Abstract
Background
Graft failure (GF) is a rare but serious complication after allogeneic hematopoietic stem cell transplantation (HSCT). Prevention of graft failure remains the most advisable approach as there is no clear recommendation for the best strategies for reversing this complication. Administration of growth factor, additional hematopoietic progenitor boost, or a salvage HSCT are current modalities recommended for the treatment of GF. Autologous recovery without evidence of disease relapse occurs rarely in patients with GF, and in the absence of autologous recovery, further salvage transplantation following a second conditioning regimen is a potential treatment option that offers the best chances of long-term disease-free survival. The preconditioning regimens of second HSCT have a significant impact on engraftment and outcome, however, currently there is no consensus on optimal conditioning regimen for second HSCT in patients who have developed GF. Furthermore, a second transplant from a different donor or the same donor is still a matter of debate.
Observations
We present our experience in managing pediatric patients with acute leukemia who encountered graft failure following stem cell transplantation.
Conclusions and relevance
Although a second transplantation is almost the only salvage method, we illustrate that some pediatric patients with acute leukemia who experience graft failure after an allogeneic stem cell transplant using Myeloablative conditioning (MAC) regimen may achieve long-term disease-free survival through autologous hematopoiesis recovery.
Similar content being viewed by others
Introduction
The successful outcome of hematopoietic stem cell transplantation (HSCT) in the treatment of malignant and non-malignant diseases relies on stable donor hematopoietic cell engraftment, which restores functional hematopoiesis and achieves immunological reconstitution. However, failure to establish persistent engraftment after HSCT remains a significant factor contributing to morbidity and mortality. Graft failure (GF) is a rare but significant complication following allogeneic HSCT, with varying incidences depending on the type of donor [1,2,3].
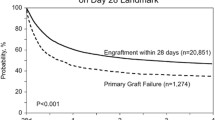
Graft failure is classically divided into primary and secondary failure. Primary graft failure is defined as the absence of initial donor cell engraftment by day + 28 if the graft source is peripheral blood (PB) or bone marrow (BM), and by day + 42 if the graft source is umblical cord blood (UCB). Secondary graft failure is characterized by the loss of donor cells after initial engraftment. Table 1 presents the definitions of hematopoietic recovery, graft rejection and failure, poor graft function, and donor chimerism in allogeneic stem cell transplantation [4,5,6].
GF is relatively uncommon in patients with leukemia who undergo HSCT from a Human leukocyte antigens (HLA) matched related donor. However, it is more commonly observed in patients with non-malignant diseases and those who receive alternative donor stem cell transplants, with incidences ranging from 4% in HLA matched unrelated donors to 20% in transplants from UCB [4, 11,12,13,14,15]. It is more frequent following haploidentical-HSCT, with an incidence of around 10% in T cell-depleted grafts, 13% in the era of post-transplant cyclophosphamide (PTCY), and 1% in Beijing protocol [16]. Promotion and failure of engraftment occure as an interaction between recipient and donor cytotoxic T lymphocytes (CTL), regulatory T cells (Tregs; CD4 + CD25 + Foxp3 + regulatory T cells) and Natural killer (NK) cells. Graft failure occurs as the result of a classical alloreactive immune response driven by residual host immunity persisting following a preparative regimen. The most prominent effector cells that induce GF are thought to be residual host CTL [7]. Conversely, donor cytotoxic T cells promote HSC engraftment. Therefore, a T-cell deficient graft would be associated with a higher prevalence of GF (Fig. 1) [17,18,19].
Recipient or donor Tregs are crucial immunomodulatory cells that provide interactions between immune and hematopoietic cells, and both are important in facilitating engraftment. Donor Tregs promote engraftment by mediating NK cell suppression, and Host Tregs help hematopoietic stem cells to maintain in the bone marrow niche [7, 20, 21]. Recipient Tregs ablation by anti-CD25 monoclonal antibodies (mAbs) has been strongly associated with inhibition of allogeneic rejection, and accordingly, adoptive transfer of host-type Tregs enhances engraftment [21,22,23,24]. NK cells represent an important part of the innate immune system and alloreactive NK cells promote engraftment following HLA-haploidentical HSCT. However, residual recipient NK cells can eliminate donor hematopoietic stem cells through perforin-mediated cytotoxicity, and result in graft rejection [7, 25, 26].
Although preventing graft failure is the most advisable approach, [3] there is no clear consensus on the best strategies to reverse this complication. However, some potential approaches to manage graft failure include the administration of growth factors, waiting for autologous reconstitution (AR), providing an additional hematopoietic progenitor boost, or undergoing a second transplant with a second preparative regimen [27]. Several reports have suggested that a second salvage transplant for graft failure in children can lead to significant transplant-related mortality and seriously compromise overall survival due to prolonged periods of aplasia when the recipient is at a higher risk of infection and hemorrhage [27]. Different factors may affect the outcome of second transplant in pediatric patients.
This review outlines our approach to this complication using our illustrative pediatric patients with acute leukemia who experienced primary and secondary GF. Additionally, it discusses the risk factors for graft failure, various approaches to manage it, salvage transplant procedure including selection of the best conditioning regimens and most appropriate donor, and also waiting for autologous recovery.
Patient 1: T cell acute lymphoblastic leukemia (T Cell ALL) with primary GF (Second transplant from a different donor)
A 9-year-old boy diagnosed with T Cell ALL received HLA-haploidentical donor stem cell transplantation in his second complete remission, from his 40-year-old father with T-cell replete granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood stem cells (PBSC) as the graft source and a cell dose of 6.3 × 106/kg CD34 + cells. The preparative regimen consisted of busulfan (16 doses; 3.8 mg/kg/day) and cyclophosphamide (a total dose of 120 mg/kg). Rabbit anti-human thymocyte globulins (ATG-Thymoglobuline, 2.5 mg/kg/day, from days − 3 to − 1) were added before transplant to prevent rejection. The graft versus host disease (GvHD) prevention included PTCY (a total dose of 80 mg/kg) plus cyclosporine A (Fig. 2). The patient and donor were ABO match and both CMV seropositive. Pre-transplantation donor-specific antibodies (DSA) were negative. On day + 28 post-HSCT, the patient's white blood cell (WBC) and platelet count were 0.1 × 109/L and 15 × 109/L, respectively, with hypoplastic bone marrow in morphologic remission. Donor chimerism by short tandem repeat polymerase chain reaction (STR-PCR) technique was also less than 5%.
