates a favorable environment for neurogenesis, axonal regeneration, and inflammation inhibition. B) Exo-loaded electroconductive hydrogel accelerating dorsal root ganglia grown on the hydrogel. Scale bars: 100 μm. C) Neuronal and oligodendrocyte differentiation of NSCs is enhanced, while astrocyte differentiation is inhibited; moreover, axon outgrowth is increased via the PTEN/PI3K/AKT/mTOR pathway. Microglia M2 polarization is promoted by the NF-𝜅B pathway. Reproduced with permission [82]. Copyright 2022, Advanced Science. NSC neural stem cell; SCI spinal cord injury
Nanofibers Containing MP (Methylprednisolone) and Exos Loaded on Hydrogel (NFs@MP-HAh@Exo) Inhibit Neuronal Apoptosis and the Inflammatory Reaction. A) NFs@MP-HAh@Exo regulate macrophage polarization. B) Immunofluorescence and hematoxylin & eosin stain images show that NFs@MP-HAh@Exo promote shrinkage of the SCI cavity in the injured lesion area after SCI. C) Immunofluorescence images show that NFs@MP-HAh@Exo promote axonal regeneration after SCI. D) Immunofluorescence images show that NFs@MP-HAh@Exo alleviate inflammation after SCI. Reproduced with permission [121]. Copyright 2023, ACS Nano. SCI spinal cord injury
Conclusions and future prospects
Exos have attracted significant interest as cell-free therapies owing to their fascinating biological properties. Increasing evidence from preclinical studies has confirmed the neuroprotective properties of Exos. On administration, Exos can specifically target and accumulate at spinal cord lesion sites and accelerate locomotor functional recovery. These beneficial effects have been mainly attributed to neurogenesis, angiogenesis, preservation of the BSCB, and anti-apoptotic and anti-inflammatory effects.
A thorough understanding of the potential mechanisms of action of Exos will facilitate their clinical application. Currently, Exos have successfully made the journey to being effectively applied in several Phase I trials [153,154,155]. However, several challenges remain regarding the establishment of the optimal application of Exos for SCI treatment. For example, several aspects are inconsistent between studies including the Exos isolation methods, application dosage, route of delivery, and treatment time points (Table S1). Additionally, SCI models have been developed using different methods (contusion injury, crush injury, clip compression injury, circular and semicircular spinal cord severed injury, and ischemia injury); moreover, the parameters of contusion and crush injuries vary, as well as SCI animal models, including both rats and mice (Table S2).
Improving the yield and purity of Exos is the most important priority since it remains the main bottleneck limiting their practical application. Although ultracentrifugation is considered the “gold standard” and is widely used in the field [25], this technology presents various limitations, including the simultaneous isolation of contaminants that are not exosomal in nature, limited reproducibility, low yield of RNA, potential damage to Exos, and insufficient capacity to process a large number of samples, making it unsuitable for clinical applications [156]. Establishing the application dosage and delivery method is also important, as these vary widely among studies. Additionally, the rapid clearance of Exos by host cells, their short half-life in vivo, and inefficient drug delivery to target tissues continue to impede Exos aggregation in the SCI area [155, 157, 158]. Combining the inherent advantages of Exos with a targeted medication has emerged as a new and potentially transformative therapeutic strategy that could significantly impact the future of SCI treatment. The remaining strategic challenges include the selection of a therapeutic agent, methods for loading cargo into Exos, enhancement of Exos stability, tissue targeting, and effective delivery of cargo to recipient cells that can utilize inherent Exo properties, such as immune modulation, regeneration promotion, and pathogen suppression [155]. Hence, it is imperative to optimize and enhance the Exo-loading capacity and techniques for improving targeting [159]. Particularly, culture conditions, including the cell type, passage number, number of cells seeded to initiate culture, and medium composition, influence not only Exos but also cargo levels [160]. Finally, a standardized animal model remains to be developed as current contusion parameters for the NYU-III weight-drop apparatus are discrepant between studies regarding height and weight, while animal model heterogeneity causes inconsistencies in SCI severity. Overall, these factors significantly influence the therapeutic effect, with the inter-study variability rendering the final curative results difficult to interpret.
In summary, although Exo-based therapeutics have been successful in numerous trials, some obstacles remain to be overcome before Exos can be tested clinically on a larger scale. Determining how Exos target specific cells and understanding the distinct physiological roles of different Exo subtypes will help facilitate the progress of Exos in the field of drug delivery.
Data availability
Not applicable.
