Abstract
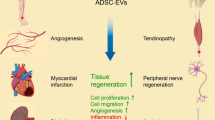
Inflammation is a key pathological feature of many diseases, disrupting normal tissue structure and resulting in irreversible damage. Despite the need for effective inflammation control, current treatments, including stem cell therapies, remain insufficient. Recently, extracellular vesicles secreted by adipose-derived stem cells (ADSC-EVs) have garnered attention for their significant anti-inflammatory properties. As carriers of bioactive substances, these vesicles have demonstrated potent capabilities in modulating inflammation and promoting tissue repair in conditions such as rheumatoid arthritis, osteoarthritis, diabetes, cardiovascular diseases, stroke, and wound healing. Consequently, ADSC-EVs are emerging as promising alternatives to conventional ADSC-based therapies, offering advantages such as reduced risk of immune rejection, enhanced stability, and ease of storage and handling. However, the specific mechanisms by which ADSC-EVs regulate inflammation under pathological conditions are not fully understood. This review discusses the role of ADSC-EVs in inflammation control, their impact on disease prognosis, and their potential to promote tissue repair. Additionally, it provides insights into future clinical research focused on ADSC-EV therapies for inflammatory diseases, which overcome some limitations associated with cell-based therapies.
Similar content being viewed by others
Introduction
Trauma, ischemia, infection, autoimmune disorders, and toxic injuries can induce tissue damage, leading to cell death, particularly through necrotic and apoptotic pathways. These events release inflammatory stimuli from the cell nucleus, mitochondria, cytoplasm, and extracellular matrix, thereby triggering an inflammatory cascade. This inflammatory response is a critical pathophysiological change in the onset and progression of diseases, such as rheumatoid arthritis and inflammatory bowel disease, serving as the body’s mechanism to counteract injury [1]. Manifesting as redness, swelling, heat, and pain, this process involves increased blood flow, vascular permeability, and leukocyte infiltration [2]. While short-term adaptive inflammatory reactions facilitate tissue repair, engaging a variety of cells, organs, and complex signaling pathways, chronic inflammation can lead to sustained tissue damage and exacerbate disease states [3] For example, in myocardial infarctions and cerebrovascular accidents, prolonged inflammatory activities cause irreversible damage in healthy tissues. Therefore, modulating inflammatory responses to slow disease progression and mitigate tissue damage is crucial, highlighting the necessity for targeted therapeutic interventions.
Extracellular vesicles (EVs) exhibit heterogeneity and are classified into three main types based on biogenesis: exosomes, microvesicles, and apoptotic bodies [4]. Exosomes, typically ranging from 30 to 150 nm in diameter, are secreted by a variety of cell types into the extracellular matrix and are crucial in mediating intercellular communication [5, 6]. In contrast, microvesicles are larger, with diameters spanning from 50 to 1000 nm [7]. Distinct from exosomes and microvesicles, apoptotic bodies form during the concluding phases of apoptosis, characterized by the condensation of chromatin and nucleus [8]. The overlapping size range between exosomes and microvesicles, combined with the lack of the specific surface protein markers, makes their differentiation challenging. Consequently, some researchers opt to study these vesicles collectively under the broad category of “EVs” to address the classification dilemma [9]. Consistent with this approach, we use the term EVs throughout this review to encompass all such vesicular entities. EVs transport various biologically active proteins, lipids, and nucleic acids from donor to recipient cells, influencing their biological functions [10]. These vesicles are integral to normal physiological process, including immune regulation [11, 12], tissue repair [13], coagulation [14], and stem cells maintenance [15]. Additionally, their cargo of bioactive components endows them with targeting and barrier-crossing capabilities, leveraging their ability to modulate cellular activities across different biological barriers [16,17,18]. This makes EVs a promising strategy for cell-free therapy across various diseases. However, specific isolation of EVs subtypes remains a major challenge, leaving the precise efficacy of each subtype unclear. We speculate that exosomes, due to their unique biological origin and relatively smaller size, may offer more specificity and efficient delivery of therapeutic components. In contrast, other types of EVs, being larger in size, could probably serve as broader therapeutic carriers containing a wider range of biologically active molecules and mechanisms. Further comparative studies are needed to fully elucidate the differences in therapeutic applications. Interestingly, a recent study from our laboratory explored subpopulations of apoptotic vesicles derived from bone marrow mesenchymal stem cells [19]. The study revealed that apoSEVs (the smaller vesicles with diameter < 1000 nm) promote stem cell proliferation, migration, and differentiation, thereby accelerating skin wound healing in a diabetic mouse model. Conversely, apoBDs (diameter > 1000 nm) exerted opposite effects on cell function and tissue regeneration. These findings highlight the functional differences among subpopulations of apoptotic vesicles. Further studies are warranted to investigate the functional differences between EV subpopulations.
To date, advanced experimental techniques for the isolation and identification of EVs have been established. Ultracentrifugation remains the predominant method for isolating EVs [20]. In addition to this, techniques such as immunoaffinity enrichment, ultrafiltration, and size exclusion chromatography are also frequently employed for the separation and purification of EVs [21]. The primary methods for EV characterization include Western blotting (WB), transmission electron microscope (TEM), and single-particle tracking (SPT). WB typically identifies markers such as CD9, CD63, CD81, Alix, and TSG101. TEM reveals EVs as having a bilayer membrane structure, appearing spherical or round. Among SPT techniques, nanoparticle tracking analysis (NTA) is extensively used, providing particle size distribution and concentration information, with EV diameters generally ranging from 50 to 1000 nm [22]. These methodologies ensure the reliable isolation, purification, and characterization of EVs, laying a solid foundation for further research and application.
Mesenchymal stem cells (MSCs), especially adipose-derived stem cells (ADSCs), play a pivotal role in cell therapy due to their ease of isolation, self-renewal, multipotency, low immunogenicity, and tissue repair efficacy [23]. ADSCs, harvested efficiently from adipose tissue via subcutaneous liposuction, stand out as ideal candidates for tissue engineering. Compared to other MSCs, they are more abundant and accessible and exhibit potent immunomodulatory capabilities, with their cytokine secretion profile exceeding that of bone marrow-derived MSCs, which is traditionally regarded as the benchmark [24]. However, their clinical application is hampered by challenges such as immune rejection, potential tumorigenicity, and ethical issues [25,26,27]. In response, ADSC-derived extracellular vesicles (ADSC-EVs) have emerged as a solution, offering comparable or even superior therapeutic benefits with fewer risks. The properties of ADSCs are significantly influenced by their secretions, which include cytokines, proteins, growth factors, and EVs containing various types of RNA. ADSC-EVs exhibit a diverse range of biological activities, including immunomodulation, anti-apoptosis, angiogenesis, and neurogenesis [28]. Moreover, the stability of ADSC-EVs in the human body presents notable advantages for therapeutic applications [29]. These vesicles facilitate targeted delivery of bioactive molecules, demonstrate stability, and bypass immunogenic and ethical challenges, representing a significant breakthrough in regenerative medicine [30]. Their capacity to modulate inflammation and enhance tissue repair [30, 31] positions ADSC-EVs as a promising avenue for cell-free therapy, mitigating the limitations associated with direct ADSC application and underscoring the necessity for further exploration of their therapeutic potential [32]. This review discusses how ADSC-EVs regulate inflammation in various diseases, thus promoting tissue repair and offering substantial clinical translation prospects.