Once primary graft failure was diagnosed, cyclosporine A was withdrawn, DSA was reassessed, and salvage HSCT was planned immediately. Performance status according to the Lansky Play-Performance scale was 90%. However, the selection of the optimal donor source and a safe conditioning regimen was of utmost importance due to the occurrence of BK virus-induced hemorrhagic cystitis. He underwent a second transplant 37 days after diagnosis of GF from his haploidentical mother (different donor) with T-cell replete G-CSF mobilized PB hematopoietic stem cells as graft source and a cell dose of 10 × 106/kg CD34 + cells. An immunoablative RIC regimen consisting of fludarabine (40 mg/m2/day × 4 days), melphalan (70 mg/m2/day × 2 days), and ATG (Hoarse; 10 mg/kg/day × 2 days) was used. PTCY (40 mg/kg/day × 2 days) and cyclosporine A administered to prevent acute and chronic GvHD (Fig. 3). Neutrophil and platelet engrafted on days + 11 and + 14 respectively with complete donor chimerism on days + 28 post-salvage transplant. BK viremia and viruria cleared post-engraftment. On the last follow-up, 18 months after re-transplant, bone marrow is in complete morphologic remission, donor chimerism by STR-PCR is 100% and measurable residual disease (MRD) level by multiparametric flow cytometry is negative. However, the patient developed steroid-resistant skin chronic GvHD and responded well to ruxolitinib.
Patient 2: acute myeloid leukemia (AML) with secondary GF (Rescue transplant from different donor)
A 3-year-old boy with high-risk AML in first complete remission received HLA-haploidentical donor allogeneic stem cell transplant from his 37-year-old father with T-cell replete G-CSF mobilized PBSC as graft source and a cell dose of 9 × 106/kg CD34 + cells. The conditioning and GvHD prophylaxis were similar to patient 1 (Fig. 2). The patient and donor were ABO match and both CMV seropositive. Engraftment occurred successfully with a complete donor chimerism. On day + 25 he presented with fever. WBC Count was 0.3 × 109/L accompanied by hypoplastic marrow and loss of full donor chimerism (less than 5%). Blood culture was positive for pseudomonas aeruginosa and DSA was negative. With the diagnosis of secondary graft failure, immunosuppressive drugs tapered off and broad-spectrum antibiotics started. Re-transplant was planned from another parent (his mother) with a RIC regimen (Fig. 3) with a cell dose of 10 × 106/kg CD34 + cells/kg. He achieved neutrophil and platelet engraftment with complete donor chimerism on day + 28 of post-salvage transplant. He experienced steroid-resistant skin chronic GvHD that was resolved by ruxolitinib. On the last follow-up, 3 years after the rescue transplant, BM is in complete morphologic remission with full donor chimerism.
Patient 3: precursor B cell acute lymphoblastic leukemia (pre B Cell ALL) with secondary GF (rescue transplant from the same donor)
A 7-year-old boy with Pre B Cell ALL in the second remission received HLA-haploidentical donor HSCT from his 13-year-old sister with T-cell replete G-CSF mobilized PBSC as graft source and a cell dose of 4.5 × 106/kg CD34 + cells. The conditioning regimen and GvHD prophylaxis were similar to patient 1 (Fig. 2). Myeloid and platelet engraftment occurred on days + 12 and + 19, respectively. Donor chimerism by STR-PCR was 100% on day + 28. On day + 40, he was hospitalized due to fever, vomiting, and malaise. WBC Count was 0.2 × 109/L accompanied by hypoplastic marrow and loss of complete donor chimerism (< 5%). CMV reactivation was detected by plasma sample using real-time PCR with a viral load of more than 2 × 106 million-copy number/ml, and blood culture was positive for pseudomonas aeruginosa, and DSA was negative. As soon as secondary graft failure was diagnosed, immunosuppressive drugs tapered off, broad-spectrum antibiotics and antifungals started, and for CMV reactivation, foscarnet was prescribed. On day + 60 post-transplant, the patient was afebrile with a negative blood culture test, and CMV by real-time PCR was also undetectable. However, the WBC count was still 0.2 × 109/L, and donor chimerism by STR-PCR still reported less than 5%. Second HSCT was planned from the same donor with the RIC regimen as described in patient 1 (Fig. 3) with a cell dose of 8 × 106/kg CD34 + cells/kg. Neutrophil and platelet engraftment occurred on days + 13 and + 17, respectively, with complete donor chimerism on days + 28 post-salvage transplant. Unfortunately, one year after transplant, he experienced bone marrow relapse.
Patient 4: philadelphia chromosome-positive (Ph +) precursor B cell acute lymphoblastic leukemia (pre B Cell ALL) with primary GF (autologous hematopoiesis recovery)
A 6-year-old boy diagnosed with Ph + Pre B Cell ALL in second remission received HLA-matched unrelated donor allogeneic HSCT with bone marrow hematopoietic stem cells as graft source and a cell dose of 3 × 106/kg CD34 + cells. The preparative regimen consisted of busulfan (16 doses; 3.8 mg/kg/day) and cyclophosphamide (a total dose of 120 mg/kg). Rabbit anti-human thymocytes globulins (ATG-Thymoglobuline, 2.5 mg/kg/day, from days − 3 to − 1) were added before transplant to prevent rejection. The GvHD prophylaxis included cyclosporine A and a short course of methotrexate. Pre-transplantation MRD level by Real Time Polymerase chain reaction (RT-PCR) for BCR-ABL1/ABL1 was undetectable, and DSA was negative. On day + 28 post-HSCT, WBC and platelet count were 0.1 × 109/L, and 10 × 109/L respectively, with hypoplastic bone marrow and donor chimerism by STR-PCR of less than 5%. Once the diagnosis of primary graft failure was established, cyclosporine A tapered off and as additional stem cells were unavailable, a conservative approach including growth factor was adopted while awaiting hematological recovery. He developed autologous hematological reconstitution 57 days after the transplant. Prophylactic Imatinib started, and MRD was assessed by RT-PCR (BCR-ABL1/ABL1) every three months.
Three years after the transplant, he feels well, and his bone marrow is in complete morphologic and molecular remission with a donor chimerism by STR-PCR of less than 5%.
Patient 5: precursor B cell acute lymphoblastic leukemia (pre B Cell ALL) with primary GF (autologous hematopoiesis recovery)
An 8-year-old girl diagnosed with Pre B Cell ALL with BM relapse underwent HLA-haploidentical donor HSCT from his 35-year-old father with T-cell replete G-CSF mobilized with a cell dose of 9 × 106/kg CD34 + cells.