Abbreviations
- SCI:
-
Spinal cord injury
- BSCB:
-
Blood–spinal cord barrier
- MSCs:
-
Mesenchymal stem cells
- BMSCs:
-
Bone marrow mesenchymal stem cells
- NSCs:
-
Neural stem cells
- CNS:
-
Central nerve system
- mRNAs:
-
Messenger RNAs
- SCs:
-
Schwann cells
- BMDMs:
-
Bone marrow-derived macrophages
- GAP-43:
-
Growth-associated protein 43
- Shh:
-
Sonic Hedgehog
- BBB:
-
Basso, Beattie, and Bresnahan
- RGMA:
-
Repulsive Guidance Molecule Family Member A
- HUVEC:
-
Human umbilical vein endothelial cell
- SPRED1:
-
Sprouty-related EVH1 domain-containing protein 1
- PIK3R2:
-
Phosphoinositide-3-kinase regulatory subunit 2
- JAK:
-
Janus kinase
- EEMs:
-
Exo-educated macrophages
- ZO-1:
-
Zonula occludens-1
- TGF-β:
-
Transforming growth factor-beta
- cAMP:
-
Cyclic AMP
- ADSCs:
-
Adipose tissue-derived stromal cells
- ROS:
-
Reactive oxygen species
- FGF2:
-
Fibroblast growth factor 2
- ER:
-
Endoplasmic reticulum
- IGF-1:
-
Insulin growth factor-1
- HDAC5:
-
Histone deacetylase 5
- FGF2:
-
Fibroblast growth factor 2
- YY1:
-
Yin yang 1
- FSP1:
-
Ferroptosis suppressor protein 1
- CSPG:
-
Chondroitin sulfate proteoglycan
- lncRNA:
-
Long non-coding RNA
- NKAP:
-
NF-κB-activating protein
- IRF5:
-
Interferon regulatory factor 5
References
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31.
Golestani A, Shobeiri P, Sadeghi-Naini M, Jazayeri SB, Maroufi SF, Ghodsi Z, Dabbagh Ohadi MA, Mohammadi E, Rahimi-Movaghar V, Ghodsi SM. Epidemiology of traumatic spinal cord Injury in developing countries from 2009 to 2020: a systematic review and Meta-analysis. Neuroepidemiology. 2022;56(4):219–39.
Furlan JC, Sakakibara BM, Miller WC, Krassioukov AV. Global incidence and prevalence of traumatic spinal cord injury. Can J Neurol Sci Le J Canadien des Sci Neurologiques. 2013;40(4):456–64.
Rahimi-Movaghar V, Sayyah MK, Akbari H, Khorramirouz R, Rasouli MR, Moradi-Lakeh M, Shokraneh F, Vaccaro AR. Epidemiology of traumatic spinal cord injury in developing countries: a systematic review. Neuroepidemiology. 2013;41(2):65–85.
Collaborators GN. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56–87.
Collaborators GSCI. Global, regional, and national burden of spinal cord injury, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Neurol. 2023;22(11):1026–47.
Jeon J, Park SH, Choi J, Han SM, Kim HW, Shim SR, Hyun JK. Association between neural stem/progenitor cells and biomaterials in spinal cord injury therapies: a systematic review and network meta-analysis. Acta Biomater. 2024;183:50–60.
Rosner J, de Andrade DC, Davis KD, Gustin SM, Kramer JLK, Seal RP, Finnerup NB. Central neuropathic pain. Nat Reviews Disease Primers. 2023;9(1):73.
Luo J, Shi X, Li L, Tan Z, Feng F, Li J, Pang M, Wang X, He L. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioactive Mater. 2021;6(12):4816–29.
Halim A, Qu KY, Zhang XF, Huang NP. Recent advances in the application of two-dimensional nanomaterials for neural tissue Engineering and Regeneration. ACS Biomaterials Sci Eng. 2021;7(8):3503–29.
Dutta D, Khan N, Wu J, Jay SM. Extracellular vesicles as an emerging Frontier in spinal cord Injury Pathobiology and Therapy. Trends Neurosci. 2021;44(6):492–506.
McDonald JW, Sadowsky C. Spinal-cord injury. Lancet (London England). 2002;359(9304):417–25.
Freund P, Seif M, Weiskopf N, Friston K, Fehlings MG, Thompson AJ, Curt A. MRI in traumatic spinal cord injury: from clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019;18(12):1123–35.
David G, Mohammadi S, Martin AR, Cohen-Adad J, Weiskopf N, Thompson A, Freund P. Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat Reviews Neurol. 2019;15(12):718–31.
David G, Seif M, Huber E, Hupp M, Rosner J, Dietz V, Weiskopf N, Mohammadi S, Freund P. In vivo evidence of remote neural degeneration in the lumbar enlargement after cervical injury. Neurology. 2019;92(12):e1367–77.
Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Reviews Disease Primers. 2017;3:17018.
Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 2014;13(12):1241–56.
Hutson TH, Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat Reviews Neurol. 2019;15(12):732–45.
Rai A, Claridge B, Lozano J, Greening DW. The Discovery of Extracellular vesicles and their emergence as a next-generation therapy. Circ Res. 2024;135(1):198–221.
Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. ExosomesNature’s lipid nanoparticles, a rising star in Drug Delivery and Diagnostics. ACS Nano. 2022;16(11):17802–46.
Jeppesen DK, Zhang Q, Franklin JL, Coffey RJ. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023;33(8):667–81.
Zhang M, Hu S, Liu L, Dang P, Liu Y, Sun Z, Qiao B, Wang C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8(1):124.