Inflammatory regulation of ADSC-EVs during wound healing
The skin, as the largest organ of the body, maintains the balance between internal and external environments. Compromised skin integrity triggers wound healing mechanisms, with inflammation being a primary early-phase response that occurs within the first 24 to 48 h post-injury [33, 34]. While moderate inflammation can be protective that facilitates debris clearance and microbes elimination to accelerate wound healing [35], excessive inflammatory activity can impede this process, leading to prolonged healing time and potential scar formation [36, 37]. ADSC-EVs, however, have been shown to regulate inflammation effectively, thereby enhancing the wound healing process. They modulate the inflammatory landscape by influencing various cellular pathways to reduce pro-inflammatory cytokine production and promoting M2 macrophage polarization, which collectively contribute to improved tissue repair and reduced scarring.
Inhibition of inflammatory mediators
ADSC-EVs can inhibit the synthesis and release of inflammatory mediators by modulating cellular signaling pathways. Multiple lines of evidence indicate that ADSC-EVs transform the pro-inflammatory microenvironment into an anti-inflammatory state in various contexts, primarily by reducing the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α [38,39,40,41], and increasing anti-inflammatory cytokines like IL-10 [42]. Additionally, the specific miRNAs enriched in ADSC-EVs also play an important role in inflammation control. Studies by Water et al. showed that miR-146a in ADSC-EVs suppresses the NF-κB signaling pathway, thereby reducing IL-1β levels and diminishing inflammation in endothelial cells to aid wound healing [43]. Further research highlights that other miRNAs like miR-10a-5p also inhibit the NF-κB signaling pathway, contributing to an anti-inflammatory milieu [44, 45].
Regulation of immune cells
Furthermore, ADSC-EVs exert significant immunomodulatory effects, notably by promoting the polarization of macrophages towards the anti-inflammatory M2 phenotype, as evidenced by the increased expression of M2 macrophage marker CD206 [42]. This shift not only enhances the release of anti-inflammatory cytokines that facilitate tissue repair and regeneration, but is also driven by delivering molecules within ADSC-EVs including miR-34a-5p, miR-124-3p, and miR-146-5p [46, 47]. Li et al. corroborated this mechanism, finding that miR-21-5p enriched in ADSC-EVs fosters M2 macrophage polarization through Krüppel-like factor 6 (KLF6) inhibition, thus ameliorating the inflammatory microenvironment in diabetic foot ulcers and promoting wound healing [48]. Moreover, ADSC-EVs treatments have been observed to decrease M1 macrophage polarization, with Zhou et al. reporting a reduction in CD68+ and CD14+ M1 macrophages following both intravenous and local application of ADSC-EVs [49]. Similarly, in diabetic models, ADSC-EVs significantly downregulate the expression levels of the M1 macrophage marker CD68 and pro-inflammatory cytokines in wound tissues, consequently enhancing the healing process [50]. Beyond macrophages, ADSC-EVs modulate other immune cells, such as B cells, attenuating their proliferation and antibody production [51]. Furthermore, miR-132 and miR-146a in ADSC-EVs act on THP-1 cells to regulate inflammation and improve vascular regeneration [52].
Modulation of oxidative stress responses
Additionally, ADSC-EVs are pivotal in modulating oxidative stress responses, thus alleviating inflammation-mediated tissue damage. These vesicles regulate the production of reactive oxygen species (ROS) and enhance redox balance, resulting in reduced oxidative stress in neutrophils and subsequent promotion of wound healing [53]. Specifically, ADSC-EVs that overexpress Nrf2 have been shown to be particularly effective in high-glucose conditions, where they lower ROS levels and decrease the concentrations of pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α [39]. This dual action not only curtails the inflammatory response but also accelerates the process of skin repair and wound closure. Complementing these findings, research by Sun et al. demonstrated that ADSC-EVs containing Early Growth Response-1 (EGR-1) inhibit neutrophil migration by affecting the expression of adhesion molecules and chemotactic factors, further supporting the role of ADSC-EVs in enhancing wound healing [54].
Inflammatory regulation of ADSC-EVs in obese adipose tissue
Obesity is a global health concern, linked to type 2 diabetes, cardiovascular conditions, osteoarthritis, and multiple types of cancer [55]. As the fifth leading cause of death worldwide [56, 57], obesity is characterized by a chronic inflammatory state, primarily in adipose tissues [58]. This inflammation leads to the release of various inflammatory mediators and cytokines, exacerbating systemic insulin resistance and contributing to metabolic disorders [59,60,61,62,63]. Therefore, targeting inflammation in adipose tissue of obese individuals is crucial for improving whole-body metabolic health.
Increased beige adipocyte biogenesis
Adaptive thermogenesis, which is also known as the browning/beiging of white adipose tissue, plays a beneficial role in enhancing energy expenditure and preventing adiposity [64,65,66]. Recent research has established a strong link between M2 macrophages and the beige fat biogenesis, with M2 macrophages facilitating this transformation by downregulating E26 transformation-specific sequence 1 (Ets1), which increases mitochondrial content in adipocytes [67]. ADSC-EVs serve as natural mediators between ADSCs and macrophages, where phosphorylated Signal Transducer and Activator of Transcription 3 (STAT3) stimulates the activation of the Arginase-1 (Arg-1) promoter/enhancer, driving macrophage polarization towards the M2 phenotype and suppressing inflammation in white adipose tissue. In this context, ADSC-EVs not only regulate macrophages to support adipose browning but also maintain metabolic homeostasis by enhancing catecholamine production, a process related to increased tyrosine hydroxylase expression in the stromal vascular fraction (SVF) induced by M2 macrophages [68]. This interaction underlines the dual role of ADSC-EVs in encouraging M2 macrophage polarization and reducing inflammation, contributing to the prevention of obesity and metabolic disorders. Zhu et al. reported that supplementing ADSC-EVs in mouse fat grafts increased the number of M2 macrophages, upregulated their infiltration, and improved vascularization of the grafts, leading to the formation of beige adipocytes [69]. Moreover, lactate is a well-documented stimulator of beige biogenesis in white adipocytes [70]. M2 macrophages promote lactate production via ADSCs, further underscoring the complex interplay in adipose browning and offering an additional mechanism to counteract inflammation and metabolic dysregulation [68] (Fig. 1).
ADSC-EVs carry phosphorylated STAT3 and are engulfed by macrophages in white adipose tissue. Phosphorylated STAT3 induces macrophage polarization towards the M2 phenotype. M2 macrophages play an anti-inflammatory role and are crucial in the browning of white adipose tissue. They induce the SVF to produce more tyrosine hydroxylase and promote ADSCs to produce more lactate. Both actions collectively contribute to the browning of white adipose tissue
Reduced pro-angiogenic potential
ADSC-EVs play a role in the inflammatory response within adipose tissue during obesity. Inflammatory adipocytes induce the expression of Vascular Cell Adhesion Molecule-1 (VCAM-1) in vascular endothelial cells through EVs secretion, leading to increased leukocyte adhesion and inflammation in adipose tissue [71]. The composition of ADSC-EVs is notably altered in obesity; they contain lower levels of Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinase-2 (MMP-2) in obese individuals compared to those who are non-obese. Furthermore, the level of miR-126, which is crucial for angiogenesis, is reduced in ADSC-EVs from obese individuals. In vitro studies have shown that ADSC-EVs from obese subjects have a diminished ability to promote migration and angiogenesis in endothelial cells, indicating a potential decrease in their pro-angiogenic capacity [72]. Given the paucity of research in this field, further investigations are imperative to explore the specific role of ADSC-EVs in modulating inflammation and their angiogenic capacity in adipose tissue during obesity.