Pre-transplantation DSA was negative. The patient and donor were ABO minor mismatch and both CMV seropositive. CMV reactivation was detected by plasma sample using real-time PCR on day + 4 and cleared by foscarnet on day + 17. On day + 28 post-transplant, WBC and platelet count were 0.1 × 109/L and 10 × 109/L respectively, with hypoplastic bone marrow and absence of donor chimerism.
Once the diagnosis of primary graft failure was confirmed, cyclosporine A was tapered off. Our preference in the same situations is a rescue second transplant with a RIC as soon as possible. However, her parents refused to accept a second transplant, and a conservative approach was adopted. She developed autologous hematological reconstitution 45 days after the transplant. MRD was assessed by multiparameter flow cytometry every three months and on the last follow-up, two years after GF, she is well, in complete morphologic remission with undetectable MRD, with and donor chimerism by STR-PCR of less than 5%.
Discussion
With the increasing number of patients eligible for allogeneic HSCT, only 25–30% of patients have an HLA-identical sibling donor, and finding a suitable HLA-compatible unrelated volunteer donor is possible for less than 70% of the remaining patients [28, 29]. In the absence of an HLA-matched donor, alternative donors of hematopoietic stem cells (HSCs), such as unrelated UCB and HLA-haploidentical relatives, are being increasingly used [30, 31]. This means that more patients may experience graft failure.
Between August 2015 to june 2022, five pediatric patients with acute leukemia (4 with ALL and 1 with AML) experienced graft failure (3 primary and 2 secondary) after allogeneic hematopoietic stem cell transplantation (HSCT) in our center. All patients had received haploidentical stem cell transplants, except for one who had been transplanted from a matched unrelated donor (MUD). Table 4 displays the characteristics of our patients who developed graft failure. Recently donor-specific antibodies (DSA) against nonshared, either major or minor donor histocompatibility antigens have been found to predict primary GF (2- to tenfold increase) in HLA haploidentical mismatched family transplants, especially in multiply transfused patients [3]. HLA disparity between the donor and recipient in haploidentical transplantation can contribute to bidirectional alloreactivity, both in graft-versus-host and host-versus-graft directions, which increases the risk of developing Primary GF [32, 33].
Although PTCY overcomes T cell- and NK cell-mediated graft rejection, antibody-mediated rejection by DSAs appears to be one of the principal mechanisms of primary GF [33]. DSAs target donor HLA antigens present on the surface of hematopoietic progenitor cells. Consequently, antigen–antibody complexes bind to C1q and activate the complement cascade, resulting in the formation of a membrane attack complex that causes donor cells lysis that leads to allograft rejection [34].
Risk factors of graft failure
Conditions associated with increased occurrence of graft failure include defects in the bone marrow microenvironment, immunological disturbances or imbalances between donor and recipient (HLA disparity, alloimmunization with anti-HLA antibodies, ABO mismatching in the donor/recipient pairs, etc.), low infused hematopoietic stem cell dose, T-cell depleted (TCD) grafts, reduced-intensity conditioning regimens (RIC), drug toxicity (myelosuppressives such as ganciclovir) and infections, especially of viral origin, such as those caused by cytomegalovirus (CMV) [16, 35,36,37].
The risk of Primary GF after haploidentical HSCT has been reported from 1% with myeloablative conditioning to 8% with non-myeloablative preparative regimens, from both BM or PB as stem cell source [38]. Moreover, in hematological malignancies, GF occurs more frequently in patients with a high-risk disease due to intensive or prolonged chemo/radiotherapy before transplant because of damage to the bone marrow microenvironment [3]. Pre-transplant transfusion-induced alloimmunization may also affect donor engraftment [39]. Table 2 illustrates conditions associated with an increased risk of graft failure.
Donor-specific anti-HLA antibodies
Donor-directed anti-human leukocyte antigen (HLA)- specific alloantibodies (DSAs) are preformed IgG antibodies against the unshared HLA molecules with the donor [43]. The strong association between DSA and graft failure after mismatched unrelated donors, cord blood, and haploidentical transplantation has been demonstrated [32, 34, 44,45,46]. Patients may form DSA as a consequence of exposure to foreign cells or a tissue, including pregnancy, previous blood product transfusion, and history of organ or blood transplantation [40]. Although DSAs against HLA class I (HLA-A and HLA-B) and class II (HLA-DRB1) antigens have an unfavorable effect on engraftment, the role of anti-HLA Abs against HLADPB1 and HLA-DQB1 is still unclear [41].
Due to high HLA disparities, the prevalence of DSA in recipients of haploidentical HSCT is higher than in matched unrelated donors, mismatched unrelated donors (mMUD), and UCB transplants [33]. Females with multiple pregnancies have a higher mean fluorescent intensity (MFI) value of DSA (86%), compared to male recipients (5%), as a consequence of alloimmunization after pregnancies against offspring antigens [47]. Several studies have shown that higher MFI values of DSA that represent the “strength” of the antibodies have been associated with an increased rate of graft failure. Although there is not a clear cut-off consensus above which the DSA is likely to cause graft failure [48, 49], in a study by Nordlander A et al., 75% of patients with MFI > 1500 before haploidentical HSCT experienced GF compared to 5% of patients without DSA [50]. Hence, frequent monitoring of DSA levels is necessary, as it is used to determine the need for pre-or post-transplant desensitization and as a decision point to consider an alternative donor against whom the patient has no DSAs, including other haploidentical related donors, UCB, and or 9/10 matched unrelated donor [40, 47, 49]. If post-transplant GF is due to DSA, second transplantation from the same donor would be at risk of engraftment failure. Therefore for retransplant in this setting, a different donor should be considered [32].
Treatment of graft failure
The management of graft failure can vary depending on center preference and experience. If autologous recovery is not possible, salvage HSCT following a second conditioning regimen is often considered the best option for achieving long-term disease-free survival (DFS) [51]. This approach aims to shorten the period of bone marrow aplasia and reduce the associated risks of infection and hemorrhage. The outcome of salvage HSCT is dependent on the comorbidities that the patient has experienced from the first HSCT [52].
The graft failure rate after salvage transplant is still high, and stable engraftment has been reported as low as 33% in the literature [51, 53]. Nevertheless, the survival rate of patients with GF after allogeneic HSCT without a second salvage transplantation is dismal, at only < 10% [54].