Jia Y, Yu L, Ma T, Xu W, Qian H, Sun Y, Shi H. Small extracellular vesicles isolation and separation: current techniques, pending questions and clinical applications. Theranostics. 2022;12(15):6548–75.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Sci (New York NY). 2020;367(6478):eaau6977.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Controlled Release: Official J Controlled Release Soc. 2015;219:396–405.
Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal stem cells for neurological disorders. Adv Sci (Weinheim Baden-Wurttemberg Germany). 2021;8(7):2002944.
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in Regenerative Medicine. Int J Mol Sci. 2017;18(9):1852.
Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21(1):179.
Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived Extracellular vesicles: toward cell-free therapeutic applications. Mol Therapy: J Am Soc Gene Therapy. 2015;23(5):812–23.
Zou J, Yang W, Cui W, Li C, Ma C, Ji X, Hong J, Qu Z, Chen J, Liu A, et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnol. 2023;21(1):14.
Mu J, Li L, Wu J, Huang T, Zhang Y, Cao J, Ma T, Chen J, Zhang C, Zhang X, et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomaterials Sci. 2022;10(7):1803–11.
Han QF, Li WJ, Hu KS, Gao J, Zhai WL, Yang JH, Zhang SJ. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022;21(1):207.
Couch Y, Buzàs EI, Di Vizio D, Gho YS, Harrison P, Hill AF, Lötvall J, Raposo G, Stahl PD, Théry C, et al. A brief history of nearly EV-erything - the rise and rise of extracellular vesicles. J Extracell Vesicles. 2021;10(14):e12144.
Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404.
Hwang J, Jang S, Kim C, Lee S, Jeong HS. Role of Stem Cell-Derived exosomes and microRNAs in spinal cord Injury. Int J Mol Sci. 2023;24(18):13849.
Li J, Luo W, Xiao C, Zhao J, Xiang C, Liu W, Gu R. Recent advances in endogenous neural stem/progenitor cell manipulation for spinal cord injury repair. Theranostics. 2023;13(12):3966–87.
Qin T, Li C, Xu Y, Qin Y, Jin Y, He R, Luo Z, Zhao J, Duan C, Lu H, et al. Local delivery of EGFR(+)NSCs-derived exosomes promotes neural regeneration post spinal cord injury via miR-34a-5p/HDAC6 pathway. Bioactive Mater. 2024;33:424–43.
Li C, Qin T, Liu Y, Wen H, Zhao J, Luo Z, Peng W, Lu H, Duan C, Cao Y, et al. Microglia-Derived Exosomal microRNA-151-3p enhances functional Healing after spinal cord Injury by attenuating neuronal apoptosis via regulating the p53/p21/CDK1 signaling pathway. Front cell Dev Biology. 2021;9:783017.
Zhou Z, Li C, Bao T, Zhao X, Xiong W, Luo C, Yin G, Fan J. Exosome-shuttled mir-672-5p from anti-inflammatory microglia repair traumatic spinal cord injury by inhibiting AIM2/ASC/Caspase-1 signaling pathway mediated neuronal pyroptosis. J Neurotrauma. 2022;39(15–16):1057–74.
Zhang B, Lin F, Dong J, Liu J, Ding Z, Xu J. Peripheral macrophage-derived exosomes promote repair after spinal cord Injury by inducing local anti-inflammatory type Microglial polarization via increasing Autophagy. Int J Biol Sci. 2021;17(5):1339–52.
Xiong W, Li C, Kong G, Zeng Q, Wang S, Yin G, Gu J, Fan J. Treg cell-derived exosomes miR-709 attenuates microglia pyroptosis and promotes motor function recovery after spinal cord injury. J Nanobiotechnol. 2022;20(1):529.
Ren J, Zhu B, Gu G, Zhang W, Li J, Wang H, Wang M, Song X, Wei Z, Feng S. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023;14(1):70.
Pan D, Li Y, Yang F, Lv Z, Zhu S, Shao Y, Huang Y, Ning G, Feng S. Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J Neuroinflamm. 2021;18(1):172.
Gao ZS, Zhang CJ, Xia N, Tian H, Li DY, Lin JQ, Mei XF, Wu C. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–23.
Peng P, Yu H, Xing C, Tao B, Li C, Huang J, Ning G, Zhang B, Feng S. Exosomes-mediated phenotypic switch of macrophages in the immune microenvironment after spinal cord injury. Biomed Pharmacotherapy = Biomedecine Pharmacotherapie. 2021;144:112311.
Huang J, Wu C, Xu G, Sun Y, Gui C, Fu J, Cui Z, Huang H. The decreased expression of miR-429 in plasma exosomes after spinal cord injury inhibits neuronal apoptosis by mediating the PTEN/PI3K/Akt pathway. Annals Translational Med. 2022;10(1):6.
Wang H, Zhao C, Rong Q, Cao J, Chen H, Li R, Zhang B, Xu P. The role of exosomes from mesenchymal stem cells in spinal cord Injury: a systematic review. Int J stem Cells 2023.