Inflammatory regulation of ADSC-EVs in cardiovascular diseases
Cardiovascular diseases are a global health concern, responsible for 17 million deaths annually, with myocardial infarction standing as a primary cause of mortality worldwide [73]. The intricate connection between adipose tissues and the cardiovascular system is significant, as both originate from the mesoderm and share developmental pathways. Moreover, adipose tissues secrete various adipokines that influence the cardiovascular system [74], while cardiac myocytes release molecules, such as atrial natriuretic peptide, to regulate adipose tissue function [75]. However, inflammatory conditions in adipose tissue, particularly during obesity, detrimentally affect cardiovascular health, evidenced by reduced angiogenic factors like VEGF and MMP-2 in ADSC-EVs [76]. Therefore, this observation has steered research towards employing adipose tissue derivatives, notably ADSC-EVs, in the therapeutic landscape of cardiovascular diseases.
Management of myocardial infarction
In mammals, myocardial infarction leads to massive cardiomyocyte death, activating the immune system and triggering a robust pro-inflammatory response. This response promotes leukocyte-endothelial cell adhesion, leading to neutrophil and monocyte infiltration. As the condition progresses, the inflammatory responses shift from pro-inflammatory to anti-inflammatory, promoting tissue repair [77]. Therefore, during the acute phase of acute myocardial infarction (AMI), it is crucial to reduce cardiomyocyte apoptosis, enhance neovascularization [78], improve myocardial perfusion, and inhibit inflammation [79]. ADSC-EVs offer cardioprotective benefits, with rapid upregulation of EGR1 in inflammation and fibrosis [80]. Studies by Liu et al. show that ADSC-EVs enriched with miR-146a exert anti-inflammatory and anti-fibrotic effects [81], effectively suppress cardiomyocyte apoptosis, inflammation, and fibrosis in myocardial injury models by inhibiting EGR1, which alleviate myocardial damage and shrink the infarct size [82]. Additionally, miR-221 and miR-222 in ADSC-EVs exhibit anti-inflammatory properties by regulating macrophage activity and attenuating the expression of inflammatory markers [83]. Injection of ADSC-EVs into the myocardium causes significantly increased miR-221/222 expression that decline the expression of P53 upregulated modulator of apoptosis (PUMA) and E26 transformation-specific-1 (ETS-1) and reduce cardiomyocyte apoptosis and hypertrophy [84]. Furthermore, ADSC-EVs improve cardiac repair post-myocardial infarction by activating the S1P/SK1/S1PR1 signaling pathway, shifting macrophage polarization towards the M2 phenotype, and decreasing the release of pro-inflammatory factors [85] (Fig. 2).
ADSC-EVs are engulfed by cardiomyocytes, where miR-221/miR-222 bind to the mRNA of PUMA and ETS-1, inhibiting their expression. Additionally, miRNA-146a binds to the mRNA of EGR-1, suppressing its expression. Collectively, these actions inhibit cardiomyocyte hypertrophy and apoptosis. Furthermore, ADSC-EVs suppress the polarization of M0 macrophages to the M1 phenotype and promote their polarization to the M2 phenotype through activation of the S1P/SK1/S1PR signaling axis, thereby reducing inflammation and damage after myocardial infarction
Improvement of vascular regeneration
ADSC-EVs play a crucial role in accelerating vascular regeneration. They convey miR-21, which activates the PI3K-AKT pathway in macrophages, and their Colony Stimulating Factor-1 (CSF-1) activates the Colony Stimulating Factor-1 Receptor (CSF-1R), encouraging a shift from the M1 to the M2 macrophage phenotype. M2 macrophages, in turn, secrete an increased array of vascular growth factors to aid vascular regeneration. In vitro studies reveal that ADSC-EVs-treated M2 macrophages can boost endothelial cell proliferation, migration, and vessel formation, thereby facilitating vascular regeneration in ischemic limbs [86].
Moreover, ADSC-EVs act as carriers to exert anti-inflammatory functions. Stanniocalcin-1 (STC-1), a conserved glycoprotein involved in mitochondrial function and inflammation suppression, plays a significant role in this process [87]. Research by Liu et al. demonstrated that ADSCs transduced with STC-1 lentiviral vectors produce STC-1-ADSC-EVs. When introduced to arterial endothelial cells, such EVs inhibit the NLRP3 inflammasome pathway, including NLRP3, Caspase-1, and IL-1β levels. Targeted siRNA against STC-1 enhanced NLRP3 inflammasome activation in mouse arterial endothelial cells, indicating that STC-1-ADSC-EVs promote the re-endothelialization of the mechanically injured carotid artery by inhibiting NLRP3 inflammasome activation [88]. These findings highlight the capacity of ADSC-EVs to improve myocardial cell damage and vascular regeneration by modulating inflammatory responses.
Inflammatory regulation of ADSC-EVs in neurological diseases
Adipose tissues, recognized as a systemic endocrine organ, play a crucial role in neuroendocrine regulation by secreting hormones like leptin, which influences diet and energy metabolism in the brain. The sympathetic nervous system interacts with adipose tissues through the release of norepinephrine, which enhances fat tissue thermogenesis and energy expenditure, and thus impacts the whole-body metabolic balance [89]. Given the intricate connections between adipose tissues and the nervous system, the potential involvement of ADSC-EVs in treating neurological disorders has garnered significant interest.
Ischemic stroke
Ischemic stroke stands as a principal cause of global disability and mortality [90]. The post-ischemic inflammation significantly contributes to the pathogenesis of brain ischemia-reperfusion injury, with microglia playing a key role in exacerbating neuronal damage. As primary immune cells in the central nervous system, microglia can induce secondary neuronal injury via the production of inflammatory mediators. Notably, ADSC-EVs have been shown to mitigate this damage by inhibiting microglial activation and reducing their cytotoxic effects, primarily through blocking the NF-κB and Mitogen-Activated Protein Kinase (MAPK) pathways [91]. Moreover, miR-126, a key regulator in endothelial cells for vascular integrity and neuro-regeneration [92], becomes downregulated in ischemia-related diseases which negatively impacts angiogenesis [93,94,95]. Research indicates that ADSC-EVs enriched with miRNA-126 can counteract this effect by reducing pro-inflammatory cytokines like TNF-α and IL-1β, inhibiting microglial activation, and thus facilitating the recovery post-stroke [96]. Human-derived ADSC-EVs further contribute to this protective mechanism by deactivating microglia/macrophages, impairing the secretion of inflammatory factors, and promoting an anti-inflammatory microenvironment. This is supported by studies, such as those by Hu et al., showing that ADSC-EVs can modulate microglia polarization and improve brain damage through targeting molecules such as STAT1 and PTEN [97,98,99] (Fig. 3).
ADSC-EVs promote the regeneration of neurons and blood vessels after a stroke by releasing various bioactive molecules, including miR-146a, miR-21-5p, and STAT3 activators. miR-146a inhibits the NF-κB pathway, reducing the production of pro-inflammatory cytokines TNF-α and IL-1β. miR-21-5p promotes M2 macrophage polarization by inhibiting PTEN. STAT3 activation in macrophages promotes the expression of Arg-1, further supporting M2 polarization. Additionally, ADSC-EVs inhibit the p38 MAPK signaling pathway. These processes collectively result in decreased inflammation and enhanced nervous tissue repair
Alzheimer’s disease
Alzheimer’s disease (AD) is becoming a prevalent neurodegenerative disorder globally, especially as the population ages [100]. It is now the seventh leading cause of death worldwide according to WHO. AD is increasing recognized as a neuroinflammatory condition, where the modulation of microglia M1 and M2 phenotypes becomes crucial [101, 102]. Research on AD mouse models has demonstrated that EVs obtained from hypoxia-treated ADSCs deliver circ-Epc1, which targets miR-770-3p to regulate Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). This interaction facilitates a shift in microglia phenotype from M1 to M2, resulting in compromised expression of pro-inflammatory factors and decreased apoptosis of hippocampal neurons. Such changes are associated with a partial reversal of the cognitive deficits characteristic of AD, underlining the therapeutic potential of ADSC-EVs in modulating neuroinflammation and ameliorating the symptoms of AD [103].