The preconditioning regimens of salvage transplant have a significant impact on engraftment and outcome [50]. However, there is currently no consensus on the optimal conditioning regimen for a second HSCT in patients who have developed GF. Most transplant centers prefer a non-myeloablative regimen that maintains sufficient immunosuppressive effects to eradicate residual host cells to promote engraftment and lessen excessive toxicity, given that patients are very fragile early after the first transplantation. On the other hand, myeloablative conditioning seems unnecessary as bone marrow is already hypocellular [8, 55,56,57].
Although different donor sources have been used for rescue HSCT after GF [58], transplantation from an immediately available donor is the optimal therapeutic option. Shortening the delay in donor procurement is of particular importance.
Several centers prefer G-CSF-mobilized PBSCs to bone marrow-derived stem cells as graft sources for salvage HSCT due to their higher engraftment rate. PBSCs have advantages such as a larger number of stem cells and higher T-cell content, which can lead to improve graft-versus-tumor effects. However, PBSC transplantation is also associated with an increased risk of GvHD [53, 59].
UCB is another important stem cell source for immediate HSCT, as it is readily available [50]. Waki et al. [60] evaluated 80 adult patients who received UCB transplants within 3 months of GF. In multivariate analysis, conditioning with fludarabine plus alkylating agents and the infusion of cord blood containing ≥ 2.5 × 107 /kg cells were associated with a higher probability of engraftment. However, transplantation-related mortality on day 100 was 45%, with 60% related to infectious complications, demonstrating the need for the earlier application of cord blood before patients complicated by infection or organ toxicity.
Second HSCT
There is no clear recommendation for the best approach to primary or secondary graft failure. No single drug or strategy has been proven to be superior to others for reversing graft failure, and current approaches to limit the detrimental impact of this complication are primarily based on its prevention. The rescue strategies are limited, and the most common approaches include recombinant growth factors (if it has not been already started as a scheduled treatment protocol), reinfusion of autologous frozen backup progenitors (if available, depending on center policy), waiting for autologous hematopoiesis recovery and salvage HSCT [61].
Survival after a second transplant has been reported to be between 10 and 30% in retrospective studies, mostly due to the poor performance of patients with GF [62]. Several key factors may contribute to successful second transplantation including a safe conditioning regimen, a short interval between GF and re-transplantation, selection of the optimal donor source, and also patient's performance status. Patients with uncontrolled active infection or GvHD, significant organ dysfunction, and poor performance are excluded from salvage transplant [32, 63].
Regarding the optimal conditioning regimen for salvage transplant, several reports have shown favorable outcomes following fludarabine-based reduced-intensity conditioning regimens [64]. A short-term reduced-intensity conditioning regimen, known as a 'one-day regimen’ including alemtuzumab developed to enhance immunosuppression by T-cell depletion. However, alemtuzumab has been associated with an increased risk of infections. Excluding alemtuzumab and a combination of fludarabine and low–dose total body irradiation (TBI) defined as a 'modified one-day conditioning regimen’ has been successful in achieving stable neutrophil engraftment [57, 65].
Fludarabine in combination with ATG as a non-myeloablative regimen and a higher number of CD34 + hematopoietic stem cells has been also associated with consistent hematopoietic reconstitution in patients with GF [51]. The immunoablative reconditioning regimens with fludarabine-based protocols and the use of a different haploidentical donor have been represented as a realistic option to rescue pediatric patients with GF [66]. Furthermore, the incorporation of alkylating agents in a preparative regimen for the second transplant has been related to survival [50]. So, a combination of alkylating agents with fludarabine may contribute to a better outcome.
We have also found that using fludarabine and melphalan as a RIC preparative regimen for a second transplant is appropriate when retransplantation is considered soon after the occurrence of graft failure.
Most of these patients lack a well-matched related or unrelated donor readily available and searching for unrelated volunteer donors from the registry bank is not practical due to the urgent need for preparation of the donor [7, 61]. In recent years, haploidentical HSCT outcomes have improved due to advances in HLA typing, GvHD prophylaxis with PTCY, wide availability of multiple donors, and also supportive care. Haploidentical progenitors are considered a valid alternative for patients who lack a suitable source of progenitors (same donor, backup progenitors, another compatible donor) for a second transplant [66, 67]. On the other hand, some studies have shown that longer intervals between graft failure and rescue transplant can be associated with better survival, probably due to enough time for recovery from first transplant-related toxic complications [67]. Guardiola et al. [68] reported that an inter-transplant time interval of more than 80 days (relative risk: 0.38, 95% confidence interval: 0.19 ± 0.76, P = 0.01) was associated with significantly improved outcomes in patients with primary or secondary graft failure.
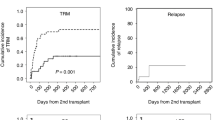
Three of our patients underwent salvage hematopoietic stem cell transplantation after an interval from the first HSCT of 37, 52, and 78 days. None of them experienced significant transplant-related complications, and in all of them, hematological recovery occurred successfully. Table 3 displays several selected studies using the second transplant in patients with graft failure.
Giammarco et al. [71] reported 19 patients with primary graft failure after haploidentical HSCT who received a second transplant. There was no statistically significant difference in the hematopoietic reconstitution rate between the patients who received a graft from the same donor (77%) and patients transplanted from another haploidentical family donor (66%) (P = 0.5). In an observational study using data from the Center for International Blood and Marrow Transplant Research (CIBMTR) database on unrelated donor transplants, 122 patients with graft failure underwent a second transplant of whom 98 patients grafted from the same donor and 24 from a different donor. One-year overall survival after the second transplant was dismal (11%), and the long-term outcome was not different between patients who transplanted from the same or different donors [74].
In a series by Grandage et al. [75] 12 pediatric patients (< 18 years) who received ex vivo T cell-depleted marrow from unrelated donors suffered graft failure (five primary, seven secondary), of whom seven patients received a second transplant from a different unrelated donor. However, the source did not affect the outcome of the second HSCT (Table 4).
In a retrospective analysis by Kato et al. [50] patients who received salvage transplants from a different donor achieved engraftment, whereas the engraftment rate of HSCT from the same donor was 42.1 ± 11.8% (P = 0.02). Nevertheless, the estimated Overall Survival (OS) probability of the two groups did not reach statistical significance (P; 0.70).