Afsartala Z, Hadjighassem M, Shirian S, Ebrahimi-Barough S, Gholami L, Hussain MF, Yaghoobi M, Ai J. Advances in management of spinal cord Injury using stem cell-derived Extracellular vesicles: a review study. Basic Clin Neurosci. 2023;14(4):443–51.
Tai W, Wu W, Wang LL, Ni H, Chen C, Yang J, Zang T, Zou Y, Xu XM, Zhang CL. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell. 2021;28(5):923–e937924.
Zheng B, Tuszynski MH. Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol. 2023;24(6):396–413.
Han M, Yang H, Lu X, Li Y, Liu Z, Li F, Shang Z, Wang X, Li X, Li J, et al. Three-dimensional-cultured MSC-Derived exosome-hydrogel hybrid microneedle array Patch for spinal cord repair. Nano Lett. 2022;22(15):6391–401.
Huang W, Lin M, Yang C, Wang F, Zhang M, Gao J, Yu X. Rat Bone Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-494 Promoting Neurofilament Regeneration and Behavioral Function Recovery after Spinal Cord Injury. Oxidative medicine and cellular longevity 2021, 2021:1634917.
Shao C, Chen Y, Yang T, Zhao H, Li D. Mesenchymal stem cell derived exosomes suppress neuronal cell Ferroptosis Via lncGm36569/miR-5627-5p/FSP1 Axis in Acute spinal cord Injury. Stem cell Reviews Rep. 2022;18(3):1127–42.
Patel P, Buchanan CN, Zdradzinski MD, Sahoo PK, Kar AN, Lee SJ, Vaughn LS, Urisman A, Oses-Prieto J, Dell’Orco M, et al. Intra-axonal translation of Khsrp mRNA slows axon regeneration by destabilizing localized mRNAs. Nucleic Acids Res. 2022;50(10):5772–92.
DeGiosio RA, Grubisha MJ, MacDonald ML, McKinney BC, Camacho CJ, Sweet RA. More than a marker: potential pathogenic functions of MAP2. Front Mol Neurosci. 2022;15:974890.
Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, Zhu J, Du X, Xiong L, Du Y, et al. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17(2):195–203.
Li S, Liao X, He Y, Chen R, Zheng WV, Tang M, Guo X, Chen J, Hu S, Sun J. Exosomes derived from NGF-overexpressing bone marrow mesenchymal stem cell sheet promote spinal cord injury repair in a mouse model. Neurochem Int. 2022;157:105339.
Jia Y, Yang J, Lu T, Pu X, Chen Q, Ji L, Luo C. Repair of spinal cord injury in rats via exosomes from bone mesenchymal stem cells requires sonic hedgehog. Regenerative Therapy. 2021;18:309–15.
Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Brazilian J Med Biol Res = Revista brasileira de pesquisas medicas e Biologicas. 2019;52(12):e8735.
Cheng J, Chen Z, Liu C, Zhong M, Wang S, Sun Y, Wen H, Shu T. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomed (London England). 2021;16(18):1567–79.
Curcio M, Bradke F. Axon Regeneration in the Central Nervous System: facing the challenges from the Inside. Annu Rev Cell Dev Biol. 2018;34:495–521.
Chen Y, Tian Z, He L, Liu C, Wang N, Rong L, Liu B. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res Ther. 2021;12(1):224.
Zhou W, Silva M, Feng C, Zhao S, Liu L, Li S, Zhong J, Zheng W. Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res Ther. 2021;12(1):174.
Sabbir MG, Fernyhough P. Muscarinic receptor antagonists activate ERK-CREB signaling to augment neurite outgrowth of adult sensory neurons. Neuropharmacology. 2018;143:268–81.
Li X, Fan C, Xiao Z, Zhao Y, Zhang H, Sun J, Zhuang Y, Wu X, Shi J, Chen Y, et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials. 2018;183:114–27.
Zhang L, Fan C, Hao W, Zhuang Y, Liu X, Zhao Y, Chen B, Xiao Z, Chen Y, Dai J. NSCs Migration Promoted and Drug Delivered Exosomes-Collagen Scaffold via a bio-specific peptide for one-step spinal cord Injury Repair. Adv Healthc Mater. 2021;10(8):e2001896.
Li D, Zhang P, Yao X, Li H, Shen H, Li X, Wu J, Lu X. Exosomes Derived from miR-133b-Modified mesenchymal stem cells promote Recovery after spinal cord Injury. Front NeuroSci. 2018;12:845.
Wang Y, Lai X, Wu D, Liu B, Wang N, Rong L. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res Ther. 2021;12(1):117.
Sun Y, Liu Q, Qin Y, Xu Y, Zhao J, Xie Y, Li C, Qin T, Jin Y, Jiang L, et al. Exosomes derived from CD271 < sup>+ CD56 < sup>+ bone marrow mesenchymal stem cell subpopoulation identified by single-cell RNA sequencing promote axon regeneration after spinal cord injury. Theranostics. 2024;14(2):510–27.
Xu L, Cao H, Xie Y, Zhang Y, Du M, Xu X, Ye R, Liu X. Exosome-shuttled miR-92b-3p from ischemic preconditioned astrocytes protects neurons against oxygen and glucose deprivation. Brain Res. 2019;1717:66–73.
Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13(9):1075–81.
Tao W, Ruan J, Wu R, Zhao M, Zhao T, Qi M, Yau SSY, Yao G, Zhang H, Hu Y, et al. A natural carotenoid crocin exerts antidepressant action by promoting adult hippocampal neurogenesis through Wnt/β-catenin signaling. J Adv Res. 2023;43:219–31.
Poplawski GHD, Lie R, Hunt M, Kumamaru H, Kawaguchi R, Lu P, Schäfer MKE, Woodruff G, Robinson J, Canete P, et al. Adult rat myelin enhances axonal outgrowth from neural stem cells. Sci Transl Med. 2018;10(442):eaal2563.
Zhou Y, Wen LL, Li YF, Wu KM, Duan RR, Yao YB, Jing LJ, Gong Z, Teng JF, Jia YJ. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regeneration Res. 2022;17(1):194–202.
Sheng X, Zhao J, Li M, Xu Y, Zhou Y, Xu J, He R, Lu H, Wu T, Duan C, et al. Bone marrow mesenchymal stem cell-derived exosomes accelerate functional recovery after spinal cord Injury by promoting the phagocytosis of macrophages to clean myelin debris. Front cell Dev Biology. 2021;9:772205.
Ma K, Xu H, Zhang J, Zhao F, Liang H, Sun H, Li P, Zhang S, Wang R, Chen X. Insulin-like growth factor-1 enhances neuroprotective effects of neural stem cell exosomes after spinal cord injury via an miR-219a-2-3p/YY1 mechanism. Aging. 2019;11(24):12278–94.
Luo Y, Xu T, Liu W, Rong Y, Wang J, Fan J, Yin G, Cai W. Exosomes derived from GIT1-overexpressing bone marrow mesenchymal stem cells promote traumatic spinal cord injury recovery in a rat model. Int J Neurosci. 2021;131(2):170–82.
Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y, Yao Y, Duan R, Jia Y. Bone mesenchymal stem cell-derived extracellular vesicles promote Recovery following spinal cord Injury via Improvement of the Integrity of the blood-spinal cord barrier. Front NeuroSci. 2019;13:209.
Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T, et al. Exosomes Derived from Bone mesenchymal stem cells repair traumatic spinal cord Injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36(3):469–84.
Huang JH, Xu Y, Yin XM, Lin FY. Exosomes Derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord Injury in rats. Neuroscience. 2020;424:133–45.
Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D, Levenberg S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord Injury. ACS Nano. 2019;13(9):10015–28.
Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H, Guan P, Lu F, Luo Y, Tan G, et al, et al. Exosomes-Loaded Electroconductive Hydrogel synergistically promotes tissue repair after spinal cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci (Weinheim Baden-Wurttemberg Germany). 2022;9(13):e2105586.
Gao L, Peng Y, Xu W, He P, Li T, Lu X, Chen G. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem cells international 2020, 2020:2853650.
Tsivelekas K, Evangelopoulos DS, Pallis D, Benetos IS, Papadakis SA, Vlamis J, Pneumaticos SG. Angiogenesis in spinal cord Injury: Progress and Treatment. Cureus. 2022;14(5):e25475.
Luo Z, Peng W, Xu Y, Xie Y, Liu Y, Lu H, Cao Y, Hu J. Exosomal OTULIN from M2 macrophages promotes the recovery of spinal cord injuries via stimulating Wnt/β-catenin pathway-mediated vascular regeneration. Acta Biomater. 2021;136:519–32.
Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB, Cao Y, Lin FY. Systemic Administration of Exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord Injury in rats. J Neurotrauma. 2017;34(24):3388–96.
Li L, Mu J, Zhang Y, Zhang C, Ma T, Chen L, Huang T, Wu J, Cao J, Feng S, et al. Stimulation by Exosomes from Hypoxia Preconditioned Human umbilical vein endothelial cells facilitates mesenchymal stem cells angiogenic function for spinal cord repair. ACS Nano. 2022;16(7):10811–23.
Zhong D, Cao Y, Li CJ, Li M, Rong ZJ, Jiang L, Guo Z, Lu HB, Hu JZ. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Experimental Biology Med (Maywood NJ). 2020;245(1):54–65.
Huang JH, He H, Chen YN, Liu Z, Romani MD, Xu ZY, Xu Y, Lin FY. Exosomes derived from M2 macrophages improve angiogenesis and functional recovery after Spinal Cord Injury through HIF-1α/VEGF Axis. Brain Sci. 2022;12(10):1322.
Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84.
Lee JR, Kyung JW, Kumar H, Kwon SP, Song SY, Han IB, Kim BS. Targeted delivery of mesenchymal stem cell-derived nanovesicles for spinal cord Injury Treatment. Int J Mol Sci. 2020;21(11):4185.
Huang JH, Chen YN, He H, Fu CH, Xu ZY, Lin FY. Schwann cells-derived exosomes promote functional recovery after spinal cord injury by promoting angiogenesis. Front Cell Neurosci. 2022;16:1077071.