Parkinson’s disease
Parkinson’s disease (PD) is a common, chronic, and progressive neurodegenerative disorder, particularly prevalent in individuals over 80 years old [104]. The pathogenesis of PD is complex, including the activation of cellular autophagy and inflammasomes. Recent studies highlight the potential of targeting these processes as new therapeutic strategies for PD [105,106,107]. Notably, NALP3 and Cyclin-Dependent Kinase 5 (CDK5) are two key regulators of cellular autophagy and inflammasomes in PD [105, 108]. Li et al. report that in a PD mouse model, ADSC-EVs overexpressing miR-188-3p target NALP3 and CDK5, which counteract the activation of cellular autophagy and inflammasomes, diminish neuronal damage in the substantia nigra and offer therapeutic benefits for PD patients [109]. These findings support the potential of ADSC-EVs in PD treatment.
The application of tissue-engineered ADSC-EVs in inflammatory diseases
Given the vital roles of ADSC-EVs in controlling inflammation across various diseases, a number of studies now have focused on their clinical application. However, systemic administration often leads to extensive accumulation of these vesicles in the liver and spleen, with rapid clearance that results in a short half-life of less than six hours, thus limiting their clinical utility [110,111,112]. To address these challenges, tissue engineering of ADSC-EVs is employed to enhance their targeting ability, effectiveness, and longevity. This engineering process encompasses pretreating ADSCs, employing bioactive scaffold materials for carrying ADSC-EVs, and editing and modifying the membrane surface of ADSC-EVs (Table 1).
Optimizing ADSC-EVs by pretreatment
Disease progression, such as in cancer, limb ischemia, and myocardial infarction often occurs under hypoxic conditions, where a metabolic shift to low oxygen level is common during inflammation. Culturing MSCs, including ADSCs, in vitro to mimic pathophysiological environments has shown that hypoxia enhances their stemness and paracrine functions [113]. For example, Qian et al. found that ADSCs on plate in low oxygen produce EVs with upregulated miR-216a-5p, which promoted stronger M2 macrophage polarization via the HMGB1/TLR4/NF-κB axis cascade, improving treatment outcomes in experimental colitis [114].
Additionally, EVs derived from hypoxia-preconditioned ADSCs have been shown to attenuate ROS production and inflammatory factor expression, aiding in the alleviation of UV-induced skin damage through circ-Ash1l delivering [115]. Free flap transplantation is a crucial method in reconstructive plastic surgery for repairing soft tissue defects, but its clinical application is often limited by ischemia/reperfusion (I/R) injury [116]. Studies have demonstrated that hypoxic preconditioning enhances the production of EVs from ADSCs compared to conventional culture conditions. Specifically, EVs collected from ADSCs under hypoxic conditions (ADSC-EVs(H)) were compared with those from conventional conditions (ADSC-EVs(N)). Results indicated that ADSC-EVs(H) were significantly more effective in mitigating hypoxia/reperfusion-induced injury. They promoted the survival of human dermal microvascular endothelial cells (HDMECs), increased autophagy levels, reduced apoptosis rates, decreased oxidative stress accumulation, and improved mitochondrial membrane potential. These findings suggest that ADSC-EVs(H) reduce HDMEC damage by activating autophagy and inhibiting apoptosis and oxidative stress, offering a promising therapeutic strategy for enhancing the survival of free flaps. However, this study is limited by its in vitro nature, and further in vivo investigations are necessary to confirm whether ADSC-EVs(H) demonstrate superior efficacy in clinical settings [117].
Culturing ADSCs under inflammatory conditions is also a common method to enhance their therapeutic properties. Researchers have cultured ADSCs under such conditions and subsequently collected EVs from these cells (iADSC-EVs). It was found that iADSC-EVs could promote the proliferation of tendon cells and enhance collagen deposition. Specifically, miR-147b within iADSC-EVs inhibits the TLR4/NF-κB signaling pathway, thus reducing M1 macrophage activation, suppressing inflammation caused by tendon injury, and promoting tendon tissue repair. However, a limitation of this study is the lack of a control group using ADSC-EVs collected under non-inflammatory conditions, which would have clarified whether inflammation induction indeed enhances the function of ADSC-EVs [118]. Moreover, the pretreated ADSC-EVs have been shown to enhance temporomandibular joint condylar cartilage regeneration. Liu et al. demonstrated that iADSC-EVs enriched with miR-27b-3p, which suppressed macrophage activation by inhibition of CSF-1 expression [119]. As such, these inflammation-responsive vesicles promote an M2 phenotype shift to assist in the regeneration of temporomandibular joint cartilage and alleviate inflammation [120].
Additionally, ultrasound pretreatment of ADSCs represents a promising approach to enhance the functionality of ADSC-EVs. Researchers have demonstrated that pre-treating ADSCs with low-intensity ultrasound at 1.5 W/cm² significantly increases the secretion of ADSC-EVs, thereby addressing the issue of insufficient EVs secretion. High-throughput sequencing revealed that low-intensity ultrasound stimulation alters the miRNA content of ADSC-EVs, enriching them with miRNAs associated with endothelial cell angiogenesis, fibroblast proliferation, and migration. These modifications further enhance the efficacy of ADSC-EVs in promoting diabetic wound healing [121].
Bioactive scaffolds for ADSC-EV delivery
In order to achieve sustained release of ADSC-EVs and prolong their efficacy, researchers utilize bioactive scaffold materials to carry ADSC-EVs. Algae, with their high biocompatibility, biodegradability, non-antigenicity, and high-water absorption, are considered effective materials for biomedical applications [122]. Shilan et al. demonstrated that algae hydrogels could achieve a sustained release of rat-derived ADSC-EVs, which enhance wound healing in rats with skin defects [123]. Similarly, compared to direct EVs injections, human-derived ADSC-EVs have been loaded onto bioengineered three-dimensional amniotic membrane scaffolds (AMS), showing improved outcomes in rat wound healing. This approach more effectively reduced inflammation, promoted collagen formation, and enhanced vascularization [124].
Additionally, Kai et al. utilized electrospinning technology to synthesize biocompatible PLGA (Poly lactic acid-co-glycolic acid)/Mg-GA MOF (Metal-organic frameworks) nanofiber scaffolds. These scaffolds were then used to carry human derived ADSC-EVs, favoring the gradual release of Mg2+, GA, and the EVs. The result was increased osteogenic differentiation, enhanced anti-inflammatory effects, and improved vascular generation, thereby facilitating bone tissue regeneration [125].
Rotator cuff injuries are among the most common musculoskeletal disorders, with current research focused on promoting tendon-bone healing (TBH) and reducing postoperative re-injury rates. Recent study reports the use of a macroporous hydrogel material (MHA) to carry ADSC-EVs, referred to as (MHA-sEVs). The macroporous hydrogel provides an aligned structure that enhances the infiltration and tenogenic differentiation of tendon-derived stem cells (TDSCs). Concurrently, the incorporated ADSC-EVs modulate inflammation by improving mitochondrial dysfunction in M1 to M0 macrophage through inhibition of the NF-κB signaling pathway and suppressing the polarization from M0 to M1 macrophages. The combined features of MHA and ADSC-EVs promote fibrocartilage formation, enhance the biomechanical strength of the regenerated supraspinatus-humerus complex, and improve tendon-bone healing in a rat model of rotator cuff injury. These findings provide new insights into the treatment of rotator cuff injuries [126].