Kongtim P et al. analyzed outcomes of patients with primary and secondary GF who received an unmanipulated haploidentical HSCT as salvage treatment and reported that using the same haploidentical donor was associated with poor OS with a hazard ratio (HR) of 2.90 (95% CI 1.07–7.92, P = 0.037) mainly due to increase in early nonrelapse mortality (NRM) [53].
In another retrospective study by Chewning et al. [51] the outcome of 16 consecutive patients who received a second HSCT following GF of initial HSCT was analyzed. Five of 10 patients transplanted from different donors survived compared with only 1 of 6 patients receiving stem cells from the same donor. Although the outcome was more favorable in the first group, this difference was not significant (P = 0.1).
Remarkably, for patients with suspected T-cell rejection as the cause of GF after haploidentical HSCT, using a different haploidentical donor with a different mismatched haplotype for the salvage transplant has been associated with a higher engraftment rate [51, 66].
Second transplant from the same or different donor
A second transplant from a different donor or the same donor is still a matter of debate [27]. While changing to a different donor may contribute to successful engraftment, there are few studies about engraftment or survival outcomes of second HSCT based on donor choice with controversial results. In the setting of graft failure after HLA-matched HSCT, it is more common to use the same previous donor for a second transplant, because another well-matched donor is rarely available. However, the engraftment failure rate has been high when using the same donor [53]. Furthermore, the risk of re-collection from the initial donor in a short period of time should be considered [32].
Autologous recovery
The recovery of host-hematopoiesis without evidence of disease relapse, known as autologous reconstitution (AR), is a rare event in patients with GF [59]. In a retrospective analysis of 1205 consecutive patients with severe aplastic anemia (SAA), conducted by the Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT–WPSAA), the cumulative incidence of AR was 4.2% (3.1–5.6) with an OS of 84% [76].
Rondon et al. [77] reported nine patients of 1726 allogeneic HSCT recipients who experienced autologous reconstitution after primary GF; seven following RIC regimens and two after myeloablative conditioning (MAC) regimen. Interestingly, patients with primary graft failure and AR had longer median survival compared to those who received a retransplant.
It is worth noting that all of our patients received a MAC regimen during their first allogeneic hematopoietic stem cell transplantation. However, two of them experienced autologous recovery.
In another retrospective cohort of 1,630 patients who underwent allogeneic HSCT for a malignant disease or severe AA, reported by Park et al. [59] primary and secondary GF occurred in 13 and 69 patients respectively. AR was observed in 11.6% (n = 8) of patients with an incidence of 0.49% of the overall study population and 11.6% among patients with secondary GF. The median time to onset of AR was 6.95 months (range, 2.3–16.7) after diagnosis of secondary GF. However, management with mobilized donor lymphocyte infusion (DLI) or rescue allogeneic HSCT was associated with a higher recovery rate compared to conservative care.
Conclusion
Graft failure is one of the most important barriers to a successful transplant that can occur early after UCB, haploidentical, and HLA-mismatched donor transplants, as well as following nonmyeloablative or RIC regimens [40, 60, 62]. According to the clinician's clinical judgment, various therapeutic approaches may be considered after graft failure [61].
Although a second transplantation is almost the only salvage method, we illustrate that some pediatric patients with acute leukemia who experience graft failure after an allogeneic stem cell transplant using a MAC regimen may achieve long-term disease-free survival through autologous hematopoiesis recovery.
Considering the second transplant, it has been shown that non-myeloablative conditioning regimens for allogeneic hematopoietic cell transplantation have led to improved outcomes over the years, with reduced morbidity and mortality from infections, organ toxicity, and graft-versus-host disease [78].
Additionally, changing to a different donor has been identified as an important factor for successful engraftment in cases of graft failure, with a higher engraftment success rate observed when using a different donor [79]
Therefore, the use of a non-myeloablative conditioning regimen and a different donor for second transplants should be carefully considered based on the individual patient's condition and prospectively assessed for its potential benefits.
Overall, we acknowledge that further experiments are needed to strengthen the decision-making process in pediatric patients with acute leukemia who experience graft failure after HSCT.
Availability of data and materials
All supporting data and materials are included in the article and additional files.
Abbreviations
- GF:
-
Graft failure
- GvHD:
-
Graft-versus-host disease
- HSCT:
-
Hematopoietic stem cell transplantation
- MAC:
-
Myeloablative conditioning
- UCB:
-
Umbilical cord blood
- BM:
-
Bone marrow
- TCD:
-
T-cell depleted
- RIC:
-
Reduced-intensity conditioning
- CMV:
-
Cytomegalovirus
- HSCs:
-
Hematopoietic stem cells
- PB:
-
Peripheral blood
- CTL:
-
Cytotoxic T lymphocytes
- DSA:
-
Donor-specific antibodies
- PTCY:
-
Post-transplant cyclophosphamide
- NK:
-
Natural killer
- G-CSF:
-
Granulocyte colony-stimulating factor
- PBSC:
-
Peripheral blood stem cells
- WBC:
-
White blood cell
- STR:
-
Short tandem repeat
- STR-PCR:
-
Short tandem repeat-polymerase chain reaction
- DFS:
-
Disease-free survival
- TBI:
-
Total body irradiation
- AML:
-
Acute myeloid leukemia
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- NRM:
-
Nonrelapse mortality
- Pre B Cell ALL:
-
Precursor B cell acute lymphoblastic leukemia
- HLA:
-
Anti-human leukocyte antigen
- MUD:
-
Matched unrelated donors
- MMUD:
-
Mismatched unrelated donors
- MRD:
-
Measurable residual disease
- MRD:
-
Matched related donors
- AR:
-
Autologous reconstitution
- SAA:
-
Severe aplastic anemia
- DLI:
-
Donor lymphocyte infusion
References
Albert MH, Sirin M, Hoenig M, Hauck F, Schuetz C, Bhattacharyya R, Stepensky P, Jacoby E, Güngör T, Beier R, Schulz A. Salvage HLA-haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for graft failure in non-malignant disorders. Bone Marrow Transplant. 2021;56(9):2248–58.
Petinati N, Drize N, Sats N, Risinskaya N, Sudarikov A, Drokov M, Dubniak D, Kraizman A, Nareyko M, Popova N, Firsova M. Recovery of donor hematopoiesis after graft failure and second hematopoietic stem cell transplantation with intraosseous administration of mesenchymal stromal cells. Stem Cells Int. 2018;10:2018.
Locatelli F, Lucarelli B, Merli P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opin Pharmacother. 2014;15(1):23–36.
Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48:537–43.