Zhang C, Zhang C, Xu Y, Li C, Cao Y, Li P. Exosomes derived from human placenta-derived mesenchymal stem cells improve neurologic function by promoting angiogenesis after spinal cord injury. Neurosci Lett. 2020;739:135399.
Li C, Qin T, Zhao J, He R, Wen H, Duan C, Lu H, Cao Y, Hu J. Bone marrow mesenchymal stem cell-derived exosome-educated macrophages promote functional Healing after spinal cord Injury. Front Cell Neurosci. 2021;15:725573.
Kim HY, Kumar H, Jo MJ, Kim J, Yoon JK, Lee JR, Kang M, Choo YW, Song SY, Kwon SP, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord Injury Treatment. Nano Lett. 2018;18(8):4965–75.
Li C, Qin T, Jin Y, Hu J, Yuan F, Cao Y, Duan C. Cerebrospinal fluid-derived extracellular vesicles after spinal cord injury promote vascular regeneration via PI3K/AKT signaling pathway. J Orthop Translation. 2023;39:124–34.
Yang Y, Xia L, Wu Y, Zhou H, Chen X, Li H, Xu M, Qi Z, Wang Z, Sun H, et al. Programmed death ligand-1 regulates angiogenesis and metastasis by participating in the c-JUN/VEGFR2 signaling axis in ovarian cancer. Cancer Commun (London England). 2021;41(6):511–27.
Folkman J. Angiogenesis and c-Jun. J Natl Cancer Inst. 2004;96(9):644.
Zhao C, Zhou T, Zhao X, Pang Y, Li W, Fan B, Li M, Liu X, Ma L, Zhang J, et al. Delayed administration of nafamostat mesylate inhibits thrombin-mediated blood-spinal cord barrier breakdown during acute spinal cord injury in rats. J Neuroinflamm. 2022;19(1):189.
Deng L, Lv JQ, Sun L. Experimental treatments to attenuate blood spinal cord barrier rupture in rats with traumatic spinal cord injury: a meta-analysis and systematic review. Front Pharmacol. 2022;13:950368.
Xin W, Qiang S, Jianing D, Jiaming L, Fangqi L, Bin C, Yuanyuan C, Guowang Z, Jianguang X, Xiaofeng L. Human bone marrow mesenchymal stem cell-derived exosomes attenuate blood-spinal cord barrier disruption via the TIMP2/MMP pathway after Acute spinal cord Injury. Mol Neurobiol. 2021;58(12):6490–504.
Nakazaki M, Morita T, Lankford KL, Askenase PW, Kocsis JD. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10(11):e12137.
Chen J, Zhang C, Li S, Li Z, Lai X, Xia Q. Exosomes Derived from Nerve Stem Cells Loaded with FTY720 Promote the Recovery after Spinal Cord Injury in Rats by PTEN/AKT Signal Pathway. Journal of immunology research 2021, 2021:8100298.
Jin LY, Li J, Wang KF, Xia WW, Zhu ZQ, Wang CR, Li XF, Liu HY. Blood-spinal cord barrier in spinal cord Injury: a review. J Neurotrauma. 2021;38(9):1203–24.
Gao P, Yi J, Chen W, Gu J, Miao S, Wang X, Huang Y, Jiang T, Li Q, Zhou W, et al. Pericyte-derived exosomal miR-210 improves mitochondrial function and inhibits lipid peroxidation in vascular endothelial cells after traumatic spinal cord injury by activating JAK1/STAT3 signaling pathway. J Nanobiotechnol. 2023;21(1):452.
Han W, Li Y, Cheng J, Zhang J, Chen D, Fang M, Xiang G, Wu Y, Zhang H, Xu K, et al. Sitagliptin improves functional recovery via GLP-1R-induced anti-apoptosis and facilitation of axonal regeneration after spinal cord injury. J Cell Mol Med. 2020;24(15):8687–702.
Zhang A, Bai Z, Yi W, Hu Z, Hao J. Overexpression of mir-338-5p in exosomes derived from mesenchymal stromal cells provides neuroprotective effects by the Cnr1/Rap1/Akt pathway after spinal cord injury in rats. Neurosci Lett. 2021;761:136124.
Zhang M, Wang L, Huang S, He X. Exosomes with high level of miR-181c from bone marrow-derived mesenchymal stem cells inhibit inflammation and apoptosis to alleviate spinal cord injury. J Mol Histol. 2021;52(2):301–11.
Xu G, Ao R, Zhi Z, Jia J, Yu B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol. 2019;234(7):10205–17.
Kang J, Li Z, Zhi Z, Wang S, Xu G. MiR-21 derived from the exosomes of MSCs regulates the death and differentiation of neurons in patients with spinal cord injury. Gene Ther. 2019;26(12):491–503.
Ji W, Jiang W, Li M, Li J, Li Z. miR-21 deficiency contributes to the impaired protective effects of obese rat mesenchymal stem cell-derived exosomes against spinal cord injury. Biochimie. 2019;167:171–8.