Surface modification of ADSC-EVs for targeted therapy
Enhancing the specificity and efficiency of ADSC-EVs for targeted therapeutic delivery is crucial for addressing complex inflammatory diseases. Therefore, researchers enhance the targeting ability of ADSC-EVs by modifying their surface structure. Surface modification of these vesicles can achieve this goal by allowing ADSC-EVs to precisely interact with cellular targets. For instance, Dong et al. utilized metabolic glycoengineering (MGE) to refine the surface of ADSC cell membranes, targeting activated macrophages in the inflamed joints of rheumatoid arthritis (RA). MGE involved attaching linear 1,6-linked dextran with sulfate groups to the ADSC membranes, generating dextran sulfate-modified ADSC-EVs (DS-ADSC-EVs). These modifications aimed at the scavenger receptor class A (SR-A), abundant in the inflamed joints of RA, with dextran sulfate serving as a targeting agent for SR-A. This approach enables DS-ADSC-EVs to specifically deliver therapeutic cargo to macrophages. In vitro studies showed that DS-ADSC-EVs significantly facilitated the transition of M1 macrophages to the M2 phenotype and prevented the reversion of M2 macrophages to the M1 phenotype. In vivo investigations revealed that mice treated with DS-ADSC-EVs displayed reduced joint cartilage erosion, neutrophil infiltration, and synovial inflammation compared to those treated with unmodified ADSC-EVs. These results indicates that DS-ADSC-EVs effectively reshaped the inflammatory microenvironment in RA by specifically targeting and modulating macrophages [127].
Although the research on the surface modification of ADSC-EVs is currently scarce, techniques from chemical, mechanical and genetic engineering could be leveraged to enhance the targeting and functional characteristics of ADSC-EVs. For instance, chemical conjugation of targeting ligands to the surface of ADSC-EVs can enable these ligands to bind to receptors on specific cell surfaces, thereby guiding ADSC-EVs to enter particular cell types or tissues and improving their specificity and efficacy. Additionally, techniques such as electroporation, covalent, and non-covalent bonding could be employed to increase the efficiency of drug loading into ADSC-EVs, significantly boosting their functionality and allowing them to deliver therapeutic agents more effectively. Moreover, genetic modifications can be used to overexpress specific proteins or miRNAs in ADSC-EVs, thereby enhancing their therapeutic efficacy and improving their bioactivity and ability to modulate disease processes. These various strategies can optimize the targeting capability, tracking ability, therapeutic activity, and stability of ADSC-EVs, providing valuable references for future research [128].
Conclusion
In summary, ADSC-EVs have emerged as potential therapeutic agents for modulating inflammation (Table 2) and promoting tissue repair (Table 3). First, ADSC-EVs play a critical role in modulating immune cell activity, central to controlling inflammation. Their bioactive cargos influence the behavior of various immune cells. For example, some miRNAs such as miR-146a in ADSC-EVs can suppress the NF-κB signaling pathway, reducing the production of pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α, while miR-21-5p promotes M2 macrophage polarization by inhibiting KLF6, enhancing anti-inflammatory responses and facilitating tissue repair. Additionally, ADSC-EVs enhance the antioxidant defense mechanisms of recipient cells by activating Nrf2, a key transcription factor that reduces ROS levels, thereby mitigating oxidative stress-induced inflammation and promoting cellular repair processes. They also promote angiogenesis, which is crucial for tissue repair and regeneration, through pro-angiogenic factors such as VEGF and miR-126, stimulating the formation of new blood vessels and enhancing the delivery of oxygen and nutrients to damaged tissues. In the context of obesity, ADSC-EVs regulate the browning of white adipose tissue, help reduce inflammation and improve metabolic health. Molecules like STAT3 in ADSC-EVs promote the polarization of macrophages towards the M2 phenotype and enhance adipocyte browning, reducing adipose tissue inflammation and metabolic dysregulation. Consequently, ADSC-EVs exhibit significant therapeutic potential in promoting wound healing, reducing neuroinflammation and fostering neuroregeneration, alleviating myocardial infarction and promoting angiogenesis, facilitating the browning of adipose tissue, and improving metabolic health in obese patients. Although currently stem cell therapy remains a mainstream cellular treatment method and the use of EVs derived from stem cells such as ADSC-EVs in clinical therapy is still limited, ADSC-EVs treatment, compared to cell therapy, reduces the risks of immune rejection and tumorigenicity, and the better stability of EVs makes them advantageous for long-term storage and transportation. Moreover, ADSC-EVs can be engineered to target specific tissues or cell types, enhancing therapeutic efficacy and reducing off-target effects, thus maximizing therapeutic outcomes.
Prospect
For future clinical research on ADSC-EVs, several critical aspects should be addressed. First, the design of clinical trials should involve defining criteria for patient selection, targeting specific conditions such as rheumatoid arthritis, osteoarthritis, diabetic wounds, and cardiovascular diseases. Establishing optimal dosages and administration routes—whether intravenous, intramuscular, or topical—is crucial to maximize therapeutic benefits while minimizing potential risks. For example, in a clinical study (Trial ID: NCT04276987), researchers treated pneumonia by administering aerosolized inhalation of allogeneic ADMSC-EVs (2 × 10^8 particles/3 ml) for 5 days. Additionally, identification of primary and secondary endpoints, such as reducing inflammation, enhancing tissue repair, and overall clinical outcomes, is vital for assessing the effectiveness of ADSC-EV. A comprehensive evaluation of the safety and efficacy of ADSC-EVs is also essential for their clinical translation. This process should begin with thorough preclinical studies in animal models to gather data on biodistribution, pharmacokinetics, and toxicity, as well as to assess long-term effects and potential for chronic use. Following this, subsequent Phase I clinical trials should determine safety, tolerability, and initial efficacy in a small patient cohort, aiming to identify adverse effects and the maximum tolerated dose. Progressing to larger Phase II and III trials is necessary to confirm efficacy and further evaluate safety, involving multiple centers to ensure reproducibility of results. Furthermore, effective management of potential side effects is crucial, including monitoring for immune responses or allergic reactions, particularly in allogeneic applications, and developing strategies to mitigate such risks. Ensuring targeted delivery of ADSC-EVs to minimize off-target effects and unintended interactions with non-target tissues is also critical, along with long-term follow-up studies to monitor delayed adverse effects and ensure sustained safety. In addition, future research should delve into the tissue engineering of ADSC-EVs to enhance targeting accuracy, increase their concentration in target organs, extend their functional duration, and leverage their capacity to deliver biopharmaceuticals for anti-inflammatory effects. Addressing these scientific and technical challenges is essential to fully realize the therapeutic potential of ADSC-EVs in treating inflammatory conditions and promoting tissue healing.
Data availability
Not applicable.