Valcarcel D, Sureda A. Graft failure. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook. EBMT; 2019, pp. 307–13.
Olsson RF, Logan BR. Chaudhury S, Zhu X, Akpek G, Bolwell BJ, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015;29(8):1754–62.
Masouridi-Levrat S, Simonetta F, Chalandon Y. Immunological basis of bone marrow failure after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;16(7):362.
Gyurkocza B, Cao TM, Storb R, et al. Salvage allogeneic hematopoietic cell transplantation with fludarabine and low-dose total body irradiation after rejection of first allografts. Biol Blood Marrow Transp. 2009;15:1314–22.
Kharfan-Dabaja MA, Kumar A, Ayala E, Aljurf M, Nishihori T, Marsh R, Burroughs LM, Majhail N, Al-Homsi AS, Al-Kadhimi ZS, Bar M. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: a report on behalf of the American society for transplantation and cellular therapy. Transp Cell Ther. 2021;27(8):642–9.
Carreras E, Dufour C, Mohty M, Kröger N (2019) The EBMT Handbook. Hematopoietic stem cell transplantation and cellular therapies. Cham, Springer
Cluzeau T, Lambert J, Raus N, et al. Risk factors and outcome of graft failure after HLA matched and mismatched unrelated donor hematopoietic stem cell transplantation: a study on behalf of SFGM-TC and SFHI. Bone Marrow Transplant. 2016;51:687–91.
Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989;73:606–13.
McCann SR, Bacigalupo A, Gluckman E, Hinterberger W, Hows J, Ljungman P, et al. Graft rejection and second bone marrow transplants for acquired aplastic anaemia: a report from the aplastic anaemia working party of the European bone marrow transplant group. Bone Marrow Transplant. 1994;13:233–7.
Kongtim P, Cao K, Ciurea SO. Donor specific anti-HLA antibody and risk of graft failure in haploidentical stem cell transplantation. Adv Hematol. 2016;2016:4025073.
Booth C, Veys P. T cell depletion in paediatric stem cell transplantation. Clin Exp Immunol. 2013;172:139–47.
Gyger M, Baron C, Forest L, et al. Quantitative assessment of hematopoietic chimerism after allogeneic bone marrow transplantation has predictive value for the occurrence of irreversible graft failure and graft-vs.-host disease. Exp Hematol. 1998;26(5):426–34.
Lapidot T, et al. Enhancement of T-cell-depleted bone marrow allografts in the absence of graft-versus-host disease is mediated by CD8++ CD4- and not by CD8+- CD4++ thymocytes. Blood. 1992;80(9):2406–11.
Martin PJ. Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med. 1993;178(2):703–12.
Gandy KL, et al. CD8+TCR+ and CD8+TCR- cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11(5):570–9.
Michael M, Shimoni A, Nagler A. Regulatory T cells in allogeneic stem cell transplantation. Clin Dev Immunol. 2013;2013: 608951.
Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103(14):5460–5. https://doi.org/10.1073/pnas.0509249103.
Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14(1):88–92. https://doi.org/10.1038/nm1688.
Pilat N, Klaus C, Gattringer M, Jaeckel E, Wrba F, Golshayan D, et al. Therapeutic efficacy of polyclonal tregs does not require rapamycin in a low- dose irradiation bone marrow transplantation model. Transplantation. 2011;92(3):280–8. https://doi.org/10.1097/TP.0b013e3182241133.
Müller AM, Poyser J, Küpper NJ, Burnett C, Ko RM, Kohrt HE, et al. Donor hematopoiesis in mice following total lymphoid irradiation requires host T-regulatory cells for durable engraftment. Blood. 2014;123(18):2882–92. https://doi.org/10.1182/blood-2013-10-530212.
Gill S, Olson JA, Negrin RS. Natural killer cells in allogeneic transplantation: effect on engraftment, graft-versus-tumor, and graft-versus-host responses. Biol Blood Marrow Transplant. 2009;15(7):765–76.
Palmer JM, Rajasekaran K, Thakar MS, Malarkannan S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J Cancer. 2013;4(1):25.
Ferrà C, Sanz J, Díaz-Pérez MA, Morgades M, Gayoso J, Cabrera JR, Villaescusa T, Sampol MA, Fernández-Avilés F, Solano C, Ortín M. Outcome of graft failure after allogeneic stem cell transplant: study of 89 patients. Leuk Lymphoma. 2015;56(3):656–62.
Rocha V, Locatelli F. Searching for alternative hematopoietic stem cell donors for pediatric patients. Bone Marrow Transplant. 2008;41(2):207–14.
Locatelli F, Bauquet A, Palumbo G, Moretta F, Bertaina A. Negative depletion of α/β+ T cells and of CD19+ B lymphocytes: a novel frontier to optimize the effect of innate immunity in HLA-mismatched hematopoietic stem cell transplantation. Immunol Lett. 2013;155(1–2):21–3.
Rocha V, Gluckman E. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1):34–41.
Locatelli F, Pende D, Maccario R, Mingari MC, Moretta A, Moretta L. Haploidentical hemopoietic stem cell transplantation for the treatment of high-risk leukemias: how NK cells make the difference. Clin Immunol. 2009;133(2):171–8.
Sun YQ, Wang Y, Wang FR, Yan CH, Cheng YF, Chen YH, Zhang YY, Han TT, Han W, Suo P, Xu LP. Graft failure in patients with hematological malignancies: a successful salvage with a second transplantation from a different haploidentical donor. Front Med. 2021:721.
Bramanti S, Calafiore V, Longhi E, Mariotti J, Crespiatico L, Sarina B, De Philippis C, Nocco A, Santoro A, Castagna L. Donor-specific anti-HLA antibodies in haploidentical stem cell transplantation with post-transplantation cyclophosphamide: risk of graft failure, poor graft function, and impact on outcomes. Biol Blood Marrow Transplant. 2019;25(7):1395–406.
Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, López AA, Yap DY, Popat U, Rondon G, Lichtiger B. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(8):1392–8.
Locatelli F. Improving cord blood transplantation in children. Br J Haematol. 2009;147(2):217–26.
Rocha V, Gluckman E. Eurocord‐Netcord registry and European Blood and Marrow Transplant group. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft‐and transplantation‐related factors. British J Haematol. 2009;147(2):262–74.
Wolff SN. Second hematopoietic stem cell transplantation for the treatment of graftfailure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant. 2002;29:545–52.
McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolaños- Meade J, Rosner GL, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–31.