Liang Y, Wu JH, Zhu JH, Yang H. Exosomes secreted by Hypoxia-pre-conditioned adipose-derived mesenchymal stem cells reduce neuronal apoptosis in rats with spinal cord Injury. J Neurotrauma. 2022;39(9–10):701–14.
Kang J, Zhang C, Zhi Z, Wang Y, Liu J, Wu F, Xu G. Stem-like cells of various origins showed therapeutic effect to improve the recovery of spinal cord injury. Artif Cells Nanomed Biotechnol. 2020;48(1):627–38.
Liu B, Zheng W, Dai L, Fu S, Shi E. Bone marrow mesenchymal stem cell derived exosomal mir-455-5p protects against spinal cord ischemia reperfusion injury. Tissue Cell. 2021;74:101678.
Li C, Jiao G, Wu W, Wang H, Ren S, Zhang L, Zhou H, Liu H, Chen Y. Exosomes from bone marrow mesenchymal stem cells inhibit neuronal apoptosis and promote motor function recovery via the Wnt/β-catenin signaling pathway. Cell Transplant. 2019;28(11):1373–83.
Kang J, Guo Y. Human umbilical cord mesenchymal stem cells derived exosomes promote neurological function recovery in a rat spinal cord Injury Model. Neurochem Res. 2022;47(6):1532–40.
Galluzzi L, Bravo-San Pedro JM, Blomgren K, Kroemer G. Autophagy in acute brain injury. Nat Rev Neurosci. 2016;17(8):467–84.
Gu J, Jin ZS, Wang CM, Yan XF, Mao YQ, Chen S. Bone marrow mesenchymal stem cell-derived Exosomes improves spinal cord function after Injury in rats by activating Autophagy. Drug Des Devel Ther. 2020;14:1621–31.
Jiang L, Zhao XH, Mao YL, Wang JF, Zheng HJ, You QS. Long non-coding RNA RP11-468E2.5 curtails colorectal cancer cell proliferation and stimulates apoptosis via the JAK/STAT signaling pathway by targeting STAT5 and STAT6. J Experimental Clin cancer Research: CR. 2019;38(1):465.
Meyer LK, Verbist KC, Albeituni S, Scull BP, Bassett RC, Stroh AN, Tillman H, Allen CE, Hermiston ML, Nichols KE. JAK/STAT pathway inhibition sensitizes CD8 T cells to dexamethasone-induced apoptosis in hyperinflammation. Blood. 2020;136(6):657–68.
Zhu B, Gu G, Ren J, Song X, Li J, Wang C, Zhang W, Huo Y, Wang H, Jin L, et al. Schwann Cell-Derived exosomes and Methylprednisolone Composite Patch for spinal cord Injury Repair. ACS Nano. 2023;17(22):22928–43.
Wang L, Pei S, Han L, Guo B, Li Y, Duan R, Yao Y, Xue B, Chen X, Jia Y. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFκB P65 subunit in spinal cord Injury. Cell Physiol Biochemistry: Int J Experimental Cell Physiol Biochem Pharmacol. 2018;50(4):1535–59.
Liu J, Lin M, Qiao F, Zhang C. Exosomes Derived from lncRNA TCTN2-Modified mesenchymal stem cells improve spinal cord Injury by miR-329-3p/IGF1R Axis. J Mol Neuroscience: MN. 2021;72(3):482–95.
Huang W, Qu M, Li L, Liu T, Lin M, Yu X. SiRNA in MSC-derived exosomes silences CTGF gene for locomotor recovery in spinal cord injury rats. Stem Cell Res Ther. 2021;12(1):334.
He X, Zhang J, Guo Y, Yang X, Huang Y, Hao D. Exosomal mir-9-5p derived from BMSCs alleviates apoptosis, inflammation and endoplasmic reticulum stress in spinal cord injury by regulating the HDAC5/FGF2 axis. Mol Immunol. 2022;145:97–108.
Chang Q, Hao Y, Wang Y, Zhou Y, Zhuo H, Zhao G. Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res Bull. 2021;170:199–210.
Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25(6):898–908.
Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19(6):323–37.
Romanelli P, Bieler L, Scharler C, Pachler K, Kreutzer C, Zaunmair P, Jakubecova D, Mrowetz H, Benedetti B, Rivera FJ, et al. Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord Injury. Front Neurol. 2019;10:1225.
Shao M, Jin M, Xu S, Zheng C, Zhu W, Ma X, Lv F. Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord Injury via shifting microglial M1/M2 polarization. Inflammation. 2020;43(4):1536–47.
Hwang J, Jang S, Kim C, Lee S, Jeong HS. Role of Stem Cell-Derived exosomes and microRNAs in spinal cord Injury. Int J Mol Sci 2023, 24(18).
Khan NZ, Cao T, He J, Ritzel RM, Li Y, Henry RJ, Colson C, Stoica BA, Faden AI, Wu J. Spinal cord injury alters microRNA and CD81 + exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain Behav Immun. 2021;92:165–83.
Li C, Li X, Zhao B, Wang C. Exosomes derived from mir-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch Physiol Biochem. 2020;126(4):369–75.