Abbreviations
- ADSCs:
-
Adipose-derived stem cells
- ADSC-EVs:
-
Extracellular vesicles secreted by Adipose-derived stem cells
- EVs:
-
Extracellular vesicles
- MSCs:
-
Mesenchymal stem cells
- MSC-EVs:
-
Extracellular vesicles secreted by Mesenchymal stem cells
- NKT cells:
-
Natural killer T cells
- SVF:
-
Stromal vascular fraction
- NF-κB:
-
Nuclear factor kappa B
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor alpha
- IL-10:
-
Interleukin-10
- EGR-1:
-
Early growth response-1
- RBP4:
-
Retinol-binding protein 4
- VCAM-1:
-
Vascular cell adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- MMP-2:
-
Matrix metalloproteinase-2
- STAT3:
-
Signal transducer and activator of transcription 3
- Arg-1:
-
Arginase-1
- AMI:
-
Acute myocardial infarction
- PUMA:
-
P53 upregulated modulator of apoptosis
- ETS-1:
-
E26 transformation-specific-1
- CSF-1:
-
Colony stimulating factor-1
- CSF-1R:
-
Colony stimulating factor-1 receptor
- STC-1:
-
Stanniocalcin-1
- MAPK:
-
Mitogen-activated protein kinase
- PTEN:
-
Phosphatase and tensin homolog
- AD:
-
Alzheimer’s disease
- TREM2:
-
Triggering receptor expressed on myeloid cells 2
- PD:
-
Parkinson’s disease
- CDK5:
-
Cyclin-dependent kinase 5
- ROS:
-
Reactive oxygen species
- OA:
-
Osteoarthritis
- TMJ:
-
Temporomandibular joint
- BMSC:
-
Bone marrow mesenchymal stem cell
- AMS:
-
Amniotic membrane scaffolds
- PLGA:
-
Poly lactic acid-co-glycolic acid
- MOF:
-
Metal-organic frameworks
- MGE:
-
Metabolic glycoengineering
- RA:
-
Rheumatoid arthritis
- FOXP3:
-
Forkhead box P3
- FoxO1:
-
Forkhead box O1
- KLF6:
-
Krüppel-like factor 6
- Ets1:
-
E26 transformation-specific sequence 1
- I/R:
-
Ischemia/reperfusion
- ADSC-EVs(N):
-
ADSC-EVs under conventional culture conditions
- ADSC-EVs(H):
-
ADSC-EVs under hypoxic conditions
- HDMECs:
-
Human Dermal Microvascular Endothelial Cells
- iADSC-EVs:
-
ADSC-EVs cultured under inflammatory conditions
- TLR4:
-
Toll-like receptor 4
- TBH:
-
Tendon-bone healing
- MHA:
-
Macroporous hydrogel material
- TDSCs:
-
Tendon-derived stem cells
- IGF-1:
-
Insulin-like growth factor 1
- WB:
-
Western blotting
- SPT:
-
Single-particle tracking
- NTA:
-
Nanoparticle tracking analysis
- TEM:
-
Transmission electron microscope
References
Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68:106–21.
Larsen GL, Henson PM. Mediators of inflammation. Annu Rev Immunol. 1983;1(1):335–59.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
Igami K, Uchiumi T, Ueda S, Kamioka K, Setoyama D, Gotoh K, Akimoto M, Matsumoto S, Kang D. Characterization and function of medium and large extracellular vesicles from plasma and urine by surface antigens and Annexin V. PeerJ Anal Chem. 2020;2:e4.
Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim et Biophys Acta (BBA)-Molecular Cell Biology Lipids. 2014;1841(1):108–20.
Pegtel DM, Gould SJ, Exosomes. Annu Rev Biochem. 2019;88:487–514.
Willms E, Cabañas C, Mäger I, Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:355368.
Zhou M, Li Y-J, Tang Y-C, Hao X-Y, Xu W-J, Xiang D-X, Wu J-Y. Apoptotic bodies for advanced drug delivery and therapy. J Controlled Release. 2022;351:394–406.
Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–43.
Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proceedings of the National Academy of Sciences 2015, 112 (12), E1433-E1442.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief C, Geuze H. J. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72.
El Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discovery. 2013;12(5):347–57.
Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia–reperfusion-induced acute and chronic kidney injury. Nephrol Dialysis Transplantation. 2011;26(5):1474–83.
Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–11.
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak M. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–56.
Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiology-Cell Physiol. 2014;306(7):C621–33.
Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1–12.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Zhang X, Yang J, Ma S, Gao X, Wang G, Sun Y, Yu Y, Wang Z, Tian W, Liao L. Functional diversity of apoptotic vesicle subpopulations from bone marrow mesenchymal stem cells in tissue regeneration. J Extracell Vesicles 2024, 13 (4), e12434.
Gardiner C, Vizio DD, Sahoo S, Théry C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles 2016, 5 (1).
Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A 2020.
Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522.
Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016;44(3):749–57.
Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Translational Med. 2013;2(6):455–63.
Zhou C, Zhang B, Yang Y, Jiang Q, Li T, Gong J, Tang H, Zhang Q. Stem cell-derived exosomes: emerging therapeutic opportunities for wound healing. Stem Cell Res Ther. 2023;14(1):107.
Liu A, Zhang X, He H, Zhou L, Naito Y, Sugita S, Lee J-W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020;20(2):125–40.
Wang Y, Cheng L, Zhao H, Li Z, Chen J, Cen Y, Zhang Z. The therapeutic role of ADSC-EVs in skin regeneration. Front Med. 2022;9:858824.
Bana-Zbczyk A. Adipose-Derived Stem Cells Secretome and Its Potential Application in Stem Cell-Free Therapy. Biomolecules 2021, 11.
An Y, Zhao J, Nie F, Qin Z, Li D. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep 2019, 9 (1).
Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay S. M. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B: Reviews. 2015;21(1):45–54.
Chen TS, Battsengel S, Kuo CH, Pan LF, Lin YM, Yao CH, Chen YS, Lin FH, Kuo WW, Huang CY. Stem cells rescue cardiomyopathy induced by P. gingivalis-LPS via miR‐181b. J Cell Physiol. 2018;233(8):5869–76.
Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10:1–12.
Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, Marks R, Naldi L, Weinstock MA, Wulf SK. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatology. 2014;134(6):1527–34.
Jia Q, Zhao H, Wang Y, Cen Y, Zhang Z. Mechanisms and applications of adipose-derived stem cell-extracellular vesicles in the inflammation of wound healing. Front Immunol. 2023;14:1214757.
Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861–85.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46.
Karppinen S-M, Heljasvaara R, Gullberg D, Tasanen K, Pihlajaniemi T. Toward understanding scarless skin wound healing and pathological scarring. F1000Research 2019, 8.
Cavallo C, Merli G, Borzì RM, Zini N, D’Adamo S, Guescini M, Grigolo B, Di Martino A, Santi S, Filardo G. Small extracellular vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-κB dependent inflammatory/catabolic environment of osteoarthritis. Sci Rep. 2021;11(1):1053.
Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14.
Wang J, Wu H, Zhao Y, Qin Y, Zhang Y, Pang H, Zhou Y, Liu X, Xiao Z. Extracellular vesicles from HIF-1α-overexpressing adipose-derived stem cells restore diabetic wounds through accelerated fibroblast proliferation and migration. Int J Nanomed 2021, 7943–57.
Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y, Xiao Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol. 2021;19:1–13.
Shi R, Jin Y, Zhao S, Yuan H, Shi J, Zhao H. Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed Pharmacother. 2022;153:113463.
Waters R, Subham S, Pacelli S, Modaresi S, Chakravarti AR, Paul A. Development of MicroRNA-146a-enriched stem cell secretome for wound-healing applications. Mol Pharm. 2019;16(10):4302–12.
Njock M-S, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E, Roufaiel M, Cybulsky MI, Schober A, Fish JE. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood J Am Soc Hematol. 2015;125(20):3202–12.
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N. Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:1–20.
Zhao AG, Shah K, Cromer B, Sumer H. Mesenchymal stem cell-derived extracellular vesicles and their therapeutic potential. Stem cells international 2020, 2020.
Heo JS, Kim S, Yang CE, Choi Y, Song SY, Kim HO. Human adipose mesenchymal stem cell-derived exosomes: a key player in wound healing. Tissue Eng Regenerative Med. 2021;18:537–48.
Li J, Wei C, Yang Y, Gao Z, Guo Z, Qi F. Apoptotic bodies extracted from adipose mesenchymal stem cells carry microRNA-21–5p to induce M2 polarization of macrophages and augment skin wound healing by targeting KLF6. Burns. 2022;48(8):1893–908.
Zhou Y, Zhao B, Zhang X-L, Lu Y-j, Lu S-T, Cheng J, Fu Y, Lin L, Zhang N-Y, Li P-X. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12(1):257.