Ruggeri A. Optimizing cord blood selection. Hematology. 2019;2019(1):522–31.
Gladstone DE, Bettinotti MP. HLA donor-specific antibodies in allogeneic hematopoietic stem cell transplantation: challenges and opportunities. Hematology. 2017;2017(1):645–50.
Brand A, Doxiadis IN, Roelen DL. On the role of HLA antibodies in hematopoietic stem cell transplantation. Tissue Antigens. 2013;81(1):1–11.
Forman SJ, Negrin RS, Antin JH, Appelbaum FR, editors. Thomas' hematopoietic cell transplantation: stem cell transplantation. Wiley. 2015.
Bailén R, Vicario JL, Solán L, Sánchez-Vadillo I, Herrera P, Calbacho M, Alenda R, López-Lorenzo JL, Humala K, Chinea A, Sánchez-Pina J. Management of donor-specific antibodies in haploidentical transplant: Multicenter experience from the Madrid group of hematopoietic transplant. Front Immunol. 2021;19(12): 674658.
Ciurea SO, de Lima M, Cano Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–27.
Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transpl. 2012;47:508–15.
Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;10:84.
Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. The European society for blood and marrow transplantation (Ebmt) consensus guidelines for the detection and treatment of donor-specific anti-hla antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:521–34. https://doi.org/10.1038/s41409-017-0062-8.
Morin-Zorman S, Loiseau P, Taupin JL, Caillat-Zucman S. Donor-specific anti-HLA antibodies in allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;12(7):307.
Sullivan HC, Gebel HM, Bray RA. Understanding solid-phase HLA antibody assays and the value of MFI. Hum Immunol. 2017;78(7–8):471–80.
Kato M, Matsumoto K, Suzuki R, Yabe H, Inoue M, Kigasawa H, Inagaki J, Koh K, Hashii Y, Tauchi H, Suminoe A. Salvage allogeneic hematopoietic SCT for primary graft failure in children. Bone Marrow Transplant. 2013;48(9):1173–8.
Chewning JH, Castro-Malaspina H, Jakubowski A, Kernan NA, Papadopoulos EB, Small TN, Heller G, Hsu KC, Perales MA, Van den Brink MR, Young JW. Fludarabine-based conditioning secures engraftment of second hematopoietic stem cell allografts (HSCT) in the treatment of initial graft failure. Biol Blood Marrow Transplant. 2007;13(11):1313–23.
Lund TC, Ahn KW, Tecca HR, Hilgers MV, Abdel-Azim H, Abraham A, Diaz MA, Badawy SM, Broglie L, Brown V, Dvorak CC. Outcomes after second hematopoietic cell transplantation in children and young adults with relapsed acute leukemia. Biol Blood Marrow Transplant. 2019;25(2):301–6.
Kongtim P, Bittencourt M, Srour SA, Ramdial J, Rondon G, Chen J, Khouri I, Betul O, Popat U, Olson A, Bashir Q. Haploidentical transplants for patients with graft failure after the first allograft. Am J Hematol. 2020;95(10):E267–9.
Fuji S, Nakamura F, Hatanaka K, Taniguchi S, Sato M, Mori SI, Sakamaki H, Yabe H, Miyamoto T, Kanamori H, Ueda Y. Peripheral blood as a preferable source of stem cells for salvage transplantation in patients with graft failure after cord blood transplantation: a retrospective analysis of the registry data of the Japanese society for hematopoietic cell transplantation. Biol Blood Marrow Transp. 2012;18(9):1407–14.
Byrne BJ, Horwitz M, Long GD, et al. Outcomes of a second non-myeloablative allogeneic stem cell transplantation following graft rejection. Bone Marrow Transp. 2008;41:39–43.
Heinzelmann F, Lang PJ, Ottinger H, et al. Immunosuppressive total lymphoid irradiation-based reconditioning regimens enable engraftment after graft rejection or graft failure in patients treated with allogeneic hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2008;70:523–8.
Sumi M, Shimizu I, Sato K, et al. Graft-failure in cord blood transplantation successfully treated with short-term reduced-intensity conditioning regimen and second allogeneic transplantation. Int J Hematol. 2010;92:744–50.
Kliman D, Bilmon I, Kwan J, Blyth E, Micklethwaite K, Panicker S, Bhattacharyya A, Deren S, Antonenas V, Huang G, Gottlieb D. Rescue haploidentical peripheral blood stem cell transplantation for engraftment failure: a single-centre case series. Intern Med J. 2018;48(8):988–91.
Park JH, Lee JH, Lee JH, Park HS, Choi EJ, Kang YA, Kang H, Woo JM, Lee YS, Jeon M, Lee KH (2021) Incidence, management, and prognosis of graft failure and autologous reconstitution after allogeneic hematopoietic stem cell transplantation. J Korean Med Sci. 36(23)
Waki F, Masuoka K, Fukuda T, Kanda Y, Nakamae M, Yakushijin K, Togami K, Nishiwaki K, Ueda Y, Kawano F, Kasai M. Feasibility of reduced-intensity cord blood transplantation as salvage therapy for graft failure: results of a nationwide survey of adult patients. Biol Blood Marrow Transp. 2011;17(6):841–51.
Singh H, Nikiforow S, Li S, Ballen KK, Spitzer TR, Soiffer R, Antin JH, Cutler C, Chen YB. Outcomes and management strategies for graft failure after umbilical cord blood transplantation. Am J Hematol. 2014;89(12):1097–101.
Ruggeri A, Labopin M, Angelucci E, Blaise D, Ciceri F, Koc Y, Chiusolo P, Diez-Martin JL, Gülbas Z, Castagna L, Bruno B. Prognostic factors for neutrophil engraftment after haploidentical cell transplantation with PT-Cy in patients with acute myeloid leukemia in complete remission, on behalf of the ALWP-EBMT. Bone Marrow Transp. 2021;56(8):1842–9.
Ozdemir ZN, Bozdağ SC. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apheres Sci. 2018;57(2):163–7.
Suma S, Yokoyama Y, Momose H, Makishima K, Kiyoki Y, Sakamoto T, Kusakabe M, Kato T, Kurita N, Nishikii H, Sakata-Yanagimoto M. Salvage cord blood transplantation using a short-term reduced-intensity conditioning regimen for graft failure. Intern Med. 2022;61(11):1673–9.
Goggins TF, Rizzeri DA, Prosnitz R, et al. One day preparative regimen for allogeneic non-myeloablative stem cell transplantation (NMSCT) using 3–5/6 HLA matched related donors. Blood. 2003;102:476b–7b.