Li L, Zhang Y, Mu J, Chen J, Zhang C, Cao H, Gao J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an Adhesive Hydrogel for Effective Treatment of spinal cord Injury. Nano Lett. 2020;20(6):4298–305.
Wang B, Chang M, Zhang R, Wo J, Wu B, Zhang H, Zhou Z, Li Z, Zhang F, Zhong C et al. Spinal cord injury target-immunotherapy with TNF-α autoregulated and feedback-controlled human umbilical cord mesenchymal stem cell derived exosomes remodelled by CRISPR/Cas9 plasmid. Biomater Adv 2021:112624.
Fan L, Dong J, He X, Zhang C, Zhang T. Bone marrow mesenchymal stem cells-derived exosomes reduce apoptosis and inflammatory response during spinal cord injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Hum Exp Toxicol. 2021;40(10):1612–23.
Zhao C, Zhou X, Qiu J, Xin D, Li T, Chu X, Yuan H, Wang H, Wang Z, Wang D. Exosomes Derived from bone marrow mesenchymal stem cells inhibit complement activation in rats with spinal cord Injury. Drug Des Devel Ther. 2019;13:3693–704.
Jiang Z, Zhang J. Mesenchymal stem cell-derived exosomes containing mir-145-5p reduce inflammation in spinal cord injury by regulating the TLR4/NF-κB signaling pathway. Cell Cycle (Georgetown Tex). 2021;20(10):993–1009.
Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflamm. 2020;17(1):47.
Sung SE, Seo MS, Kim YI, Kang KK, Choi JH, Lee S, Sung M, Yim SG, Lim JH, Seok HG, et al. Human epidural AD-MSC exosomes improve function recovery after spinal cord Injury in rats. Biomedicines. 2022;10(3):678.
Wang H, Chen FS, Zhang ZL, Zhou HX, Ma H, Li XQ. MiR-126-3p-Enriched Extracellular vesicles from Hypoxia-Preconditioned VSC 4.1 neurons attenuate Ischaemia-Reperfusion-Induced Pain Hypersensitivity by regulating the PIK3R2-Mediated pathway. Mol Neurobiol. 2021;58(2):821–34.
Xi T, Jin F, Zhu Y, Wang J, Tang L, Wang Y, Liebeskind DS, He Z. MicroRNA-126-3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem Biophys Res Commun. 2017;494(1–2):144–51.
Romanelli P, Bieler L, Heimel P, Škokić S, Jakubecova D, Kreutzer C, Zaunmair P, Smolčić T, Benedetti B, Rohde E, et al. Enhancing functional recovery through Intralesional Application of Extracellular vesicles in a rat model of traumatic spinal cord Injury. Front Cell Neurosci. 2021;15:795008.
Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823.
Van Broeckhoven J, Sommer D, Dooley D, Hendrix S, Franssen A. Macrophage phagocytosis after spinal cord injury: when friends become foes. Brain. 2021;144(10):2933–45.
Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS ONE. 2018;13(1):e0190358.
Liu C, Hu F, Jiao G, Guo Y, Zhou P, Zhang Y, Zhang Z, Yi J, You Y, Li Z, et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J Nanobiotechnol. 2022;20(1):65.
Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, Hu JG, Lü HZ. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–70.
Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204.
Yuan F, Peng W, Yang Y, Xu J, Liu Y, Xie Y, Huang T, Shi C, Ding Y, Li C, et al. Endothelial progenitor cell-derived exosomes promote anti-inflammatory macrophages via SOCS3/JAK2/STAT3 axis and improve the outcome of spinal cord injury. J Neuroinflamm. 2023;20(1):156.
Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Therapy. 2020;22(1):75.
Rahimifard M, Maqbool F, Moeini-Nodeh S, Niaz K, Abdollahi M, Braidy N, Nabavi SM, Nabavi SF. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11–9.
Zhang Y, Dou Y, Liu Y, Di M, Bian H, Sun X, Yang Q. Advances in therapeutic applications of Extracellular vesicles. Int J Nanomed. 2023;18:3285–307.
Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Communication Signaling: CCS. 2022;20(1):145.
György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–64.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–707.
Xin L, Lin X, Zhou F, Li C, Wang X, Yu H, Pan Y, Fei H, Ma L, Zhang S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020;113:252–66.
Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–56.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, Storage, Diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917–34.
Ludwig N, Whiteside TL, Reichert TE. Challenges in Exosome Isolation and analysis in Health and Disease. Int J Mol Sci. 2019;20(19):4684.
Acknowledgements
The authors thank the Home for Researchers and Figdraw.
Funding
This research was funded by the National Natural Science Foundation of China (82271414 and 82401619), Youth Support Programmed Project of China-Japan Union Hospital of Jilin University (2022qnpy09 and 2022qnpy11), Jilin Provincial Finance Program (2023SCZ41).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization, drafting, and editing of this manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Luo, W., Meng, C. et al. Exosomes as promising bioactive materials in the treatment of spinal cord injury. Stem Cell Res Ther 15, 335 (2024). https://doi.org/10.1186/s13287-024-03952-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03952-5