Zhao B, Zhang X, Zhang Y, Lu Y, Zhang W, Lu S, Fu Y, Zhou Y, Zhang J, Zhang J. Human exosomes accelerate cutaneous wound healing by promoting collagen synthesis in a diabetic mouse model. Stem Cells Dev. 2021;30(18):922–33.
Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22(2):369–79.
Heo JS, Kim S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci Rep. 2022;12(1):2776.
Monsel A, Zhu Y-g, Gennai S, Hao Q, Hu S, Rouby J-J, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324–36.
Sun Y, Ju Y, Fang B. Exosomes from human adipose-derived mesenchymal stromal/stem cells accelerate angiogenesis in wound healing: implication of the EGR-1/lncRNA-SENCR/DKC1/VEGF-A axis. Hum Cell. 2022;35(5):1375–90.
Perdomo CM, Cohen RV, Sumithran P, Clément K, Frühbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023;401(10382):1116–30.
Collaborators GO. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Smith KB, Smith MS. Obesity statistics. Prim care: Clin Office Pract. 2016;43(1):121–35.
Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circul Res. 2020;126(11):1549–64.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91.
Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith C. W. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115(8):1029–38.
Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-κB and c-Jun NH2-terminal kinase pathways. Diabetes. 2009;58(1):104–15.
Christiansen T, Richelsen B, Bruun J. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes. 2005;29(1):146–50.
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiological reviews 2018.
Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63.
Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Investig. 2011;121(1):96–105.
Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L, MacPherson KA, Spurgin SB, Sun K, Holland WL. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metabol. 2016;23(6):1167–84.
Wu S, Qiu C, Ni J, Guo W, Song J, Yang X, Sun Y, Chen Y, Zhu Y, Chang X. M2 macrophages independently promote beige adipogenesis via blocking adipocyte Ets1. Nat Commun. 2024;15(1):1646.
Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235–47.
Zhu Y-z, Zhang J, Hu X, Wang Z-h, Wu S, Yi. Y.-y. supplementation with extracellular vesicles derived from adipose-derived stem cells increases fat graft survival and browning in mice: a cell-free approach to construct beige fat from white fat grafting. Plast Reconstr Surg. 2020;145(5):1183–95.
Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63(10):3253–65.
Wadey RM, Connolly KD, Mathew D, Walters G, Rees DA, James PE. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis. 2019;283:19–27.
Togliatto G, Dentelli P, Gili M, Gallo S, Deregibus C, Biglieri E, Iavello A, Santini E, Rossi C, Solini A. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes. 2016;40(1):102–11.
Jee SH, Kivimaki M, Kang H-C, Park IS, Samet JM, Batty GD. Cardiovascular disease risk factors in relation to suicide mortality in Asia: prospective cohort study of over one million Korean men and women. Eur Heart J. 2011;32(22):2773–80.
Liberale L, Bonaventura A, Vecchiè A, Matteo C, Dallegri F, Montecucco F, Carbone F. The role of adipocytokines in coronary atherosclerosis. Curr Atheroscler Rep. 2017;19:1–12.
Souza SC, Chau MD, Yang Q, Gauthier M-S, Clairmont KB, Wu Z, Gromada J, Dole WP. Atrial natriuretic peptide regulates lipid mobilization and oxygen consumption in human adipocytes by activating AMPK. Biochem Biophys Res Commun. 2011;410(3):398–403.
Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Reviews Cardiol. 2019;16(2):83–99.
Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circul Res. 2016;119(1):91–112.
Hynes B, Kumar AH, O’Sullivan J, Klein Buneker C, Leblond A-L, Weiss S, Schmeckpeper J, Martin K, Caplice NM. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J. 2013;34(10):782–9.
Valina C, Pinkernell K, Song Y-H, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28(21):2667–77.
Pan J, Alimujiang M, Chen Q, Shi H, Luo X. Exosomes derived from miR-146a‐modified adipose‐derived stem cells attenuate acute myocardial infarction – induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 2019;120(3):4433–43.
Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci. 2006;103(33):12481–6.
Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, Jin M. Mir-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Therapy-Nucleic Acids. 2018;11:103–15.
Seeley JJ, Baker RG, Mohamed G, Bruns T, Hayden MS, Deshmukh SD, Freedberg DE, Ghosh S. Induction of innate immune memory via microRNA targeting of chromatin remodelling factors. Nature. 2018;559(7712):114–9.
Lai T, Lee T, Chang Y, Chen Y, Lin S, Lin S, Pu C, Tsai J, Chen Y. MicroRNA-221/222 mediates ADSC-exosome-induced cardioprotection against ischemia/reperfusion by targeting PUMA and ETS-1. Front Cell Dev Biol. 2020; 8: 569150. 2020.
Deng S, Ge Z, Song Y, Wang H, Liu X, Zhang D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564.
Zhu D, Johnson TK, Wang Y, Thomas M, Huynh K, Yang Q, Bond VC, Chen YE, Liu D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res Ther. 2020;11:1–14.
Oh JY, Ko JH, Lee HJ, Yu JM, Choi H, Kim MK, Wee WR, Prockop DJ. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells. 2014;32(6):1553–63.
Liu K, Shi H, Peng Z, Wu X, Li W, Lu X. Exosomes from adipose mesenchymal stem cells overexpressing stanniocalcin-1 promote reendothelialization after carotid endarterium mechanical injury. Stem Cell Reviews Rep. 2022;18(3):1041–53.
Martinez-Sanchez N, Sweeney O, Sidarta-Oliveira D, Caron A, Stanley SA, Domingos A. I. The sympathetic nervous system in the 21st century: Neuroimmune interactions in metabolic homeostasis and obesity. Neuron. 2022;110(21):3597–626.
Barr TL, Simpkins J. Ischemic stroke: a consequence of a diseased immune system? Aging Disease. 2014;5(5):292.
Feng N, Jia Y, Huang X. Exosomes from adipose-derived stem cells alleviate neural injury caused by microglia activation via suppressing NF-kB and MAPK pathway. J Neuroimmunol. 2019;334:576996.
Berezikov E, van Tetering G, Verheul M, van de Belt J, van Laake L, Vos J, Verloop R, van de Wetering M, Guryev V, Takada S. Many novel mammalian microRNA candidates identified by extensive cloning and RAKE analysis. Genome Res. 2006;16(10):1289–98.
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–84.
Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010;184(1):21–5.
Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circul Res. 2010;107(6):810–7.
Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, Hong W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Translational Res. 2019;11(2):780.
Fan H, Tang H-B, Shan L-Q, Liu S-C, Huang D-G, Chen X, Chen Z, Yang M, Yin X-H, Yang H. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflamm. 2019;16:1–15.
Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, Pu H, Li W-W, Tang B, Wang Y. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci. 2015;112(9):2853–8.
Hu X, Pan J, Li Y, Jiang Y, Zheng H, Shi R, Zhang Q, Liu C, Tian H, Zhang Z. Extracellular vesicles from adipose-derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res Ther. 2022;13(1):21.
Idda ML, Munk R, Abdelmohsen K, Gorospe M. Noncoding RNAs in Alzheimer’s disease. Wiley Interdisciplinary Reviews: RNA 2018, 9 (2), e1463.
Maezawa I, Nguyen HM, Di Lucente J, Jenkins DP, Singh V, Hilt S, Kim K, Rangaraju S, Levey AI, Wulff H. Kv1. 3 inhibition as a potential microglia-targeted therapy for Alzheimer’s disease: preclinical proof of concept. Brain. 2018;141(2):596–612.
Yao K, Zu H-b. Microglial polarization: novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology. 2020;28(1):95–110.