Lang P, Mueller I, Greil J, Bader P, Schumm M, Pfeiffer M, Hoelle W, Klingebiel T, Heinzelmann F, Belka C, Schlegel PG. Retransplantation with stem cells from mismatched related donors after graft rejection in pediatric patients. Blood Cells Mol Dis. 2008;40(1):33–9.
Prata PH, Resche-Rigon M, Blaise D, Socié G, Rohrlich PS, Milpied N, Turlure P, Nguyen S, Sirvent A, Bulabois CE, Berceanu A. Outcomes of salvage haploidentical transplant with post-transplant cyclophosphamide for rescuing graft failure patients: a report on behalf of the francophone society of bone marrow transplantation and cellular therapy. Biol Blood Marrow Transp. 2019;25(9):1798–802.
Guardiola P, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukaemia, chronic myeloid leukaemia and aplastic anaemia. French Soc Bone Marrow Transp Br J Haematol. 2000;111:292–302.
Nagler A, Labopin M, Kulagin AD, Velardi A, Sanz J, Labussière-Wallet H, Potter V, Kuball J, Sica S, Parovichnikova EN, Bethge W. Long-term outcome of second allogeneic stem cell transplantation (HSCT2) for primary graft failure in patients with acute leukemia in remission: a study on behalf of the acute leukemia working party of the European society for blood and marrow transplantation. Blood. 2022;140(Supplement 1):7578–9.
Subburaj D, Li AM, Rozmus J, Schultz KR. Successful rescue transplant for children with primary graft failure using early intervention with a single day preparative regimen and related haploidentical donor. Bone Marrow Transplant. 2021;56(8):2031–3.
Giammarco S, Raiola AM, Di Grazia C, Bregante S, Gualandi F, Varaldo R, Chiusolo P, Sora F, Sica S, Laurenti L, Metafuni E. Second haploidentical stem cell transplantation for primary graft failure. Bone Marrow Transp. 2021;56(6):1291–6.
Harada K, Fuji S, Seo S, Kanda J, Ueki T, Kimura F, Kato K, Uchida N, Ikegame K, Onizuka M, Matsuoka KI. Comparison of the outcomes after haploidentical and cord blood salvage transplantations for graft failure following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55(9):1784–95.
Mochizuki K, Sano H, Akaihata M, Kobayashi S, Waragai T, Ohara Y, Takahashi N, Ito M, Ikeda K, Ohto H, Kikuta A. T cell replete–haploidentical second hematopoietic stem cell transplantation for primary graft failure in pediatric patients with hematologic malignancies. Pediatr Transplant. 2017;21(7): e13043.
Schriber J, Agovi MA, Ho V, Ballen KK, Bacigalupo A, Lazarus HM, Bredeson CN, Gupta V, Maziarz RT, Hale GA, Litzow MR. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol Blood Marrow Transp. 2010;16(8):1099–106.
Grandage VL, Cornish JM, Pamphilon DH, Potter MN, Steward CG, Oakhill A, Marks DI. Second allogeneic bone marrow transplants from unrelated donors for graft failure following initial unrelated donor bone marrow transplantation. Bone Marrow Transp. 1998;21(7):687–90.
Piccin A, McCann S, Socie G, Oneto R, Bacigalupo A, Locasciulli A, Marsh J, Schrezenmeier H, Tichelli A, Hand E, Lawler M. Survival of patients with documented autologous recovery after SCT for severe aplastic anemia: a study by the WPSAA of the EBMT. Bone Marrow Transp. 2010;45(6):1008–13.
Rondón G, Saliba RM, Khouri I, Giralt S, Chan K, Jabbour E, McMannis J, Champlin R, Shpall E (2008) Long-term follow-up of patients who experienced graft failure postallogeneic progenitor cell transplantation. Results of a single institution analysis. Biol Blood Marrow Transp. 14(8):859–866
Cooper JP, Storer BE, Granot N, Gyurkocza B, Sorror ML, Chauncey TR, Shizuru J, Franke GN, Maris MB, Boyer M, Bruno B. Allogeneic hematopoietic cell transplantation with non-myeloablative conditioning for patients with hematologic malignancies: improved outcomes over two decades. Haematologica. 2021;106(6):1599.
Sun YQ, Wang Y, Wang FR, Yan CH, Cheng YF, Chen YH, Zhang YY, Han TT, Han W, Suo P, Xu LP. Graft failure in patients with hematological malignancies: a successful salvage with a second transplantation from a different haploidentical donor. Front Med. 2021;4(8): 604085.
Acknowledgements
Not applicable.
Funding
The authors would like to express their gratitude to the Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT) at Tehran University of Medical Sciences (TUMS), Tehran, Iran, for providing financial support through a small grant (TUMS, Grant No. 1400-2-107-54776, issued on 10.30.2021).
Author information
Authors and Affiliations
Contributions
TR and AK contributed to the design and conceived of the program, directed the project, reviewed the studies, wrote and edited the manuscript. HA assessed the studies, extracted the data, wrote, edited, and critically revised the manuscript. MRR, AHM, NK, and SR assessed the studies, extracted the data, and wrote the part of the manuscript. AKa wrote the part of the manuscript and directed the project. MN and SM contributed to this work. SAM, GJ, and AK critically revised the manuscript. All authors read and approved the final manuscript and accept accountability for all aspects of this work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This project is part of a larger initiative titled “Unmanipulated Peripheral Blood Stem Cell Transplantation with non-TBI Myeloablative Conditioning Regimen from Haploidentical and Unrelated versus Related Donors for Acute Leukemia in Children, Adolescents and Young Adults (CAYA): A Competing Risk Analysis” aimed at advancing research and development in the field of oncology, hematology, and cell therapy. The ethical committee of Hematology, Oncology and Stem Cell Transplantation, Tehran University of Medical Sciences has approved and issued this study under the code IR.TUMS.HORCSCT.REC.1400.032 on 12.23.2021. The authors are committed to protecting the data of all patients and donors involved in this study, and no individual data will be reported.
Consent for publication
Consent for publication was given by the parents of the patients.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rostami, T., Rostami, M.R., Mirhosseini, A.H. et al. Graft failure after allogeneic hematopoietic stem cell transplantation in pediatric patients with acute leukemia: autologous reconstitution or second transplant?. Stem Cell Res Ther 15, 111 (2024). https://doi.org/10.1186/s13287-024-03726-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03726-z