Liu H, Jin M, Ji M, Zhang W, Liu A, Wang T. Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer’s disease mice model. Aging. 2022;14(7):3070.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE. Parkinson disease. Nat Reviews Disease Primers. 2017;3(1):1–21.
Li D, Yang H, Ma J, Luo S, Chen S, Gu Q. MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Hum Cell. 2018;31(2):106–15.
Qiao C, Zhang L-X, Sun X-Y, Ding J-H, Lu M, Hu G. Caspase-1 deficiency alleviates dopaminergic neuronal death via inhibiting caspase-7/AIF pathway in MPTP/p mouse model of Parkinson’s disease. Mol Neurobiol. 2017;54:4292–302.
Rivero-Ríos P, Madero-Pérez J, Fernández B, Hilfiker S. Targeting the autophagy/lysosomal degradation pathway in Parkinson s disease. Curr Neuropharmacol. 2016;14(3):238–49.
Su L-Y, Li H, Lv L, Feng Y-M, Li G-D, Luo R, Zhou H-J, Lei X-G, Ma L, Li J-L. Melatonin attenuates MPTP-induced neurotoxicity via preventing CDK5-mediated autophagy and SNCA/α-synuclein aggregation. Autophagy. 2015;11(10):1745–59.
Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from mir-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol Therapy-Nucleic Acids. 2021;23:1334–44.
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4(1):26316.
Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Controlled Release. 2015;199:145–55.
Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8(1):483–94.
Shin H-S, Lee S, Kim Y-M, Lim J-Y. Hypoxia-activated adipose mesenchymal stem cells prevents irradiation-induced salivary hypofunction by enhanced paracrine effect through fibroblast growth factor 10. Stem Cells. 2018;36(7):1020–32.
Qian W, Huang L, Xu Y, Lu W, Wen W, Guo Z, Zhu W, Li Y. Hypoxic ASCs-derived exosomes attenuate colitis by regulating macrophage polarization via miR-216a-5p/HMGB1 axis. Inflamm Bowel Dis. 2023;29(4):602–19.
Zha J, Pan Y, Liu X, Zhu H, Liu Y, Zeng W. Exosomes from hypoxia-pretreated adipose‐derived stem cells attenuate ultraviolet light‐induced skin injury via delivery of circ‐Ash1l. PhotoDermatol PhotoImmunol PhotoMed. 2023;39(2):107–15.
Knight KR. Review of postoperative pharmacological infusions in ischemic skin flaps. Microsurgery; 1994.
Zhao MY, Shi MY, Lin H. Extracellular vesicles from hypoxia-pretreated adipose-derived stem cells regulate hypoxia/reoxygenation-induced human dermal microvascular endothelial apoptosis and autophagy in vitro. Heliyon 2023, 9 (2).
Shen H, Lane RA. Extracellular vesicles from primed adipose-derived stem cells enhance Achilles tendon repair by reducing inflammation and promoting intrinsic healing. Stem Cells. 2023;41(6):617–27.
Li W, Chang N, Tian L, Yang J, Ji X, Xie J, Yang L, Li L. miR-27b-3p, miR-181a-1-3p, and mir-326-5p are involved in the inhibition of macrophage activation in chronic liver injury. J Mol Med. 2017;95:1091–105.
Liu Y, Zhang Z, Wang B, Dong Y, Zhao C, Zhao Y, Zhang L, Liu X, Guo J, Chen Y. Inflammation-stimulated MSC‐Derived Small Extracellular Vesicle miR‐27b‐3p regulates macrophages by targeting CSF‐1 to promote Temporomandibular Joint Condylar Regeneration. Small. 2022;18(16):2107354.
Zheng Y, Xu P, Pan C, Wang Y, Liu Z, Chen Y, Chen C, Fu S, Xue K, Zhou Q. Production and Biological effects of Extracellular vesicles from adipose-derived stem cells were markedly increased by Low-Intensity Ultrasound Stimulation for promoting Diabetic Wound Healing. Stem Cell Reviews Rep. 2023;19(3):784–806.
Khanmohammadi M, Nemati S, Ai J, Khademi F. Multipotency expression of human adipose stem cells in filament-like alginate and gelatin derivative hydrogel fabricated through visible light-initiated crosslinking. Mater Sci Engineering: C. 2019;103:109808.
Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S, Akbariqomi M, Sanikhani NS, Absalan M, Tavoosidana G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J Biomedical Mater Res Part A. 2020;108(3):545–56.
Khalatbary AR, Omraninava M, Nasiry D, Akbari M, Taghiloo S, Poorhassan M, Ebrahimpour-Malekshah R, Asadzadeh M, Raoofi A. Exosomes derived from human adipose mesenchymal stem cells loaded bioengineered three-dimensional amniotic membrane-scaffold-accelerated diabetic wound healing. Arch Dermatol Res. 2023;315(10):2853–70.
Kang Y, Xu C, Dong X, Qi M, Jiang D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioactive Mater. 2022;18:26–41.
Song W, Ma Z, Wang X, Wang Y, Wu D, Wang C, He D, Kong L, Yu W, Li JJ. Macroporous Granular Hydrogels Functionalized with Aligned Architecture and small extracellular vesicles stimulate osteoporotic Tendon-To‐Bone Healing. Adv Sci. 2023;10(34):2304090.
You DG, Lim GT, Kwon S, Um W, Oh BH, Song SH, Lee J, Jo D-G, Cho YW, Park JH. Metabolically engineered stem cell–derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7(23):eabe0083.
Yang C, Xue Y, Duan Y, Mao C, Wan M. Extracellular vesicles and their engineering strategies, delivery systems, and biomedical applications. J Controlled Release. 2024;365:1089–123.
Tavasolian F, Hosseini AZ, Soudi S, Naderi M. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. 2020;20(4):297–312.
Shang Y, Sun Y, Xu J, Ge X, Hu Z, Xiao J, Ning Y, Dong Y, Bai C. Exosomes from mmu_circ_0001359-modified ADSCs attenuate airway remodeling by enhancing FoxO1 signaling-mediated M2-like macrophage activation. Mol Therapy-Nucleic Acids. 2020;19:951–60.
Eirin A, Zhu X-Y, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman L. O. Mesenchymal stem cell–derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–24.
Virla F, Turano E, Scambi I, Schiaffino L, Boido M, Mariotti R. Administration of adipose-derived stem cells extracellular vesicles in a murine model of spinal muscular atrophy: effects of a new potential therapeutic strategy. Stem Cell Res Ther. 2024;15(1):94.
Mou S, Zhou M, Li Y, Wang J, Yuan Q, Xiao P, Sun J, Wang Z. Extracellular vesicles from human adipose-derived stem cells for the improvement of angiogenesis and fat-grafting application. Plast Reconstr Surg. 2019;144(4):869–80.
Bian Z, Wang X, Zhu R, Chen S. Mir-21-5p in extracellular vesicles obtained from adipose tissue-derived stromal cells facilitates tubular epithelial cell repair in acute kidney injury. Cytotherapy. 2023;25(3):310–22.
Acknowledgements
The authors extend their sincere gratitude to Chengcheng Liao and Liang Cheng for their helpful discussions, which greatly enhanced the quality of this manuscript.
Funding
National Natural Science Foundation of China 82100959.
Author information
Authors and Affiliations
Contributions
B.Z., Q.C., and Q.Z. wrote and edited the manuscript. W.T., T.C., and Z.L. designed and revised the manuscript. All authors approved the final version of the work to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, B., Chen, Q., Zhang, Q. et al. Therapeutic potential of adipose-derived stem cell extracellular vesicles: from inflammation regulation to tissue repair. Stem Cell Res Ther 15, 249 (2024). https://doi.org/10.1186/s13287-024-03863-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03863-5